Abstract
Over 90% of the human listeriosis cases are caused by Listeria monocytogenes serotypes 1/2a, 1/2b and 4b strains. As an alternative to antigen-antibody based serotyping, a PCR-based method for serogrouping has been developed and validated. In this communication, we report an in-depth analysis of five 4b variant strains, four clinical isolates from Australia and one environmental isolate from USA. Although these five strains were serotype 4b by classical serotyping method, the serogrouping PCR profiles of these strains show the presence of a 1/2a-3a specific amplicon in addition to the standard 4b-4d-4e specific amplicons. These strains were further analyzed by pulsed field gel electrophoresis, binary gene typing, multi-locus variable-number-tandem-repeat analysis and a high density pan-genomic Listeria microarray. Using these sub-typing results, the clinical isolates were grouped into two distinct genomic groups- one of which could be part of an unidentified outbreak. The microarray results when compared with our database of other 4b outbreak isolates indicated that the serotype 4b variant strains represent very different genotypic profiles than the known reported 4b outbreak strains representing major epidemic clones. The acquisition of serotype 1/2a gene clusters by the 4b variant strains appears to be independent in origin, spanning large areas of geographical and temporal space and may indicate predisposition of some 4b strains towards accepting DNA from related organisms.
Introduction
Listeria monocytogenes continues to cause foodborne diseases with 20–30% mortality and >95% hospitalization. The incidence of foodborne listeriosis in the United States alone is about 1,600 cases per year [1]. The incidence in most of the European countries and Canada is similar with slightly higher rates in Scandinavian countries [2]. Although the majority of listeriosis outbreaks and sporadic cases have been associated with deli meats and dairy products, recent listeriosis outbreaks involving fresh fruits and vegetables, including the cantaloupe associated outbreak in the US, are indicative of the fact that L. monocytogenes can survive and multiply in foods other than those commonly reported as a vehicle for foodborne listeriosis [3], [4]. Also interesting is the noticeable shift in demography of the individuals contracting listeriosis. During 1980–2000, most of the listeriosis cases were pregnancy associated while recent outbreaks show that the majority of the cases were non-pregnancy associated affecting elderly individuals [5], [6]. These observations underline the importance of in-depth genomic characterization and their significance in understanding the emergence of newer pathotypes, association with newer food groups and the shift in demography.
The value of molecular sub-typing for Lisetria and other foodborne pathogens during outbreak and traceback investigations cannot be overemphasized. In addition to epidemiological investigation, accurate determination of the source/s of foodborne outbreaks by comparing molecular sub-typing patterns of clinical, food and environmental isolates provides the scientific basis for quick determination of contaminated food/s thereby reducing the spread and burden of the outbreaks. In addition, molecular sub-typing is also important for understanding the pathophysiology [7], [8] of the organisms, source attribution [9] and for understanding of genomic evolution and emergence of newer traits. For example, that there may be specific genetic footprints in strains causing febrile gastroenteritis and invasive listeriosis was evident from the DNA microarray based sub-typing of L. monocytogenes [7] but the significance of such findings is far from clear. Previous studies have also indicated that different sub-types of L. monocytogenes are not equally distributed among food, environmental and clinical samples and different sub-types may pose different amount of risks [10], [11]. In order to identify the genetic diversity among the outbreak strains, several molecular approaches have been utilized [12]. These efforts clearly show the usefulness of detailed genotypic characterization of the outbreak associated L. monocytogenes strains not only for epidemiological and trace-back investigations but also for understanding the diversity and evolution of this organism. It is anticipated that in-depth genomic characterization of L. monocytogenes strains will help formulate intelligent hypotheses for its diverse pathophysiology, adaptation to newer food matrices and change in disease demography.
Serotyping of L. monocytogenes constitutes the very first step of sub-typing. Based on somatic and flagellar antigens, L. monocytogenes can be classified into 13 serotypes [13] of which serotypes 1/2a, 1/2b and 4b represent the vast majority of the disease causing strains [14]–[16]. The classical serotyping based on antigen-antibody reaction is time consuming, complicated and subjective [15], [17]. A simpler version of the classical serotyping is the determination of serogroups 1 and 4 by slide agglutination assay. The test, although simple, does not identify serotypes nor does it identify serogroup 3. Additionally, the test is variable and subject to interpretation. To avoid the usual pitfalls of the antigen-antibody based serotyping, several genome sequence-based serotyping methods have been developed [18]. Of all these sequence-based methods, a simple multiplex PCR based method by Doumith et al (2004) [19] appears to hold the maximum promise. The PCR-based assay uses five primer pairs of which four of them are serogroup specific and the fifth one, primers for prs gene, is Listeria genus specific [17], [19]. A modified version of this assay using hly specific primers instead of prs gene specific primers for L. monocytogenes has been reported by Burall et al [17]. Using this scheme, while majority of the strains can be properly classified in 1/2a-3a, 1/2b-3b, 1/2c-3c and 4b, 4d, 4e groups, a small group of strains showed PCR banding patterns that could not be classified by this scheme. These 4b variant strains, termed IVb-v1, produced a serotype 1/2a specific lmo0737 amplicon in addition to standard serotype 4b,4d, 4e specific bands for ORF 2110 and ORF 2819 [17], [20]. Recently, Leclercq et al (2011) [21] and Lee et al (2012) [22] reported the characterization of 45 serotype 4b L. monocytogenes strains collected over a long period from the different parts of the world. These IVb-v1 strains have been isolated from a variety of food, human and environmental sources separated by time and space. The multiplex serotyping PCR [19] of these strains also resulted in an lmo0737 specific band in addition to serogroup 4b specific bands. LeClercq et al. analyzed 22 IVb-v1 strains by ApaI/AscI generated PFGE profiles and grouped these strains into six different profiles, although 14 out of 22 strains were indistinguishable from each other indicating that there are clonal groups among these IVb-v1 strains. A sub-set (n = 7) of these strains were also analyzed by a multi locus sequence typing (MLST) protocol which revealed two MLST types [21]. The twenty three strains from clinical and processing plants from US was similarly analyzed by Lee et al. [22] by MLGT and susceptibility to Sau3A/MboI digestion and found to form three clonal groups.
In this paper, we report an in-depth genetic analysis of a group of five IVb-v1 strains originated in two different continents, Australia and North America, by a variety of sub-typing methods including pulsed field gel electrophoresis, binary typing, multi-locus variable tandem repeat, restriction enzyme digestion and a custom made pan-genomic DNA microarray. These techniques with varied discriminatory indices [23] provided us with a unique opportunity to compare the usefulness of multiple sub-typing techniques for their use during outbreak investigation and other purposes. Our results showed that the three of the four IVb-v1 strains from Australia probably represent an undocumented outbreak cluster. These three IVb-v1 strains also appear to form a separate clonal group, distinct from other clonal groups reported for the IVb-v1 strains [22]. Results from these molecular sub-typing assays identified unique genetic footprints of these strains and discuss the value of such analyses to understand genomic diversity, evolution and biology of L. monocytogenes.
Materials and Methods
Serotyping by Antisera and by PCR
The L. monocytogenes serotype 4b variant strains (Table 1) were serotyped by multiplex PCR and antisera agglutination as described previously [17]. Briefly, overnight cultures grown on BHI agar at 37°C were used to make lysates for multiplex PCR analysis as well as for agglutination assay using Difco Listeria types 1 and 4 antisera (BD Diagnostic Systems, Sparks, MD) following the manufacturer’s protocol. A commercially available L. monocytogenes serotyping kit (Denka Seiken Co., Tokyo, Japan) was also used to serotype some of these isolates using the manufacturer’s instruction.
Table 1. L. monocytogenes strains used in this study and their serotypic profiles.
| Strains | Source/Symptom | Multiplex PCR | Serotype** | |||||
| ORF 2819 | ORF 2110 | Lmo 0737 | Lmo 1118 | prs | hlyA | |||
| LS406 | Human/Febrile Gastroenteritis | + | + | − | − | + | + | 4b |
| LS412 | Human/Invasive | + | + | − | − | + | + | 4b |
| LS542 | Environmental Swab | + | + | + | − | + | + | 4b |
| LS642(10M127*) | Human/Invasive | + | + | + | − | + | + | 4b |
| LS643(10M130*) | Human/Invasive | + | + | + | − | + | + | 4b |
| LS644(10M138A*) | Human/Invasive | + | + | + | − | + | + | 4b |
| LS645(10M198*) | Human/Invasive | + | + | + | − | + | + | 4b |
*Alternate designation [20].
**Antibody- based serotyping.
+/− indicates presence/absence of the band.
Binary Typing
A binary typing method was developed based on the presence or absence of selected genes among the L. monocytogenes isolates [24]. Out of 44 screened candidate genes, an eight loci panel showed more significant variations than others. This eight loci panel combined with PCR-based serotyping [19] provided 95.4% Simpson index (SI) as a typing tool [23].
Multilocus Variable Tandem Repeats Analysis (MLVA)
Multi-locus variable-number-tandem-repeat analysis (MLVA) is a widely used typing method for L. monocytogenes [25]–[28]. An optimized MLVA typing panel was developed recently by selection of the optimal combination of loci from the previously reported panels [29]. Therefore, we used this new method for typing of the 4b variant strains.
Pulsed-field Gel Electrophoresis (PFGE) Typing
Pulsed-field gel electrophoresis (PFGE) analysis was performed according to the protocol developed by the Centers for Disease Control and Prevention (CDC, Atlanta, GA; http://www.cdc.gov/pulsenet/protocols.htm), using Salmonella braenderup H9812 as the control strain. PFGE results were analyzed using the BioNumerics Software (Applied-Maths, Kortrijk, Belgium). Banding pattern similarity was compared using an average of two-enzyme analysis with a 1.5% band position tolerance. All PFGE profiles generated were compared to isolates from clinical human Listeriosis cases in the CDC national PulseNet database.
DNA Microarray Analysis
The L. monocytogenes serotype 4b variant strains (Table 1) were grown in brain heart infusion (BHI) broth and/or BHI agar at 37°C. The L. monocytogenes 4b strains used in the comparison of genomic contents were obtained from various sources previously described [7]. Genomic DNA was isolated from 10 ml of cultures grown overnight in a shaking incubator at 170 rpm using the Qiagen DNeasy Blood and Tissue kit (Qiagen, Valencia, CA), followed by DNA fragmentation and 3′-end labeling as previously described [7]. The labeled product was then used for hybridization onto the Listeria GeneChip. Array hybridization, washing, staining and scanning were performed from the labeled DNA according to the Affymetrix GeneChip Expression Analysis Technical Manual and Laksanalamai et al. 2012 [7], [30].
All Affymetrix CEL files generated in this study were parsed and analyzed using algorithms including MAS5.0 [30]–[32] with a Tau value as reported previously [7]. Robust Multi Array (RMA) method to identify summarized probe-set intensities was implemented by the Affy package of R and Bioconductor [33]–[36]. The gene present/absent binary nucleotide calls were performed as described previously [7] and the genetic relationship among these strains were analyzed using Splitstree 4.11.3 [37]. A neighbor-net or neighbor joining phylogeny highlighting the distribution of the L. monocytogenes serotype 4b variant strains was constructed using the uncorrected p-distance in Splitstree 4.11.3.
Restriction Enzyme Digestion Analysis
Genomic DNA, prepared as described previously, was used for restriction digest analysis with MboI and Sau3A1 (New England Biolabs, Ipswich, MA) according to the manufacturers protocols, similar to prior work [38]. Reactions, containing 0.5 ug of genomic DNA, were incubated for 1hr at 37°C and then loaded on a 1% agarose gel for visualization of DNA digestion.
Results and Discussion
The three clinical isolates, LS642, LS643 and LS644, were from listeriosis patients in New South Wales, Australia and the fourth clinical isolate, LS645, was from Victoria, Australia in 2009. The isolates were collected as a part of routine surveillance of human listeriosis cases in Australia and no epidemiological link was identified among these four cases [20]. The fifth isolate, LS542, was from a soft cheese manufacturing facility environment, collected as a part of monitoring of ready to eat food facilities by USFDA [17]. The isolates were serotyped by antisera serotyping kit as 4b. However, the results of the multiplex PCR serotyping appeared to be a combination of serogroup 4b-4d-4e and 1/2a-3a (Fig. 1). These strains produced three serogroup specific bands of lmo0737, ORF2110 and ORF2819 in addition to Listeria genus specific prs band and L. monocytogenes specific hly band confirming that the isolates were indeed L. monocytogenes. The additional PCR band, lmo0737 is characteristic of serogroup 1/2a-3a. The amplified band for lmo0737 from these five strains were purified and sequenced and the sequence comparison between these fragments and bona fide lmo0737 fragment from a 1/2a strain showed that the sequences of all six strains were identical (data not shown) establishing that the additional band was indeed from lmo0737 and not from any other sequences resulting from any mis-priming or other artifacts during the amplification. In order to further characterize these five IVb-v1 strains, we conducted binary gene typing [24] of these strains. Table 2 shows the results from this assay. LS643, LS644 and LS645, clinical isolates from NSW and Victoria formed a single binary type 158 while LS642, the single clinical isolate from NSW and the environmental isolate from the USA, formed their own types; LS542 appeared to be genetically closer to LS642 as they differed by a single locus LC32. Similar genotypic variability was also observed by MLVA (Table 3). The MLVA analysis [29] grouped LS643, LS644 and LS645 into a single pattern (04-17-24-05-02-0-15-0-21) while LS542 and LS642 varied in five out of nine loci analyzed in this assay. These results clearly indicate that LS643, LS644 and LS645 represent a clonal group. Although no epidemiological link could be established, the results strongly indicate the possibility of a common source for these cases.
Figure 1. Multiplex PCR profiles of L. monocytogenes strains.
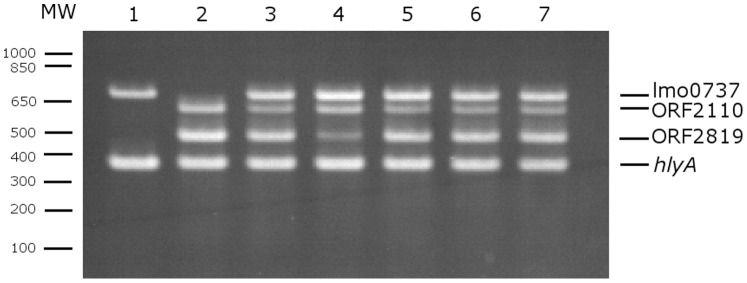
Lanes: MW, Molecular weight markers. Lane 1: LS1 serotype 1/2a, Lane 2: LS402, serotype 4b; Lane3-7: serotype IVb-v1 strains LS542; LS642; LS643; LS644; LS645, respectively.
Table 2. The binary gene typing profiles of the L. monocytogenes strains.
| Strains | Genetic | Loci | Binary Type | ||||||
| LC3 | LN4 | LB10 | LC32 | LB50 | LC52 | LC68 | LN1 | ||
| LS542 | + | − | + | − | + | + | + | + | 175 |
| LS642 | + | − | + | + | + | + | + | + | 191 |
| LS643 | + | − | − | + | + | + | + | − | 158 |
| LS644 | + | − | − | + | + | + | + | − | 158 |
| LS645 | + | − | − | + | + | + | + | − | 158 |
+/− indicates presence/absence of band.
Table 3. The MLVA profiles of the L. monocytogenes strains.
| Strains | Genetic | Loci | |||||||
| LMV6 | LMV1 | LMV2 | Lm11 | Lm10 | LMV7 | Lm32 | LMTR6 | Lm23 | |
| LS542 | 05 | 06 | 23 | 05 | 02 | 07 | 14 | 0 | 14 |
| LS642 | 03 | 12 | 14 | 05 | 03 | 07 | 14 | 0 | 16 |
| LS643 | 04 | 17 | 24 | 05 | 02 | 0 | 15 | 0 | 21 |
| LS644 | 04 | 17 | 24 | 05 | 02 | 0 | 15 | 0 | 21 |
| LS645 | 04 | 17 | 24 | 05 | 02 | 0 | 15 | 0 | 21 |
Numbers in the boxes represent the numbers of repeats in each of these loci.
The PulseNet database stores and analyzes PFGE profiles of various foodborne bacterial pathogens including L. monocytogenes [39]. Since its inception, PulseNet has become the mainstay in foodborne outbreak investigations in the USA and rest of the world. Currently, the US PulseNet L. monocytogenes database contains more than 13,000 PFGE patterns, including isolates from human (n = 7576), animal (n = 46), food (n = 2863) and environment (n = 2799). In order to further characterize the genotypic variability of these IVb-v1 strains, we performed PFGE analysis of these strains using a standard PFGE protocol [40]. Figure 2 shows the graphical representation of the ApaI/AscI PFGE profiles of five IVb-v1 and two serotype 4b strains (Table 1) representing an invasive outbreak LS412 and a gastroenteritis outbreak LS406. The PFGE profiles were analyzed and the dendrogram was drawn as described in the Materials and Methods. Again, it is clear that the PFGE profiles of LS643, LS644 and LS645 were indistinguishable from each other but they are quite distinct from LS542 and LS642 (Fig. 3). A query of the PulseNet database of the L. monocytogenes PFGE profiles did not reveal any match with any of these three patterns arising from the IVb-v1 strains although a few closely matched patterns with LS542 have been observed (data not shown). Overall, the PFGE data mirrored the binary typing and MLVA data indicating that LS643, LS644 and LS645 are genotypically very similar to each other and may indicate a common source for all three human cases. Although we did not see any significant difference among these three typing methods, the PFGE based typing provided an opportunity to compare these strains with other strains collected over the years and stored in the PulseNet dataset.
Figure 2. ApaI/AscI PFGE profiles of L. monocytogenes strains 4b and IVb-v1.
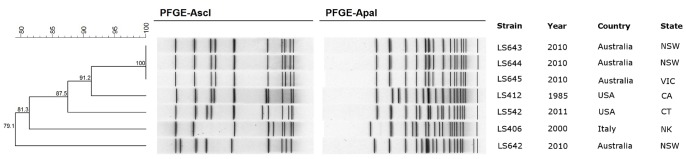
The dendrogram was calculated and drawn using Bionumerics software. NSW; New South Wales, VIC; Victoria, CA; California, CT; Connecticut, and NK;Not known.
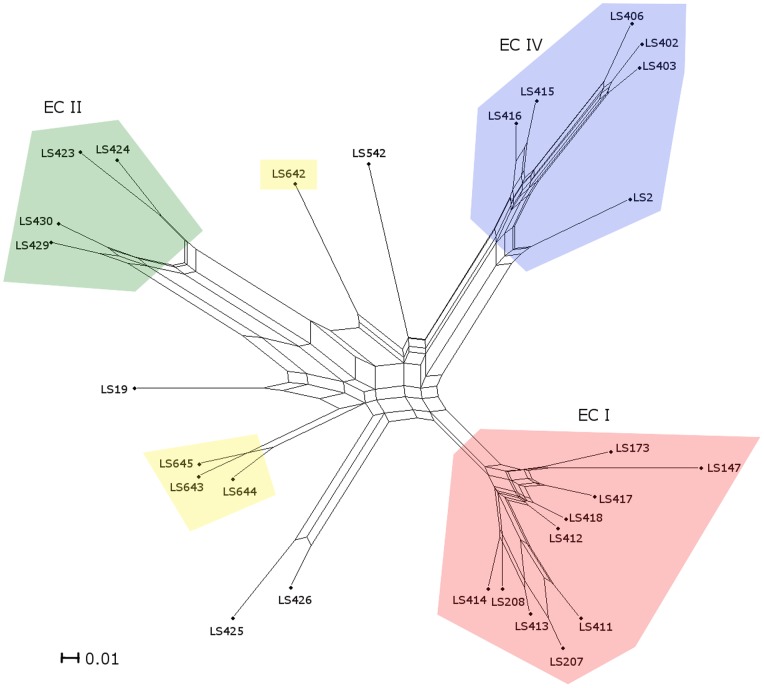
Figure 3. A neighbor-net constructed from the gene contents of 28 strains belonging to serotype 4b.
The parallel edges represent incompatible signals indicative of independent gene loss or gain due to the multiple transduction or recombination. Serotypes and epidemic clones are grouped in different color as indicated. Node labels refer to strain names (Table 1 and Laksanalamai et al 2012 (7). Scale bar represents number of gene differences (present or absent) per gene site.
High density DNA microarray has been successfully utilized for species identification [41], [42], serotypic and lineage determination [43], [44], virulence assessment [45] and epidemiological investigations [46], [47]. A pan-genomic microarray, Listeria GeneChip, has been used to reveal the genomic contents of L. monocytogenes outbreak strains [7] including strains involved in the 2011 cantaloupe associated listeriosis outbreak [4]. In this study, we also used the same Listeria GeneChip to analyze the gene contents and genomic architecture of the IVb-v1 strains.
The comparison of the probe-set data (present/absent) between the serotypes 4b and IVb-v1 by MAS5.0 algorithm revealed that strains LS643, LS644 and LS645 are clustered together forming a new group that is distinct from other serotype 4b outbreak strains. The genetic content analysis of strain LS642 clearly indicated that it is branched away from these three strains (Fig. 3) suggesting extensive genetic variability. Although the strain LS642 was isolated from the same state of New South Wales, it is more closely related to the strain LS542 than to its Australian counterparts. The microarray results combined with the PFGE, binary genotyping and MLVA data again support the notion the strains LS643, LS644, and LS645 could be part of a common source outbreak even though no epidemiological link was established. The microarray data also allowed us to identify if these strains belonged to any of the known epidemic clones (EC). Previously, extensive genomic information has led to the establishment of five distinct ECs of L. monocytogenes [7], [14] of which serotype 4b strains belonged to ECI, ECII and ECIV. Our microarray data clearly shows that the IVb-v1 strains are genotypically distinct and do not belong to any of these three ECs (Fig. 3). Comparison of the gene contents between LS642 and the group of LS643, LS644 and LS645 revealed that 2.2% (415) of all the probe-sets are uniquely present in strain LS642 but absent in all of LS643, LS644 and LS645 strains (Table S1). On the other hand, 1.5% (273) of all the probe-sets are uniquely present in LS643, LS644 and LS645 (Table S2) but absent in LS642. It is interesting to note that of all the unique probe-sets present in LS642 (Table S1) about 8% uniqueness is derived from phage sequences compared to about 1% phage sequences attributed to the uniqueness of LS643, LS644 and LS645 (Table S2). Such difference may indicate different ancestry of these two groups of IVb-v1 strains.
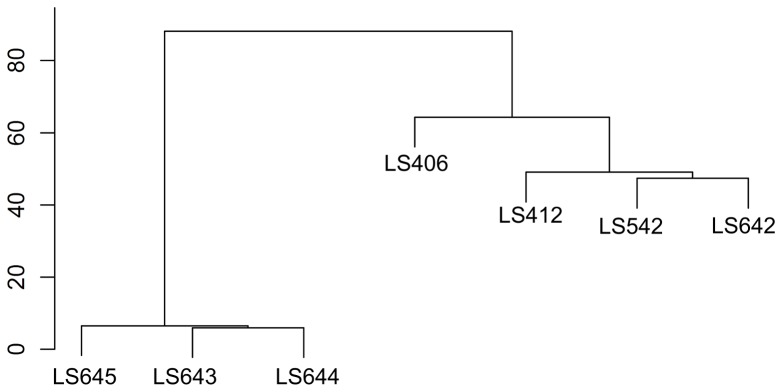
The microarray data was also used to investigate the genetic variability of the serotypes 4b and IVb-v1 strains using a Robust Multi Array (RMA) algorithm to assess the individual probe-set intensity without utilizing the mismatched-probe intensity information [33], [35], [36]. The hierarchical clustering (Fig. 4) based on the summarized probe-set intensity among the serotypes 4b and IVb-v1 strains were consistent with the MAS5.0 analysis for genetic contents (Fig. 3). The RMA analysis (Fig. 4) divides the seven strains into two groups. LS643, LS644 and LS645 formed a close cluster while the other four strains (LS406, LS412, LS542 and LS642) formed a separate group where the IVb-v1 strains (LS542 and LS642) and LS412 were much closer to each other than LS406. In term of pathophysiology it is interesting to note that the strains that were associated with invasive listeriosis (LS412, and LS642) were clustered closely while LS406, associated with a gastroenteritis outbreak [48], was branched away from this cluster.
Figure 4. Hierarchical clustering based on the Robust Multi Array (RMA) analysis of the L. monocytogenes strains serotypes 4b (LS406, LS412) and IVb-v1 (LS542, LS642, LS643, LS644, LS645).
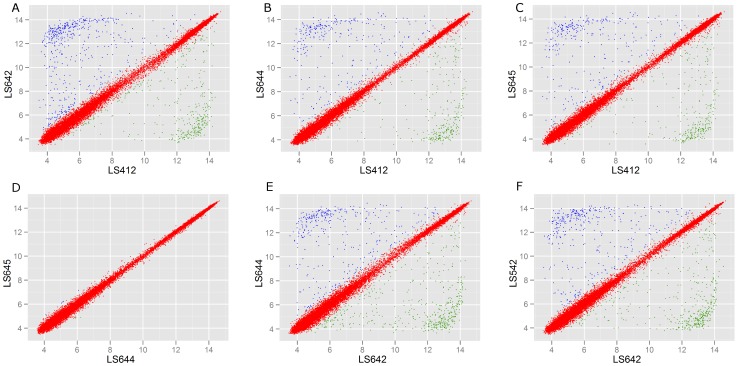
The variation of probe-set intensity (higher and lower than two-fold) between strains is a measure of pan-genomic variability that can be visually assessed by scatter plot. The plot also allows us to quantify the extent of variability and identify the genes that are different between the strains. In order to further assess the genomic differences between 4b and IVb-v1 strains and among IVb-v1 strains, we analyzed the scatter plots of various groups of strains (Fig. 5). Comparison of a serotype 4b strain, LS412, with the IVb-v1 strains (LS642, LS644 and LS645) revealed (Fig. 5-A, B, and C) that the numbers of probe-sets different between the paired strains out of a total of 18630 probe-sets analyzed are 846, 572 and 587 for LS642, LS644 and LS645, respectively. Similar analysis between LS412 and LS644 (Fig. 5B, n = 572) and between LS412 and LS645 (Fig. 5C, n = 587) revealed that 95–97% of probe-sets are identical, suggesting the close relationship between the two serotype IVb-v1 strains. In addition, the identical genetic make-up between LS644 and LS645 is also confirmed when a similar analysis was done between these two strains (Fig. 5D). The comparisons between LS642 and LS644 (Fig. 5E) and between LS642 and LS542 (Fig. 5F) revealed that there are 842 and 787 probe-sets different, respectively. These analyses clearly show the extent of genetic variability among the IVb-v1 strains. These results, however, are slightly different from the PFGE based clustering, possibly due to the differences in the amount of information between two methods. In addition, partial analysis of these Australian IVb-v1 strains based on the whole genome sequences [49] also revealed that LS542 and LS642 are more diverse from LS643, LS644 and 645 (data not shown) in agreement with other subtyping methods.
Figure 5. Scatter plots of the summarized Robust Multi-Array Averaging (RMA) intensities.
A–C, between serotypes 4b (LS412) and IVb-v1 (LS642, A; LS644, B; LS645, C). D–E, between the same serotype, IVb-v1, isolated from Australia; from the different states (LS644 and LS645, D); from the same state (LS642 and LS644, E). F, between the same serotype, IVb-v1, isolated from the different countries (LS642; Australia and LS542; USA).
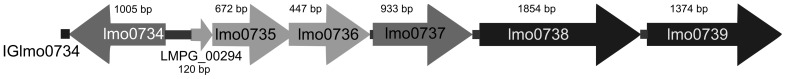
Our in-depth analysis of the extent of genetic variability of the IVb-v1 strains agrees with the previous findings that, although these strains share a common serotype IVb-v1, due to the presence of a serotype 1/2a specific locus lmo0737, the extent of genetic difference can be substantial. In order to investigate the extent of gene sharing between the serotype 1/2a and IVb-v1 strains, we explored the possibility of the existence of any other genomic footprints shared only by these IVb-v1 and serotype 1/2a strains. The comparison of the genetic contents of 84 strains in our microarray database clearly indicated that the serotype IVb-v1 strains are more closely related to the serotype 4b than to 1/2a. Serotypes IVb-v1 and 1/2a strains shared a total of ten unique probe-sets, representing two intergenic region and eight genes (Table 4). Multiple probe-sets analysis using Listeria GeneChip confirms that lmo0734– lmo0739 (Fig. 6) are present only in the L. monocytogenes serotypes IVb-v1 and 1/2a strains. Recently, Lee et al (2012) [22] reported the presence of the same gene cassette (lmo0734-lmo0739) in 23 serotype IVb-v1 strains. The total length of this region is approximately 6.3 kb. Two of these genes code for enzymes in pentose-phosphate pathway, one gene codes for an enzyme in the glycolysis/gluconeogenesis while lmo0734 codes for a lacI type transcription regulator and lmo0738 codes for a component of phosphotransferase system. At this point, we do not know the biological significance of these genes or how these genes were acquired by a set of 4b strains. A similar conclusion was also reached by Lee et al (2012) [22] from their study with 23 IVb-v1 strains collected in the US. Mutants with an in-frame deletion of this gene cassette in EGDe did not show any growth defect in vitro or in vivo [50] although these genes were up-regulated in the intestine of the infected mice and lmo0737 was down regulated in blood indicating the involvement during infection process [51]. Further experiments with isogenic constructs of IVb-v1 strains would be needed to understand the biological role of this cassette.
Table 4. Unique probe-sets in 1/2a and IVb-v1 L. monocytogenes strains.
| Serotype | |||||
| Probe-ID | Annotation (100% homology) | 1/2a(n = 34) | 1/2b(n = 20) | 4b(n = 25) | IVb-v1(n = 5) |
| IGlmo0734_at | Intergenic region | + | − | − | + |
| IGlmo0735_x_at | Intergenic region | + | − | − | + |
| AARI_0596_s_at | LacI family-transcriptional regulator (lmo0734) | + | − | − | + |
| AARM_0745_S_at | LacI family-transcriptional regulator(lmo0734) | + | − | − | + |
| AARY_1549_s_at | 6-phospho-beta-glucosidase (lmo0739) | + | − | − | + |
| lmo0735_s_at | Ribulose-5-Phosphate 3-Epimerase | + | − | − | + |
| lmo0736_at | Ribose 5-phosphate isomerase | + | − | − | + |
| lmo0737_s_at | Hypothetical protein | + | − | − | + |
| lmo0738_s_at | Phosphotransferase system (PTS) beta-glucoside-specificenzyme IIABC component | + | − | − | + |
| LMPG_00294_s_at | 39 amino acid hypothetical protein (IGlmo0735) | + | − | − | + |
Figure 6. The organization of the genomic region containing the lmo0737 to lmo0739 cassette that is present only in the L. monocytogenes serotypes IVb-v1 and 1/2a strains, confirmed by microarray analysis.
Origin of this highly conserved region (LMOf2365_0734– LMOf2365_0739 cassette in IVb-v1 strains remains highly speculative at this point. Absence of this gene cassette in serotype 1/2b and majority of the serotype 4b strains and presence of this cassette in serotype 1/2a strains suggest horizontal gene transfer from serotype 1/2a to certain groups of serotype 4b strains [21], [22] although we could not locate any signs of phage genome or any transposon-like sequences flanking this region. It has been indicated that serotype 1/2a strains are more promiscuous in terms acquiring phage genomes [52]. It is conceivable that these 4b variant strains (IVb-v1) have some unique traits that make them more disposed to accepting genes from other organisms. Further experiments are however needed to prove or disprove this hypothesis. In addition, the analysis of this conserved region based on the whole genome sequence of these IVb-v1 strains [49] revealed that there are a few conserved SNPs between the 1/2a and IVb-v1 strains(data not shown) These nucleotide changes mostly resulted in silent mutations. However, there is a SNP in lmo0737, a hypothetical regulatory protein, resulting in a proline substituted serine in all four Australian strains. This change may be significant as the protein secondary structure could be altered leading to a different function of the protein.
One of the distinguishing features of the genomic contents of various epidemic clones of L. monocytogenes is the presence/absence of a restriction-modification (RM) cassette [53]. This cassette is characterized by the presence of a gene coding for Sau3A restriction enzyme (LMOf2365_0325), a DNA binding site (LMOf2365_0326) and a DNA methylase gene (LMOf2365_0327). Presence of these genes have been shown to be responsible for the resistance of the genomic DNA to Sau3A digestion while same genomic DNA remains sensitive to Mbo1 cleavage as cytosine methylation does not affect this enzyme. This RM gene cassette is present in all the ECI strains but absent in other 4b strains. Using the resistance/sensitivity to Sau3A and Mbo1 phenotype, Lee et al. [38] have shown that the collection of their IVb-v1 strains could be classified into two groups of which clonal groups 1 and 2 were sensitive to both Sau3A and Mbo1 while the third group was sensitive to Sau3A but resistant to Mbo1 indicating adenine rather than cytosine methylation of GATC sites in this group. As RM systems form the hallmark of bacterial genomic evolution, we decided to study the Sau3A and Mbo1 digestion pattern of the five IVb-v1 strains and also analyzed the presence/absence of the genes in the RM cassette region by microarray hybridization. Our results (Table 5) show that LS643, LS644 and LS645 genomic DNA were resistant to Sau3A digestion but sensitive to Mbo1 digestion indicating cytosine methylation at the GATC sites of these strains. This is also supported by the positive hybridization signals from the probe-sets representing LMOf2365_0325 (Sau3A enzyme), LMOf2365_0326 (DNA binding protein) and LMOf2365_0327 (DNA methylase) and also two downstream genes LMOf2365_0328 and LMOf2365_0329. The other two IVb-v1 strains, LS542 and LS642 were sensitive to both Sau3A and Mbo1 and were lacking genes from LMOf2365_0325 to LMOf2365_0328. The representative strains from 4b, 1/2a and 1/2b serotypes showed Mbo1 susceptibility while Sau3A results and presence/absence of the genes in the RM cassette were mixed (data not shown). The results further substantiated other genomic comparison data that LS643, LS644 and LS645 belonged to a genomic group while LS542 and LS642 belonged to separate groups. The results also showed that LS643, LS644 and LS645 represent a clonal group distinctly different from the three clonal groups of IVb-v1 strains described previously [22].
Table 5. Susceptibility to Mbo1 and Sau3A digestion and distribution of the genes in the restriction-modification cassette in selected L. monocytogenes strains.
| Probe ID | |||||||||
| Strain | Serotype | Mbo | Sau3A1 | LMOf2365 | LMOf2365 | LMOf2365 | LMOf2365 | LMOf2365 | LMOf2365 |
| 0325_at | 0326_at | 0327_at | 0328_s_at | IG0329_at | 0329_s_at | ||||
| LS412 | 4b | + | − | P | P | P | P | P | P |
| LS429 | 4b | + | + | A | A | A | A | A | P |
| LS406 | 4b | + | + | A | A | A | A | A | P |
| LS542 | IVb-v1 | + | + | A | A | A | A | A | P |
| LS642 | IVb-v1 | + | + | A | A | A | A | A | P |
| LS643 | IVb-v1 | + | − | P | P | P | P | P | P |
| LS644 | IVb-v1 | + | − | P | P | P | P | P | P |
| LS645 | IVb-v1 | + | − | P | P | P | P | P | P |
| LS146 | 1/2b | + | − | P | P | P | P | P | P |
| LS484 | 1/2b | + | + | A | A | A | A | A | A |
| LS686 | 1/2b | + | + | A | A | A | A | A | P |
| LS787 | 1/2a | + | − | A | A | A | P | P | P |
| LS120 | 1/2a | + | + | A | A | A | A | A | A |
+: cut; − uncut; P: presence and A: absence of hybridization signals with the gene specific probe.
Dispersion of unique genotypes of L. monocytogenes throughout the world has been illustrated by the occurrence of multiple epidemic clones (EC) and multiple sequence types (ST) [54], [55]. The multilocus sequence typing (MLST) or simply sequence typing (ST) based subtyping has resulted in the formulation of clonal complex (CC), which unlike the ECs, does not require any epidemic outbreak association and thus represent a more overarching way to investigate the clonality of this organism. In several instances, ECs and CCs overlap with each other while in other cases they are distinct genogroups. For example all the known ECIs, ECIVs and ECIIs appear to fall under CC1, CC2, and CC6 respectively [55]. Using the draft whole genome sequences of the five IVb-v1 strains [49], we found that LS643, LS644 and LS645belong to ST240 while LS542 and LS642 belong to ST554 and ST572, respectively. The assignment of the same ST for LS643, LS644 and LS645 bolsters our previous assertions (Table 2, 3 and Figure 3) that these strains are very closely related to each other. A query of the MLST database at the Institute Pasteur (http://www.pasteur.fr/recherché/genopole/PF8/mlst/Lmono.html) revealed that the ST240 and ST693 are different by just one allele (dapE) and therefore by definition [56], these strains could be part of a clonal complex. Similarly, LS642 with ST572 match all but dat gene sequence with ST373 and thus could be part of a clonal group. It is interesting to note that LS643, LS644 and LS645 share ST240 with another IV-v1 strain isolated in 1959 in Switzerland from a human bacterimic patient [21].
In summary, our analyses clearly show that the IVb-v1 strains are genetically distinct from 4b strains and also among each other. These differences go beyond the presence/absence of a 6.3 kb DNA cassette as shown by microarray scatter plot analysis (Fig. 5) and by comparing the trees created among these strains with and without the 6.3 kb DNA specific probe-sets (data not shown). Three of the four human L. monocytogenes IVb-v1 strains from Australia shared a very extensive genetic homology indicating that these strains could be part of an outbreak cluster. These three strains also formed a different clonal group not reported previously [22]. The IVb-v1 pattern among geographically, temporally and genetically unrelated strains indicates that such variability can originate independently and the events are not of recent origin. The acquisition of approximately 6.3kb DNA from 1/2a serotype indicates that some 4b strains probably are more prone to genetic exchanges, a crucial requirement for emergence of newer traits.
Supporting Information
Probe-sets uniquely present in LS642 and absent in LS643, LS644 and LS645.
(DOCX)
Probe-sets uniquely present in LS643, LS644 and LS645 but absent in LS642.
(DOCX)
Funding Statement
The authors have no support or funding to report.
References
- 1. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, et al. (2011) Foodborne illness acquired in the United States - major pathogens. EmergInfectDis 17: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Notermans S, Todd ECD (2011) Surveillance of listeriosis and its causative pathogen, Listeria monocytogenes . Food Control 22: 1484–1490. [Google Scholar]
- 3. Cosgrove S, Cronquist A, Wright G, Ghosh T, Vogt R, et al. (2011) Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe–United States, August-September 2011. MMWR Morb Mortal Wkly Rep 60: 1357–1358. [PubMed] [Google Scholar]
- 4. Laksanalamai P, Joseph LA, Silk BJ, Burall LS, L Tarr C, et al. (2012) Genomic characterization of Listeria monocytogenes strains involved in a multistate listeriosis outbreak associated with cantaloupe in US. PLoS One 7: e42448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mook P, O’Brien SJ, Gillespie IA (2011) Concurrent conditions and human listeriosis, England, 1999–2009. Emerg Infect Dis 17: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silk BJ, Date KA, Jackson KA, Pouillot R, Holt KG, et al. (2012) Invasive listeriosis in the Foodborne Diseases Active Surveillance Network (FoodNet), 2004–2009: further targeted prevention needed for higher-risk groups. Clin Infect Dis 54 Suppl 5: S396–404. [DOI] [PubMed] [Google Scholar]
- 7. Laksanalamai P, Jackson SA, Mammel MK, Datta AR (2012) High Density Microarray Analysis Reveals New Insights into Genetic Footprints of Listeria monocytogenes Strains Involved in Listeriosis Outbreaks. PLoS One 7: e32896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts AJ, Williams SK, Wiedmann M, Nightingale KK (2009) Some Listeria monocytogenes outbreak strains demonstrate significantly reduced invasion, inlA transcript levels, and swarming motility in vitro. Appl Environ Microbiol 75: 5647–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferreira V, Barbosa J, Stasiewicz M, Vongkamjan K, Moreno Switt A, et al. (2011) Diverse geno- and phenotypes of persistent Listeria monocytogenes isolates from fermented meat sausage production facilities in Portugal. Appl Environ Microbiol 77: 2701–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kathariou S (2002) Listeria monocytogenes virulence and pathogenicity, a food safety perspective. JFood Prot 65: 1811–1829. [DOI] [PubMed] [Google Scholar]
- 11.Liu D (2008) Epidemiology. In: Liu D, editor. Handbook of Listeria monocytogenes Boca Raton: CRC Press. 27–59.
- 12.Datta AR, Laksanalamai P, Solomotis M (2012) Recent developments in molecular sub-typing of Listeria monocytogenes. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. [DOI] [PubMed]
- 13.Seeliger HP, Hohne K (1979) Serotyping of Listeria monocytogenes and related species. Methods Microbiol: 31–49.
- 14.Cheng Y, Siletzky RM, Kathariou S (2008) Genomic division/lineages, epidemic clones and population structure. In: Liu D, editor. Handbook of Listeria monocytogenes Boca Raton: CRC Press. 337–357.
- 15.Graves LM, Swaminathan B, Hunter SB (2007) Subtyping Listeria monocytogenes In: Ryser ET, Marth EH, editors. Listeria, listeriosis, and food safety. Boca Raton: CRC Press. 283–304.
- 16. Mead PS, Dunne EF, Graves L, Wiedmann M, Patrick M, et al. (2006) Nationwide outbreak of listeriosis due to contaminated meat. Epidemiol Infect 134: 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burall LS, Simpson AC, Datta AR (2011) Evaluation of a Serotyping Scheme Using a Combination of an Antibody-Based Serogrouping Method and a Multiplex PCR Assay for Identifying the Major Serotypes of Listeria monocytogenes JFood Prot. 74: 403–409. [DOI] [PubMed] [Google Scholar]
- 18. Jadhav S, Bhave M, Palombo EA (2012) Methods used for the detection and subtyping of Listeria monocytogenes . J Microbiol Methods. [DOI] [PubMed] [Google Scholar]
- 19. Doumith M, Buchrieser C, Glaser P, Jacquet C, Martin P (2004) Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. JClinMicrobiol 42: 3819–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang B, Fang N, Dimovski K, Wang X, Hogg G, et al. (2011) Observation of a new pattern in serogroup-related PCR typing of Listeria monocytogenes 4b isolates. J Clin Microbiol 49: 426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leclercq A, Chenal-Francisque V, Dieye H, Cantinelli T, Drali R, et al. (2011) Characterization of the novel Listeria monocytogenes PCR serogrouping profile IVb-v1. Int J Food Microbiol 147: 74–77. [DOI] [PubMed] [Google Scholar]
- 22. Lee S, Ward TJ, Graves LM, Wolf LA, Sperry K, et al. (2012) Atypical Listeria monocytogenes serotype 4b strains harboring a lineage II-specific gene cassette. Appl Environ Microbiol 78: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunter PR, Gaston MA (1988) Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol 26: 2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang B, Schleehauf JK, Eglezos S, Bates J (2007) Binary typing of Listeria monocytogenes isolates from patients and food through multiplex PCR and reverse line hybridisation; SA, Australia. 136–137.
- 25. Lindstedt BA, Tham W, Danielsson-Tham ML, Vardund T, Helmersson S, et al. (2008) Multiple-locus variable-number tandem-repeats analysis of Listeria monocytogenes using multicolour capillary electrophoresis and comparison with pulsed-field gel electrophoresis typing. J Microbiol Methods 72: 141–148. [DOI] [PubMed] [Google Scholar]
- 26. Miya S, Kimura B, Sato M, Takahashi H, Ishikawa T, et al. (2008) Development of a multilocus variable-number of tandem repeat typing method for Listeria monocytogenes serotype 4b strains. Int J Food Microbiol 124: 239–249. [DOI] [PubMed] [Google Scholar]
- 27. Murphy M, Corcoran D, Buckley JF, O’Mahony M, Whyte P, et al. (2007) Development and application of Multiple-Locus Variable number of tandem repeat Analysis (MLVA) to subtype a collection of Listeria monocytogenes . Int J Food Microbiol 115: 187–194. [DOI] [PubMed] [Google Scholar]
- 28. Sperry KE, Kathariou S, Edwards JS, Wolf LA (2008) Multiple-locus variable-number tandem-repeat analysis as a tool for subtyping Listeria monocytogenes strains. J Clin Microbiol 46: 1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X, Huang B, Eglezos S, Graham T, Blair B, et al. (2013) Identification of an optimized panel of variable number tandem-repeat (VNTR) loci for Listeria monocytogenes typing. Diagn Microbiol Infect Dis 75: 203–206. [DOI] [PubMed] [Google Scholar]
- 30.Affymetrix (2005) Affymetrix GeneChip Expression Analysis Technical Manual http://media.affymetrix.com/support/downloads/manuals/expression_analysis_technical_manual.pdf.Accessed 2014 Jan 22.
- 31. Hubbell E, Liu WM, Mei R (2002) Robust estimators for expression analysis. Bioinformatics 18: 1585–1592. [DOI] [PubMed] [Google Scholar]
- 32. Jackson SA, Patel IR, Barnaba T, LeClerc JE, Cebula TA (2011) Investigating the global genomic diversity of Escherichia coli using a multi-genome DNA microarray platform with novel gene prediction strategies. BMCGenomics 12: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193. [DOI] [PubMed] [Google Scholar]
- 34. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, et al. (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Irizarry RA, Wu Z, Jaffee HA (2006) Comparison of Affymetrix GeneChip expression measures. Bioinformatics 22: 789–794. [DOI] [PubMed] [Google Scholar]
- 37. Huson DH (1998) SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14: 68–73. [DOI] [PubMed] [Google Scholar]
- 38. Lee S, Ward TJ, Siletzky RM, Kathariou S (2012) Two novel type II restriction-modification systems occupying genomically equivalent locations on the chromosomes of Listeria monocytogenes strains. Appl Environ Microbiol 78: 2623–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gerner-Smidt P, Hise K, Kincaid J, Hunter S, Rolando S, et al. (2006) PulseNet USA: a five-year update. Foodborne Pathog Dis 3: 9–19. [DOI] [PubMed] [Google Scholar]
- 40. Halpin JL, Garrett NM, Ribot EM, Graves LM, Cooper KL (2010) Re-evaluation, optimization, and multilaboratory validation of the PulseNet-standardized pulsed-field gel electrophoresis protocol for Listeria monocytogenes FoodbornePathogDis. 7: 293–298. [DOI] [PubMed] [Google Scholar]
- 41. Call DR, Borucki MK, Loge FJ (2003) Detection of bacterial pathogens in environmental samples using DNA microarrays. JMicrobiolMethods 53: 235–243. [DOI] [PubMed] [Google Scholar]
- 42. Volokhov D, Rasooly A, Chumakov K, Chizhikov V (2002) Identification of Listeria species by microarray-based assay. JClinMicrobiol 40: 4720–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Call DR, Borucki MK, Besser TE (2003) Mixed-genome microarrays reveal multiple serotype and lineage-specific differences among strains of Listeria monocytogenes JClinMicrobiol. 41: 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang C, Zhang M, Ju J, Nietfeldt J, Wise J, et al. (2003) Genome diversification in phylogenetic lineages I and II of Listeria monocytogenes: identification of segments unique to lineage II populations. JBacteriol 185: 5573–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doumith M, Cazalet C, Simoes N, Frangeul L, Jacquet C, et al. (2004) New aspects regarding evolution and virulence of Listeria monocytogenes revealed by comparative genomics and DNA arrays. InfectImmun 72: 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borucki MK, Kim SH, Call DR, Smole SC, Pagotto F (2004) Selective discrimination of Listeria monocytogenes epidemic strains by a mixed-genome DNA microarray compared to discrimination by pulsed-field gel electrophoresis, ribotyping, and multilocus sequence typing. JClinMicrobiol 42: 5270–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Orsi RH, Borowsky ML, Lauer P, Young SK, Nusbaum C, et al. (2008) Short-term genome evolution of Listeria monocytogenes in a non-controlled environment. BMCGenomics 9: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aureli P, Fiorucci GC, Caroli D, Marchiaro G, Novara O, et al. (2000) An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes N Engl J Med. 342: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 49. Laksanalamai P, Steyert SR, Burall LS, Datta AR (2013) Genome Sequences of Listeria monocytogenes Serotype 4b Variant Strains Isolated from Clinical and Environmental Sources. Genome Announc 1(5): e00771–00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Milillo SR, Badamo JM, Wiedmann M (2009) Contributions to selected phenotypic characteristics of large species- and lineage-specific genomic regions in Listeria monocytogenes . Food Microbiol 26: 212–223. [DOI] [PubMed] [Google Scholar]
- 51. Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, et al. (2009) The Listeria transcriptional landscape from saprophytism to virulence. Nature 459: 950–956. [DOI] [PubMed] [Google Scholar]
- 52. Chen J, Novick RP (2009) Phage-mediated intergeneric transfer of toxin genes. Science 323: 139–141. [DOI] [PubMed] [Google Scholar]
- 53. Yildirim S, Elhanafi D, Lin W, Hitchins AD, Siletzky RM, et al. (2010) Conservation of genomic localization and sequence content of Sau3AI-like restriction-modification gene cassettes among Listeria monocytogenes epidemic clone I and selected strains of serotype 1/2a. Appl Environ Microbiol 76: 5577–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chenal-Francisque V, Lopez J, Cantinelli T, Caro V, Tran C, et al. (2011) Worldwide distribution of major clones of Listeria monocytogenes . Emerg Infect Dis 17: 1110–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cantinelli T, Chenal-Francisque V, Diancourt L, Frezal L, Leclercq A, et al. (2013) “Epidemic clones” of Listeria monocytogenes are widespread and ancient clonal groups. J Clin Microbiol 51: 3770–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ragon M, Wirth T, Hollandt F, Lavenir R, Lecuit M, et al. (2008) A new perspective on Listeria monocytogenes evolution. PLoS Pathog 4: e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Probe-sets uniquely present in LS642 and absent in LS643, LS644 and LS645.
(DOCX)
Probe-sets uniquely present in LS643, LS644 and LS645 but absent in LS642.
(DOCX)