SUMMARY
Objective
The purpose of this work was to review the current literature on cartilage and meniscal T2 relaxation time.
Methods
Electronic searches in PubMed were performed to identify relevant studies about T2 relaxation time measurements as non-invasive biomarker for knee osteoarthritis (OA) and cartilage repair procedures.
Results
Initial osteoarthritic changes include proteoglycan loss, deterioration of the collagen network, and increased water content within the articular cartilage and menisci. T2 relaxation time measurements are affected by these pathophysiological processes.
It was demonstrated that cartilage and meniscal T2 relaxation time values were significantly increased in subjects with compared to those without radiographic OA and focal knee lesions, respectively. Subjects with OA risk factors such as overweight/obesity showed significantly greater cartilage T2 values than normal controls. Elevated cartilage and meniscal T2 relaxation times were found in subjects with vs without knee pain. Increased cartilage T2 at baseline predicted morphologic degeneration in the cartilage, meniscus, and bone marrow over 3 years. Furthermore, cartilage repair tissue could be non-invasively assessed by using T2 mapping. Reproducibility errors for T2 measurements were reported to be smaller than the T2 differences in healthy and diseased cartilage indicating that T2 relaxation time may be a reliable discriminatory biomarker.
Conclusions
Cartilage and meniscal T2 mapping may be suitable as non-invasive biomarker to diagnose early stages of knee OA and to monitor therapy of OA.
Keywords: Osteoarthritis, Knee, Magnetic resonance imaging, Cartilage and meniscal T2 relaxation time
Introduction
Osteoarthritis (OA) is the most common form of arthritis. The most affected joints are the knee, hip, and hands. Nearly 27 million individuals in the United States have clinically symptomatic OA, including about 9.2 million adults having symptomatic knee OA1. The predominant clinical symptoms of knee OA are pain and disability2. OA is characterized by progressive loss of articular cartilage, osteophyte formation, synovitis, and subchondral bone changes. Radiography and morphologic magnetic resonance imaging (MRI) of the knee joint are limited in their ability to detect early stages of OA and subtle changes due to therapy response, e.g., after cartilage repair procedures3,4.
The initial osteoarthritic changes include proteoglycan loss and deterioration of the collagen network within the cartilage and menisci, which cause increased mobility of water and consequentially increased water content5,6. Quantitative MRI techniques including cartilage and meniscal T2 relaxation time measurements reflect these pathophysiological changes as outlined in previous review articles about OA imaging7–11. Due to promising results, cartilage T2 measurements were included in the National Institutes of Health (NIH) sponsored Osteoarthritis Initiative (OAI), a 9 year longitudinal, observational multicenter study with 4796 participants12,13.
Thus, the purpose of this work was to review the current literature on cartilage and meniscal T2 relaxation time.
Cartilage and meniscal T2 relaxation time
Identification and selection of the literature
Electronic searches in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) were performed up to April 2013 to identify relevant studies for this review. No starting date was entered for the electronic searches to obtain the entire literature available in PubMed. Search terms used included “Osteoarthritis”, “Knee”, “Magnetic Resonance Imaging”, and “Cartilage T2”. The search was restricted to studies about humans. The reference lists of relevant articles were also screened.
Background on T2 measurements and comparison with other quantitative MRI techniques
Cartilage is composed by chondrocytes surrounded by the extracellular matrix, which consists primarily of water, proteoglycans (core protein with attached glycosaminoglycan (GAG) chains), and collagen fibers. These macromolecules restrict the motion of water in the extracellular matrix. Pathophysiological processes of early cartilage degeneration are characterized by GAG loss and deterioration of the collagen network causing increased mobility of water and consequentially increased water content within the cartilage5,6. These changes in the extracellular matrix can be detected by quantitative MRI techniques including sodium-23 (23Na) MRI, glycosaminoglycan chemical exchange saturation transfer (gagCEST), delayed gadolinium enhanced MRI of the cartilage (dGEMRIC), diffusion-weighted and diffusion tensor imaging (DWI and DTI, respectively), T1rho and T2 relaxation time measurements.
GAG has a negative fixed-charge density resulting in the attraction of positive counterions such as sodium. Thus, a loss of GAG due to early cartilage degeneration results in the loss of sodium ions, which can be measured by 23Na MRI14. Furthermore, Ling et al. demonstrated that the GAG concentration closely correlates with measures using gagCEST15. 23Na MRI and gagCEST have shown promising results in the assessment of cartilage repair procedures16–18. However, 23Na MRI and gagCEST are technically challenging and have been performed mostly on 7 T MRI systems to obtain a sufficient signal-to-noise ratio (SNR) and spatial resolution19. Therefore, these techniques are not available at the moment for broad clinical use.
The dGEMRIC technique requires an injection of the negatively charged contrast agent gadopentate dimeglumine (Gd-DTPA2–). The contrast agent penetrates into the cartilage and distributes within the cartilage in an inverse relationship to the concentration of negatively charged GAG20. Thus, the GAG content of the articular cartilage can be estimated from measuring the T1 values after penetration of Gd-DTPA2–. It has been demonstrated that dGEMRIC improves the differentiation of disease status and may predict the development of knee OA21,22. The drawbacks of the dGEMRIC technique are related to the need for the contrast agent injection and the relatively long examination time.
DWI, DTI, T1rho and T2 relaxation time measurements do not require a contrast agent injection and can be performed on most clinical 1.5 and 3.0 T MRI systems.
The increased mobility of water in early diseased cartilage can be assessed by DWI. Apparent diffusion coefficients (ADCs) were elevated in degenerated compared to healthy cartilage and provided additional information about cartilage repair tissue beyond morphological MRI23–25. DTI of the cartilage measures diffusion anisotropy (e.g., fractional anisotropy), which is an indirect measurement of the collagen architecture26. Raya et al. reported decreased fractional anisotropy in samples with cartilage degeneration, suggesting a good performance of DTI for detection of early cartilage degeneration27.
In contrast to DWI and DTI, the performance of cartilage and meniscal T1rho and T2 relaxation time measurements as non-invasive biomarker for early stages of knee OA have been investigated in a considerable number of studies. In vitro studies observed correlations of T2 relaxation time measurements with the mechanical, histological and biochemical properties of cartilage28,29. In one of the early in-vivo studies, Dardzinski et al. performed cartilage T2 measurements in asymptomatic volunteers and observed a reproducible pattern of increasing T2 values that was proportional to the known spatial variation in cartilage water and inversely proportional to the GAG distribution30. In a subsequent study, Mosher et al. reported elevated T2 relaxation times in damaged compared to healthy articular cartilage31. While T2 measurements mostly characterize the deterioration of collagen network, T1rho relaxation time is a more specific indicator of the GAG content32. However, Li et al. found high correlations between T1rho and T2 relaxation times33. Although T1rho measurements have been developed over 20 years ago for in-vivo imaging and have been prototyped by many groups, this method remains work-in-progress.
Image acquisition
In-vivo knee cartilage T2 mapping is typically performed at clinical 1.5 and 3.0 T MRI systems. Several different sequences have been used for T2 quantification of articular cartilage including spin echo (SE), multi-echo SE, fast spin echo (FSE), and T2-prepared 3D spoiled gradient recalled (SPGR) acquisitions. Pai et al. compared these sequences and reported a considerable variability in scan time, SNR and reproducibility34.
SE sequences are most commonly used and are provided by most manufactures of MRI systems. They can be applied with different numbers and values of echo times (TE). For example, a multi-slice multi-echo (MSME) SE sequence with seven echoes (TE = 10, 20, 30, 40, 50, 60, and 70 ms), repetition time (TR) of 2700 m, and total acquisition time of 10.6 min is performed in the OAI13. However, it is important to note that MSME SE sequences acquire multi-slice data using slice-selective refocusing pulses. In the presence of miscalibration or inhomogeneities of the transmit radiofrequency field, slice-selective refocusing pulses do not result in rectangular slice profiles, leading to imperfect refocusing throughout the slice and causing stimulated echo contributions to the measured signal35,36. The occurrence of the stimulated echoes causes therefore overestimation of T2 values in MSME sequences. Excluding the first echo from the later fitting process is one way to reduce the effects from stimulated echo signal contribution in the calculated T2 values35,36. Furthermore, off-resonance effects can be generated in multi-slice acquisitions by applying refocusing pulses for other slices. This introduces magnetization transfer contrast into the images, resulting in reduced signal intensity in cartilage and thus possibly in inaccuracy of the T2 measurements. To investigate the effect of magnetization transfer on multi-slice T2 measurements, Watanabe et al. measured T2 value with single-slice and multi-slice acquisition37. They reported a substantial drop in T2 values when obtained with the multi-slice compared to the single-slice acquisition. However, no apparent interslice variation in T2 values was observed when the multi-slice acquisition was used. They concluded that multi-slice acquisition of cartilage T2 measurements is clinically applicable when inaccuracies caused by multi-slice acquisition are taken into account.
T2 mapping based on 3D SPGR overcomes the error sources of an MSME SE sequence. The sequence consists of a nonselective T2 preparation and a 3D SPGR acquisition during the transient signal evolving towards steady state34. However, this sequence has been used only in a limited number of research studies and is not included in the image protocol of large trials, e.g., the OAI38–41.
Cartilage T2 mapping has to be differentiated from cartilage T2* mapping. T2* and T2 values are related by the equation , where γ is the gyromagnetic ratio of the observed nucleus and DB0 is the magnetic field inhomogeneity. Based on the assumption that the applied static magnetic field B0 is uniform, γΔB0 is only influenced by local magnetic susceptibility fields present at the cartilage—bone interface or within the cartilage microstruc-ture42. It has been hypothesized that T2* measurements may provide a greater sensitivity to cartilage degeneration, in particular close to the cartilage—bone interface. T2* mapping may be advantageous compared to T2 mapping, since it lacks radiofrequency refocusing pulses, thus avoiding errors resulting from simulated echoes and magnetization transfer. Significant association between T2 and T2* measurements have been reported in previous studies, indicating that both measurements reflect the microstructural composition of the cartilage42–44. However, T2* mapping has not shown clear advantages beyond T2 mapping so far.
Compared to hyaline articular cartilage, menisci have shorter T2 relaxation times (5–8 m). Therefore, T2 relaxation time measurements of the menisci have been performed using acquisitions with shorter TEs, e.g., TE = 4.1, 14.5, 25.0, and 45.9 ms, as reported by Rauscher et al.40. A promising alternative to quantitatively characterize the menisci is ultra-short echo time (UTE) imaging, in particular by using high-field MRI systems45.
Image post-processing
The T2 relaxation time value for each pixel is computed by fitting the measured signal intensity S at each TE to a mono-exponential decay function S(TEi) = S0 • e(−TEi/T2), where S0 is the signal intensity at zero TE. Different fitting methods such as linear least squares, weighted linear least squares or non-linear least squares algorithms have been used to solve the equation for T2 and S046. Since the accuracy and precision of T2 is substantially affected in images with low SNR, it was suggested to use noise-corrected fitting methods, which showed better accuracy and precision in phantom and in-vivo measurements with low SNR47. Finally, T2 maps displaying the computed T2 values of each pixel are generated.
Segmentation is required to extract T2 values from distinct cartilage compartments. Two different techniques have been used for cartilage segmentation: either direct segmentation on the T2 maps or segmentation on spoiled gradient echo (SPGR) or double-echo steady-state (DESS) images for cartilage volume measurements with later superimposition of these segmentations on the T2 maps.
Using the direct segmentation technique, regions of interest (ROIs) aremanually drawntodelineatecartilageareasontheT2maps.These ROIs are simultaneously defined on the T2 map and first echo of the MSME sequence in order to exclude fluid and chemical shift artifacts from the ROIs. This is done by opening separate image panels at the same time with synchronized cursor, slice number, and zoom as outlined by Stehling et al.48. The direct segmentation technique allows a fast and reliable, but manual segmentation of cartilage compartments for T2 quantifications. In contrast, (semi-) automatic segmentation software programs were developed for cartilage volume measurements using edge detection or graph-cuts algo-rithms49,50. In case of already existing segmentations for cartilage volume measurements, these can be superimposed on T2 maps to avoid a complete new segmentation for T2 quantifications. This requires software solutions to adjust for the different slice thickness of SPGR/DESS images and T2 maps, and registration of T2 maps on the SPGR/DESS images due to patient movement between DESS/SPGR and T2 mapping acquisitions50,51. After superimposition on the T2 maps, the segmented compartments often have to be manually corrected due to accidentally included fluid with elevated T2 relaxation time values.
Segmentation and T2 quantification of the articular cartilage of one knee is performed in about 60 min using the direct segmentation technique compared to a time effort up to 300 min using the segmentation superimposition techniques (including cartilage volume measurements)48. Therefore, the direct segmentation technique is recommended if only T2 quantifications are the research purpose of a study, since good agreement between the two segmentation techniques regarding T2 values have been reported48.
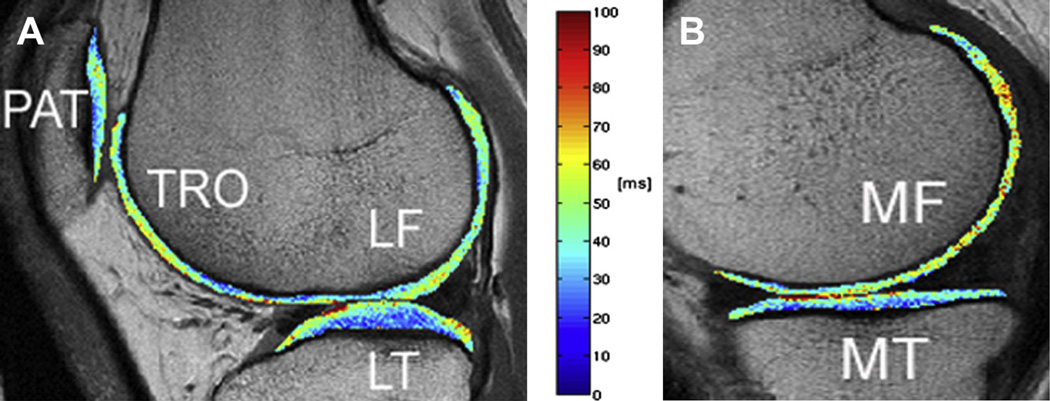
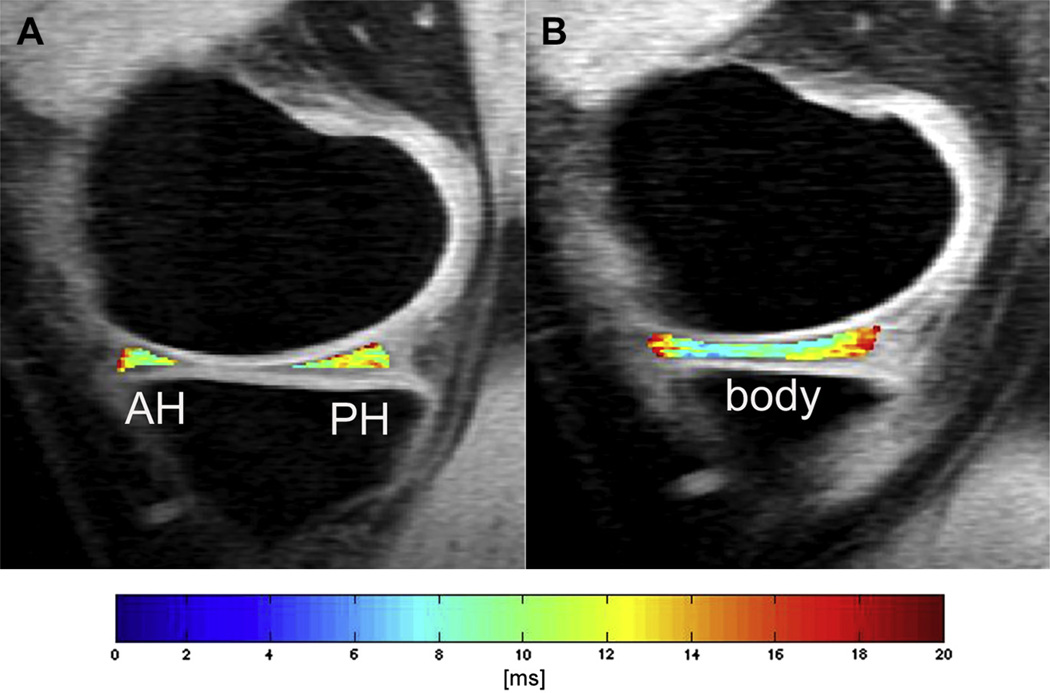
Independent of the segmentation technique, the following compartments of articular cartilage are usually investigated: patella (PAT), trochlea (TRO), medial femur (MF), lateral femur (LF), medial tibia (MT), and lateral tibia (LT). The LF/MF compartment can be subdivided in a central and posterior sub-region, the TRO and PAT in a medial, central, and lateral sub-region. The medial and lateral sub-regions of the TRO are often added to the MF and LF compartment, respectively. Representative T2 maps with the segmented PAT, TRO, MF/LF/MT/LT compartments are displayed in Fig. 1. The medial/lateral meniscus is usually divided in anterior, posterior, and body compartment as shown in Fig. 2.
Fig. 1.
Representative color-coded, sagittal T2 maps with segmented PAT, TRO, LF, and LT compartments in A, and MF and MT compartments in B. A sagittal 2D MSME SE sequence was used for T2 mapping (TR of 2700 m; seven TEs of 10 ms, 20 ms, 30 ms, 40 ms, 50 ms, 60 ms, and 70 ms; slice thickness of 3 mm with 0.5 mm gap, in-plane spatial resolution of 0.313 × 0.446 mm2, acquisition time of 10.6 min).
Fig. 2.
Representative color-coded, sagittal T2 maps with segmented anterior horn (AH) and posterior horn (PH) of the medial meniscus in A, and segmented body of medial meniscus in B. A nonselective T2 preparation and a 3D SPGR acquisition during the transient signal evolving towards steady state was used for T2 mapping (TR of 2000 ms; four TEs of 4.1 ms, 14.5 ms, 25.0 ms, and 45.9 ms; slice thickness of 3 mm; in-plane spatial resolution of 0.547 × 0.729 mm2, acquisition time of 10.6 min).
Mean T2 of all pixels included in the segmented compartment is the standard parameter of cartilage T2 mapping40,41,52–54. To account for the spatial distribution of T2 relaxation times30,36, laminar (i.e., zonal) and texture analyses were introduced as more sophisticated parameters for T2 quantifications of the articular cartilage. Laminar analysis can be automatically performed and subdivides the segmented compartment, e.g., into a superficial and deeper cartilage layer38,55,56. The superficial layer is orientated to the articular surface, the deeper layer to the cartilage–bone interface. Carballido-Gamio et al. reported significantly greater T2 relaxation times in the superficial compared to the deep cartilage layer and suggested that laminar analysis could lead to better and probably earlier identification of cartilage matrix degeneration38,55. Texture analysis was developed by Haralick et al.57. Based on grey level co-occurrence matrix (GLCM), information about the spatial distribution of T2 relaxation time values is extracted by analyzing their co-occurrences at a certain orientation and inter-pixel distance. Two texture parameters from the contrast group (contrast and homogeneity), three from the orderliness group (angular second moment, energy, and entropy) and three from the stats group (mean, variance, and correlation) were computed in previous studies38,51,58–60. To give some examples about the meaning of these texture parameter: contrast is a measure of the differences in neighboring pixel values. High T2 contrast signifies that many pixels with different T2 values are neighboring. Entropy is a measure of disorder in an image. Higher T2 entropy signifies less uniform distribution of probabilities of T2 relaxation time co-occurrences. Variance is a measure of the distribution of pixels about the mean. Higher T2 contrast, T2 entropy, and T2 variance were observed in subjects with OA risk factors compared to normal controls, thus providing additional information with respect to early cartilage degeneration59.
Reproducibility of T2 measurements
T2 relaxation time is only a discriminatory biomarker if the reproducibility error for T2 values is lower than the T2 differences in healthy and diseased cartilage. In previous studies, normal controls had between 3% and 12% lower global T2 values than subjects with OA risk factors and OA, respectively52,54,59. Glaser et al. examined the reproducibility of mean T2 values in the PAT in replicate scans56. They reported reproducibility errors for patellar mean T2 values of 3–7%. Lower reproducibility errors of mean T2 values were observed in all articular cartilage compartments for repetitive segmentation in the same T2 maps (0.3–3.3%)48. Mosher et al. demonstrated moderate to excellent reproducibility in a clinical trial network (ACRIN-PA 4001 multicenter trial)61. Reproducibility measurements were performed in subjects without as well with mild and severe radiographic OA. In total, 50 subjects underwent cartilage T2 measurements multiple times. Reproducibility errors for cartilage T2 ranged from 4% to 14%.
T2 texture parameters showed greater reproducibility errors compared to mean T2 (between 1% and 7% for T2 entropy, contrast, and variance)58,60. Reproducibility errors of meniscal T2 mapping ranged from 4.55 to 13.71% as reported by Rauscher et al.40.
The use of different radiofrequency coils and different MR scanners impact the reproducibility and comparison of T2 map-ping62,63. With higher SNR, significantly longer T2 values were measured in the deep cartilage layer of all compartments and in the whole cartilage of the MT and central MF compartments63. These error sources have to be taken into account when the clinical relevance of T2 mapping is discussed.
T2 and OA risk factors
Age, female gender, increased body mass index (BMI), and knee malalignment are well-known risk factors for knee OA64–66. Previous studies have found associations between these OA risk factors and T2 relaxation time measurements.
Elevated cartilage T2 values are associated with aging. Mosher et al. obtained patellar cartilage T2 relaxation time values from 30 asymptomatic women67. T2 values were significantly greater in the superficial 40% of cartilage in the 46–65 year old sub-group and over the entire cartilage in the 66–86 year old sub-group than in the 18–30 year old sub-group. These findings are consistent with the hypothesis that senescent changes of cartilage matrix begin near the articular surface and progress to the deeper cartilage with advancing age68. Meniscal T2 values of the posterior horn (PH) of both menisci combined were significantly greater in women aged 50–70 (1.777 ms) and 35–49 (0.741 ms) compared to women aged 20–34 (0.088 ms) (study population: 30 women)69. However, corresponding differences in men were not significant (study population: 30 men). Furthermore, Rauscher et al. observed only a relatively low correlation of meniscal T2 values with age in 23 healthy subjects (R2 = 0.18)40. Therefore, the association of meniscal T2 values and age may be considered as relatively weak.
The differences in cartilage T2 between genders have been investigated by Mosher et al., who measured cartilage T2 relaxation times in young healthy males (n = 7) and females (n = 10)70. They observed no significant differences in meanT2 and spatial variation of T2 values between males and females. However, meniscal T2 values of the PH of both menisci combined were greater in asymptomatic women compared to men69,71. In the future, additional studies or the analysis of existing OAI data are needed comparing T2 measurements of older males and females to further our knowledge about the association of cartilage T2 relaxation times and the known fact that females are at increased risk for OA.
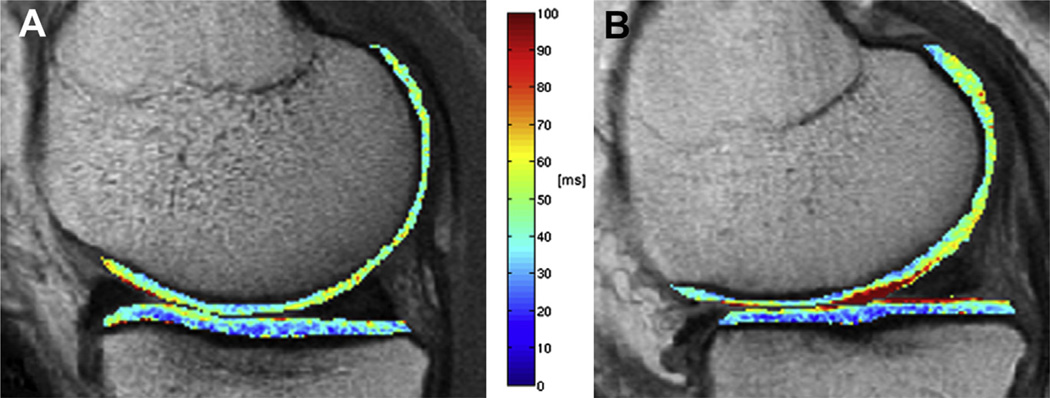
The effect of body mass on cartilage biochemical composition as assessed by T2 mapping has been investigated by Baum et al.58. They performed cartilage T2 measurements in 267 subjects aged 45–55 years consistingof 36 normal controls and 231 subjects with OA risk factors, including subjects with normal weight (n = 78), overweight (n = 84), and obesity (n = 69), respectively. They reported that obese subjects had the highest mean T2 values and the most heterogeneous cartilage as assessed by T2 texture analysis, while normal controls had the lowest meanT2 values and the most homogeneous cartilage at baseline. In addition, T2 entropy was constantly elevated over 36 months. These results indicate advanced cartilage matrix degeneration due to increased BMI and underline the importance of overweight and obesity as risk factor for knee OA (Fig. 3).
Fig. 3.
Representative color-coded, sagittal T2 maps with segmented MF and MT compartments of a normal control (A) and a subject with the OA risk factor obesity (B). Note the elevated cartilage T2 values in the obese subject compared to the normal control. A sagittal 2D multi-slice multi-echo (MSME) SE sequence was used for T2 mapping (TR of 2700 m; seven TEs of 10 ms, 20 ms, 30 ms, 40 ms, 50 ms, 60 ms, and 70 ms; slice thickness of 3 mm with 0.5 mm gap, in-plane spatial resolution of 0.313 × 0.446 mm2, acquisition time of 10.6 min).
Knee malalignment has been identified as risk factor for the progression of knee OA, but controversial findings have been reported for the association between knee malalignment and incident knee OA66. Friedrich et al. observed greater T2 values in subjects with medial knee OA and varus compared to those with valgus malalignment in all compartments of the femoro-tibial joint, adding support to the hypothesis of an association between OA and knee malalignment (study population: n = 24)72. However, longitudinal studies are needed investigating the relation of knee malalignment and early cartilage matrix degeneration as assessed by T2 mapping.
T2 and physical activity
Acute loading of the knee during MR scanning resulted in a significant decrease in T2 relaxation times of the articular knee cartilage due to a loss of water content (study population: n = 30)73. Similarly, T2 values directly measured after running were decreased, while temporary non-weight bearing of the articular cartilage was associated with elevated T2 relaxation times74–76.
Stehling et al. obtained meniscal T2 measurements of 13 marathon runners before, 48–72 h after, and 3 months after competition and compared them with those of 10 controls77. All marathon runners showed a significant increase in T2 values after competition in all meniscus compartments, which decreased after 3 months. Similar results were reported for articular cartilage T2 measurements by Luke et al.78. It remains to be investigated whether these changes are completely reversible or whether the biochemical composition of cartilage is partly irreversibly altered due to heavy loading conditions.
Regarding long-term loading, recent studies examined the association of physical activity and T2 measurements in subjects with risk factors for knee OA79,80. Physical activity was assessed by using the Physical Activity Scale for the Elderly (PASE). Subjects with high PASE scores perform greater amount of physical activity than subjects with low PASE scores. In addition to higher prevalence and severity of meniscal and cartilage lesions, significantly higher patellar T2 values were observed in subjects with high PASE scores compared to subjects with low PASE scores (study population: n = 120)79. Hovis et al. reported that light exercise was associated with low T2 values, whereas moderate/strenuous exercise in women with OA risk factors was associated with high T2 values (study population: n = 161)80.
Future studies are needed to determine the causality of long-term loading and (pathophysiologic) adaptation processes of the cartilage.
T2 and morphologic findings of OA
Mean T2 and T2 texture parameters of the articular cartilage adequately differentiated subjects with radiographic OA and normal controls with AUC values up to 0.8238,81. Similar findings were reported for meniscal T2 measurements40. It is important to note that these studies had limitations. The studies by Rauscher et al. (study population: n = 60) and Carballido-Gamio et al. (study population: n = 36) investigating meniscal and cartilage T2 values had a cross-sectional study design38,40. In contrast, Blumenkrantz et al. assessed mean T2 and T2 texture parameter over 9 months81. However, the study population was relatively small (eight mild OA patients and 10 age-matched controls).
Interestingly, early cartilage matrix degeneration due to prevalent OA risk factors could be sensitively detected by T2 mapping, but not with radiography. Joseph et al. compared normal controls and subjects, who had diverse OA risk factors including history of knee injury or surgery, but no radiographic OA (Kellgren–Lawrence (KL) score of zero) (study population: n = 145)59. They found higher and more heterogeneous knee cartilage T2 in subjects with OA risk factors than in normal controls (mean T2 averaged over all compartments: 32.65 ± 1.55 ms vs 32.07 ± 1.38 ms). Baum et al. reported similar findings in a longitudinal study over 24 months52: subjects with OA risk factors, but no radiographic OA (n = 101) showed elevated T2 values compared to normal controls (n = 41) at baseline and 24 month follow-up (MF compartment at baseline: 37.9 ± 2.3 ms vs 36.9 ± 2.3 ms; MF compartment at 24 month follow-up: 38.2 ± 2.7 vs 36.8 ± 2.1). Both studies included only subjects with a BMI of 19–27 kg/m2 to exclude obesity as an OA risk factor. Furthermore, differences in T2 values between subjects with and without OA risk factors were assessed by using multivariate linear regression models adjusting for age, gender, and BMI. Therefore, the elevated and more heterogeneous cartilage T2 in subjects with compared to those without OA risk factors are not associated with overweight/obesity, but with the other OA risk factors as defined by the OAI study protocol (history of knee injury, history of knee surgery, etc.)12.
Furthermore, Baum et al. assessed in this study cartilage lesions by using the whole-organ magnetic resonance imaging score (WORMS) and observed significantly higher T2 values in subjects with compared to those without cartilage lesions (WORMS grade >0 vs WORMS grade of 0) at baseline and 24 month follow-up52,82. Similar findings were reported for meniscal lesions and alterations of the subchondral bone. The prevalence of meniscal lesions was associated with elevated meniscal T2 as well as increased T2 of the adjacent articular cartilage41,83,84. Bining et al. compared articular cartilage T2 values of 88 subjects with vs 60 subjects without bone marrow edema pattern (BMEP)85. They demonstrated that subjects with BMEP had significantly increased T2 values in the adjacent articular cartilage. Quantitative assessment of the subchondral bone microstructure revealed further insights in the complex pathophysiology of knee OA. Bolbos et al. compared articular cartilage T2 values and microstructure parameters of the underlying trabecular bone of 16 healthy controls and 16 patients with early OA86. Early OA patients had significantly greater T2 and apparent trabecular separation values as well as lower apparent bone volume fraction than normal controls. In consistency, a recent study demonstrated that subjects with lesions in the PH of the medial meniscus had lower apparent bone volume fraction and greater apparent trabecular thickness in the subchondral bone as well as higher T2 relaxation times in the deep layer of the articular cartilage (study population: n = 59)87. These findings suggest that changes of bone, cartilage, and meniscus may be intimately related.
Furthermore, longitudinal studies demonstrated that cartilage T2 measurements at baseline predicted progression of focal knee lesions over 24 and 36 months, respectively39,60. Joseph et al. analyzed focal knee lesions as assessed by the WORMS, mean T2, and T2 texture parameters in 289 subjects with OA risk factors at baseline and 36 month follow-up60,82. They reported that elevated T2 and more heterogeneous T2 (based on T2 texture analysis) at baseline were associated with longitudinal morphologic degeneration in the cartilage, meniscus, and bone marrow over 3 years. Prasad et al. made similar observations with increased T2 measurements at baseline as predictor of progression of cartilage abnormalities over a period of 2 years (study population: n = 55)39. These results demonstrate that T2 may be an early biomarker for future morphologic degeneration associated with OA.
T2 and OA associated knee pain
It was reported previously that focal knee lesions including meniscal tears, BMEP as well as synovitis and joint effusion were associated with pain severity in subjects with radiographic OA88. Baum et al. investigated the association between focal knee lesions and cartilage T2 with knee pain status in subjects without radiographic OA, but with OA risk factors53. They selected 42 subjects aged 45–55 years, right knee pain (Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score >5), and no left knee pain (WOMAC pain score of 0). Two comparison groups consisting of 42 subjects with no knee pain (WOMAC pain score 0 in both knees) and 42 subjects with bilateral knee pain (WOMAC pain score >5 in both knees) were also recruited. The groups were frequency matched by sex, age, BMI, and KL score. Prevalence and severity of focal knee lesions as assessed by the WORMS grading and cartilage T2 values of the three groups were compared. Only cartilage lesions were significantly associated with knee pain status. However, elevated cartilage T2 values were observed in subjects with knee pain compared to asymptomatic subjects (mean cartilage T2 averaged over all compartments in the right knee: subjects with without knee pain: 32.4 ± 1.8 ms; subjects with right knee pain: 34.4 ± 1.8 ms; subjects with bilateral knee pain: 34.7 ± 4.7 ms). Similarly, Zarins et al. observed correlations of r = 0.55 for meniscal T2 values and pain score (study population: n = 63)41.
Cartilage and meniscal T2 relaxation times reflect knee pain prevalence. However, cartilage itself cannot directly generate pain, since it does not contain nerve fibers89. Subchondral bone and synovium may be responsible for nociceptive stimuli in OA and not the cartilage itself. Therefore, the pathomechanism between cartilage matrix degeneration as assessed by T2 mapping and knee pain status remains unclear. In the future, the association of T2 mapping and knee pain has to be investigated in longitudinal studies.
T2 and cartilage repair tissue
Cartilage repair procedures are of interest due to their potential to provide pain relief and alter the progression of OA90. It was hypothesized that T2 measurements provide additional information about the outcome of cartilage repair procedures.
Healthy cartilage and cartilage repair tissue could be adequately differentiated by dGEMRIC, T2, and T2* mapping43,44. In particular, zonal T2 measurements revealed differences between healthy cartilage and cartilage repair tissue in subjects after matrix-associated autologous chondrocyte transplantation (MACT). While healthy cartilage showed a significant increase from deep to superficial cartilage zones, cartilage repair tissue did not show a significant stratification of T2 values.
T2 measurements were also able to detect compositional differences in cartilage repair tissue following different repair procedures. Welsch et al. compared cartilage T2 values after microfracture therapy (MFX) and MACT in two studies (study population: n = 20 and n = 34, respectively)91,92. Global T2 of control articular cartilage areas were similar in the MFX group (57.8 ± 8.7 ms) and MACT group (56.7 ± 6.0 ms)91. Compared to patients after MACT, global mean T2 in the cartilage repair area was significantly reduced in patients after MFX. The global T2 of cartilage repair tissue in patients after MFX amounted 47.3 ± 10.3 ms, whereas those in patients after MACT amounted of 56.4 ± 9.6. Furthermore, repair tissue after MACT showed a zonal variation in T2 values with a significant increase from deep to superficial zones. In contrast, no significant trend between different zones was observed for repair tissue after MFX. Previous histologic evaluation of repair tissue after MFX revealed disorganized fibrocartilage, whereas repair tissue after MACT was described as hyaline-like with a normal zonal collagen organization90. Thus, the T2 measurements of cartilage repair tissue performed by Welsch et al. were able to characterize these histologic differences with reduced global T2 values and less zonal T2 variation in subjects after MFX compared to those after MACT91,92.
Subsequent studies confirmed the ability of T2 mapping to detect differences in cartilage repair tissue after different repair procedures. Salzmann et al. obtained T2 maps of 18 patients, who underwent MACT or osteochondral autograft transplantation (OCT), and reported significantly lower T2 values in cartilage repair tissue after MACT compared to OCT93. Welsch et al. obtained T2 measurements of cartilage repair tissue after MACT using either a hyaluronic-based or a collagen-based scaffold (study population: n = 20)94. They observed greater T2 values in the cartilage repair tissue using the collagen-based scaffold, indicating differences in the composition of the repair tissue even 2 years post-implantation.
Ideally, cartilage repair tissue develops over time a collagen network with a zonal organization similar to normal hyaline cartilage. It was demonstrated that zonal T2 measurements may be sensitive to characterize the maturation of cartilage repair tissue95. Welsch et al. obtained T2 measurements in 15 patients treated MACT 19.7 ± 12.1 months after surgery and 1 year later. Global T2 values showed no significant difference between sites of healthy cartilage and cartilage repair tissue. While healthy cartilage showed a significant T2 increase from the deep to superficial cartilage zone at both time points, a significant zonal T2 stratification in the repair tissue could be observed only in the later MRI exam. Similar findings were reported by Theologis et al.96. Thus, zonal T2 mapping may be able to visualize the maturation process of cartilage repair tissue.
In the lights of these results, Welsch et al. compared the Lysholm score (patient report on knee function) and the magnetic resonance observation of cartilage repair tissue (MOCART) score with T2 measurements in their ability to assess differences between cartilage repair tissue after MFX and MACT25. While no differences between MFX and MACT patients were observed by using the Lysholm and MOCART score, T2 measurements were lower in the cartilage repair tissue of patients after MFX compared to those after MACT. Therefore, T2 measurements may be a promising tool to assess non-invasively the different ultra-structural outcome and efficacy of cartilage repair techniques, which are currently incompletely evaluated with clinical and morphological information. However, it is important to note that up to now clinical outcome and T2 values showed only a weak to moderate correlation (mean r = 0.53) according to a recent review article by de Windt et al.97.
Limitations and future developments
Cartilage and meniscal T2 mapping have a number of limitations, which do not allow the routine clinical utilization of T2 values at the moment. The major limitations and future developments that could advance the use of T2 are discussed in this section.
T2 values obtained with different acquisition methods and at different MRI scanners showed substantial variations34. The MSME SE sequence has been most commonly used. Using this sequence, error sources including stimulated echoes and magnetization transfer have to be considered. Furthermore, more than two echoes are preferable for an acceptable SNR and accurate fitting process46,47. Thus, the same T2 acquisition method and calibration procedures are mandatory to assure a reliable comparison of T2 measurements longitudinally and across different MRI scanners. The quality assurance methods from the OAI are a step into this direction98.
Practical issues have to be considered for T2 acquisition. Significant differences between cartilage T2 values obtained at the beginning and at the end of the MRI examination were reported by Apprich et al. resulting from the different states of unloading of the knee in the course of the MRI examination due to the supine position of the patient99. Therefore, the timepoint of T2 acquisition has to be considered in the MRI protocol. Apprich et al. recommended to measure T2 after unloading, i.e., at the end of the MRI examination.
Zonal T2 measurements, particularly important for the assessment of cartilage repair tissue, and T2 texture analysis may be affected by partial volume effects. Thus, the results concerning zonal T2 variation of cartilage repair tissue or cartilage T2 heterogeneity in general have to be interpreted carefully.
The reproducibility errors for T2 mapping (repetitive analyses as well as measurements) were all obtained in a research setting. Given the working conditions of a radiologist in clinical routine, T2 reproducibility errors are expected to increase when a radiologist performs the segmentation in a clinical setting. Therefore, a fully-automated segmentation algorithm for T2 maps seems to be the best way to implement T2 relaxation time measurements in clinical practice. Alternatively, T2 maps may be inspected visually to detect elevated cartilage T2 values. Kijowski et al. demonstrated that the addition of T2 mapping to the routine MRI protocol could improve the diagnostic performance in the detection of surgically confirmed cartilage lesions100.
Lastly, T2 mapping has been used for the assessment of cartilage repair procedures, but has not been included in clinical trials with pharmaceutical intervention. Based on the published studies, it may be justified to adopt T2 relaxation time measurements in such a trial.
Conclusions
T2 relaxation time measurements showed promising results in multiple studies to assess non-invasively early cartilage degeneration reflecting changes of the biochemical composition of the articular cartilage and menisci. Most importantly, cartilage T2 mapping showed the potential to (1) predict longitudinal morphologic degeneration in the cartilage, meniscus, and bone marrow and (2) monitor subtle changes due to therapy response after cartilage repair procedures.
Acknowledgments
Role of the funding source
This work was in parts supported by NIH U01AR059507 (to TML), P50 (project 3, AR060752-01) (to TML) and F32AR059478-01 (to GBJ).
Footnotes
Authors’ contributions
Conception and design: TB, TML, JSB; Analysis and interpretation of the available literature: TB, GBJ, DCK, PMJ, TML, JSB; Drafting of the article: TB; Critical revision of the article for important intellectual content: GBJ, DCK, PMJ, TML, JSB; Final approval of the article: TB, GBJ, DCK, PMJ, TML, JSB.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
T. Baum, Email: thbaum@gmx.de.
G.B. Joseph, Email: gabby.joseph@ucsf.edu.
D.C. Karampinos, Email: dimitrios.karampinos@tum.de.
P.M. Jungmann, Email: pia.jungmann@tum.de.
T.M. Link, Email: thomas.link@ucsf.edu.
J.S. Bauer, Email: jsb@tum.de.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoar-thritis and the genesis of pain. Rheum Dis Clin North Am. 2008;34:623–643. doi: 10.1016/j.rdc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012;14:212. doi: 10.1186/ar3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Li L, et al. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage. 2011;19:557–588. doi: 10.1016/j.joca.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dijkgraaf LC, de Bont LG, Boering G, Liem RS. Normal cartilage structure, biochemistry, and metabolism: a review of the literature. J Oral Maxillofac Surg. 1995;53:924–929. doi: 10.1016/0278-2391(95)90283-x. [DOI] [PubMed] [Google Scholar]
- 6.Roughley PJ, Lee ER. Cartilage proteoglycans: structure and potential functions. Microsc Res Tech. 1994;28:385–397. doi: 10.1002/jemt.1070280505. [DOI] [PubMed] [Google Scholar]
- 7.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 8.Roemer FW, Crema MD, Trattnig S, Guermazi A. Advances in imaging of osteoarthritis and cartilage. Radiology. 2011;260:332–354. doi: 10.1148/radiol.11101359. [DOI] [PubMed] [Google Scholar]
- 9.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31:37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burstein D, Gray ML. Is MRI fulfilling its promise for molecular imaging of cartilage in arthritis? Osteoarthritis Cartilage. 2006;14:1087–1090. doi: 10.1016/j.joca.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Quatman CE, Hettrich CM, Schmitt LC, Spindler KP. The clinical utility and diagnostic performance of magnetic resonance imaging for identification of early and advanced knee osteoarthritis: a systematic review. Am J Sports Med. 2011;39:1557–1568. doi: 10.1177/0363546511407612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteo-arthritis imaging e the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012;8:622–630. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterfy CG, Schneider E, Nevitt M. The Osteoarthritis Initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium MRI of human articular cartilage in vivo. Magn Reson Med. 1998;39:697–701. doi: 10.1002/mrm.1910390505. [DOI] [PubMed] [Google Scholar]
- 15.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci U S A. 2008;105:2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krusche-Mandl I, Schmitt B, Zak L, Apprich S, Aldrian S, Juras V, et al. Long-term results 8 years after autologous osteochondral transplantation: 7 T gagCEST and sodium magnetic resonance imaging with morphological and clinical correlation. Osteoarthritis Cartilage. 2012;20:357–363. doi: 10.1016/j.joca.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt B, Zbyn S, Stelzeneder D, Jellus V, Paul D, Lauer L, et al. Cartilage quality assessment by using glycosamino-glycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology. 2011;260:257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 18.Trattnig S, Welsch GH, Juras V, Szomolanyi P, Mayerhoefer ME, Stelzeneder D, et al. 23Na MR imaging at 7 T after knee matrix-associated autologous chondrocyte transplantation preliminary results. Radiology. 2010;257:175–184. doi: 10.1148/radiol.10100279. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Haris M, Cai K, Kassey VB, Kogan F, Reddy D, et al. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3 T and 7 T. Magn Reson Med. 2012;68:588–594. doi: 10.1002/mrm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray ML, Burstein D, Kim YJ, Maroudas A. 2007 Elizabeth Winston Lanier Award Winner. Magnetic resonance imaging of cartilage glycosaminoglycan: basic principles, and clinical applications. J Orthop Res. 2008;26:281–291. doi: 10.1002/jor.20482. [DOI] [PubMed] [Google Scholar]
- 21.Owman H, Tiderius CJ, Neuman P, Nyquist F, Dahlberg LE. Association between findings on delayed gadolinium-enhanced magnetic resonance imaging of cartilage and future knee osteoarthritis. Arthritis Rheum. 2008;58:1727–1730. doi: 10.1002/art.23459. [DOI] [PubMed] [Google Scholar]
- 22.Williams A, Sharma L, McKenzie CA, Prasad PV, Burstein D. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage in knee osteoarthritis: findings at different radiographic stages of disease and relationship to malalign-ment. Arthritis Rheum. 2005;52:3528–3535. doi: 10.1002/art.21388. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich KM, Mamisch TC, Plank C, Langs G, Marlovits S, Salomonowitz E, et al. Diffusion-weighted imaging for the follow-up of patients after matrix-associated autologous chondrocyte transplantation. Eur J Radiol. 2010;73:622–628. doi: 10.1016/j.ejrad.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Mlynarik V, Sulzbacher I, Bittsansky M, Fuiko R, Trattnig S. Investigation of apparent diffusion constant as an indicator of early degenerative disease in articular cartilage. J Magn Reson Imaging. 2003;17:440–444. doi: 10.1002/jmri.10276. [DOI] [PubMed] [Google Scholar]
- 25.Welsch GH, Trattnig S, Domayer S, Marlovits S, White LM, Mamisch TC. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthritis Cartilage. 2009;17:1219–1227. doi: 10.1016/j.joca.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Filidoro L, Dietrich O, Weber J, Rauch E, Oerther T, Wick M, et al. High-resolution diffusion tensor imaging of human patellar cartilage: feasibility and preliminary findings. Magn Reson Med. 2005;53:993–998. doi: 10.1002/mrm.20469. [DOI] [PubMed] [Google Scholar]
- 27.Raya JG, Melkus G, Adam-Neumair S, Dietrich O, Mutzel E, Reiser MF, et al. Diffusion-tensor imaging of human articular cartilage specimens with early signs of cartilage damage. Radiology. 2013;266:831–841. doi: 10.1148/radiol.12120954. [DOI] [PubMed] [Google Scholar]
- 28.Lammentausta E, Kiviranta P, Nissi MJ, Laasanen MS, Kiviranta I, Nieminen MT, et al. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: relationships with tissue mechanical properties. J Orthop Res. 2006;24:366–374. doi: 10.1002/jor.20041. [DOI] [PubMed] [Google Scholar]
- 29.Nissi MJ, Toyras J, Laasanen MS, Rieppo J, Saarakkala S, Lappalainen R, et al. Proteoglycan and collagen sensitive MRI evaluation of normal and degenerated articular cartilage. J Orthop Res. 2004;22:557–564. doi: 10.1016/j.orthres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205:546–550. doi: 10.1148/radiology.205.2.9356643. [DOI] [PubMed] [Google Scholar]
- 31.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2 – preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 32.Regatte RR, Akella SV, Borthakur A, Reddy R. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging. 2003;17:114–121. doi: 10.1002/jmri.10228. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929–936. doi: 10.1002/mrm.20609. [DOI] [PubMed] [Google Scholar]
- 34.Pai A, Li X, Majumdar S. A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging. 2008;26:1215–1220. doi: 10.1016/j.mri.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging. 2003;17:358–364. doi: 10.1002/jmri.10263. [DOI] [PubMed] [Google Scholar]
- 36.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe A, Boesch C, Obata T, Anderson SE. Effect of mul-tislice acquisition on T1 and T2 measurements of articular cartilage at 3 T. J Magn Reson Imaging. 2007;26:109–117. doi: 10.1002/jmri.20962. [DOI] [PubMed] [Google Scholar]
- 38.Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009;36:4059–4067. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T(1rho) and T(2) relaxation times predict progression of knee oste-oarthritis. Osteoarthritis Cartilage. 2013;21:69–76. doi: 10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rauscher I, Stahl R, Cheng J, Li X, Huber MB, Luke A, et al. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249:591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteo-arthritis. Osteoarthritis Cartilage. 2010;18:1408–1416. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamisch TC, Hughes T, Mosher TJ, Mueller C, Trattnig S, Boesch C, et al. T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol. 2012;41:287–292. doi: 10.1007/s00256-011-1171-x. [DOI] [PubMed] [Google Scholar]
- 43.Welsch GH, Mamisch TC, Hughes T, Zilkens C, Quirbach S, Scheffler K, et al. In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest Radiol. 2008;43:619–626. doi: 10.1097/RLI.0b013e31817e9122. [DOI] [PubMed] [Google Scholar]
- 44.Welsch GH, Trattnig S, Hughes T, Quirbach S, Olk A, Blanke M, et al. T2 and T2* mapping in patients after matrix-associated autologous chondrocyte transplantation: initial results on clinical use with 3.0-Tesla MRI. Eur Radiol. 2010;20:1515–1523. doi: 10.1007/s00330-009-1669-y. [DOI] [PubMed] [Google Scholar]
- 45.Robson MD, Bydder GM. Clinical ultrashort echo time imaging of bone and other connective tissues. NMR Biomed. 2006;19:765–780. doi: 10.1002/nbm.1100. [DOI] [PubMed] [Google Scholar]
- 46.Koff MF, Amrami KK, Felmlee JP, Kaufman KR. Bias of cartilage T2 values related to method of calculation. Magn Reson Imaging. 2008;26:1236–1243. doi: 10.1016/j.mri.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C. T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med. 2010;63:181–193. doi: 10.1002/mrm.22178. [DOI] [PubMed] [Google Scholar]
- 48.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging – data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bae KT, Shim H, Tao C, Chang S, Wang JH, Boudreau R, et al. Intra- and inter-observer reproducibility of volume measurement of knee cartilage segmented from the OAI MR image set using a novel semi-automated segmentation method. Osteoarthritis Cartilage. 2009;17:1589–1597. doi: 10.1016/j.joca.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carballido-Gamio J, Bauer J, Lee KY, Krause S, Majumdar S. Combined image processing techniques for characterization of MRI cartilage of the knee. Conf Proc IEEE Eng Med Biol Soc. 2005;3:3043–3046. doi: 10.1109/IEMBS.2005.1617116. [DOI] [PubMed] [Google Scholar]
- 51.Carballido-Gamio J, Link TM, Majumdar S. New techniques for cartilage magnetic resonance imaging relaxation time analysis: texture analysis of flattened cartilage and localized intra- and inter-subject comparisons. Magn Reson Med. 2008;59:1472–1477. doi: 10.1002/mrm.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the Osteoarthritis Initiative. J Magn Reson Imaging. 2012;35:370–378. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64:248–255. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Benjamin MC, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteo-arthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carballido-Gamio J, Blumenkrantz G, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T(2) knee cartilage laminar organization in a subset of patients from the Osteo-arthritis Initiative. Magn Reson Med. 2010;63:465–472. doi: 10.1002/mrm.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glaser C, Mendlik T, Dinges J, Weber J, Stahl R, Trumm C, et al. Global and regional reproducibility of T2 relaxation time measurements in human patellar cartilage. Magn Reson Med. 2006;56:527–534. doi: 10.1002/mrm.21005. [DOI] [PubMed] [Google Scholar]
- 57.Haralick R, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;SMC-3:610–621. [Google Scholar]
- 58.Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six-month followup data from a longitudinal observational multicenter study. Arthritis Care Res (Hoboken) 2013;65:23–33. doi: 10.1002/acr.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls – data from the Osteoarthritis Initiative. Arthritis Res Ther. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean heterogeneity of MRcartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years – data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20:727–735. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee articular cartilage damage in osteo-arthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258:832–842. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balamoody S, Williams TG, Wolstenholme C, Waterton JC, Bowes M, Hodgson R, et al. Magnetic resonance transverse relaxation time T2 of knee cartilage in osteoarthritis at 3-T: a cross-sectional multicentre, multivendor reproducibility study. Skeletal Radiol. 2013;42:511–520. doi: 10.1007/s00256-012-1511-5. [DOI] [PubMed] [Google Scholar]
- 63.Dardzinski BJ, Schneider E. Radiofrequency (RF) coil impacts the value and reproducibility of cartilage spin-spin (T2) relaxation time measurements. Osteoarthritis Cartilage. 2013;21:710–720. doi: 10.1016/j.joca.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brennan SL, Cicuttini FM, Pasco JA, Henry MJ, Wang Y, Kotowicz MA, et al. Does an increase in body mass index over 10 years affect knee structure in a population-based cohort study of adult women? Arthritis Res Ther. 2010;12:R139. doi: 10.1186/ar3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459–467. doi: 10.1002/art.24336. [DOI] [PubMed] [Google Scholar]
- 67.Mosher TJ, Liu Y, Yang QX, Yao J, Smith R, Dardzinski BJ, et al. Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum. 2004;50:2820–2828. doi: 10.1002/art.20473. [DOI] [PubMed] [Google Scholar]
- 68.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoar-thritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiang SW, Tsai PH, Chang YC, Wang CY, Chung HW, Lee HS, et al. T2 values of posterior horns of knee menisci in asymptomatic subjects. PLoS One. 2013;8:e59769. doi: 10.1371/journal.pone.0059769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mosher TJ, Collins CM, Smith HE, Moser LE, Sivarajah RT, Dardzinski BJ, et al. Effect of gender on in vivo cartilage magnetic resonance imaging T2 mapping. J Magn Reson Imaging. 2004;19:323–328. doi: 10.1002/jmri.20013. [DOI] [PubMed] [Google Scholar]
- 71.Tsai PH, Chou MC, Lee HS, Lee CH, Chung HW, Chang YC, et al. MR T2 values of the knee menisci in the healthy young population: zonal and sex differences. Osteoarthritis Cartilage. 2009;17:988–994. doi: 10.1016/j.joca.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Friedrich KM, Shepard T, Chang G, Wang L, Babb JS, Schweitzer M, et al. Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur Radiol. 2010;20:1532–1538. doi: 10.1007/s00330-009-1689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Souza RB, Stehling C, Wyman BT, Hellio Le Graverand MP, Li X, Link TM, et al. The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Oste-oarthritis Cartilage. 2010;18:1557–1563. doi: 10.1016/j.joca.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234:245–249. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- 75.Souza RB, Baum T, Wu S, Feeley BT, Kadel N, Li X, et al. Effects of unloading on knee articular cartilage T1rho and T2 magnetic resonance imaging relaxation times: a case series. J Orthop Sports Phys Ther. 2012;42:511–520. doi: 10.2519/jospt.2012.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010;18:358–364. doi: 10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stehling C, Luke A, Stahl R, Baum T, Joseph G, Pan J, et al. Meniscal T1rho and T2 measured with 3.0 T MRI increases directly after running a marathon. Skeletal Radiol. 2011;40:725–735. doi: 10.1007/s00256-010-1058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luke AC, Stehling C, Stahl R, Li X, Kay T, Takemoto S, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does longdistance running lead to cartilage damage? Am J Sports Med. 2010;38:2273–2280. doi: 10.1177/0363546510372799. [DOI] [PubMed] [Google Scholar]
- 79.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the Osteoarthritis Initiative. Radiology. 2010;254:509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, et al. Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum. 2011;63:2248–2256. doi: 10.1002/art.30419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Lu Y, Munoz T, et al. The feasibility of characterizing the spatial distribution of cartilage T(2) using texture analysis. Osteo-arthritis Cartilage. 2008;16:584–590. doi: 10.1016/j.joca.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Friedrich KM, Shepard T, de Oliveira VS, Wang L, Babb JS, Schweitzer M, et al. T2 measurements of cartilage in osteo-arthritis patients with meniscal tears. AJR Am J Roentgenol. 2009;193:411–415. doi: 10.2214/AJR.08.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kai B, Mann SA, King C, Forster BB. Integrity of articular cartilage on T2 mapping associated with meniscal signal change. Eur J Radiol. 2011;79:421–427. doi: 10.1016/j.ejrad.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 85.Bining HJ, Santos R, Andrews G, Forster BB. Can T2 relaxation values and color maps be used to detect chondral damage utilizing subchondral bone marrow edema as a marker? Skeletal Radiol. 2009;38:459–465. doi: 10.1007/s00256-008-0629-y. [DOI] [PubMed] [Google Scholar]
- 86.Bolbos RI, Zuo J, Banerjee S, Link TM, Ma CB, Li X, et al. Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis Cartilage. 2008;16:1150–1159. doi: 10.1016/j.joca.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar D, Schooler J, Zuo J, McCulloch CE, Nardo L, Link TM, et al. Trabecular bone structure and spatial differences in articular cartilage MR relaxation times in individuals with posterior horn medial meniscal tears. Osteoarthritis Cartilage. 2013;21:86–93. doi: 10.1016/j.joca.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:1033–1040. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 89.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 90.Gomoll AH, Filardo G, de GL, Espregueira-Mendes J, Marcacci M, Rodkey WG, et al. Surgical treatment for early osteoarthritis. Part I: cartilage repair procedures. Knee Surg Sports Traumatol Arthrosc. 2012;20:450–466. doi: 10.1007/s00167-011-1780-x. [DOI] [PubMed] [Google Scholar]
- 91.Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kut-scha-Lissberg F, Marlovits S, et al. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedureseinitial experience. Radiology. 2008;247:154–161. doi: 10.1148/radiol.2471070688. [DOI] [PubMed] [Google Scholar]
- 92.Welsch GH, Trattnig S, Scheffler K, Szomonanyi P, Quirbach S, Marlovits S, et al. Magnetization transfer contrast and T2 mapping in the evaluation of cartilage repair tissue with 3 T MRI. J Magn Reson Imaging. 2008;28:979–986. doi: 10.1002/jmri.21516. [DOI] [PubMed] [Google Scholar]
- 93.Salzmann GM, Paul J, Bauer JS, Woertler K, Sauerschnig M, Landwehr S, et al. T2 assessment and clinical outcome following autologous matrix-assisted chondrocyte and osteochondral autograft transplantation. Osteoarthritis Cartilage. 2009;17:1576–1582. doi: 10.1016/j.joca.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Welsch GH, Mamisch TC, Zak L, Blanke M, Olk A, Marlovits S, et al. Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med. 2010;38:934–942. doi: 10.1177/0363546509354971. [DOI] [PubMed] [Google Scholar]
- 95.Welsch GH, Mamisch TC, Marlovits S, Glaser C, Friedrich K, Hennig FF, et al. Quantitative T2 mapping during follow-up after matrix-associated autologous chondrocyte transplantation (MACT): full-thickness and zonal evaluation to visualize the maturation of cartilage repair tissue. J Orthop Res. 2009;27:957–963. doi: 10.1002/jor.20835. [DOI] [PubMed] [Google Scholar]
- 96.Theologis AA, Schairer WW, Carballido-Gamio J, Majumdar S, Li X, Ma CB. Longitudinal analysis of T1rho and T2 quantitative MRI of knee cartilage laminar organization following microfracture surgery. Knee. 2012;19:652–657. doi: 10.1016/j.knee.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Windt TS, Welsch GH, Brittberg M, Vonk LA, Marlovits S, Trattnig S, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee?: a systematic review and meta-analysis. Am J Sports Med. 2013 doi: 10.1177/0363546512473258. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 98.Schneider E, Nessaiver M. The Osteoarthritis Initiative (OAI) magnetic resonance imaging quality assurance update. Osteoarthritis Cartilage. 2013;21:110–116. doi: 10.1016/j.joca.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Apprich S, Welsch GH, Mamisch TC, Szomolanyi P, Mayerhoefer M, Pinker K, et al. Detection of degenerative cartilage disease: comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 Tesla. Osteoarthritis Cartilage. 2010;18:1211–1217. doi: 10.1016/j.joca.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 100.Kijowski R, Blankenbaker DG, Munoz Del RA, Baer GS, Graf BK. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology. 2013;267:503–513. doi: 10.1148/radiol.12121413. [DOI] [PubMed] [Google Scholar]