Abstract
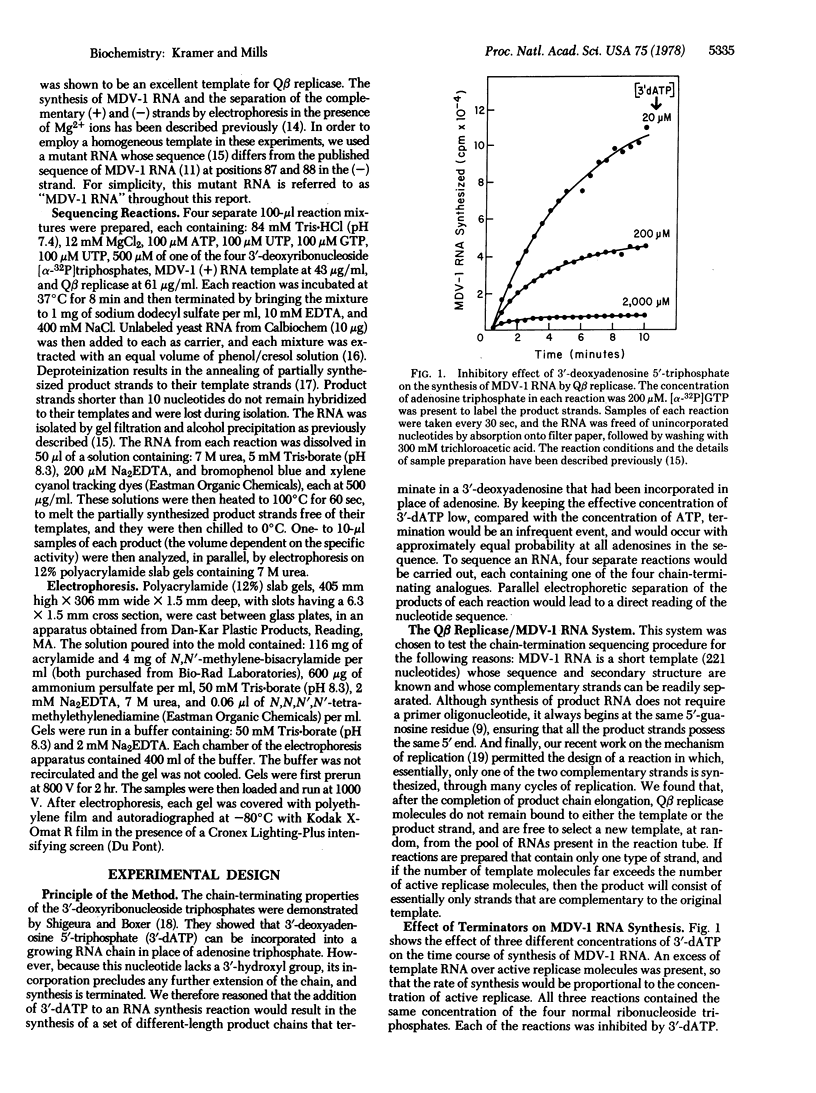
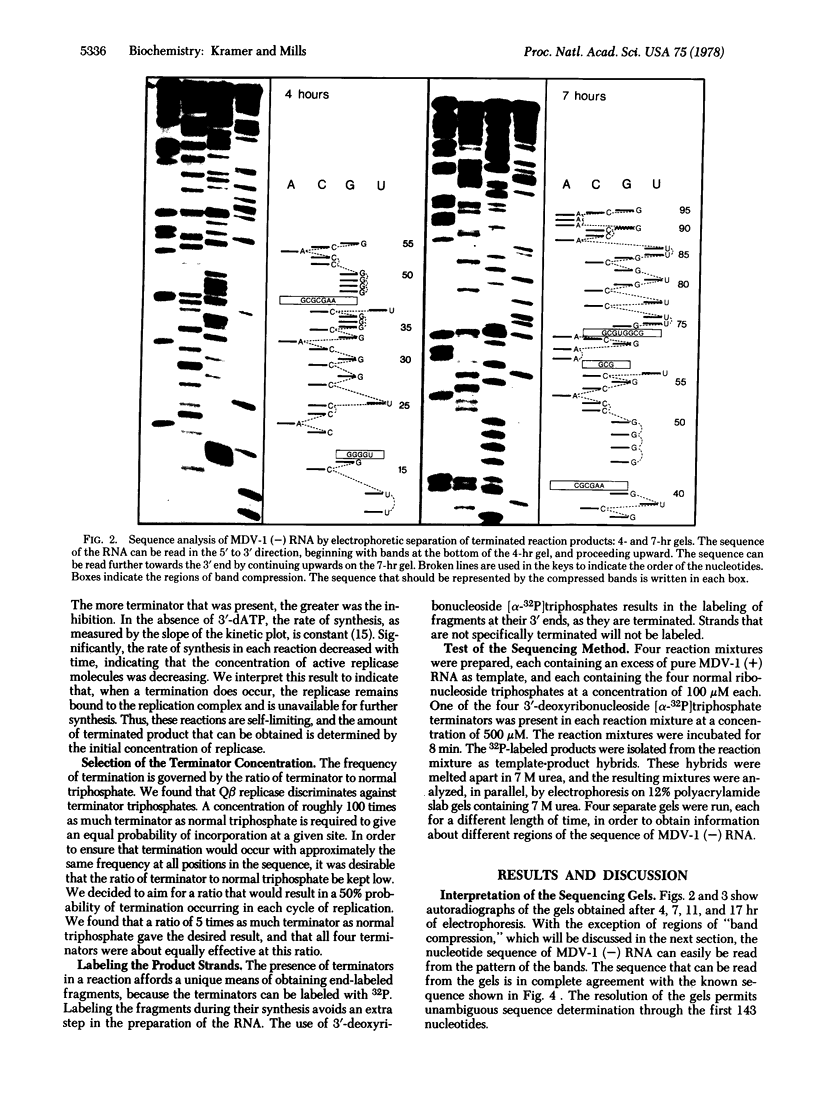
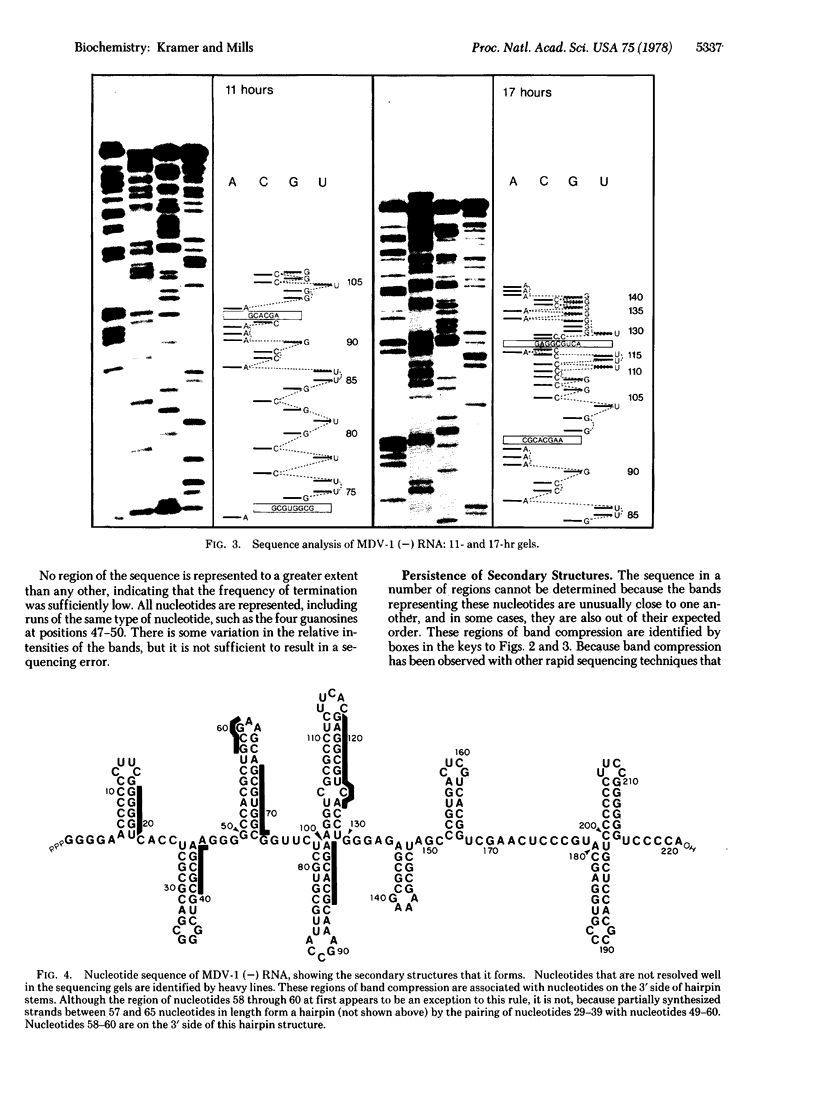
A rapid method for determining nucleotide sequences in RNA is described. It employs the 3'-deoxy analogues of the ribonucleoside triphosphates as specific chain terminators during RNA synthesis. For example, the inclusion of 3'-deoxyuridine 5'-triphosphate in an RNA synthesis reaction in addition to the four usual ribonucleoside triphosphate precursors results in the synthesis of a set of different-length product strands that terminate in a 3'-deoxyuridine that has been incorporated in place of uridine. To sequence an RNA, four separate reactions are run, each employing a different 3'-deoxy terminator. Parallel electrophoretic analysis of the resulting four sets of specifically terminated product chains leads to a direct reading of the nucleotide sequence. We tested this method by sequencing MDV-1 (-) RNA, a molecule that is synthesized in vitro by phage Qbeta replicase. The sequence read from the resulting gels agreed completely with the known sequence of MDV-1 (-) RNA. The bands in some regions of the sequencing gels were unusually close to one another, as has also been observed in other rapid sequencing procedures, making order assignment in these regions very difficult. Because the secondary structure of MDV-1 (-) RNA was known, it was shown that the compression of the bands is due to the persistence of secondary structures during electrophoresis. Thus, structured regions of nucleic acids may introduce difficulties for sequencing techniques that employ the currently available methods of gel electrophoresis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. DNA sequencing by partial ribosubstitution. J Mol Biol. 1978 Feb 15;119(1):83–99. doi: 10.1016/0022-2836(78)90271-1. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feix G., Slor H., Weissmann C. Replication of viral RNA. 13. The early product of phage RNA synthesis in vitro. Proc Natl Acad Sci U S A. 1967 May;57(5):1401–1408. doi: 10.1073/pnas.57.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruna I., Spiegelman S. Specific template requirments of RNA replicases. Proc Natl Acad Sci U S A. 1965 Aug;54(2):579–587. doi: 10.1073/pnas.54.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Mills D. R., Kramer F. R., Spiegelman S. A replicating RNA molecule suitable for a detailed analysis of extracellular evolution and replication. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3038–3042. doi: 10.1073/pnas.69.10.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer F. R., Mills D. R., Cole P. E., Nishihara T., Spiegelman S. Evolution in vitro: sequence and phenotype of a mutant RNA resistant to ethidium bromide. J Mol Biol. 1974 Nov 15;89(4):719–736. doi: 10.1016/0022-2836(74)90047-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Dobkin C., Kramer F. R. Template-determined, variable rate of RNA chain elongation. Cell. 1978 Oct;15(2):541–550. doi: 10.1016/0092-8674(78)90022-3. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R., Spiegelman S. Complete nucleotide sequence of a replicating RNA molecule. Science. 1973 Jun 1;180(4089):916–927. doi: 10.1126/science.180.4089.916. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Donelson J. E., Coulson A. R., Kössel H., Fischer D. Use of DNA polymerase I primed by a synthetic oligonucleotide to determine a nucleotide sequence in phage fl DNA. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1209–1213. doi: 10.1073/pnas.70.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Van Kreijl C. F., Beelen R. H., Borst P. On the mechanism of oligonucleotide-primed RNA synthesis. II. Synthesis of specific primer-initiated RNA copies suitable for DNA sequence analysis. Nucleic Acids Res. 1977 Feb;4(2):445–455. doi: 10.1093/nar/4.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]




