Abstract
Ten percent of all strokes occurring in the USA are caused by intracranial arterial stenosis (IAS). Symptomatic IAS carries one of the highest rates of recurrent stroke despite intensive medical therapy (25 % in high-risk groups). Clinical results for endovascular angioplasty and stenting have been disappointing. The objectives of this study were to review the contemporary understanding of symptomatic IAS and present potential alternative treatments to resolve factors not addressed by current therapies. We performed a literature review on IAS pathophysiology, natural history, and current treatment. We present an evaluation of the currently deficient aspects in its treatment and explore the role of alternative surgical approaches. There is a well-documented interrelation between hemodynamic and embolic factors in cerebral ischemia caused by IAS. Despite the effectiveness of medical therapy, hemodynamic factors are not addressed satisfactorily by medications alone. Collateral circulation and severity of stenosis are the strongest predictors of risk for stroke and death. Indirect revascularization techniques, such as encephaloduroarteriosynangiosis, offer an alternative treatment to enhance collateral circulation while minimizing risk of hemorrhage associated with hyperemia and endovascular manipulation, with promising results in preliminary studies on chronic cerebrovascular occlusive disease. Despite improvements in medical management for IAS, relevant aspects of its pathophysiology are not resolved by medical treatment alone, such as poor collateral circulation. Surgical indirect revascularization can improve collateral circulation and play a role in the treatment of this condition. Further formal evaluation of indirect revascularization for IAS is a logical and worthy step in the development of intracranial atherosclerosis treatment strategies.
Keywords: Atherosclerosis, Encephaloduroarteriosynangiosis, Indirect revascularization, Intracranial arterial stenosis, Ischemia, Stroke
Introduction
Of the 900,000 cases of stroke or transient ischemic attack (TIA) that occur each year in the USA, approximately 90,000 to 100,000 are caused by intracranial arterial stenosis [44]. Symptomatic intracranial arterial stenosis carries one of the highest rates of recurrent stroke despite medical therapy, with annual recurrence rates for ischemic stroke reported in the Stenting Versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial as high as 12.2 % in the intensive medical therapy arm, treated with double antiplatelet medications (aspirin and clopidogrel), intensive management of risk factors for vascular disease, in particular high blood pressure and hyperlipidemia, and inclusion in a life-coaching program [11]. The incidence of recurrent stroke can be even higher in some high-risk groups, up to 25 % in African-Americans and females [55].
Despite improvements in the technical success of endovascular angioplasty and stenting, the clinical results of stenting have been disappointing. In fact, SAMMPRIS was stopped early because of negative results, with stroke or death within the first 30 days of enrolment occurring in 14 % of patients treated with angioplasty and stenting compared to 5.8 % of patients treated with medical therapy alone [11].
The high rates of stroke and death associated with intensive medical management and the failure of recent attempts with endovascular interventions demand novel strategies for the treatment of patients with this condition. In this paper, we review the current knowledge of intracranial atherosclerosis pathophysiology and the role of medical and endovascular treatments. We also explore the theoretical arguments in support of using alternative surgical techniques such as indirect revascularization by encephaloduroarteriosynangiosis (EDAS) as a potential approach to symptomatic intracranial stenosis.
Epidemiology
Approximately 100,000 patients every year suffer from ischemic events related to intracranial atherosclerotic disease in the USA, with an estimated prevalence of 20 to 40 persons per 100,000 people worldwide [40]. Limited data on the prevalence of asymptomatic intracranial atherosclerosis are available in the general population. In asymptomatic predominantly white US patients referred for carotid Doppler ultrasound, intracranial atherosclerotic stenosis was identified by transcranial Doppler ultrasound in 13 % of individuals [18]. Baker and colleagues [8] evaluated 5,035 intracranial arteries during autopsy at 22 predefined anatomic areas and identified severe intracranial atherosclerosis in 43 % of individuals age 60 to 69, 65 % on those age 70 to 79, and 80 % on those older than 80 years.
The prevalence of this disorder in asymptomatic patients has great relevance from the preventive perspective; however, the major clinical burden is produced by symptomatic intracranial arterial stenosis, which carries one of the highest rates of recurrent stroke despite medical therapy, with annual recurrence rates for ischemic stroke reported in the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial as high as 15 % in the aspirin arm [12] and in the intensive medical treatment arm of SAMMPRIS as high as 12.2 % [11]. There are racial, ethnic, and gender variances in this disease, most severely affecting certain minorities. Intracranial arterial stenosis is responsible for up to 10 % of ischemic strokes in Whites, 29 % of ischemic strokes in Blacks, 11 % of ischemic strokes in Hispanics, and 26 % of ischemic strokes in Asians [55]. The risk of stroke and death with intracranial arterial stenosis is disproportionally elevated in women, and it has been reported to be close to 29 % [54].
Pathophysiology of intracranial atherosclerosis
Intracranial atherosclerosis is a disease of the arterial wall for which the etiology and pathophysiology have not been completely elucidated. Traditionally, the process has been considered an age-related, degenerative fibroproliferative disorder of the arterial wall that would eventually induce ischemia of the tissues irrigated by those vessels [47]. However, today, intracranial atherosclerosis can be considered more as a multifactorial process in which risk factors for endothelial injury and lipid deposition in the arterial wall work in concert with inflammation from the genesis to the development and dynamic variations in the stability of the arterial atherosclerotic plaque (Fig. 1).
Fig. 1.
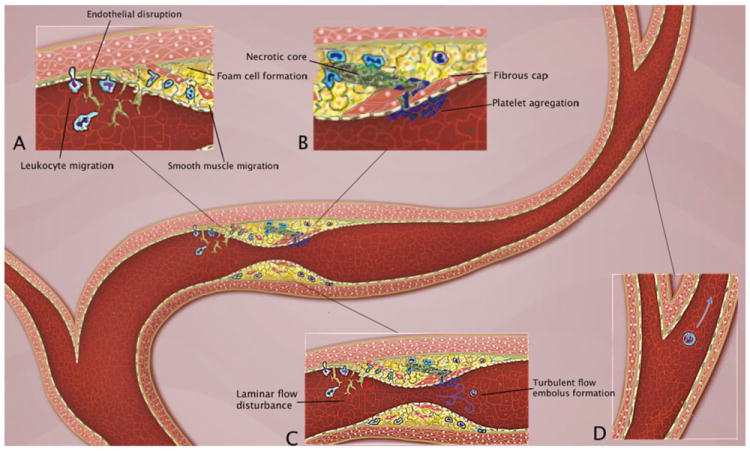
Pathophysiology of intracranial atherosclerosis. A The endothelial injury and increased permeability induced by factors such as hypertension and smoking generates adhesion of monocytes and lymphocytes to the disrupted vessel lining, followed by migration beneath the endothelial surface and cellular aggregation in the subendothelial tissue. Macrophages take up lipid, forming foam cells, and these and other macrophages are activated and cytokines and growth factors are released, leading to smooth muscle cell migration and proliferation. B There is formation of a fibrous cap that covers a mixture of leukocytes, lipid, and necrotic tissue. Eventually the fibrous cap ruptures and ulcerates generating platelet aggregation and embolus formation. C The early endothelial injury generates disturbances of the laminar flow, which creates flow separation and oscillatory shear stress. This precipitates a cycle of further endothelial and arterial wall damage. Stenosis of the vessel lumen, in concert with embolus generation, produces strokes in a complementary occurrence of hypoperfusion and embolism (D)
More than 20 years ago, Reaven introduced the concept that a series of related factors such as hypercholesterolemia, hyperinsulinemia, hypertension, and hypertriglyceridemia tended to co-exist in the same patients and that this risk factor clustering could be of critical importance in the underlying etiology of cardiovascular disease [41]. This clustering of factors, also known as metabolic syndrome, has been found consistently to be an important risk factor for cardiovascular disease incidence and mortality [21]. Lipoprotein(a), which is an atherogenic lipoprotein rich in cholesterol, similar to the low-density lipoprotein, has been found to promote cholesterol depositions in the arterial wall and to inhibit endogenous endothelial fibrinolysis [48]. Diabetes mellitus type 2 has been independently associated with higher rates of intracranial atherosclerosis in Caucasian individuals, and it appears to have a synergetic action with lipoprotein(a) in increasing the development of intracranial atherosclerosis in patients with both risk factors [3].
Although abdominal obesity is a well-recognized risk factor for cardiovascular disease, the actual mechanisms of its role in the genesis of atherosclerosis are not well known. The discovery of the secretory role of adipose tissue and of adipokines has opened the exploration of new mechanistic hypotheses. It appears that out of the known adipokines, leptin could be related to stroke risk, while the data on adiponectin and resistin remain conflicting [46].
Hypertension and smoking induce direct damage in the vessels by producing endothelial dysfunction. This process generates disturbances of the laminar flow, which creates flow separation and oscillatory shear stress [32]. In turn, the flow abnormalities precipitate further endothelial and arterial wall damage in a cycle that, in concert with the risk factors mentioned above, results in the inception and progression of atherosclerotic lesions [20]. In this dynamic process, from the genesis of the first arterial wall abnormalities to the development and progression of the atheromatous plaque, inflammation is believed to play a crucial role.
In addition to the hemodynamic phenomena described above, the endothelial injury generates a series of immunologically mediated events. According to the inflammatory theory of atherosclerosis, the endothelial injury generates adhesion of monocytes and lymphocytes to the endothelial surface, migration of those cells beneath the endothelial surface, and cellular aggregation in the subendothelial tissue. Macrophages then take up lipid, forming foam cells, and these and other macrophages are activated and cytokines and growth factors are released. This leads to smooth muscle cell proliferation and fibrous plaque formation [43]. In support of this mechanism, several inflammatory biomarkers, and particularly high sensitivity C-reactive protein, have been identified as predictors of the risk of recurrent stroke in individuals with intracranial atherosclerosis [5]. The role of these markers is, however, less clear in predicting a first stroke in individuals with asymptomatic intracranial atherosclerotic stenosis [53].
Pathophysiology of ischemic stroke in intracranial arterial stenosis
The pathophysiology of ischemic stroke secondary to intracranial arterial stenosis involves multiple mechanisms. Intracranial arterial stenosis may incite downstream ischemia in a specific arterial territory due to hypoperfusion, in situ thrombosis, artery-to-artery emboli, perforator vessel occlusion by the atherosclerotic plaque, or combined mechanisms [9]. There is an interrelated and complementary occurrence of hypoperfusion and embolism, in which reduced blood perfusion distal to high-grade stenosis limits the ability of emboli from the stenosis to be washed out of the cerebral circulation and therefore may accumulate in the lowest perfusion pressure regions. Investigations using MRI and transcranial Doppler studies have corroborated this hypothesis and have suggested additional mechanisms in which stenosis and embolism act synergistically to produce ischemia [29, 49]. Kasner et al. demonstrated that the strongest predictive factor of subsequent stroke in a territory of a symptomatic intracranial artery was severe stenosis ≥70 % (HR 2.03; 95 % CI 1.29 to 3.22; p=0.0025), which underlines the pathophysiologic significance of the degree of stenosis [25]. While aggressive medical therapy can have a significant impact in reducing the risk of embolic stroke, in cases of severe intracranial arterial stenosis with hypoperfusion of the involved territory, there is a persistent risk of ischemia [57]. Hemodynamic impairment is a risk factor for stroke in patients with intracranial occlusive disease, just as in those with extracranial carotid artery occlusive disease [2]. Amin-Hanjani et al. measured quantitative flow in the basilar artery and its branches in 50 patients with symptomatic vertebrobasilar disease. Forty-seven of the 50 patients were followed for a mean of 28 months. None of the 31 patients with normal distal flow had a recurrent event. Several of the 16 patients with low flow suffered recurrent strokes prior to intervention [2].
Angiogenesis and intracranial arterial stenosis
Angiogenesis is a complex process triggered by hypoxia that consists of creation of new vessels from preexisting vascular structures [34]. Angiogenesis involves a delicate balance between pro-angiogenic and anti-angiogenic factors that may play important roles at different stages of cerebral ischemia and in the mechanisms of both atherosclerotic plaque instability and collateral circulation formation [42].
In a study of 109 stroke subjects treated with intravenous standard thrombolytic therapy, Navarro-Sobrino et al. showed that a pro-angiogenic predominance in plasma at admission, before any intervention, was associated with milder short-term neurological deficits while an acute anti-angiogenic status, primarily determined by a high endostatin level, predicted a worse long-term functional outcome. The admission hepatocyte growth factor (HGF) level and the ratio of HGF/endostatin were inversely associated with NIHSS score at 2 and 12 h after treatment (p=0.02, R=−0.4 and p=0.04, R=−0.4, respectively). Other ratios of pro-/anti-angiogenic factors, such as keratinocyte growth factor (KGF)/endostatin (p=0.04, R=−0.4) or KGF/thrombospondin-1, were also higher in patients with mild neurological deficits (p=0.001, R=−0.7; p=0.04, R=−0.4; and p=0.01, R=−0.5 at 1, 2, and 12 h, respectively). In addition, vascular endothelial growth factor (VEGF) and VEGF/endostatin ratio 1 h after tPA treatment were strongly associated with a lower NIHSS score from 2 h to discharge (p<0.05 at all time points). This association was also found for platelet-derived growth factor-BB/angiostatin at 1 h (p<0.05) [38].
The actual mechanisms by which this pro-angiogenic status may induce beneficial effects in the outcome of patients with stroke are not clear. Two theories have been postulated: (1) the elevation of angiogenic factors induced by hypoxia facilitates the generation of collateral channels to improve tissue perfusion in the areas of the penumbra [6], or (2) the new vessels formed facilitate the migration of inflammatory cells, such as granulocytes and macrophages, that can more efficiently “clean-up” the necrotic tissue [33].
The role of angiogenesis in the intracranial plaque destabilization is less clear. This effect that has been demonstrated in the extracranial vessels [10] usually occurs in the setting of large atherosclerotic plaques that may exceed the limit at which they require additional sources of perfusion, which would be rare in the relatively small size of intracranial arteries and atherosclerotic lesions [4].
Collateral circulation and intracranial arterial stenosis
The cerebral collateral circulation can be defined as the supplementary network of vascular channels that stabilize cerebral blood flow when the principal conduits fail [30]. The arterial anatomy of the collateral circulation is quite extensive and encompasses both intracranial and extracranial sources of auxiliary vascular channels. Cases of moyamoya disease clearly demonstrate the resourcefulness and capacity of the collateral network in a chronic arterial steno-occlusive process, recruiting not only readily available primary collaterals from the structural configuration of the circle of Willis, but secondary intracranial paths, such as those provided by lenticulo-striate vessels and thalamoperforating arteries, and extensive extracranial connections from dural and ethmoidal vessels (Fig. 2).
Fig. 2.
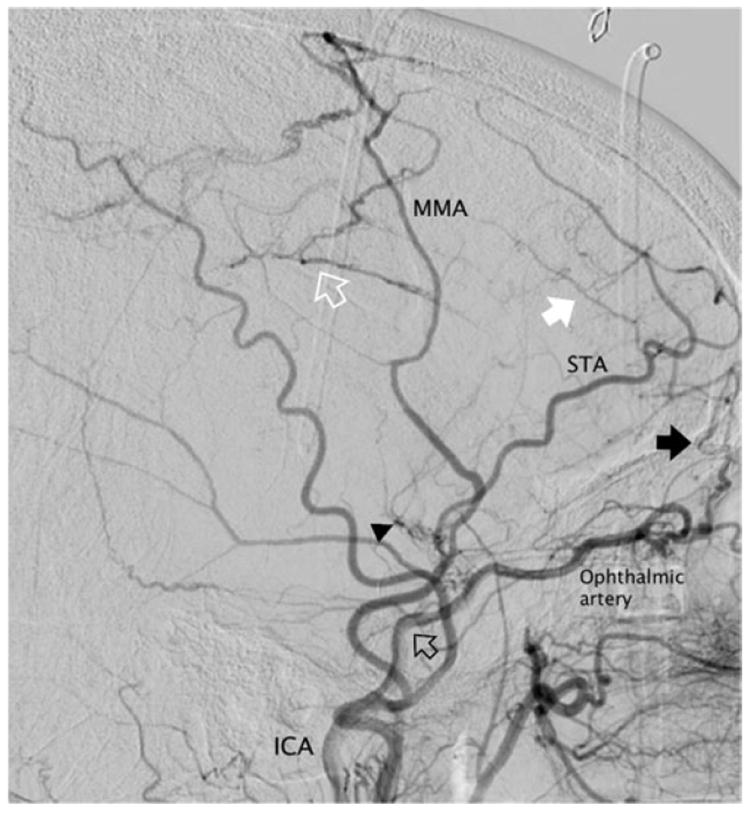
Collaterals in moyamoya disease. Lateral angiogram after common carotid injection in a 46-year-old female with advanced moyamoya disease, Suzuki stage 6. The internal carotid artery (ICA) is completely occluded above the origin of the ophthalmic artery (empty black arrow). Moyamoya lenticulo-striate vessels are regressing (arrowhead) and the cerebral circulation depends exclusively on collaterals. There are spontaneous transosseous anastomoses from the anterior branch of the superficial temporal artery (STA) to frontal cortical vessels (white arrow), anterior meningeal branches (black arrow) from the ophthalmic artery, and the middle meningeal artery (MMA) to the pericallosal and callosomarginal arteries in the parietal region (empty white arrow)
In the setting of chronic ischemia, preexisting collateral vessels are recruited and, according to the demand of flow, enlarged [15, 36]. However, new vessels sprouting from available and in-proximity arterial sources can also be formed to build the supplementary network, as is clearly observed from the meningeal collaterals to the brain parenchyma in patients with moyamoya [39] and postoperatively following indirect revascularization [28, 37]. In other words, new collaterals can develop by angiogenic processes to form new pathways for circulation.
The magnitude of collateral flow available for the territory irrigated by a compromised artery is crucial. In a recent analysis of the role of collaterals in modifying stroke risk in the WASID population, Liebeskind et al. demonstrated that when the degree of arterial luminal stenosis was severe (≥70 %), collaterals had a dramatic role in averting stroke. The absence of collaterals or presence of only poor collaterals increased the risk of stroke in the compromised vascular territory sixfold (HR no collaterals or poor versus good, 6.05; 95 % CI 1.41–25.92; log rank p=0.0056) [31].
Current treatment of symptomatic intracranial arterial stenosis
The results of two recent clinical trials exploring interventions for the management of cerebrovascular disease in the setting of carotid occlusion with bypass surgery (Carotid Occlusion Surgery Study—COSS) and intracranial stenosis with angioplasty and stenting (SAMMPRIS) have shown improvement in the efficacy of aggressive medical treatment. The estimated rate of recurrent stroke in patients with severe intracranial arterial stenosis (≥70 %) based on the WASID trial was 24.7 % [13]; however, in the medical arm of the two recently halted COSS and SAMMPRIS trials, the rates of stroke and death were 21 % at 2 years in COSS and 12.2 % at 1 year in SAMMPRIS. Patients in the medical arm of SAMMPRIS were managed with aggressive medical treatment including antiplatelet medications (aspirin 325 mg and clopidogrel 75 mg daily) and intensive management of vascular risk factors (blood pressure control to systolic below 140 or 130 mm Hg if diabetic, and LDL<70 mg/dL). As supported by other studies, aggressive medical management appears to reduce the risk of embolisms [56]; however, medical management alone may not address the progression of intracranial arterial stenosis and the pathophysiologic components of hypoperfusion and poor collateral circulation [14, 16, 19, 31].
Since the first report of angioplasty for the treatment of intracranial stenosis in 1980 [52], several advances in stent technology and endovascular navigation led to a growing interest in using angioplasty and stenting for the management of these lesions [22, 23, 26]. Percutaneous transluminal angioplasty and stenting (PTAS) was tested versus best medical therapy in the SAMMPRIS trial [11]. This study aimed to compare the safety and efficacy of intensive medical therapy plus stenting with intensive medical therapy only in preventing stroke. After only 59 % (451 of 764) of the planned patients had been enrolled, the study was stopped early because 14 % of patients treated with angioplasty and stenting experienced stroke or died within the first 30 days after enrolment, compared to 5.8 % of patients treated with medical therapy alone. This included five stroke-related deaths within 30 days of enrolment, all in the stent arm. The results of this study indicate that PTAS has a high risk of peri-procedural complications. The rate of cerebral hemorrhage within 30 days in the PTAS group was quite high. Ten out of the 46 patients that had events within 30 days had intracerebral hemorrhages, four of which were fatal. Intracerebral hemorrhage alone represented more than 20 % of the adverse events occurring within 30 days after angioplasty, and it was responsible for 80 % of the fatalities (four out of five) in the PTAS group. These hemorrhages were subarachnoid hemorrhages due to injury of the intracranial vessels and intraparenchymal bleeding due to the sudden hyperperfusion produced after reopening of severely stenotic arteries [11]. Clearly, any future therapeutic approach to the treatment of symptomatic intracranial arterial stenosis should have lower peri-procedural risks than those reported for PTAS and reduce the chances of intracerebral hemorrhage.
The surgical options for intracranial arterial stenosis have been limited. In particular, direct revascularization techniques with bypass surgery have not been demonstrated to improve clinical outcomes compared to the best medical management in terms of subsequent risk for stroke [7]. The extracranial–intracranial (EC-IC) trial found that patients with intracranial arterial stenosis, rather than complete occlusion, can actually fare worse following bypass [7, 24]. Direct bypass induces an abrupt increase in flow that consequently may produce hyperperfusion and hemorrhage. In addition to this effect, the competitive flow introduced by the bypass on the stenotic arterial segment can precipitate stasis of flow and thrombosis of adjacent perforators and the stenotic segment itself (Fig. 3). The more recent COSS trial aimed to determine if EC-IC bypass could reduce subsequent ischemic stroke compared to medical management alone in patients with arterial occlusion. This study did not include individuals with symptomatic stenosis. The final COSS results have not been published yet, but during the “Late Breaking Abstract” presentation in the International Stroke Meeting in Los Angeles on February 10, 2011, the COSS investigators showed that despite good patency of grafts and improvement in PET oxygen extraction fraction in the bypass patients, the peri-operative stroke rate (within 30 days) was 15 %, not significantly different than the earlier EC-IC bypass trial. The 2-year stroke and death rate in the surgical arm was not significantly better than that in the nonsurgical arm (21 versus 23 %, respectively, p=0.7).
Fig. 3.
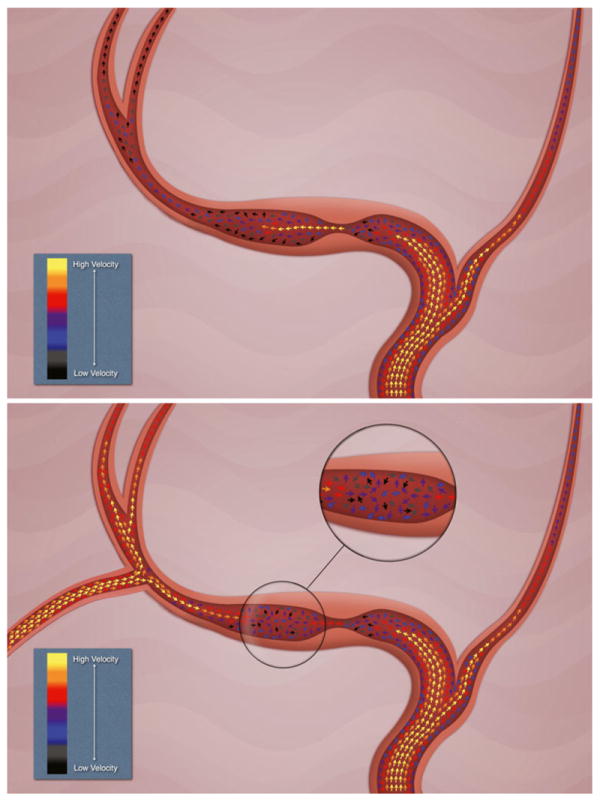
Flow changes associated with EC-IC bypass. A qualitative depiction of flow patterns in intracranial stenosis with reduction of flow velocities associated with arterial narrowing before the stenosis and acceleration of flow in the stenotic segment. Distal flow can reach critical low velocities (blue and black arrows). After bypass placement, a theoretical competitive flow introduced by the bypass on the post-stenotic arterial segment can precipitate increased turbulence, stasis of flow and thrombosis of adjacent perforators, and the stenotic segment itself
Alternative surgical perspectives for the treatment of intracranial arterial stenosis
Indirect revascularization techniques, such as EDAS, have been used for the treatment of moyamoya disease, especially in children [1, 35, 45, 50]. The EDAS procedure aims to bring scalp (superficial temporal artery) and dural (middle meningeal artery branches) arteries into close approximation with the arteries on the brain surface, encouraging new blood vessel growth to supply the brain from the extracranial carotid artery circulation. It is considered a form of indirect revascularization because a direct bypass anastomosis is not created. Instead, revascularization occurs where the brain needs it by endogenous angiogenesis from the extracranial arteries, creating new blood vessel supply to the brain or even spontaneous anastamoses to the intracranial circulation in some cases. The details of the EDAS technique, as practiced at our institution, have been described in detail previously in the literature [17].
The EDAS technique offers several advantages: (1) it obviates the need for temporary occlusion of cerebral vessels, which is inherent to bypass; (2) it avoids hyperperfusion since a direct anastomosis with immediate increase in blood flow is not established; (3) it prevents the development of competing areas of flow in or close to the stenosis, which could generate focal thrombosis and embolisms; (4) it provides a source of extracranial collaterals that develops as needed; and (5) it is technically less demanding than bypass surgery. The use of EDAS in adults was controversial in the past, chiefly because some authors doubted the ability of mature blood vessels to exhibit robust neovascularity [51]. In a recently published study, we have demonstrated that in adults with moyamoya disease, EDAS offers robust revascularization with a quantifiable increase in collateral circulation that develops gradually, avoiding the potential problems of abrupt flow restoration of direct bypass and stenting [17]. In that study, we showed that 97 % (32 of 33) of adults (≥18 years of age) and 89 % (eight of nine) of pediatric patients had a good response to EDAS without new ischemic symptoms 1 month postoperatively. Additionally, a comparison of adult and pediatric patients presenting with ischemic symptoms and at least 1 year of follow-up showed that 91 % (10 of 11) of adults and 80 % (four of five) of children had a good response to indirect revascularization. The oldest patients had a good response to treatment (patients ≥50 years of age remained symptom-free at the last follow-up). Using the Matsushima grading scale for revascularization, grades A, B, and C occurred in 62.5, 37.5, and 0 % of children and in 50, 40, and 10 % in adults, respectively, which were not statistically different.
The use of indirect revascularization for patients with non-moyamoya intracranial arterial stenosis has been only tested in a very limited fashion. Komotar et al. studied 12 symptomatic patients, 11 with intracranial occlusion and 1 with stenosis, including two with carotid dissections and several with severe bilateral disease [27]. Ten out of the 12 patients developed strokes. However, it is impossible to make conclusive assessments in this series, as occlusion most likely represents a completely different disease state for which the gradual formation of collaterals is not effective. Other aspects of their patient selection make their results impossible to generalize; for example, the presence of bilateral severe disease blurred any potential effect of the revascularization as 5 out of 10 strokes occurred in the hemisphere contralateral to the one being treated. Finally, there was no uniform application of intensive medical management along with the surgical procedure, which today is recognized to clearly provide protection against embolic events.
In a pilot study of EDAS revascularization for non-moyamoya intracranial stenosis, we have demonstrated a clinical efficacy of 84 % in protecting from TIAs, with no strokes or deaths in the treated population at a mean follow-up of 54 months. None of the enrolled patients suffered complications associated with intracerebral hemorrhage and the procedural complication rate was very low. As seen in patients with moyamoya, this study also demonstrated robust collateral formation at 6 months and 1 year in cerebral angiograms (in press) (Fig. 4).
Fig. 4.
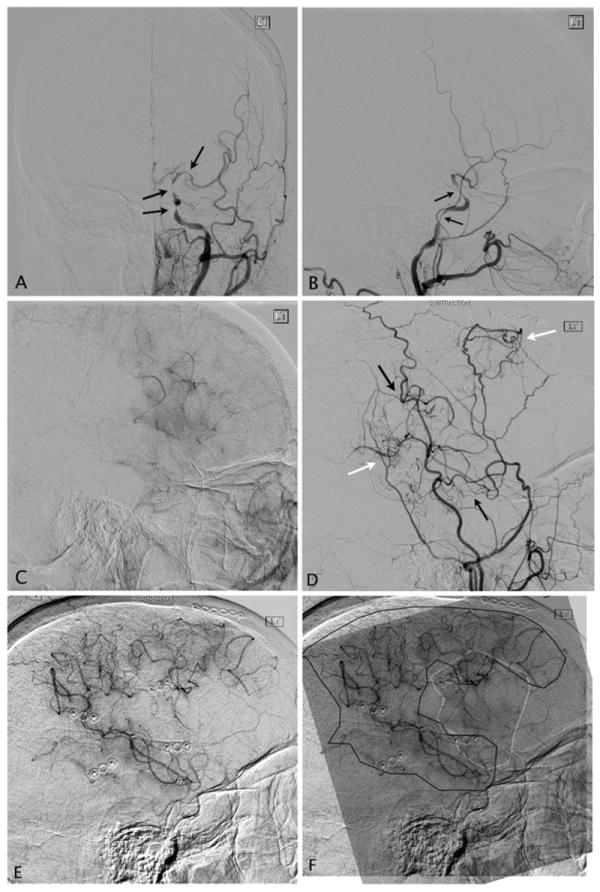
Post-EDAS development of collaterals. a, b Anteroposterior and lateral preoperative angiograms on a 62-year-old female post-angioplasty and stenting of atherosclerotic stenosis of the cavernous left internal carotid artery, who developed re-stenosis of the treated segment and additional tandem stenosis of the petrous ICA and left MCA (black arrows). c Capillary phase angiogram demonstrates a significantly reduced area of the MCA territory being perfused by the left ICA injection. d Six months post-EDAS, lateral angiogram of the external carotid artery (ECA) demonstrates multiple new collaterals developed from the superficial temporal artery (black arrows) and the middle meningeal artery (white arrows) with direct anastomosis to the MCA and cortical blush in the MCA territory. e Capillary phase of left ECA angiography demonstrates a significant contribution to the irrigation of the left MCA territory by the new collaterals from the ECA branches. f Composite image of the postoperative ECA capillary phase (black outline) supraimposed on the preoperative ICA injection (white outline) demonstrates complementary development of collaterals in the preoperatively hypoperfused cortical MCA territory
Summary
In summary, there is evidently a need for alternatives to current medical management that can address the hemodynamic issues associated with intracranial arterial stenosis and poor collateral circulation, without inducing elevated procedural complication rates, in particular preventing intracerebral hemorrhages. EDAS revascularization may potentially address some of the challenges faced in the treatment of these patients by providing alternative pathways of collateral circulation that grow on demand, producing sustainable collaterals from the external carotid circulation while avoiding the hemorrhagic complications associated with sudden hyperperfusion and manipulation of diseased arteries.
The clinical and angiographic results of the technique in both moyamoya and non-moyamoya patients with symptomatic intracranial stenosis are promising and should be formally tested versus intensive medical management in a clinical trial. Populations with unilateral severe intracranial stenosis with poor collateral circulation are at the highest risk of recurrent stroke and the EDAS revascularization could potentially modify the powerful negative predictive factor of collateral paucity and act in concert with intensive medical management in reducing the risk of stroke and death in these patients.
Acknowledgments
This study received funding from the American Heart Association Science Innovation Award (NRG) and the Ruth and Raymond Stotter Chair Endowment (NRG).
Footnotes
Conflict of interest None
Contributor Information
Nestor R. Gonzalez, Email: ngonzalez@mednet.ucla.edu, Department of Neurosurgery, David Geffen School of Medicine at UCLA, 10833 LeConte Ave., Rm 18-251 Semel, Los Angeles, CA 90095-7039, USA; Department of Radiology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; UCLA Stroke Center, Los Angeles, CA, USA.
David S. Liebeskind, UCLA Stroke Center, Los Angeles, CA, USA Department of Neurology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Joshua R. Dusick, Department of Neurosurgery, David Geffen School of Medicine at UCLA, 10833 LeConte Ave., Rm 18-251 Semel, Los Angeles, CA 90095-7039, USA
Fernando Mayor, Department of Radiology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Jeffrey Saver, UCLA Stroke Center, Los Angeles, CA, USA; Department of Neurology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
References
- 1.Adelson PD, Scott RM. Pial synangiosis for moyamoya syndrome in children. Pediatr Neurosurg. 1995;23(1):26–33. doi: 10.1159/000120932. [DOI] [PubMed] [Google Scholar]
- 2.Amin-Hanjani S, Du X, Zhao M, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36(6):1140. doi: 10.1161/01.STR.0000166195.63276.7c. [DOI] [PubMed] [Google Scholar]
- 3.Arenillas J, Molina C, Chacon P, Rovira A, Montaner J, Coscojuela P, Sanchez E, Quintana M, Alvarez-Sabin J. High lipoprotein (a), diabetes, and the extent of symptomatic intracranial atherosclerosis. Neurology. 2004;63(1):27. doi: 10.1212/01.wnl.0000132637.30287.b4. [DOI] [PubMed] [Google Scholar]
- 4.Arenillas JF, Alvarez-Sabin J. Basic mechanisms in intracranial large-artery atherosclerosis: advances and challenges. Cerebrovasc Dis. 2005;20(2):75–83. doi: 10.1159/000089359. [DOI] [PubMed] [Google Scholar]
- 5.Arenillas JF, Alvarez-Sabin J, Molina CA, Chacon P, Montaner J, Rovira A, Ibarra B, Quintana M. C-reactive protein predicts further ischemic events in first-ever transient ischemic attack or stroke patients with intracranial large-artery occlusive disease. Stroke. 2003;34(10):2463. doi: 10.1161/01.STR.0000089920.93927.A7. [DOI] [PubMed] [Google Scholar]
- 6.Arenillas JF, Alvarez-Sabin J, Montaner J, Rosell A, Molina CA, Rovira A, Ribo M, Sanchez E, Quintana M. Angiogenesis in symptomatic intracranial atherosclerosis. Stroke. 2005;36(1):92–97. doi: 10.1161/01.STR.0000149617.65372.5d. [DOI] [PubMed] [Google Scholar]
- 7.Awad I, Furlan AJ, Little JR. Changes in intracranial stenotic lesions after extracranial–intracranial bypass surgery. J Neurosurg. 1984;60(4):771–776. doi: 10.3171/jns.1984.60.4.0771. [DOI] [PubMed] [Google Scholar]
- 8.Baker A, Flora G, Resch J, Loewenson R. The geographic pathology of atherosclerosis: a review of the literature with some personal observations on cerebral atherosclerosis. Journal of Chronic Diseases. 1967;20(9):685–706. [Google Scholar]
- 9.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55(11):1475. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 10.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nature medicine. 2001;7(4):425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 11.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 13.Chimowitz MI, Lynn MJ, Turan TN, Fiorella D, Lane BF, Janis S, et al. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. J Stroke Cerebrovasc Dis. 2011;20(4):357–368. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JW, Kim JK, Choi BS, Lim HK, Kim SJ, Kim JS, Suh DC. Angiographic pattern of symptomatic severe M1 stenosis: comparison with presenting symptoms, infarct patterns, perfusion status, and outcome after recanalization. Cerebrovasc Dis. 2010;29(3):297–303. doi: 10.1159/000275508. [DOI] [PubMed] [Google Scholar]
- 15.Coyle P, Heistad D. Development of collaterals in the cerebral circulation. J Vasc Res. 1991;28(1–3):183–189. doi: 10.1159/000158860. [DOI] [PubMed] [Google Scholar]
- 16.Derdeyn CP. Mechanisms of ischemic stroke secondary to large artery atherosclerotic disease. Neuroimaging Clinics of North America. 2007;17(3):303–311. doi: 10.1016/j.nic.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Dusick J, Gonzalez NR, Martin NA. Clinical and angiographic outcomes from indirect revascularization surgery for Moyamoya disease in adults and children: a review of 63 procedures. Neurosurgery. 2011;68(1):34–43. doi: 10.1227/NEU.0b013e3181fc5ec2. discussion 43. [DOI] [PubMed] [Google Scholar]
- 18.Elmore EM, Mosquera A, Weinberger J. The prevalence of asymptomatic intracranial large vessel occlusive disease: the role of diabetes. J Neuroimaging. 2003;13(3):224–227. [PubMed] [Google Scholar]
- 19.Famakin BM, Chimowitz MI, Lynn MJ, Stern BJ, George MG. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke. 2009;40(6):1999–2003. doi: 10.1161/STROKEAHA.108.546150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46(6):937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 21.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119(10):812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Gomez CR, Misra VK, Campbell MS, Soto RD. Elective stenting of symptomatic middle cerebral artery stenosis. Am J Neuroradiol. 2000;21(5):971. [PMC free article] [PubMed] [Google Scholar]
- 23.Gress DR, Smith WS, Dowd CF, Halbach VV, Finley RJ, Higashida RT. Angioplasty for intracranial symptomatic vertebrobasilar ischemia. Neurosurgery. 2002;51(1):23. doi: 10.1097/00006123-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Investigator E-IB. Failure of extracranial–intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313(19):1191–1200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 25.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Benesch CG. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113(4):555. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 26.Kim DJ, Lee BH, Kim DI, Shim WH, Jeon P, Lee TH. Stent-assisted angioplasty of symptomatic intracranial vertebrobasilar artery stenosis: feasibility and follow-up results. Am J Neuroradiol. 2005;26(6):1381. [PMC free article] [PubMed] [Google Scholar]
- 27.Komotar RJ, Starke RM, Otten ML, Merkow MB, Garrett MC, Marshall RS, Elkind MS, Connolly ES. The role of indirect extracranial–intracranial bypass in the treatment of symptomatic intracranial atheroocclusive disease. J Neurosurg. 2009;110(5):896–904. doi: 10.3171/2008.9.JNS17658. [DOI] [PubMed] [Google Scholar]
- 28.Kono S, Oka K, Sueishi K, Sonobe M. Histopathological studies on spontaneous vault moyamoya and revascularized collaterals formed by encephalomyosynangiosis. Clin Neurol Neurosurg. 1997;99:S204–S207. [PubMed] [Google Scholar]
- 29.Lee DK, Kim JS, Kwon SU, Yoo SH, Kang DW. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: early diffusion-weighted imaging study. Stroke. 2005;36(12):2583. doi: 10.1161/01.STR.0000189999.19948.14. [DOI] [PubMed] [Google Scholar]
- 30.Liebeskind DS. Collateral circulation. Stroke. 2003;34(9):2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 31.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, Chimowitz MI. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69(6):963–974. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA: the journal of the American Medical Association. 1999;282(21):2035. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 33.Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab. 2001;21(10):1223–1231. doi: 10.1097/00004647-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Marti H, Risau W. Angiogenesis in ischemic disease. Thrombosis and haemostasis. 1999;82:44. [PubMed] [Google Scholar]
- 35.Matsushima T, Inoue T, Ikezaki K, Matsukado K, Natori Y, Inamura T, Fukui M. Multiple combined indirect procedure for the surgical treatment of children with moyamoya disease. A comparison with single indirect anastomosis with direct anastomosis. Neurosurg Focus. 1998;5(5):6. doi: 10.3171/foc.1998.5.5.7. [DOI] [PubMed] [Google Scholar]
- 36.Meyer JS, Denny-Brown D. The cerebral collateral circulation. I. Factors influencing collateral blood flow. Neurology. 1957;7(7):447. doi: 10.1212/wnl.7.7.447. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura M, Imai H, Konno K, Kubota C, Seki K, Puentes S, Faried A, Yokoo H, Hata H, Yoshimoto Y. Experimental investigation of encephalomyosynangiosis using gyrencephalic brain of the miniature pig: histopathological evaluation of dynamic reconstruction of vessels for functional anastomosis. Journal of Neurosurgery: Pediatrics. 2009;3(6):488–495. doi: 10.3171/2008.6.PEDS0834. [DOI] [PubMed] [Google Scholar]
- 38.Navarro-Sobrino M, Rosell A, Hernández-Guillamon M, Penalba A, Boada C, Domingues-Montanari S, Ribó M, Alvarez-SabÌn J, Montaner J. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216(1):205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 39.Piao R, Oku N, Kitagawa K, Imaizumi M, Matsushita K, Yoshikawa T, Takasawa M, Osaki Y, Kimura Y, Kajimoto K. Cerebral hemodynamics and metabolism in adult moyamoya disease: comparison of angiographic collateral circulation. Ann Nucl Med. 2004;18(2):115–121. doi: 10.1007/BF02985101. [DOI] [PubMed] [Google Scholar]
- 40.Qureshi AI, Suri MFK, Ziai WC, Yahia AM, Mohammad Y, Sen S, Agarwal P, Zaidat OO, Suarez JI, Wityk RJ. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: a multicenter study. Neurosurgery. 2003;52(5):1033. [PubMed] [Google Scholar]
- 41.Reaven GM. Banting lecture 1988 Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 42.Rosell-Novel A, Montaner J, Alvarez-SabÌn J. Angiogenesis in human cerebral ischemia. Rev Neurol. 2004;38(11):1076. [PubMed] [Google Scholar]
- 43.Ross R. Atherosclerosis -- An Inflammatory Disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 44.Sacco RL, Kargman D, Gu Q, Zamanillo M. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: the Northern Manhattan Stroke Study. Stroke. 1995;26(1):14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 45.Sainte-Rose C, Oliveira R, Puget S, Beni-Adani L, Boddaert N, Thorne J, Wray A, Zerah M, Bourgeois M. Multiple bur hole surgery for the treatment of moyamoya disease in children. Journal of Neurosurgery: Pediatrics. 2006;105(6):437–443. doi: 10.3171/ped.2006.105.6.437. [DOI] [PubMed] [Google Scholar]
- 46.Savopoulos C, Michalakis K, Apostolopoulou M, Miras A, Hatzitolios A. Adipokines and stroke: A review of the literature. Maturitas. 2011;70(4):322–327. doi: 10.1016/j.maturitas.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Saz-Saucedo P, Maestre-Moreno JF, Arenillas-Lara JF. Ateromatosis intracraneal. Medicina clÌnica. 2008;131(4):141–152. doi: 10.1157/13124100. [DOI] [PubMed] [Google Scholar]
- 48.Scanu AM, Lawn RM, Berg K. Lipoprotein(a) and atherosclerosis. Ann Intern Med. 1991;115(3):209. doi: 10.7326/0003-4819-115-3-209. [DOI] [PubMed] [Google Scholar]
- 49.Schreiber S, Serdaroglu M, Schreiber F, Skalej M, Heinze HJ, Goertler M. Simultaneous occurrence and interaction of hypoperfusion and embolism in a patient with severe middle cerebral artery stenosis. Stroke. 2009;40(7):e478–e480. doi: 10.1161/STROKEAHA.109.549378. [DOI] [PubMed] [Google Scholar]
- 50.Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004;100(2 Suppl Pediatrics):142–149. doi: 10.3171/ped.2004.100.2.0142. [DOI] [PubMed] [Google Scholar]
- 51.Starke RM, Komotar RJ, Connolly ES. Optimal surgical treatment for moyamoya disease in adults: direct versus indirect bypass. Neurosurg Focus. 2009;26(4):E8. doi: 10.3171/2009.01.FOCUS08309. [DOI] [PubMed] [Google Scholar]
- 52.Sundt TM, Smith H, Campbell J, Vlietstra R, Cucchiara R, Stanson A. Transluminal angioplasty for atherosclerosis disease of the vertebral and basilar arteries. Mayo Clin Proc. 1980;55:673–680. [PubMed] [Google Scholar]
- 53.Takahashi W, Ohnuki T, Ohnuki Y, Kawada S, Takagi S. The role of high-sensitivity C-reactive protein in asymptomatic intra-and extracranial large artery diseases. Cerebrovasc Dis. 2008;26(5):549–555. doi: 10.1159/000160212. [DOI] [PubMed] [Google Scholar]
- 54.Williams JE, Chimowitz MI, Cotsonis GA, Lynn MJ, Waddy SP. Gender differences in outcomes among patients with symptomatic intracranial arterial stenosis. Stroke. 2007;38(7):2055–2062. doi: 10.1161/STROKEAHA.107.482240. [DOI] [PubMed] [Google Scholar]
- 55.Wityk R, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27(11):1974. doi: 10.1161/01.str.27.11.1974. [DOI] [PubMed] [Google Scholar]
- 56.Wong KSL, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, Han Z, Tan KS, Ratanakorn D, Chollate P. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9(5):489–497. doi: 10.1016/S1474-4422(10)70060-0. [DOI] [PubMed] [Google Scholar]
- 57.Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Ueno M, Nishizawa S, Konishi J, Shio H. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive diseases. J Nucl Med. 1999;40(12):1992. [PubMed] [Google Scholar]


