Abstract
Aquaporin 1 (AQP1) is a plasma membrane water-transporting protein expressed strongly in tumor microvascular endothelia. We previously reported impaired angiogenesis in implanted tumors in AQP1-deficient mice and reduced migration of AQP1-deficient endothelial cells in vitro. Here, we investigated the consequences of AQP1 deficiency in mice that spontaneously develop well-differentiated, luminal-type breast adenomas with lung metastases [mouse mammary tumor virus-driven polyoma virus middle T oncogene (MMTV-PyVT)]. AQP1+/+ MMTV-PyVT mice developed large breast tumors with total tumor mass 3.5 ± 0.5 g and volume 265 ± 36 mm3 (se, 11 mice) at age 98 d. Tumor mass (1.6±0.2 g) and volume (131±15 mm3, 12 mice) were greatly reduced in AQP1−/− MMTV-PyVT mice (P<0.005). CD31 immunofluorescence showed abnormal microvascular anatomy in tumors of AQP1−/− MMTV-PyVT mice, with reduced vessel density. HIF-1α expression was increased in tumors in AQP1−/− MMTV-PyVT mice. The number of lung metastases (5±1/mouse) was much lower than in AQP1+/+ MMTV-PyVT mice (31±8/mouse, P<0.005). These results implicate AQP1 as an important determinant of tumor angiogenesis and, hence, as a potential drug target for adjuvant therapy of solid tumors.—Esteva-Font, C., Jin, B.-J., Verkman, A. S. Aquaporin-1 gene deletion reduces breast tumor growth and lung metastasis in tumor-producing MMTV-PyVT mice.
Keywords: AQP1, water channel, endothelia
Aquaporin 1 (AQP1) is a member of a family of water-transporting proteins with 13 homologous isoforms in mammals (1). AQP1 is a small integral membrane protein present in cell plasma membranes as tetramers, in which each ∼30-kDa monomer contains a pore for selective, single-file transport of water. AQP1 is expressed broadly in microvascular endothelia, except in the central nervous system, as well as in nonvascular corneal, lymphatic, and cardiac endothelia, and in some epithelia, including kidney tubules, choroid plexus, and ciliary body (2, 3). Phenotype analysis of mice lacking AQP1 has shown its involvement in urinary concentrating function (4), aqueous humor (5), cerebrospinal fluid secretion (6), and corneal transparency (7). AQP1 functions as a plasma membrane water channel that facilitates solute-free water transport in response to a transmembrane osmotic gradient, which accounts for its role in fluid absorption and secretion.
AQP1 is strongly expressed in microvascular endothelia in all tumors that have been studied (8, 9). Postulating the involvement of AQP1 in tumor angiogenesis, we found reduced growth of implanted tumors in AQP1-null mice with abnormal vasculature, as well as impaired microvessel formation in implanted growth factor-containing Matrigel pellets (10). Evidence was found for impaired migration of AQP1-deficient endothelial cells in culture as responsible for impaired tumor angiogenesis. A mechanism was proposed in which AQP1-facilitated water influx in lamellipodia at the leading edge of migrating cells enhances lamellipodial extension and cell migration (11). A recent knockdown study further supports the involvement of AQP1 in angiogenesis (9).
In addition to the role of AQP1 in tumor angiogenesis, AQPs are expressed in various tumors (12), with AQP expression often correlating with tumor grade (13, 14). We previously found increased metastasis and local invasion of AQP-expressing tumors (15), which is probably related to increased tumor cell migration. There is also evidence for involvement of glycerol-transporting AQPs (aquaglyceroporins) in the proliferation of certain tumors, such as AQP3 in skin cancer, by a mechanism involving enhanced cell glycerol uptake and metabolism (16). AQPs are thus potential drug targets in oncology to inhibit angiogenesis, as well as to inhibit tumor cell invasion and metastasis.
The present study was done to investigate the role of AQP1 in tumor angiogenesis using a mouse model in which tumors develop spontaneously. Tumor angiogenesis involves the action of proangiogenic factors, including vascular endothelial growth factor (VEGF-A), which is released by growing tumor cells in response to nutrient and oxygen deprivation, as well as from stromal cells, macrophages, and mast cells (17). During angiogenesis, endothelial cells become motile, invasive and extend filopodia, which we propose is facilitated by AQP1 water permeability. We measured tumor growth, angiogenesis, and metastasis in mouse mammary tumor virus-driven polyoma virus middle T oncogene (MMTV-PyVT) mice, which spontaneously develop multiple primary breast tumors with lung metastases (18). MMTV-PyVT mice have been used to model estrogen receptor-negative late-stage carcinomas and breast cancer metastasis to lungs (19, 20). Following transfer of the AQP1-null genotype, we found reduced tumor growth and abnormal tumor microvasculature in AQP1-deficient MMTV-PyVT mice, and greatly reduced lung metastasis.
MATERIALS AND METHODS
Generation of MMTV-PyVT, AQP1-deficient mice
FVB/N-Tg(MMTV-PyVT)634Mul/J transgenic male mice (referred to as PyVT; ref. 18) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and crossed with AQP1−/− females in a CD1 genetic background. AQP1−/− mice were produced by targeted gene disruption and lack of AQP1 protein in all tissues (21). F1 PyVT/+ male mice were then mated with CD1 AQP1−/− or CD1 AQP1+/+ mice to produce F2 PyVT/+; AQP1−/− and PyVT/+; AQP1+/+ mice. Male PyVT/+; AQP1−/− or AQP1+/+ mice were back-crossed with CD1 AQP1−/− or CD1 AQP1+/+ females for >5 generations to obtain the study cohort. Breedings were done using male PyVT transgenic mice because female mice, although able to become pregnant and deliver litters, are unable to nurse and feed their offspring. Mouse genotype was determined by PCR analysis of tail DNA for PyVT (Laragen, Culver City, CA, USA) and for AQP1, as described previously (21). Animal protocols were approved by the University of California–San Francisco Committee on Animal Research.
Female PyVT mice were used for this study, as their reported tumor latency is 73 d, much less than 137 d in males (22). At age 98 d, mice were anesthetized, weighed, and perfused through the left cardiac ventricle with 5 ml PBS, and then 20 ml of PBS containing 4% paraformaldehyde. Breast tumors and lungs were removed, and wet weight was obtained. Breast tumor size was measured using digital calipers, and tumor volume (expressed in cubic millimeters) was calculated as π/6 × height × width × depth. Tissues were postfixed for 24 h in 4% PFA and processed for paraffin embedding.
Histology and immunohistochemistry
Paraffin-embedded breast tumors and lungs were cut in 8-μm-thick transverse sections and stained with hematoxylin and eosin (H&E). For lungs, 5 sections 300 μm apart were used for H&E staining and immunohistochemistry. For immunofluorescence, 8-μm-thick paraffin sections were deparaffinized and rehydrated in serial ethanols. Antigen retrieval was performed with citrate buffer using microwaves. After permeabilization with 0.3% Triton X-100 for 10 min and blocking with 1% BSA for 1 h, tissue sections were immunostained for 1 h with rabbit anti-AQP1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD31 (1:200; Santa Cruz Biotechnology), VEGF (1:200; Santa Cruz Biotechnology), hypoxia-inducible factor 1α (HIF-1α; 1:200; Santa Cruz Biotechnology), or VEGF receptor 2 (VEGFR2; 1:100; Cell Signaling Technology, Danvers, MA, USA). Sections were then rinsed with PBS and incubated for 1 h with 4 μg/ml donkey anti-rabbit IgG-conjugated Alexa Fluor 555, donkey anti-mouse IgG-conjugated Alexa Fluor 555, or donkey anti-rabbit IgG-conjugated Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA). Immunofluorescence was visualized on a Leica DM 4000 B microscope and captured using RT-KEISE 6 (Leica Microsystems, Mannheim, Germany) camera and Nikon Eclipse FN1 confocal microscope with D Ellipse C1 camera (Nikon Metrology, Tokyo, Japan). VEGF and HIF-1α protein expression were quantified using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Analysis of tumor microvasculature
CD31 immunofluorescence was imaged using a ×10 objective lens (30 micrographs/group). Tumor microvascular was analyzed using MatLab (23). Image analysis involved extraction of features from raw images to generate microvessel areas and lengths. Because of the complex and heterogeneous shape of tumor microvessels and the intrinsic noise in immunofluorescence micrographs, several image processing steps were required to extract meaningful information. Three image processing steps (depicted in Fig. 3A, see Results) were used: image adjustment by background noise elimination and conversion into a binary image by thresholding; closed-object recognition; and extraction of the skeleton image. Image process details are provided in Supplemental Material. Data are presented as number histograms of microvessel area and length.
Figure 3.
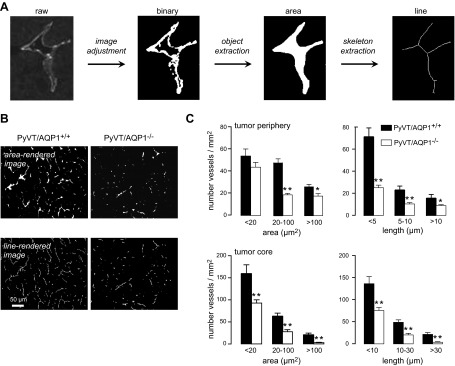
Abnormal tumor microvessels in AQP1−/− PyVT mice. A) Approach for quantification of microvessel area and length by image analysis (conversion to a binary image, object extraction, and skeleton extraction). B) Representative rendered area- and line-rendered images of CD31-stained microvessels. C) Histogram summary of microvessel areas and lengths, analyzed in micrographs from tumor periphery (top panels) and tumor core (bottom panels). Bars represent means ± se, 30 sections analyzed per group. *P < 0.05, **P < 0.005.
Statistical analysis
Statistical analysis was done using Prism 4 software (GraphPad Software, San Diego, CA, USA). Comparisons between 2 groups used Student's t test. Statistical significance was determined as values of P < 0.05.
RESULTS
Reduced breast tumor growth in AQP1−/− PyVT mice
Following backcross of the AQP1-null genotype into PyVT mice, we measured tumor size and microvasculature at 98 d, which, based on prior reports (22, 24), is sufficient for spontaneous generation of robust primary tumors with many lung metastases. A total of 11 AQP1+/+ PyVT mice and 12 AQP1−/− PyVT mice were studied. Gross visual inspection showed smaller tumors in the AQP1−/− PyVT mice. Representative photographs in Fig. 1A show well-demarcated tumor borders. Histological examination by H&E staining showed typical adenocarcinoma luminal-type tumor pathology, which was morphologically similar in the primary breast tumors of AQP1+/+ and AQP1−/− PyVT mice (Fig. 1B).
Figure 1.
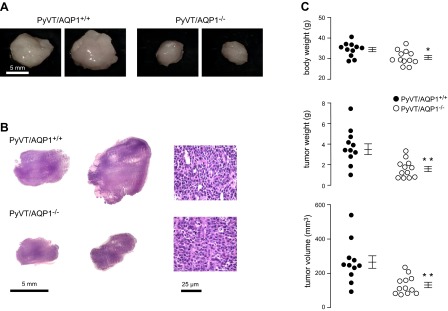
Reduced size of spontaneously formed breast tumors in AQP1-deficient PyVT female mice. A) Gross appearance of primary breast tumors from 2 PyVT/AQP1+/+ and 2 PyVT/AQP1−/− mice at age 98 d. B) Low (left panel) and high (right panel) magnification H&E staining of breast tumors. C) Body weight (top panel), total breast tumor weight (middle panel), and total breast tumor volume (bottom panel) at 98 d, with data shown for each mouse (means ± se). *P < 0.05, **P < 0.005.
Figure 1C summarizes body weight, and total tumor weight and volume of each mouse. Body weight was slightly lower (by 10±3%) in AQP1−/− than AQP1+/+ PyVT mice, which probably reflects the reduced tumor burden. Total tumor weight and volume were greatly reduced in AQP1−/− vs. AQP1+/+ PyVT mice, by 46 ± 7 and 50 ± 6%, respectively.
Abnormal tumor microvasculature in AQP1−/− PyVT mice
Tumor microvessels were visualized by CD31 immunofluorescence. Figure 2A shows colocalization of CD31 and AQP1 immunofluorescence on microvessels from primary breast tumors in AQP1+/+ PyVT mice. AQP1 showed a plasma membrane localization pattern, as expected. AQP1 immunofluorescence was absent in AQP1−/− PyVT mice. Though AQP1 was expressed in all CD31-positive tumor microvacular endothelia in AQP1+/+ PyVT mice, AQP1 was not expressed in the tumor cells (Fig. 2B). Figure 2C shows representative CD31 immunofluorescence in breast tumors from AQP1+/+ and AQP1−/− PyVT mice. At low magnification (Fig. 2C, left panels), there appeared to be fewer and smaller microvessels in tumors from AQP1−/− PyVT mice, with no obvious differences in vessel structure seen at high magnification (Fig. 2C, right panels).
Figure 2.
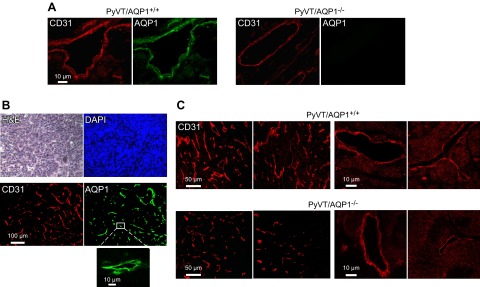
Tumor microvasculature in primary breast tumors of female AQP1+/+ and AQP1−/− PyVT mice at 98 d. A) CD31 (red) and AQP1 (green) immunofluorescence showing colocalization in tumor of AQP1+/+ PyVT mouse. B) H&E, DAPI, CD31 and AQP1 in tumor of AQP1+/+ PyVT mouse showing absence of AQP1 expression in tumor cells. C) Examples of CD31 immunofluorescence of breast tumors from 2 AQP1+/+ and AQP1−/− PyVT mice at low (left panels) and high (right panels) magnifications.
Microvascular anatomy of CD31-immunostained tumor sections was quantified by image analysis. The approach, as shown in Fig. 3A, was to apply threshold criteria to visualize CD31-positive pixels in the image, which, together with cluster/noise analysis, were used to identify microvessels and determine their area and length in the image plane. Representative area- and line-rendered images are shown in Fig. 3B. Number histograms summarizing microvessel areas and lengths at the tumor periphery and tumor core are provided in Fig. 3C. There were fewer tumor microvessels in AQP1−/− vs. AQP1+/+ PyVT mice in both the periphery and core of the tumor, with smaller area and length.
As VEGF is a major driver of tumor angiogenesis, we examined sections of primary tumors for expression of VEGF and its receptor VEGFR2, as well as HIF-1α, which increases in response to relative tumor hypoxia and stimulates VEGF production (25). Figure 4A shows comparable VEGF immunofluorescence in breast tumors of AQP1+/+ and AQP1−/− mice, and a small but significant increase in HIF-1α immunofluorescence. VEGFR2 immunofluorescence, which is expected to be concentrated in a subset of proliferating endothelial cells where the angiogenic sprout is initiated, showed greater staining in AQP1+/+ PyVT mice compared to AQP1−/− PyVT mice, with a larger number of VEGFR2-positive vessels as quantified by area and length histogram analysis (Fig. 4B). Figure 4C shows that all VEGFR2-positive tumor microvessels were also CD31-positive, but many CD31-positive microvessels were VEGFR2-negative, as expected.
Figure 4.
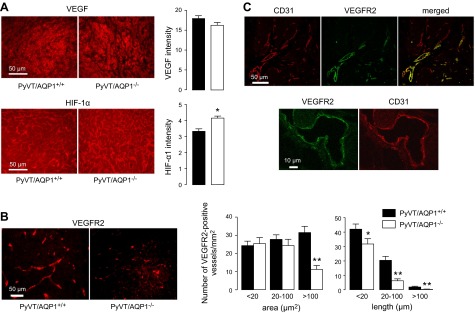
Tumor VEGF, HIF-1α, and VEGFR and immunofluorescence. A) VEGF and HIF-1α immunofluorescence in primary breast tumors of 98-d-old female PyVT mice shown with quantification of (background-subtracted) fluorescence intensity. B) VEGFR2 immunofluorescence (left panels) with quantification of stained microvessel areas and lengths shown as number histograms (right panels). Bars represent means ± se, 30 sections analyzed per group. *P < 0.05, **P < 0.005. C) CD31 and VEGFR2 immunofluorescence shown at low and high magnifications. Note VEGRF2-negative, CD31-positive tumor microvessels in merged image.
Greatly reduced lung metastases in AQP1−/− PyVT mice
Lung metastasis is a consistent finding in PyVT mice. Figure 5A (top panel) shows examples of surface tumor nodules seen in AQP1+/+ and AQP1−/− PyVT mice at age 98 d, which were not seen in control AQP1+/+ or AQP1−/− mice. Histological examination by H&E staining showed remarkably fewer metastatic nodules in lungs from AQP1−/− PyVT mice (Fig. 5A, bottom panel). Tumor histology was similar in the lung nodules from AQP1+/+ and AQP1−/− mice, and nodule size appeared smaller in AQP1−/− PyVT mice (Fig. 5B). AQP1 is expressed strongly in normal lung tissue in AQP1+/+ PyVT mice (in alveolar microvessels and erythrocytes), as expected, but is absent in the tumor cells (Fig. 5C).
Figure 5.
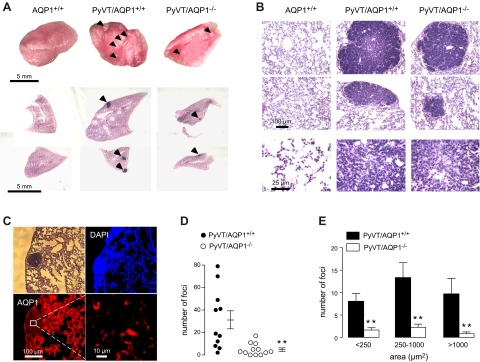
Reduced tumor metastases in lung in AQP1−/− PyVT mice. A) Gross anatomy (top panel) and low-magnification H&E-stained images of lungs showing surface tumor metastases (arrows; bottom panel) in 98-d-old female PyVT mice. B) H&E staining of lung at high magnification. C) Absence of AQP1 in tumor cells in lung metastases. D) Number of metastatic foci in AQP1+/+ and AQP1−/− PyVT mice. E) Histogram distribution summarizing metastatic nodule size (area). Values are presented as means ± se. **P < 0.005.
Figure 5D summarizes the total number of metastatic foci seen in lungs of each mouse, showing significantly few nodules in the AQP1−/− mice. Nodule size, summarized as number histogram plots (Fig. 5E), showed fewer nodules of all sizes in AQP1−/− PyVT mice than in AQP1−/− PyVT mice.
DISCUSSION
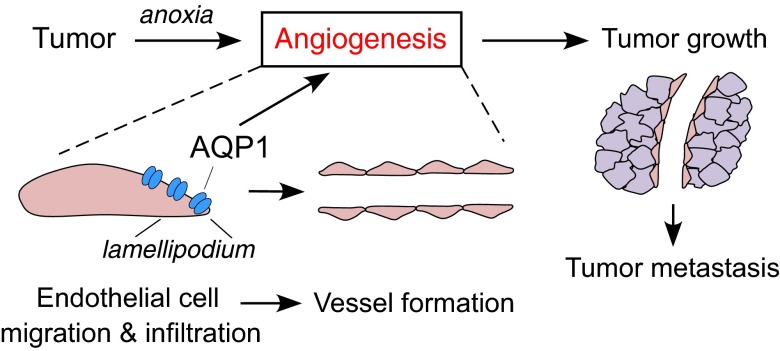
Angiogenesis is required to supply oxygen and nutrients to rapidly growing tumor cells, as well as to remove waste products. Endothelial cells comprising the tumor microvasculature originate from surrounding normal tissue in which they migrate, proliferate, and form vascular structures in response to proangiogenic factors, such as VEGF, fibroblast growth factors, HIF, platelet-derived growth factors, and angiopoietin-2 (17, 26). The initial step in angiogenesis is the endothelial cell migration. Figure 6 diagrams our proposed mechanism for involvement of AQP1 in tumor angiogenesis and growth. AQP1-facilitated plasma membrane water influx at the leading edge of migrating endothelial cells promotes lamellipodial extension and cell migration. Multiple lines of evidence support the involvement of AQPs in cell migration: facilitation of migration in many cell types; polarization of AQPs to the leading edge of migrating cells; enhanced lamellipodial dynamics in AQP-expressing cells; and influence of small osmotic gradients on cell migration (10, 15, 27–29). The slowed migration of endothelial cells in AQP1 deficiency results in impaired angiogenesis with a reduced number of microvessels and an abnormal vascular pattern with smaller and shorter vessels in AQP1-deficient mice compared to wild-type mice. The slowed tumor growth in AQP1 deficiency is a consequence of impaired angiogenesis, which reduces the delivery of oxygen and nutrients to tumor cells
Figure 6.
Proposed mechanism of AQP1-dependent tumor angiogenesis and growth. Tumor angiogenesis requires endothelial cell migration and invasion, which is facilitated by AQP1 water transport at the leading edge (lamellipodia) of migrating endothelial cells. See Discussion for further explanation.
Our results support the potential of AQP1 as a therapeutic target to reduce tumor and nontumor angiogenesis, involving the creation of AQP1-expressing microvessels. As an example of nontumor angiogenesis, AQP1 has been shown to facilitate hepatic angiogenesis in association with hepatic fibrosis and portal hypertension in cirrhosis (30). Inhibition of AQP1 function and/or expression is predicted to reduce angiogenesis. AQP1 water transport is inhibited by sulfhydryl-reactive, heavy metal-containing compounds, such as HgCl2 and organomercurials (31); however, these compounds are toxic and nonselective in their actions. Various small-molecule AQP1 inhibitors have been reported (32–34); however, many of the reported AQP1s have not been confirmed on reevaluation (35, 36), and others await corroboration using definitive assays of water permeability. Small molecules that down-regulate AQP1 expression in actively proliferating microvessels might be an alternative strategy. One study reported reduced angiogenesis in implanted melanomas injected with AQP1 siRNA (9), although the data are difficult to interpret because of unusually large effects seen and the difficulty in delivering siRNA by intratumoral injection. Monoclonal antibodies targeting AQP1 represent an alternative strategy; antibodies against extracellular epitopes on a different water channel, AQP4, are in development for therapy of the neuroinflammatory demyelinating disease neuromyelitis optica in which anti-AQP4 antibodies damage astrocytes in the central nervous system (37). AQP1-targeted antiangiogenesis therapeutics could be useful alone or together with established drugs acting on different targets, such as anti-VEGF antibodies, in inhibiting tumor angiogenesis.
We found a greatly reduced number of lung metastases in the AQP1−/− MMTV-PyVT mice. Tumor metastasis involves tumor cell migration and invasion to access and exit the vasculature, interactions with the extracellular matrix, and survival at a distant site from the primary tumor mass, as well as tumor cell proliferation and angiogenesis at the site of metastasis. The effect of AQP1 deletion here is probably the consequence of reduced primary tumor mass and/or impaired tumor angiogenesis in the lung metastases. As the epithelial tumor cells in this model do not express AQP1, the reduced number of lung metastases in the AQP1-deficient mice is unlikely to be related to tumor cell migration or invasion.
Although the preponderance of evidence supports a role of AQP1 in tumor angiogenesis, there are potential caveats in the interpretation of the data from AQP1−/− mice. In general, phenotype findings in knockout mice could represent a primary or second effect of gene deletion. AQP1−/− mice appear phenotypically normal and have the same growth as AQP+/+ mice (21), although they are polyuric, with ∼3-fold increased daily urine output compared to matched AQP+/+ mice. At baseline, mean arterial pressure in AQP1−/− mice is slightly lower than in AQP+/+ mice (4), although blood chemistries, including renal function, do not differ. We reported previously that retinal angiogenesis in a neonatal mouse model of oxygen-induced retinopathy was not different in AQP+/+ vs. AQP−/− mice, as AQP1 is absent from proliferating microvessels in retina (38). It may be informative to establish mouse models with selective manipulation of AQP1 expression in tumor microvessels, as well as to test small-molecule AQP1 inhibitors, when available.
Several drugs have been approved by the U.S. Food and Drug Administration as antiangiogenic therapy, most targeting VEGF (bevacizumab and ranibizumab) or its receptors (vatalanib, sorafenib, and sunitinib). One issue in VEGF-targeted therapies is the development of drug resistance. As a different target, AQP1-directed therapeutics might be useful in combination with VEGF-targeted or other antiangiogenic therapeutics to reduce tumor vessel formation. Impairment of endothelial cell motility by blocking AQP1 would reduce migration and invasion of endothelial cells, reducing tumor angiogenesis. It is well known that hypoxia activates VEGF signaling leading to angiogenesis; recently, it was found that AQP1 expression is also responsive to HIF, which may represent a VEGF-independent signaling mechanism driving angiogenesis under hypoxic conditions (39). An earlier study of in vitro tube formation by retinal vascular endothelia showed additive effects reduced AQP1 expression and inhibition of VEGF signaling (40), supporting independent antiangiogenic mechanisms and, hence, the possibility of combined drug therapy.
In summary, our data in a spontaneously tumor-producing mouse model provide further evidence for AQP1 as an important determinant in tumor angiogenesis. As current evidence supports a unique role of AQP1 in angiogenesis by a mechanism involving endothelial cell migration and invasion, pharmacological inhibition of AQP1 water transport function or expression may have utility in cancer therapy.
Supplementary Material
Acknowledgments
The authors thank Liman Qian for help in mouse breeding and genotyping analysis.
This study was supported by grants DK35124, EY13574, DK72517, and EB00415 from the U.S. National Institutes of Health. C.E.-F. was supported by a grant from the Fulbright Program and the Ministry of Education, Culture, and Sports of Spain.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AQP1
- aquaporin 1
- H&E
- hematoxylin and eosin
- HIF-1α
- hypoxia-inducible factor 1α
- MMTV-PyVT
- mouse mammary tumor virus-driven polyoma virus middle T oncogene
- VEGF
- vascular endothelial growth factor
- VEGFR
- vascular endothelial growth factor receptor
REFERENCES
- 1. Carbrey J. M., Agre P. (2009) Discovery of the aquaporins and development of the field. Handb. Exp. Pharmacol. 190, 3–28 [DOI] [PubMed] [Google Scholar]
- 2. Verkman A. S. (2006) Aquaporins in endothelia. Kidney Int. 69, 1120–1123 [DOI] [PubMed] [Google Scholar]
- 3. Mobasheri A., Marples D. (2004) Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am. J. Physiol. Cell Physiol. 286, C529–C537 [DOI] [PubMed] [Google Scholar]
- 4. Schnermann J., Chou C. L., Ma T., Traynor T., Knepper M. A., Verkman A. S. (1998) Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc. Natl. Acad. Sci. U. S. A. 95, 9660–9664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang D., Vetrivel L., Verkman A. S. (2002) Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J. Gen. Physiol. 119, 561–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oshio K., Watanabe H., Song Y., Verkman A. S., Manley G. T. (2005) Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. FASEB J. 19, 76–78 [DOI] [PubMed] [Google Scholar]
- 7. Thiagarajah J. R., Verkman A. S. (2002) Aquaporin deletion in mice reduces corneal water permeability and delays restoration of transparency after swelling. J. Biol. Chem. 277, 19139–19144 [DOI] [PubMed] [Google Scholar]
- 8. Endo M., Jain R. K., Witwer B., Brown D. (1999) Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc. Res. 58, 89–98 [DOI] [PubMed] [Google Scholar]
- 9. Nicchia G. P., Stigliano C., Sparaneo A., Rossi A., Frigeri A., Svelto M. (2013) Inhibition of aquaporin-1 dependent angiogenesis impairs tumour growth in a mouse model of melanoma. J. Mol. Med. (Berl.) 91, 613–623 [DOI] [PubMed] [Google Scholar]
- 10. Saadoun S., Papadopoulos M. C., Hara-Chikuma M., Verkman A. S. (2005) Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 434, 786–792 [DOI] [PubMed] [Google Scholar]
- 11. Papadopoulos M. C., Saadoun S., Verkman A. S. (2008) Aquaporins and cell migration. Pflügers Arch. 456, 693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mobasheri A., Airley R., Hewitt S. M., Marples D. (2005) Heterogeneous expression of the aquaporin 1 (AQP1) water channel in tumors of the prostate, breast, ovary, colon and lung: a study using high density multiple human tumor tissue microarrays. Int. J. Oncol. 26, 1149–1158 [PubMed] [Google Scholar]
- 13. Verkman A. S., Hara-Chikuma M., Papadopoulos M. C. (2008) Aquaporins—new players in cancer biology. J. Mol. Med. (Berl.) 86, 523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ribatti D., Ranieri G., Annese T., Nico B. (2013) Aquaporins in cancer. [E-pub ahead of print] Biochim. Biophys. Acta 21, 10.1016/j.bbagen.2013.09.025 [DOI] [PubMed] [Google Scholar]
- 15. Hu J., Verkman A. S. (2006) Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 20, 1892–1894 [DOI] [PubMed] [Google Scholar]
- 16. Hara-Chikuma M., Verkman A. S. (2008) Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol. Cell. Biol. 28, 326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gomes F. G., Nedel F., Alves A. M., Nör J. E., Tarquinio S. B. (2013) Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci. 92, 101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guy C. T., Cardiff R. D., Muller W. J. (1992) Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell. Biol. 12, 954–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin E. Y., Jones J. G., Li P., Zhu L., Whitney K. D., Muller W. J., Pollard J. W. (2003) Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 163, 2113–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Almholt K., Juncker-Jensen A., Laerum O. D., Danø K., Johnsen M., Lund L. R., Rømer J. (2008) Metastasis is strongly reduced by the matrix metalloproteinase inhibitor galardin in the MMTV-PymT transgenic breast cancer model. Mol. Cancer Ther. 7, 2758–2767 [DOI] [PubMed] [Google Scholar]
- 21. Ma T., Yang B., Gillespie A., Carlson E. J., Epstein C. J., Verkman A. S. (1998) Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J. Biol. Chem. 273, 4296–4299 [DOI] [PubMed] [Google Scholar]
- 22. Lam J. B., Chow K. H., Xu A., Lam K. S., Liu J., Wong N. S., Moon R. T., Shepherd P. R., Cooper G. J., Wang Y. (2009) Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS One 4, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonzalez R. C., Woods R. E., Eddins S. L., (2004) Digital Imaging Processing Using MATLAB, Prentice-Hall, Upper Saddle River, NJ, USA [Google Scholar]
- 24. Martin M. D., Carter K. J., Jean-Philippe S. R., Chang M., Mobashery S., Thiolloy S., Lynch C. C., Matrisian L. M., Fingleton B. (2008) Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res. 68, 6251–6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fraisl P. (2013) Crosstalk between oxygen- and nitric oxide-dependent signaling pathways in angiogenesis. Exp. Cell Res. 319, 1331–1339 [DOI] [PubMed] [Google Scholar]
- 26. Hanahan D., Weinberg R. A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 27. Saadoun S., Papadopoulos M. C., Watanabe H., Yan D., Manley G. T., Verkman A. S. (2005) Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell Sci. 118, 5691–5698 [DOI] [PubMed] [Google Scholar]
- 28. Auguste K. I., Jin S., Uchida K., Yan D., Manley G. T., Papadopoulos M. C., Verkman A. S. (2007) Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 21, 108–116 [DOI] [PubMed] [Google Scholar]
- 29. Levin M. H., Verkman A. S. (2006) Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest. Ophthalmol. Vis. Sci. 47, 4365–4372 [DOI] [PubMed] [Google Scholar]
- 30. Yokomori H., Oda M., Yoshimura K., Kaneko F., Hibi T. (2011) Aquaporin-1 associated with hepatic arterial capillary proliferation on hepatic sinusoid in human cirrhotic liver. Liver Int. 31, 1554–1564 [DOI] [PubMed] [Google Scholar]
- 31. Macey R. I., Farmer R. E. (1970) Inhibition of water and solute permeability in human red cells. Biochim. Biophys. Acta 211, 104–106 [DOI] [PubMed] [Google Scholar]
- 32. Migliati E., Meurice N., DuBois P., Fang J. S., Somasekharan S., Beckett E., Flynn G., Yool A. J. (2009) Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 76, 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seeliger D., Zapater C., Krenc D., Haddoub R., Flitsch S., Beitz E., Cerdà J., de Groot B. L. (2013) Discovery of novel human aquaporin-1 blockers. ACS Chem. Biol. 8, 249–256 [DOI] [PubMed] [Google Scholar]
- 34. Brooks H. L., Regan J. W., Yool A. J. (2000) Inhibition of aquaporin-1 water permeability by tetraethylammonium, involvement of the loop E pore region. Mol. Pharmacol. 57, 1021–1026 [PubMed] [Google Scholar]
- 35. Yang B., Kim J. K., Verkman A. S. (2006) Comparative efficacy of HgCl2 with candidate aquaporin-1 inhibitors DMSO, gold, TEA+ and acetazolamide. FEBS Lett. 580, 6679–6684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sogaard R., Zeuthen T. (2008) Test of blockers of AQP1 water permeability by a high-resolution method: no effects of tetraethylammonium ions or acetazolamide. Pflügers Arch. 456, 285–292 [DOI] [PubMed] [Google Scholar]
- 37. Papadopoulos M. C., Verkman A. S. (2012) Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 11, 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruiz-Ederra J., Verkman A. S. (2007) Aquaporin-1 independent microvessel proliferation in a neonatal mouse model of oxygen-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 48, 4802–4810 [DOI] [PubMed] [Google Scholar]
- 39. Abreu-Rodriguez I., Sanchez Silva R., Martins A. P., Soveral G., Toledo-Aral J. J., Lopez-Barneo J., Echevarria M. (2011) Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1α. PLoS One 6, e28385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaneko K., Yagui K., Tanaka A., Yoshihara K., Ishikawa K., Takahashi K., Bujo H., Sakurai K., Saito Y. (2008) Aquaporin 1 is required for hypoxia-inducible angiogenesis in human retinal vascular endothelial cells. Microvasc. Res. 75, 297–301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.