Abstract
The bone morphogenetic protein (BMP) signaling pathways have important roles in embryonic development and cellular homeostasis, with aberrant BMP signaling resulting in a broad spectrum of human disease. We report that BMPs unexpectedly signal through the canonical transforming growth factor β (TGF-β)-responsive Smad2 and Smad3. BMP-induced Smad2/3 signaling occurs preferentially in embryonic cells and transformed cells. BMPs signal to Smad2/3 by stimulating complex formation between the BMP-binding TGF-β superfamily receptors, activin receptor-like kinase (ALK)3/6, and the Smad2/3 phosphorylating receptors ALK5/7. BMP signaling through Smad2 mediates, in part, dorsoventral axis patterning in zebrafish embryos, whereas BMP signaling through Smad3 facilitates cancer cell invasion. Consistent with increased BMP-mediated Smad2/3 signaling during cancer progression, Smad1/5 and Smad 2/3 signaling converge in human cancer specimens. Thus, the signaling mechanisms used by BMPs and TGF-β superfamily receptors are broader than previously appreciated.—Holtzhausen, A., Golzio, C., How, T., Lee, Y.-H., Schiemann, W. P., Katsanis, N., Blobe, G. C. Novel bone morphogenetic protein signaling through Smad2 and Smad3 to regulate cancer progression and development.
Keywords: TGF-β, tumorigenesis, invasion, dorsoventral axis
The transforming growth factor β (TGF-β) superfamily, including TGF-βs, bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs), and activins, regulate many cellular processes, including proliferation, differentiation, migration, apoptosis, angiogenesis, and embryonic development (1, 2). TGF-β superfamily pathways are also involved in the pathophysiology of a broad spectrum of human disease, including cardiovascular disease, cancer, musculoskeletal disease, and reproductive and developmental disorders (1).
TGF-β superfamily ligands regulate cellular processes by binding to 3 classes of cell-surface receptors: the type I receptors [activin receptor-like kinase (ALK)-1–7], the type II receptors [type II transforming growth factor receptor (TβRII), type II bone morphogenetic receptor (BMPRII), type II activin receptor (ActRII), and ActRIIB[ (1, 3), and coreceptors [type III transforming growth factor receptor (TβRIII), endoglin, and repulsive guidance molecules; ref. 2] (Fig. 1A). The type I and II receptors are single-pass transmembrane proteins, each containing a cytoplasmic serine-threonine protein kinase domain, that participate in a kinase cascade to initiate signaling through the Smad family of transcription factors and through non-Smad pathways (refs. 1, 4 and Fig. 1A). Homozygous deletion of each TGF-β superfamily receptor, with the exception of ALK6, induces embryonic lethality, whereas homozygous deletion of ALK6 causes developmental defects, suggesting that these receptors and their signaling pathways all have essential and nonredundant roles in embryonic development (1). Among other functions, BMP2b and BMP7 are necessary for patterning of the dorsoventral axis during zebrafish embryonic development (5–7).
Figure 1.
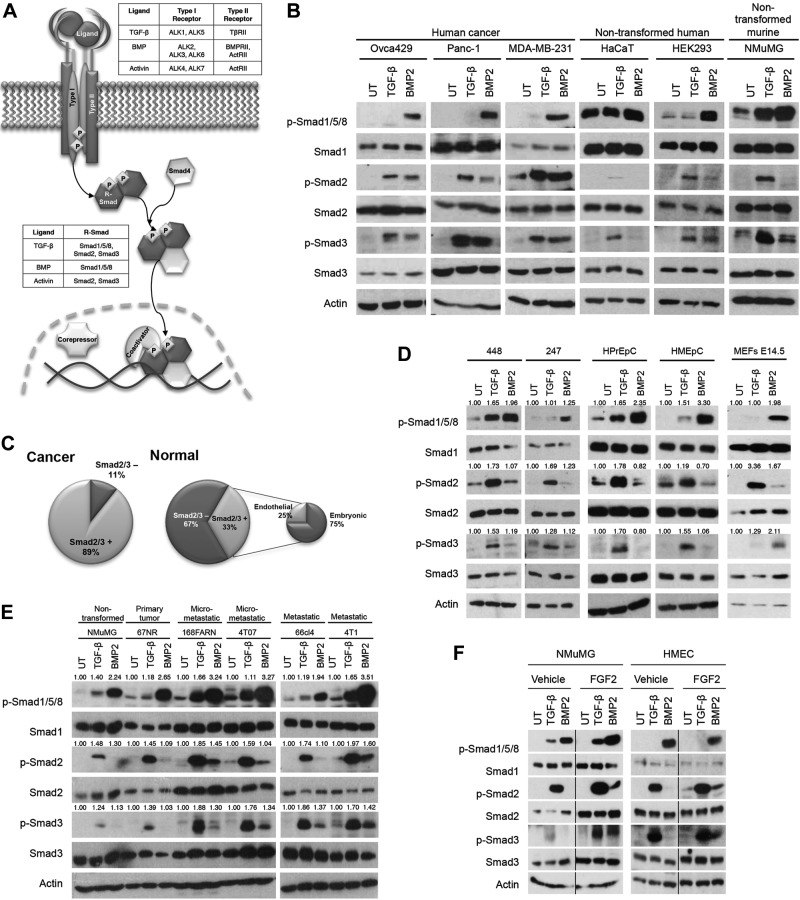
BMP2 induces Smad2/3 phosphorylation preferentially in embryonic and transformed cell lines. A) TGF-β superfamily ligands bind to a type I or type II cell surface receptor that recruits the reciprocal receptor. The type II receptor phosphorylates and activates the type I receptor, which then phosphorylates an R-Smad. Phosphorylated R-Smads form a complex with the common Smad, Smad4, translocate into the nucleus, and mediate gene transcription. B) Indicated human cancer or nontransformed human or murine cell lines were left untreated (UT) or stimulated with 100 pM TGF-β or 10 nM BMP2 for 40 min, lysed, and analyzed by Western blot with the indicated antibodies. Data are representative of 2 independent experiments. C) Of the screened cancer cell lines, 89% were positive for BMP2-induced Smad2/3 phosphorylation, whereas 11% did not induce Smad2/3 phosphorylation in response to BMP treatment. Of the nontransformed or normal cell lines, 67% were negative for BMP2-induced Smad2/3 phosphorylation, whereas 33% were positive. Of the positive nontransformed cell lines, 25% were endothelial and 75% were embryonic. D) Cells isolated from ascites of ovarian cancer patients, designated 448 and 247, primary human epithelial cells isolated from the prostate (HPrEpC) and breast (HMEpC), and mouse embryonic fibroblasts (MEFs) were UT or were treated as in B. Lysates were analyzed by Western blot with the indicated antibodies. E) A murine breast cancer progression cell line series was UT or treated and analyzed as in B. Data are representative of 2 independent experiments. F) NMuMG and HMECs were vehicle treated or treated with 10 ng/ml FGF2 for 72 h, serum starved for 6 h, and UT or treated and analyzed as in B. Data are representative of 2 independent experiments. G) MDA-MB-231 cells were UT, or treated with 100 pM TGF-β or 10 nM BMP2 for the indicated times and analyzed. H) MDA-MB-231 cells were pretreated with 10 μg/ml cycloheximide for 1 h then UT or treated and analyzed as in B. I) MDA-MB-231 cells were pretreated with increasing concentrations of the pan-TGF-β-neutralizing antibody 2G7 for 30 min, then UT or treated and analyzed as in B. J) MDA-MB-231 cells were UT or treated with the indicated doses of BMP2 or TGF-β for 40 min and analyzed. K) NOSE7, Panc-1, or MDA-MB-231 cells were UT or treated and analyzed as in (B). Data are representative of 3 independent experiments.
In canonical signaling, TGF-β binds to TβRII, resulting in complex formation with ALK5 and, in certain cell types, ALK1. Upon complex formation, TβRII phosphorylates and activates ALK5 and/or ALK 1, which enables recruitment and phosphorylation of Smad2/3 and Smad1/5/8, respectively (4, 8). In contrast, BMP associates with a type I receptor (ALK3, ALK6, or ALK2), which induces complex formation with BMPRII or ActRII (3, 9). Upon complex formation, BMPRII or ActRII phosphorylates and activates ALK3, ALK6, or ALK2, which enables recruitment and phosphorylation of Smad1/5/8. In all cases, the phosphorylated Smads form a complex with the common Smad, Smad4, and these Smad complexes accumulate in the nucleus where they interact with specific transcription factors, coactivators, and corepressors to regulate gene transcription (2). More recently, TGF-β was shown to induce phosphorylation of Smad1/5 through ALK5, causing increased migration (10). Additional work demonstrated that TGF-β-induced Smad1/5 is dependent on TβRII, ALK5, and ALK3 or ALK2 complexes, forming mixed Smad1/2 complexes that mediate anchorage-independent growth (11).
In most species, the number of TGF-β superfamily ligands exceeds the number of available receptors. TGF-β superfamily signaling specificity may be mediated through combinatorial receptor complex formation, leading to activation of specific downstream pathways. Some TGF-β superfamily ligands, including the TGF-βs and activins, bind with high affinity to their respective type II receptors (4). In contrast, the BMPs bind with a greater affinity to their respective type I receptors (12). In both cases, type I and II receptors are necessary to stabilize the receptor complex (13). Despite these insights, the molecular basis for the specificity of TGF-β superfamily receptor complex formation remains to be elucidated.
While investigating BMP signaling, we unexpectedly observed that BMPs, in addition to activating Smad1/5/8, induced phosphorylation of Smad2/3. In this study we investigated the mechanism, function, and significance of BMP2-induced Smad2/3 phosphorylation in the context of human disease.
MATERIALS AND METHODS
Cell culture
Cell lines used in this study are described in Table 1.
Table 1.
Cell lines
| Line | Description | Growth medium |
|---|---|---|
| 247 | Primary human ovarian cancer cells isolated from ascites | RPMI-1640 + 10% FBS |
| 3T3 | Murine embryonic fibroblasts | DMEM + 10% FBS |
| 448 | Primary human ovarian cancer cells isolated from ascites | RPMI 1640 + 10% FBS |
| 4T1 | Murine breast carcinoma | DMEM + 10% FBS |
| 5637 | Human bladder carcinoma | RPMI 1640 + 10% FBS |
| 5Y | Human neuroblastoma | MEM:F12 + 2 mM l-glutamine + NEAA + 10% FBS |
| 67NR, 168FARN, 4T07, 66cl4 | Murine breast cancer progression series. 67NR forms primary tumors; 168FARN forms micrometastases in lymph nodes and 4T07 micrometastases to blood, lymph nodes and lungs. 66cl4 and 4T1 are fully metastatic and forms visible lung nodules (14, 15) | DMEM + 10% FBS |
| BE2 | Human neuroblastoma | MEM:F12 + 2 mM l-glutamine + NEAA + 10% FBS |
| Cos-7 | African green monkey kidney fibroblast-like | DMEM + 10% FBS |
| DOV13 | Human ovarian cancer | RPMI-1640 + 10% FBS |
| HaCaT | Human nontransformed keratinocyte | DMEM + 10% FBS |
| Hek293 | Human nontransformed embryonic kidney | DMEM + 10% FBS |
| Hep3B | Human hepatocellular carcinoma | MEM + 10% FBS |
| HepG2 | Human hepatocellular carcinoma | MEM + 10% FBS |
| HEY | Human ovarian cancer | RPMI-1640 + 10% FBS |
| HMEC | Nontransformed human mammary epithelial | DMEM + 10 μg/ml insulin + 10% FBS |
| HMEC-1 | Human dermal microvascular endothelial | MCDB-131 + 10% FBS + 1 μg/ml hydrocortisone + 10 ng/ml EGF + 2 mM l-glutamine |
| HMEpC | Primary human mammary epithelial cells (purchased from Cell Applications, Inc.) | Mammary epithelial growth medium (Cell Applications, Inc.) |
| HPrEpC | Primary human prostate epithelial cells (purchased from Cell Applications, Inc.) | Epi growth medium (Cell Applications, Inc.) |
| HT-29 | Human colorectal adenocarcinoma | McCoy's 5A + 10% FBS |
| IGROV | Human ovarian cancer | RPMI-1640 + 10% FBS |
| L6 | Rat myoblast | DMEM + 10% FBS |
| Mammary epithelial cells (TβRII WT and KO) | Nontransformed murine mammary epithelial | DMEM:F12 + 2.5 mM l-glutamine + 10% adult bovine serum (ABS) |
| Mammary fibroblast cells (TβRII WT and KO) | Nontransformed murine mammary fibroblast | DMEM:F12 + 2.5 mM l-glutamine + 10% ABS |
| MCF10A | Nontransformed human mammary epithelial | F12:DMEM + 10 μg/ml insulin + 0.5 μg/ml hydrocortisol + 20 ng/ml EGF + 100 ng/ml cholera toxin + 5% horse serum |
| MCF10A T1k, Ca1h, Ca1a | Human breast cancer progression series. MCF10A is nontumorigenic, T1k forms ducts that can develop hyperplastic lesions; Ca1h forms low-grade, well-differentiated carcinomas; and Ca1a forms poorly differentiated, metastatic carcinomas (16). | DMEM:F12 + 10 μg/ml insulin + 0.5 μg/ml hydrocortisol + 20 ng/ml EGF + 100 ng/ml cholera toxin + 5% horse serum |
| MCF7 | Human breast adenocarcinoma | MEM + 0.01 mg/ml insulin + 10% FBS |
| MDA-MB-231 | Human breast cancer | MEM + sodium pyruvate + nonessential amino acids (NEAA) + 10% FBS |
| MDCK | Nontransformed canine kidney epithelial | MEM + 10% FBS |
| MEEC | Murine embryonic endothelial | MCDB-131 + 15% FBS + 2 mM l-glutamine + 1 mM sodium pyruvate + 100 μg/ml heparin + 15 μg/ml endothelial cell growth supplement |
| Mv1Lu, DR, R1b | Nontransformed mink lung epithelial Mv1Lu is wild-type, DR lacks TβRII and ALK5, and R1b lacks ALK5 (17) | DMEM + 10% FBS |
| NMuMG | Murine nontransformed mammary epithelial | DMEM + 4.5g/l-glucose + 10 mg/ml insulin + 10% FBS |
| NOSE7 | Normal ovarian surface epithelial | RPMI-1640 + 10% FBS |
| Ovca 429 | Human ovarian cancer | RPMI + 10% FBS |
| Panc-1 | Human pancreatic cancer | DMEM + 10% FBS |
| PASMC | Murine pulmonary artery smooth muscle cells | RPMI-1640 + 10% FBS |
| PC3 | Human prostate adenocarcinoma | F12 + 10% FBS |
| RT4 | Human transitional cell papilloma | McCoy's 5A + 10% FBS |
| SHEP | Human neuroblastoma | DMEM + 2 mM l-glutamine + NEAA + 10% FBS |
| SJD.1 | Adult zebrafish caudal fin fibroblast | High glucose DMEM + 15% heat inactivated FBS, 28 °C |
| SKNAS | Human neuroblastoma | DMEM + 2 mM l-glutamine + NEAA + 10% FBS |
| SW480 | Human colorectal adenocarcinoma | DMEM + 10% FBS |
| SW620 | Human colorectal adenocarcinoma | DMEM + 10% FBS |
| T24 | Human transitional cell carcinoma | McCoy's 5A + 10% FBS |
| ZF4 | Zebrafish embryonic fibroblast | DMEM:F12 + 10% FBS, 28°C |
Reagents
SB431542 was purchased from Sigma-Aldrich (St. Louis, MO, USA), and dorsomorphin from Tocris (Minneapolis, MN, USA). LDN193189 was a gift from Dr. Paul Yu (Massachusetts General Hospital, Boston, MA, USA). The pan-TGF-β-neutralizing antibody 2G7 was a gift from Genentech (South San Francisco, CA, USA). Cycloheximide was purchased from Sigma-Aldrich. Ligands (TGF-β1, BMP2, BMP3, BMP4, BMP7, BMP9, GDF5, and activin A) were purchased from R&D Systems (Minneapolis, MN, USA).
Immunoprecipitation (IP)
Cells were lysed in coimmunoprecipitation (co-IP) lysis buffer (20 mM Hepes, 2 mM EDTA, 10 mM NaF, 150 mM NaCl, 10% glycerol, 0.5% Nonidet P-40) and lysates were precleared. Lysates were incubated with PAS or PGS beads and the appropriate primary antibody on a rotator overnight at 4°C. Immunoprecipitates were washed 3 times in co-IP lysis buffer and subjected to Western blot analysis.
Antibodies
Primary antibodies [p-Smad1/5/8 (9511), Smad1 (9743), p-Smad2 (3101), Smad2 (3103), p-Smad3 (9520), Smad3 (9523), and Smad5 (9517)] were purchased from Cell Signaling Technology (Danvers, MA, USA). Secondary anti-mouse-HRP antibody (95017-332) was purchased from Amersham (Pittsburgh, PA, USA) and secondary anti-rabbit-HRP (7074) from Cell Signaling Technology. Alexa Fluor 488 goat anti-rabbit, Alexa Fluor 594 goat anti-rabbit, Alexa Fluor 488 goat anti-mouse, and Alexa Fluor 594 goat anti-mouse were purchased from Molecular Probes (Eugene, OR, USA). Monoclonal β-actin antibody was purchased from Sigma-Aldrich (A5441). The following antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA): TβRII (sc-17792), BMPRII (sc-5682, sc-20737), ALK1 (sc-2897), ALK2 (sc-25449), ALK3 (sc-20736), ALK4 (sc-31297), ALK5 (sc-9048), ALK6 (sc-25455), and ALK7 (sc-135001).
Constructs
The authors thank the following investigators for their generous gifts: Dr. Liliana Attisano [University of Toronto, Toronto, ON, Canada; dominant-negative (DN) BMPRII], Dr. Chun Peng (York University, Toronto, ON, Canada; DN ALK7, myc-ALK7), Dr. Patricia Donahoe (Harvard Medical School, Boston, MA, USA; DN ALK3, DN ALK6), Dr. Xiao-Fan Wang (Duke University; pLKO NTC, shALK5, VSVG, Gag/Pol, pSuperRetro NTC), Dr. Bozena Kaminska (Nencki Institute of Experimental Biology, Warsaw, Poland; shTβRII), Dr. Sabine Mazerbourg (Université Henri Poincaré, Nancy, France; shBMPRII, shALK6), Dr. Xin-Hua Feng (Baylor College of Medicine, Houston, TX, USA; shSmad2, shSmad3), Dr. Jean Zhao (Flag-ALK2, Addgene plasmid 20412), and Dr. Jeff Wrana (ALK4, Addgene plasmid 11756). shALK7, shSmad1, and shSmad5 were purchased from Sigma-Aldrich. shALK3 was purchased from the Duke RNAi facility.
Nuclear translocation
Cells were left untreated or treated with 100 pM TGF-β or 10 nM BMP2 for 40 min, then washed and processed as described in the Immunofluorescence section. The cells were stained for total Smad1, Smad2, or Smad3. Three random fields were imaged, and the percentages of cells with predominantly nuclear Smads were determined and graphed.
Proximity ligation assays (PLAs)
Duolink II PLAs were performed as described in the manufacturer's protocol (Olink Bioscience, Uppsala, Sweden). Three random images were taken at ×40 with a fluorescence microscope. PLA signals were quantified by using the count function of NIS-Elements BR microscopy software (Nikon, Tokyo, Japan).
Microarray gene expression analyses
Genes under control of Smad1/5/8, Smad2, or Smad3 were selected on the basis of previous characterization (14–17). Microarray data sets were retrieved from Oncomine 4.4 (Life Technologies-Compendia Bioscience, Ann Arbor, MI, USA; https://www.oncomine.com/resource/login.html) to test the correlation of coincident expression between TGF-β- and BMP-responsive genes in normal and malignant tissues. Genes designated TGF-β-responsive included ATF3, IgA, CDKN1A, JUNB, Myc, and TGM2; those designated BMP-responsive included DLX2, ID2, ID4, Smad6, TLX2, and VENTX. Dual TGF-β- and BMP-responsive genes included Smad7, ID1, ID3, CXXC5, and CRABP2. Pearson correlation coefficients between individual TGF-β- and BMP-regulated genes were calculated for each data set and subsequently were used to determine the global significance of coincidently regulated genes by Fisher's combined probability test. In all cases, P < 0.05 was considered to be statistically significant.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton-X 100. The samples were blocked in 5% BSA, incubated with primary antibodies at a 1:500 dilution, washed again, and incubated with secondary antibodies at a 1:500 dilution. After another wash, the samples were mounted in Prolong gold (Invitrogen-Life Technologies, Carlsbad, CA, USA).
Reverse transcription and real-time PCR
RNA was isolated with the RNAEasy kit (Qiagen, Valencia, CA, USA). cDNA was made with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Real-time PCR was performed with iQ SYBR Green Supermix (Bio-Rad) and primers specific for human GAPDH (sense: GAGTCAACGGATTTGTCGT, antisense: TTGATTTTGGAGGGATCTCG). Data were analyzed by the ΔCT method, with GAPDH used as a reference gene.
Luciferase reporter assay
Cells were transfected by Lipofectamine LTX and Plus Reagent (Invitrogen-Life Technologies),with SV40-Renilla and XVent (Smad1 reporter), ARE/FAST (Smad2 reporter), or pE2.1 (Smad3 reporter). At 24 h after transfection, the cells were serum starved and treated with 100 pM TGF-β or 10 nM BMP2 or left untreated as a negative control. The cells were washed, and luciferase activity was assayed with the Dual Luciferase Assay kit (Promega, Madison, WI, USA), according to the manufacturer's instructions. Luminescence was determined with a plate reader.
Binding and cross-linking
BMP2 was purchased from R&D Systems and labeled with 125I, according to the chloramine-T method (18), and binding and cross-linking were performed (19). Briefly, cells were incubated with KRH buffer [50 mM HEPES (pH7.5), 130 mM NaCl, 5 mM MgSO4, 1 mM CaCl2 and 5 mM KCl] containing 0.5% BSA for 30 min at 37°C and then with 20 nM 125I-BMP2 for 3 h at 4°C. 125I-BMP2 was cross-linked with 0.5 mg/ml disuccinimidyl suberate and quenched with 20 mM glycine. The cells were then washed with KRH buffer, lysed, and analyzed by SDS-PAGE and phosphorimaging of dried gels.
Flow cytometry
Cells were harvested and washed in flow buffer (0.5% BSA in PBS) and incubated with 1 μg primary antibody for 1 h. After they were washed, the cells were incubated with 0.5 μg fluorescently labeled secondary antibody for 30 min on ice in the dark, washed, and fixed in 0.5% paraformaldehyde.
In vitro kinase assay
The assay was performed as described elsewhere (10). Briefly, receptors were immunoprecipitated from HEK293 cells transfected with epitope-tagged receptors. The immunoprecipitates were washed in lysis buffer, then in kinase buffer (5 mM Tris, 1 mM MgCl2, 0.1 mM CaCl2, pH 7.4). They were incubated with bacterially expressed GST-Smad for 30 min at room temperature in kinase buffer containing 100 μM ATP. The reaction was quenched with 2× sample buffer, subjected to SDS-PAGE, and analyzed by Western blot with phospho-specific antibodies.
Epithelial–mesenchymal transition (EMT) assay
NMuMG cells were treated with 10 ng/ml fibroblast growth factor (FGF)-2 for 72 h to induce EMT.
Matrigel invasion assay
Cells (50,000) were seeded in serum-free medium on a Matrigel-coated filter placed in a cell migration chamber (BD Biosciences, San Jose, CA, USA) and allowed to migrate. The cells were fixed in methanol and stained with DRAQ5 (BioStatus, Shepshed, UK) and Sapphire 700 (Li-Cor Biosciences, Lincoln, NE, USA), each diluted 1:1000. The filters were rinsed in PBS, dried, and scanned and quantified with a Li-Cor Odyssey scanner.
Morpholino (MO) and embryo manipulations
Zebrafish (Danio rerio) embryos were raised and maintained as previously (20). Translational MO against bmp2b (5′-GTCTGCGTTCCCGTCGTCTCCTAAG-3′; ref. 21) was obtained from Gene Tools, LLC (Philomath, OR, USA). We injected 0.7 ng of MO and/or 100 pg RNA into wild-type zebrafish embryos at the 1- to 2-cell stage. Injected embryos were scored at 1 d postfertilzation and classified into 3 groups: normal and dorsalized groups, compared with an age-matched control group from the same clutch. For DN and constitutively active (CA) rescue experiments, site-directed mutagenesis was performed to convert the human wild-type SMAD2 and SMAD3 transcript into a DN or CA form (22). These CA or DN forms of Smad2 and SMAD3 were cloned into the pCS2 vector and transcribed in vitro using the SP6 Message Machine kit (Ambion, Austin, TX, USA). All the experiments were repeated 3 times, and a t test was used to determine the significance of the morphant phenotype and the rescue.
RESULTS
BMP2 induces Smad2/3 phosphorylation preferentially in embryonic and transformed cell lines
In canonical signaling, BMP2 induces phosphorylation of Smad1/5/8. In response to BMP2 stimulation, in addition to phosphorylation of Smad1/5/8 we unexpectedly observed phosphorylation of Smad2/3 (Fig. 1B, Supplemental Fig. S1A, and Table 2). In addition to inducing phosphorylation of Smad1/5/8 in all but 1 cell line evaluated (39/40), BMP2 robustly induced phosphorylation of Smad2 and/or Smad3 in 89% (25/28) of the cancer cell lines tested (Fig. 1C), including murine and human cancer cell line models of bladder, breast, colon, ovarian, pancreatic, and prostate cancers (Fig. 1B, Supplemental Fig. S1A, and Table 2). Of note, whereas BMP2 induced phosphorylation of Smad1/5/8 in all but one of the nontransformed cell lines tested (11/12), BMP2 induced phosphorylation of Smad2 and/or Smad3 in only 33% (4/12; Fig. 1C) of nontransformed cell lines (Fig. 1B, Supplemental Fig. S1A, and Table 2). Notably, 3 of the 4 nontransformed cell lines exhibiting BMP2-induced Smad2 and/or Smad3 phosphorylation were of embryonic origin, and the 4th was an endothelial cell line (Fig. 1C). In addition, BMP2-induced Smad2/3 phosphorylation occurred in primary ovarian cancer cells obtained from the ascites of ovarian cancer patients and in mouse embryonic fibroblasts (Fig. 1D), but not in primary human epithelial cells isolated from prostate and breast tissues (Fig. 1D). These data suggest that BMP2-induced Smad2/3 phosphorylation is not a result of immortalization.
Table 2.
Smad phosphorylation summary
| Cell line | TGF-β1 |
BMP2 |
||||
|---|---|---|---|---|---|---|
| p-Smad1/5/8 | p-Smad2 | p-Smad3 | p-Smad1/5/8 | p-Smad2 | p-Smad3 | |
| 168FARN | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 247 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 3T3 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 448 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 4T07 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4T1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 5637 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 5Y | ✓ | ✓ | ✓ | |||
| 66cl4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 67NR | ✓ | ✓ | ✓ | ✓ | ✓ | |
| BE2 | ✓ | ✓ | ✓ | ✓ | ||
| Ca1a | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Ca1h | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cos-7 | ✓ | ✓ | ✓ | ✓ | ||
| DOV13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| HaCaT | ✓ | ✓ | ✓ | |||
| Hek293 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Hep3B | ✓ | ✓ | ✓ | ✓ | ✓ | |
| HepG2 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| HEY | ✓ | ✓ | ✓ | ✓ | ||
| HMEC | ✓ | ✓ | ✓ | |||
| HMEC-1 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| HMEpC | ✓ | ✓ | ✓ | ✓ | ||
| HPrEpC | ✓ | ✓ | ✓ | ✓ | ||
| HT-29 | ✓ | ✓ | ✓ | ✓ | ||
| IGROV | ✓ | ✓ | ✓ | ✓ | ||
| MCF10A | ✓ | ✓ | ✓ | ✓ | ||
| MCF7 | ✓ | ✓ | ✓ | |||
| MDA-MB-231 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| MDCK | ✓ | ✓ | ✓ | ✓ | ||
| MEEC | ✓ | ✓ | ✓ | |||
| MEFs | ✓ | ✓ | ✓ | ✓ | ✓ | |
| NMuMG | ✓ | ✓ | ✓ | ✓ | ||
| Ovca 492 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Panc-1 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| PC3 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| RT4 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| SHEP | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| SJD.1 | ✓ | ✓ | ✓ | ✓ | ||
| SKNAS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| SW480 | ✓ | ✓ | ✓ | |||
| SW620 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| T1k | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| T24 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| ZF4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Smad phosphorylation profile in response to 40 min of 100 pM TGF-β or 10 nM BMP2 treatment.
As BMP2-induced Smad2/3 phosphorylation preferentially occurs in embryonic or transformed cells, we sought to investigate whether dedifferentiation during cancer progression promotes this novel mode of BMP signaling. In a murine breast cancer cell line progression series (23, 24), where the isogenic lines recapitulate cancer progression from normal epithelium, to locally invasive cancer, then to metastatic cancer, BMP2-induced Smad1/5/8 and Smad3 phosphorylation increased as the model progressed from recapitulation of benign to metastatic disease, whereas BMP2-induced Smad2 phosphorylation oscillated (Fig. 1E), suggesting that dedifferentiation during cancer progression promotes BMP2-induced Smad1/5/8 and Smad3 phosphorylation. Although BMP2 induced Smad2 and Smad3 in a human breast cancer cell line progression series (Supplemental Fig. S1B), there was no trend corresponding with the aggressiveness of this progression series.
The EMT process promotes cell migration and invasion. EMT is considered a crucial part of embryogenesis, wound healing, and metastasis (25). We therefore investigated whether EMT promotes BMP-stimulated Smad2/3 signaling. Indeed, although BMP did not stimulate Smad2/3 phosphorylation in vehicle-treated NMuMG and human mammary endothelial cells (HMECs; Fig. 1A, F and Supplemental Fig. S1A), we observed robust phosphorylation of Smad2/3 in response to BMP2 in NMuMG and HMECs after FGF2-mediated induction of EMT (Fig. 1F and Supplemental Fig. S1C).
BMP superfamily ligands induce Smad2/3 phosphorylation, nuclear translocation, and gene transcription
In MDA-MB-231 human breast cancer cells, where BMP signals to both Smad1/5/8 and Smad2/3, BMP2 induced Smad1/5/8 phosphorylation 10 min after BMP2 treatment, peaking at 60 min, and TGF-β1 induced Smad2/3 phosphorylation 5 min after TGF-β1 treatment, peaking at 40–60 min (Fig. 1G). BMP2 induced Smad2 phosphorylation 5 min after BMP2 treatment, peaking at 40–60 min, and BMP2 induced Smad3 phosphorylation 10 min after BMP2 treatment, peaking at 40 min. Thus, BMP2-induced Smad2/3 phosphorylation occurred rapidly and with the same kinetics as TGF-β1-induced Smad2/3 phosphorylation. In addition, cycloheximide-mediated inhibition of new protein synthesis had no effect on BMP2-induced Smad2/3 phosphorylation (Fig. 1H). Furthermore, the pan-TGF-β1/2/3-neutralizing antibody 2G7 had no effect on BMP2-induced Smad2/3 phosphorylation at concentrations that effectively inhibited TGF-β1-induced Smad2/3 phosphorylation (Fig. 1I). Although having no effect on BMP2-induced Smad1/5/8 or Smad2 phosphorylation, 100 μg/ml 2G7 inhibited BMP2-induced Smad3 phosphorylation, potentially through steric hindrance caused at this high concentration. Taken together, these data suggest that BMP2 directly induces Smad2/3 phosphorylation.
Despite their homology, the TGF-β and BMP receptors have very different affinities for their cognate ligands (1), with TβRII and -III binding to their ligands, the TGF-βs, with a Kd in the picomolar range and the type I BMP receptors binding to the BMP ligands with a Kd in the nanomolar range. Specifically, TGF-β1 binds to TβRII with a Kd of 5–50 pM (26), with the concentration of TGF-β1 in human serum ranging from 20 to 1400 pM (27). In contrast, BMP4 binds to ALK3 with a Kd of 25.4 nM (28), BMP2 binds to ALK3/6 with a Kd of ∼1 nM, and BMP7 binds to ALK6 with a Kd ∼5–10 nM (29), with the circulating concentration of BMP7 ranging between 3 and 9 nM (30) and the concentration of BMP4 in human serum ranging from 17 to 48 nM (31). Consistent with a Kd in the nanomolar range and these circulating BMP concentrations, BMP2 induced Smad2/3 phosphorylation at physiologically relevant BMP2 levels, with BMP2 inducing dose-dependent Smad1/5/8 and Smad3 phosphorylation from 250 pM to 10 nM and dose-dependent Smad2 phosphorylation from 500 pM to 10 nM (Fig. 1J), whereas TGF-β1 induced dose-dependent Smad/3 phosphorylation from 1 to 100 pM (Fig. 1J). Of note, because BMP2 and other BMPs were used at nanomolar concentrations, a TGF-β1 ELISA assay was used to confirm that these recombinant proteins had less than 42.5 pg/ml (<1.7 pM) TGF-β1, supporting a direct role for BMP2 in mediating Smad2/3 phosphorylation.
BMP2 is a member of the large BMP subfamily of TGF-β superfamily ligands. To determine the scope of BMP-induced Smad2/3 phosphorylation, we screened representative members of the BMP family for their ability to induce Smad2/3 phosphorylation. As expected, BMP4/7/9 and GDF5, but not BMP3, which is known to function as a BMP antagonist (32), stimulated Smad1/5/8 phosphorylation in human ovarian NOSE7, Panc-1, and MDA-MB-231 cells (Fig. 1K). In NOSE7 cells, all BMP family members tested, including BMP3, also stimulated Smad2/3 phosphorylation (Fig. 1K). The effect of BMP3 was cell-line dependent, as BMP3 was unable to induce Smad2/3 phosphorylation in the Panc-1 or MDA-MB-231 cell lines (Fig. 1K). These data support BMP-mediated Smad2/3 phosphorylation as a general function of BMP superfamily ligands, with BMP3's ability to induce Smad2/3 phosphorylation suggesting a distinct mechanism from canonical BMP-induced Smad1/5/8 phosphorylation.
To mediate TGF-β superfamily signaling, the Smads form a complex with Smad4, accumulate in the nucleus, and regulate transcriptional events. Consistent with the ability of BMP2 to evoke Smad2/3 phosphorylation, BMP2 induced the nuclear accumulation of Smad2/3, with clear nuclear localization at 20 min after BMP2 stimulation (Supplemental Fig. S1D, E). Nuclear localization of Smad2 peaked at 40 min and decreased to near basal levels at 60 min, whereas Smad3 nuclear localization gradually increased and remained predominantly nuclear at 60 min (Supplemental Fig. S1D, E). The kinetic differences in BMP2-induced Smad2 vs. Smad3 nuclear localization suggest a divergent mechanism for phosphorylation and/or nuclear translocation. Further, BMP2 stimulated transcription of the canonical BMP/Smad1 reporter XVent2 and the Smad2-responsive ARE reporter, in conjunction with the coactivator FAST and the PAI-1-based, Smad3-responsive pE2.1 reporter (Supplemental Fig. S2A), to an extent similar to that of TGF-β1. These results support a physiologically relevant role for BMP ligands signaling through Smad2/3.
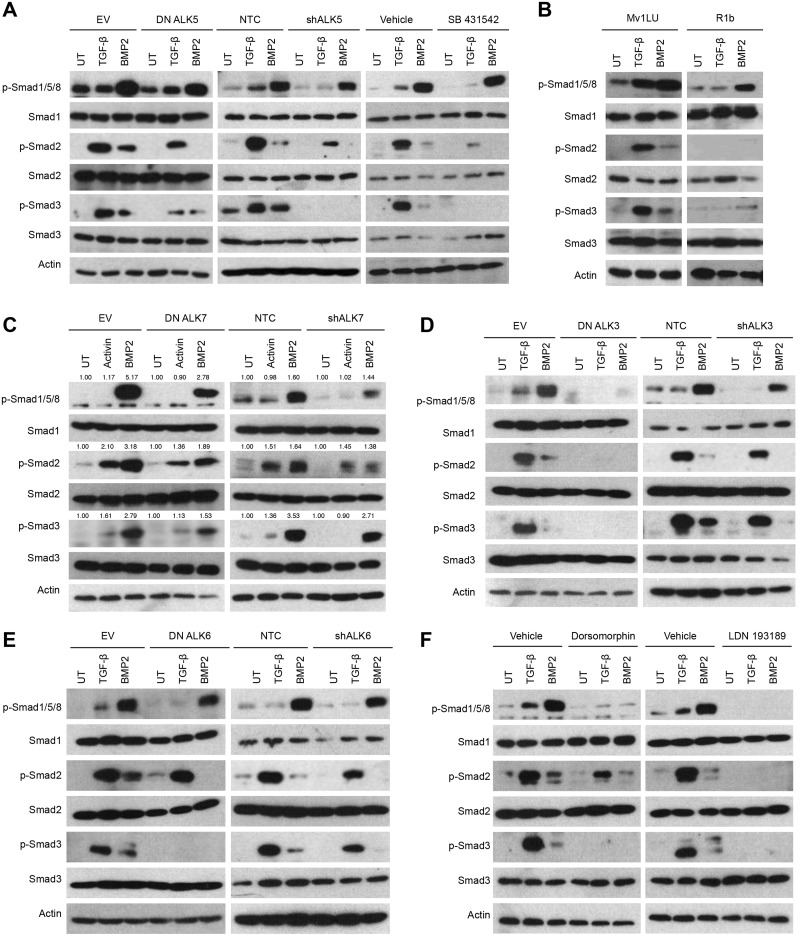
BMP2-induced Smad2/3 phosphorylation is differentially regulated by type II TGF-β superfamily receptors
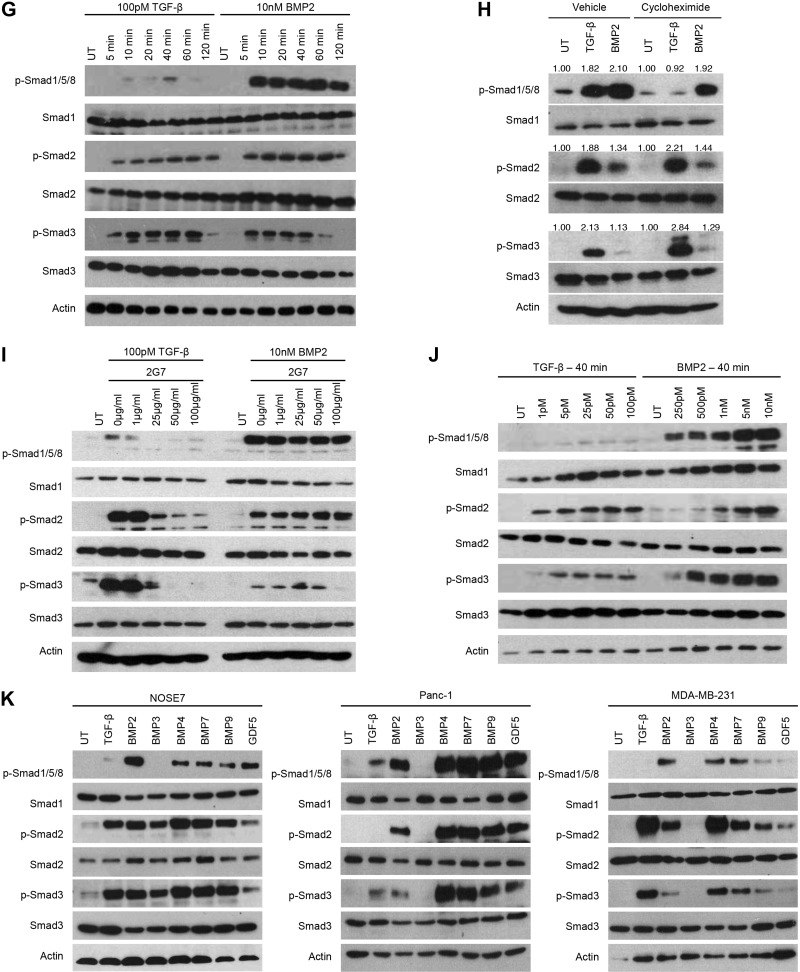
Expression of DN TβRII abrogated TGF-β-induced Smad2 phosphorylation, while attenuating TGF-β induced Smad3 phosphorylation, confirming the efficacy of DN TβRII (Fig. 2A and Supplemental Fig. S2B). Of note, while DN TβRII attenuated BMP2-induced Smad1/5/8 phosphorylation and abrogated that of Smad2, BMP2-induced Smad3 phosphorylation was not affected (Fig. 2A and Supplemental Fig. S2B). Similar results were obtained with shRNA-mediated silencing of TβRII expression, and this phenotype was rescued by expression of shRNA-resistant TβRII (Fig. 2A and Supplemental Fig. S2B). Further, in TβRII-null murine mammary epithelial cells (33), BMP2-induced Smad1/5/8 and Smad2 phosphorylation were decreased relative to TβRII wild-type mammary epithelial cells, whereas BMP2-induced Smad3 phosphorylation was not affected (Fig. 2B). In contrast, in TβRII-null murine mammary fibroblasts (34), while TGF-β-induced Smad2/3 phosphorylation was attenuated, BMP2-induced Smad1/5/8 and Smad2/3 phosphorylation were all unchanged or increased (Fig. 2C), suggesting context-dependent roles for TβRII. Finally, in the mink lung Mv1Lu-DR cell line, which lacks TβRII expression (35), TGF-β1-induced Smad2/3 phosphorylation was abrogated, whereas BMP2-induced Smad2 phosphorylation was decreased and BMP2-mediated Smad3 phosphorylation was unchanged (Fig. 2D). These findings support a specific role for TβRII in mediating BMP2-induced Smad2 but not Smad3 signaling in epithelial cells and suggest a different mechanism operating in mesenchymal cells.
Figure 2.
BMP2-induced Smad2/3 phosphorylation is differentially regulated by type II TGF-β superfamily receptors. A) MDA-MB-231 cells were transfected with a DN TβRII or an empty vector (EV) control. TβRII expression was silenced by transfection of a shRNA to TβRII (shTβRII) and a nontargeting control (NTC) shRNA was used as the control. shRNA-mediated silencing of TβRII was rescued by transfecting in shRNA-resistant TβRII (shTβRII+TβRII). Transfected cells were UT, stimulated with 100 pM TGF-β or 10 nM BMP2 for 40 min, lysed, and analyzed by Western blot with the indicated antibodies. B, C) TβRII wild-type (WT) mammary epithelial cells (B) or fibroblast cells (C) and TβRII-null (KO) mammary epithelial cells (B) or fibroblast cells (C) were UT or treated and analyzed as in A. D) Mv1Lu and DR mink lung cells were UT or treated and analyzed as in A. E) MDA-MB-231 cells were transfected with an EV, a DN mutant of BMPRII (DN BMPRII), nontargeting control (NTC), or shRNA against BMPRII (shBMPRII). Silencing of BMPRII was rescued by transfection of shRNA-resistant BMPRII (shBMPRII+BMPRII). Transfected cells were UT or treated and analyzed as in A. F) Wild-type pulmonary artery smooth muscle cells (WT) and BMPRII null PASMCs (KO) were UT or treated and analyzed as in A. Data are representative of 2 independent experiments.
DN BMPRII significantly attenuated BMP2-induced Smad1/5/8 (Fig. 2E). In contrast to the results with DN TβRII, DN BMPRII abrogated BMP2-induced Smad3 phosphorylation, while having no effect on BMP2-induced Smad2 phosphorylation (Fig. 2E and Supplemental Fig. S2C). Similar results were obtained with shRNA-mediated silencing of BMPRII expression and the attenuation of BMP2-induced Smad3 phosphorylation was rescued by expression of shRNA-resistant BMPRII (Fig. 2E and Supplemental Fig. S2C). Further, in BMPRII-null pulmonary artery smooth muscle cells (36), BMP-induced Smad1/5/8 phosphorylation was decreased but not abrogated, probably because of compensation by the type II activin receptors. Nonetheless, in BMPRII-null cells, BMP-induced Smad3 phosphorylation was attenuated, whereas Smad2 phosphorylation was unaffected (Fig. 2F). These findings support a specific role for BMPRII in mediating BMP2-induced Smad3 phosphorylation, but not that of Smad2. In addition, in contrast to BMP-induced Smad1/5/8 phosphorylation, the type II activin receptors did not compensate for loss of BMPRII in facilitating Smad3 phosphorylation by BMP2. Taken together, these results suggest that BMP2 signaling to Smad2 and Smad3 is differentially mediated by TβRII and BMPRII, respectively.
Heteromeric ALK complexes mediate BMP-induced Smad2/3 signaling
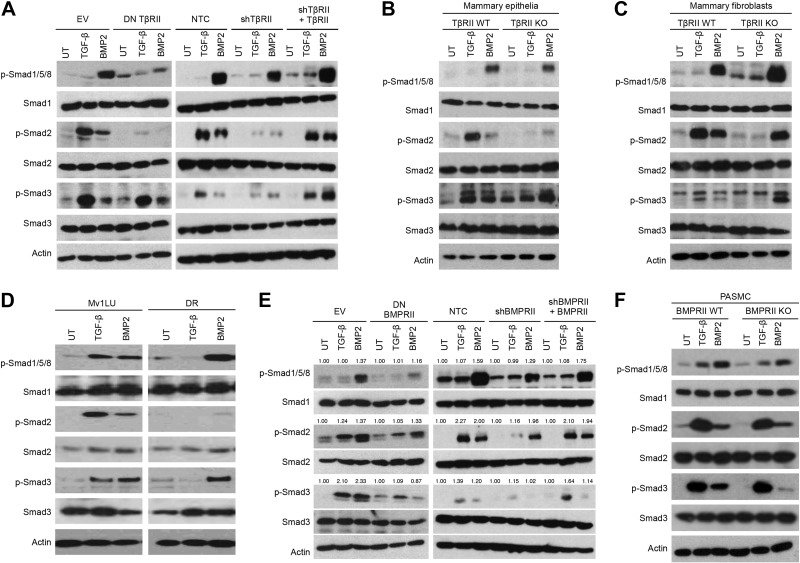
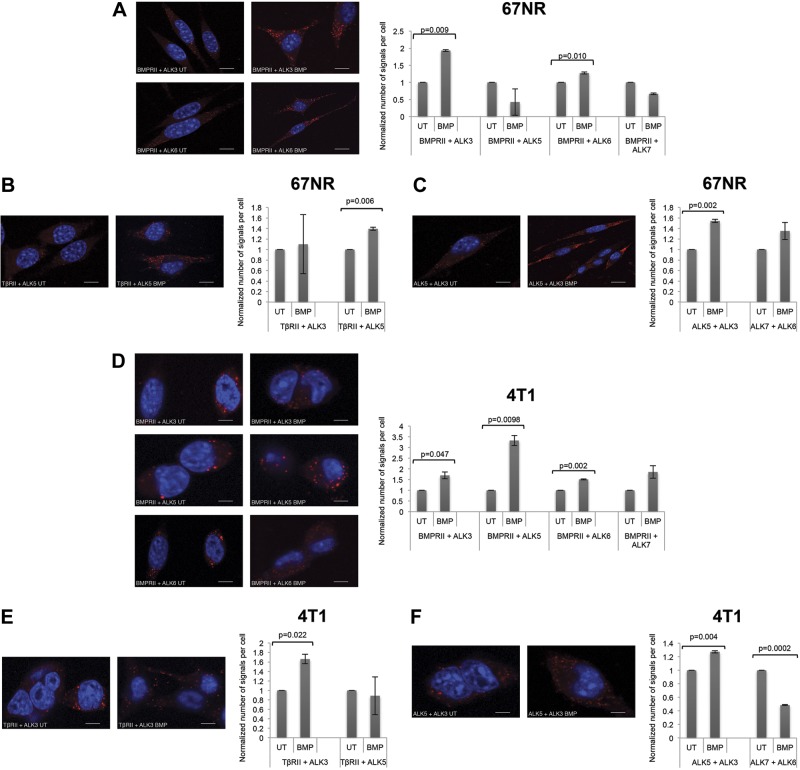
The type I TGF-β superfamily receptors capable of phosphorylating Smad2/3 (i.e., ALK5/7; ref. 4, 8 and Supplemental Fig. S2D) differed from the type I TGF-β superfamily receptors capable of binding BMPs (i.e., ALK2/3/6; ref. 9 and Supplemental Fig. S2E). Therefore, we hypothesized that BMPs induce Smad2/3 phosphorylation by inducing heterodimeric complexes containing 1 type I receptor that can bind to BMP and another type I receptor capable of phosphorylating Smad2 and/or Smad3. Utilizing PLAs with TGF-β superfamily receptor specific antibodies (Supplemental Fig. S2F), we established that, as expected, BMP2 stimulated complex formation between BMPRII and ALK3, as well as between BMPRII and ALK6 (Fig. 3A). BMP2 also unexpectedly stimulated complex formation between TβRII and ALK5 (Fig. 3B), between BMPRII and ALK5, and between BMPRII and ALK7 (Fig. 3A), suggesting crosstalk between canonical BMP and TGF-β receptors. BMP2 also stimulated complex formation between ALK5 and ALK3 and between ALK6 and ALK7 (Fig. 3C). As expected, PLAs with isotype controls showed no detectable signal (Supplemental Fig. S2G). However, ALK5/ALK2, ALK5/ALK6, ALK4/ALK2, ALK4/ALK6, and ALK7/ALK3 complexes all decreased with BMP stimulation (Fig. 3C), consistent with previous reports suggesting that preformed complexes (PFCs) are present on the cell surface (37) and dissociate in favor of high-affinity complexes in the presence of ligand.
Figure 3.
Heteromeric complexes form between canonical TGF-β and BMP receptors. Panc-1 cells were untreated (UT) or treated with 10 nM BMP2, and PLAs were performed with antibodies against endogenous receptors BMPRII + ALK1–7 (A), TβBRII + ALK1–7 (B), and ALK5 + ALK2/3/6, ALK4 + ALK2/3/6, and ALK7 + ALK2/3/6 (C). Representative immunofluorescence images of the indicated receptor pairs (left). Images of 3 random fields were quantified by using the count function of NIS-Br-Elements microscopy software. Data are expressed relative to untreated control cells. Scale bar = 10 μm. Results are means ± se of 3 independent experiments. *P < 0.05 (Student's t test).
To determine whether type II receptors are necessary for noncanonical ALKs to form a complex, we performed PLAs in cell lines lacking either TβRII (Supplemental Fig. S2H) or BMPRII (Supplemental Fig. S2I). Although BMP2 stimulated complex formation between ALK3 and ALK5 in TβRII-expressing cells (Supplemental Fig. S2H), there was no induction in TβRII-knockout (KO) cells (Supplemental Fig. S2H). In a similar manner, although BMP2 stimulated complex formation between ALK3 and ALK5 and between ALK6 and ALK7 in BMPRII-expressing cells (Supplemental Fig. S2I), there was no induction in BMPRII-KO cells (Supplemental Fig. S2I). These results support an important role for these type II receptors in BMP-stimulated, heteromeric ALK complex formation.
As the results from the PLAs suggested several potential mechanisms by which BMP could stimulate signaling through the canonical TGF-β Smads, we investigated the role of specific type I TGF-β superfamily receptors. DN ALK5 inhibited TGF-β1-induced Smad2/3 phosphorylation, and, consistent with studies with the small-molecule inhibitor, attenuated BMP2 stimulated Smad1/5/8 and Smad2/3 phosphorylation (Fig. 4A and Supplemental Fig. S2J). Similar results were obtained with shRNA-mediated silencing of ALK5 expression. As expected, the small-molecule inhibitor SB431542, which inhibits ALK5 and, to a lesser extent, ALK4/7 (38), inhibited TGF-β1-induced Smad2/3 phosphorylation (Fig. 4A). Of note, SB431542 also attenuated BMP2-induced Smad1/5/8, while abrogating BMP2-induced Smad2/3 phosphorylation. Finally, in the mink lung Mv1Lu-R1b cell line, which lacks ALK5 expression (35), TGF-β1-induced Smad2/3 phosphorylation was abrogated and BMP2-induced Smad2/3 phosphorylation was decreased, whereas BMP2-mediated Smad1/5/8 phosphorylation was not affected (Fig. 4B). Taken together, these results support an unexpected role for ALK5 in BMP2-mediated signaling to Smad2/3.
Figure 4.
Heteromeric ALK complexes mediate BMP-induced Smad2/3 signaling. A) MDA-MB-231 cells were transfected with an empty vector (EV), DN ALK5, nontargeting control (NTC), or shRNA against ALK5 (shALK5). Transfected cells were untreated (UT), stimulated with 100 pM TGF-β or 10 nM BMP2, and then lysed. MDA-MB-231 cells were pretreated with 10 μM SB431542 for 30 min, then UT, stimulated with 100 pM TGF-β or 10 nM BMP2, and lysed. Lysates were analyzed by Western blot with the indicated antibodies. B) Mv1Lu and R1b mink lung cells were UT, treated with 100 pM TGF-β or 10 nM BMP2 for 40 min, lysed, and analyzed by Western blot with the indicated antibodies. C) MDA-MB-231 cells were transfected with an EV, DN ALK7, an NTC, or shALK7. Transfected cells were UT or treated and analyzed as in B. D, E) MDA-MB-231 cells were transfected with an EV, DN ALK3, an NTC, or shALK3 (D) or with an EV, DN ALK6, an NTC, or shALK6 (E). Cells were serum starved overnight and then UT or treated and analyzed as in B. F) MDA-MB-231 cells were pretreated with 10 μM dorsomorphin or LDN 193189 for 30 min. Cells were UT or treated as in B. Data are representative of 3 independent experiments.
Consistent with the ability of ALK7 to phosphorylate Smad2/3 in response to activin, and form BMP-stimulated complexes with BMPRII and ALK6 (Fig. 3A, C), DN ALK7 inhibited activin-induced Smad2/3 phosphorylation and attenuated BMP2-induced Smad2/3 and Smad1/5/8 phosphorylation (Fig. 4C and Supplemental Fig. S2K). Similar effects were observed with shRNA-mediated silencing of ALK7 expression (Fig. 4C and Supplemental Fig. S2K), supporting a role for ALK7 in BMP2-mediated Smad2/3 signaling.
Further, consistent with the ability of ALK3 to form BMP-stimulated complexes with ALK5/6, to form BMP-stimulated complexes with ALK7 (Fig. 3C), either DN or shRNA-mediated silencing of ALK3 or ALK6 attenuated BMP2-induced Smad1/5/8 signaling, whereas abrogating BMP2 induced Smad2/3 signaling (Fig. 4D, E and Supplemental Fig. S2L, M). Similarly, inhibition of ALK3/ALK6 with the small-molecule inhibitors dorsomorphin (39) and LDN 193189 (39) also abrogated BMP2-induced Smad1/5/8 and Smad2/3 phosphorylation (Fig. 4F). These results suggest that the canonical BMP type I receptors ALK3 and ALK6 facilitate both canonical BMP signaling to Smad1/5/8 and noncanonical BMP signaling to Smad2/3.
Taken together, these results suggest a mechanism by which BMP superfamily ligands can signal to the canonical TGF-β-responsive Smads, namely Smad2/3, via heteromeric complexes containing type I receptors that bind BMPs (ALK3/6), type I receptors that phosphorylate Smad2/3 (ALK5/7), and type II TGF-β superfamily receptors.
BMP2 induces formation of noncanonical receptor complexes that correspond to Smad phosphorylation profiles
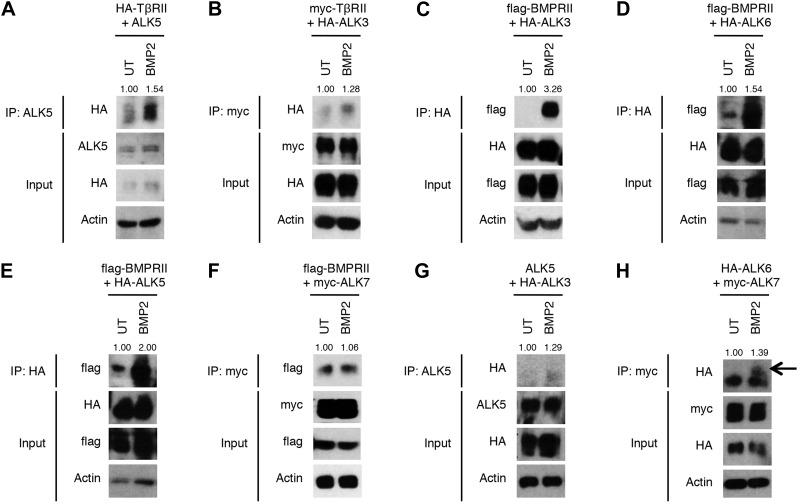
To provide additional support for this provocative model, we initially investigated these heteromeric complexes with an independent co-IP approach. Consistent with the PLA results, BMP2 stimulated complex formation between TβRII and ALK5 (Fig. 5A) and between TβRII and ALK3 (Fig. 5B). As expected, BMP2 stimulated complex formation between BMPRII and ALK3 (Fig. 5C) and between BMPRII and ALK6 (Fig. 5D). Further, BMP2 stimulated complex formation between BMPRII and ALK5 (Fig. 5E), whereas the interaction between BMPRII and ALK7 appeared to be constitutive and not increased by BMP2 treatment (Fig. 5F), contrary to Panc-1 cells, where we found an increase in ALK6/7 association with BMP treatment (Fig. 3C). Finally, BMP2 stimulated complex formation between ALK5 and ALK3 (Fig. 5G), as well as between ALK6 and ALK7 (Fig. 5H). These results are consistent with the PLA results and support the hypothesis that BMPs induce heteromeric complex formation between BMP binding receptors and Smad2/3 phosphorylating receptors.
Figure 5.
BMP2 induces formation of heteromeric receptor complexes. HEK293 cells were transfected with the indicated epitope-tagged receptors, serum starved, and treated with 10 nM BMP2 or UT. Cells were lysed, and receptor complexes were immunoprecipitated with anti-ALK5 (A, G), anti-myc (B, F, H) or anti-HA (C–E) antibodies. Western blot analysis was performed with the indicated antibodies. Data are representative of 2 independent experiments.
We then explored whether the cell receptor complex profile would correspond to the Smad phosphorylation profile. In the matched, isogenic 67NR and 4T1 cell lines, BMP stimulated Smad1/5/8 phosphorylation to an equivalent extent, whereas BMP stimulated only Smad2 phosphorylation in the 67NR cell line, and both Smad2 and Smad3 phosphorylation in the 4T1 cell line (Fig. 1E). Consistent with the ability of BMP to stimulate Smad1/5/8 phosphorylation in both cell lines, BMP increased BMPRII/ALK3 and BMPRII/ALK6 complexes in both cell lines (Fig. 6A, D). In addition, consistent with the ability of BMP to stimulate Smad2 phosphorylation in both cells lines, it increased TβRII/ALK5 and TβRII/ALK3 complex formation (Fig. 6B, E) as well as ALK3/5 complexes in both cell lines (Fig. 6C, F). However, consistent with the higher degree of BMP-induced Smad2 phosphorylation in the 4T1 cell line, 4T1 cells exhibited increased BMP2-induced TβRII/ALK3 complex formation (Fig. 6E). Finally, complexes mediating BMP-induced Smad3 phosphorylation, including BMPRII and ALK5 or BMPRII and ALK7 were induced by BMP only in the 4T1 cell line (Fig. 6D) and not in the 67NR cell line (Fig. 6A). Taken together, these data strongly support a model wherein BMP induces complex formation between TGF-β and BMP receptors to mediate noncanonical Smad2/3 phosphorylation.
Figure 6.
Noncanonical receptor complexes form specifically in cells exhibiting BMP2-induced Smad2/3 phosphorylation. A–C) 67NR cells were untreated (UT) or treated with 10 nM BMP2, and PLAs were performed with antibodies against the endogenous receptors BMPRII + ALK3/5–7 (A), TβRII +ALK3/5 (B), and ALK3/5 and ALK7/6 (C). Left panels: representative immunofluorescence images of the indicated receptor pairs. Right panels: images of 3 random fields were quantified by the count function of NIS-Br-Elements microscopy software. Data are expressed relative to UT control cells. D–F) 4T1 cells were UT or treated with 10 nM BMP2, and PLAs were performed with antibodies against the endogenous receptors BMPRII + ALK3/5–7 (D), TβRII +ALK3/5 (E), and ALK3/5 and ALK7/6 (F), and quantified as in A. Data are expressed relative to untreated control cells. Scale bars = 10 μm. Results are means ± se of 4 independent experiments. *P < 0.05 (Student's t test).
BMP2 induces cell invasion through Smad3
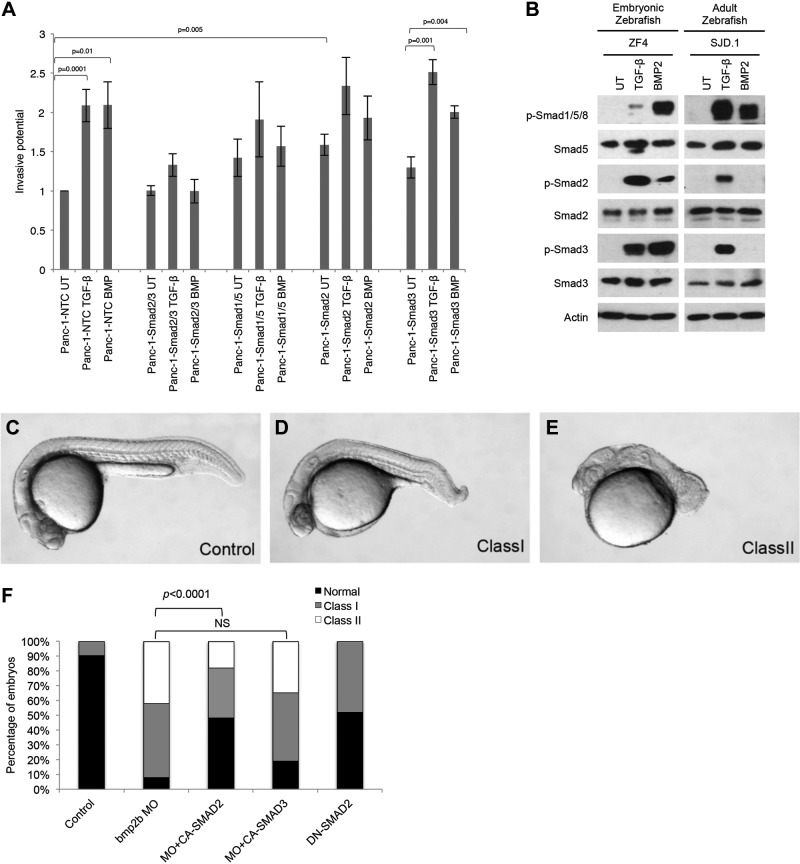
The biological effects of BMPs, including regulation of cell proliferation, migration, and invasion, as well as aberrant BMP signaling in human disease, have been largely attributed to BMP-induced Smad1/5/8 signaling (40–43). As BMP-mediated Smad2/3 signaling occurred preferentially in transformed cells, to investigate the role of BMP-mediated Smad2/3 signaling, we used shRNA to silence Smad1 and Smad5, Smad2 and Smad3, or Smad1 and Smad5 along with either Smad2 or Smad3 (Supplemental Fig. S2N, O) and assessed the effects on BMP-mediated cellular responses in the BMP-responsive MDA-MB-231 human breast cancer and Panc-1 pancreatic cancer cells. In the context of the full complement of receptor-activated Smads, BMP and TGF-β increased pancreatic cancer cell (Fig. 7A) and BMP2-induced breast cancer cell (Supplemental Fig. S2P) invasion. However, after shRNA-silencing of Smad1/5, with signaling occurring through Smad2/3 (Panc-1-Smad2/3 and MDA-MB-231-Smad2/3), BMP failed to increase cell invasion (Fig. 7A and Supplemental Fig. S2P), whereas TGF-β still increased invasion (Fig. 7A). When Smad2/3 are silenced, with signaling occurring through Smad1/5 (Panc-1-Smad1/5 and MDA-MB-231-Smad1/5), neither TGF-β nor BMP increased cancer cell invasion (Fig. 7A and Supplemental Fig. S2P), suggesting that BMP-mediated invasion is regulated by the ratio of Smad1/5 vs. Smad2/3 signaling. Although resulting in higher basal invasion, silencing Smad1/5/3 while retaining Smad2 expression (Panc-1-Smad2, MDA-MB-231-Smad2) failed to restore BMP- or TGF-β-mediated invasion (Fig. 7A and Supplemental Fig. S2P). Further, consistent with the specific role for Smad3 in cancer progression suggested by increased BMP2-stimulated Smad3 phosphorylation in the breast cancer progression series (Fig. 1E), silencing of Smad1/5/2, while retaining Smad3 expression (Panc-1-Smad3, MDA-MB-231-Smad3) restored BMP- and TGF-β-mediated invasion (Fig. 7A and Supplemental Fig. S2P). Taken together, these data suggest that BMP-induced Smad3 signaling mediates the effects of BMP on breast and pancreatic cancer cell invasiveness.
Figure 7.
BMP2 induces invasion through Smad3 and mediates dorsoventral axis formation through Smad2. A) Panc-1-nontargeting control (NTC), Panc-1-Smad2/3, Panc-1-Smad1/5, Panc-1-Smad2, and Panc-1-Smad3 cells were untreated (UT) or treated with 10 nM BMP2, allowed to invade through Matrigel-coated filters for 24 h, and quantified. Data are expressed relative to untreated MDA-MB-231-NTC cells. Results are means ± sem of 2 independent experiments. *P < 0.05 (Student's t test). B) Embryonic (ZF4) and adult (SJD.1) zebrafish cell lines were UT or stimulated with 100 pM TGF-β or 10 nM BMP2 for 40 min as indicated, lysed, and analyzed by Western blot with the indicated antibodies. C) Wild-type embryo at 1 d after fertilization. D, E) Zebrafish embryos injected with bmp2b MO had variable phenotypes, ranging from mildly dorsalized (D) to strongly dorsalized (E) (class I and II, respectively). F) Quantification of dorsalization in embryo batches injected with bmp2b MO (targeting the translational start site) alone or with 100 pg human CA SMAD2, or CA SMAD3 mRNA or injected with 100 pg DN SMAD2 mRNA (n=80–98 embryos/injection). Representative photographs of live embryos at ∼22–24 h.
BMP2 mediated Smad2 signaling mediates, in part, dorsoventral axis specification in zebrafish embryos
The BMP signaling pathway has a well-defined and distinct role in specifying dorsoventral axis formation during embryonic development. Specifically, during zebrafish (Danio rerio) embryonic development, BMP2b and BMP7 establish a dorsoventral gradient, with the BMPs specifying the ventral axis and disruption in BMP signaling resulting in a dorsalized phenotype (5–7). Consistent with the results obtained in human and murine cell lines, in addition to inducing Smad1/5/8 phosphorylation, BMP2 induced Smad2/3 phosphorylation in the embryonic zebrafish cell line ZF4, but not in the adult zebrafish cell line SJD.1 (Fig. 7B). As expected, compared to normal (Fig. 7C) embryos at the same stage of development, MO-induced silencing of bmp2b resulted in moderate (Fig. 7D) to severe (Fig. 7E) dorsalization. To investigate the role of BMP2-induced Smad2 or Smad3 in BMP-mediated dorsoventral axis specification, CA human SMAD2 (CA-SMAD2) or SMAD3 (CA-SMAD3) was coinjected with the bmp2b-silencing MO. Although expression of CA-SMAD3 failed to rescue the dorsalized phenotype (Fig. 7F), CA-SMAD2 effectively and significantly rescued bmp2b-MO-induced dorsalization, with an increase in the number of normal embryos (P<0.0001) and a decrease in the number of severely and moderately dorsalized embryos (Fig. 7F). Furthermore, expression of DN SMAD2 (DN-SMAD2) partially replicated the bmp2b-MO–induced knockdown dorsalized phenotype (Fig. 7F). These data suggest that BMP signals, in part, through Smad2, but not Smad3, to specify dorsoventral axis formation during zebrafish development.
Canonical Smad1/5 and Smad2/3 signaling converge in human cancer specimens
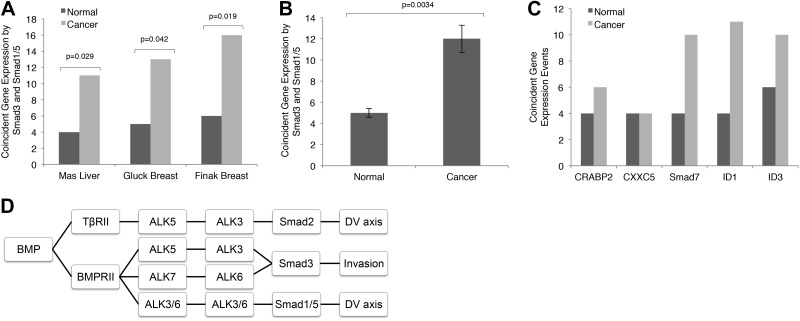
Our survey of cancer cell line models, primary epithelial cells, and primary cancer cells suggested that BMP-induced Smad2/3 signaling occurs preferentially in transformed cells. To investigate whether BMP-induced Smad2/3 signaling was occurring in the context of human cancers in vivo, we analyzed publicly available datasets for coexpression of canonical Smad1/5/8- and Smad2/3-responsive genes. In both patients with breast cancer and patients with liver cancer, relative to normal control samples, we observed a significant increase in coincident expression of genes that are known to be regulated by both Smad1/5/8 and Smad2/3 in tumor samples (Fig. 8A–C and Supplemental Tables S1–S6). In a similar manner, we observed a significant increase in coincident expression of canonical Smad1/5/8-responsive genes with canonical Smad2/3-responsive genes in tumor samples (Fig. 8A–C and Supplemental Tables S1–S6). Taken together, these data support a convergence of canonical Smad1/5 and Smad2/3 signaling in human cancers, suggesting that the BMP-induced Smad2/3 signaling defined herein occurs in the context of human cancers in vivo.
Figure 8.
Tumorigenesis elicits coincident regulation of TGF-β- and BMP-responsive genes. A) Data demonstrate the convergence of TGF-β- and BMP-regulated Smads in liver and breast tumors (dark bars) as compared to corresponding normal tissue (light bars). Pearson correlation coefficients were used in the Fisher's combined probability test to identify significant and coincidentally expressed genes. Results are the number of coincident events that occurred between normal and malignant tissues. B) Mean ± se summary data from A showing a significant convergence of Smad3 and Smad1/5 signaling in cancer tissues, as compared to that in corresponding normal tissues. P values were calculated by Student's t test. C) Data demonstrating that the expression levels of genes under control of both Smad1/5/8 and Smad2/3 were upregulated in cancer (dark bars) vs. normal (light bars) samples. Pearson correlation coefficients were used in the Fisher's combined probability test to identify significant and coincidentally expressed genes. Results are number of coincident events that occurred in normal and malignant tissues. D) BMP2 signaled through heteromeric receptor complexes to regulate tumorigenesis and development. It signaled through TβRII/ALK5/ALK3, leading to Smad2 phosphorylation to regulate dorsoventral axis specification in zebrafish, and through BMPRII/ALK5/ALK3 or BMPRII/ALK7/ALK6 to induce Smad3 phosphorylation to regulate cancer cell invasion.
DISCUSSION
In this study, we demonstrated and elucidated the mechanisms of two novel BMP signaling pathways. In addition to the canonical BMP-Smad1/5/8 pathway, BMP2 and other BMP family ligands induce direct, rapid, and potent phosphorylation and signaling via Smad2/3. Mechanistically, we found that BMPs activated Smad2/3 via heteromeric receptor complex formation between the BMP-binding cell surface receptors ALK3/6, the Smad2/3 phosphorylating receptors ALK5/7, and type II TGF-β and BMP receptors (Fig. 8D). Further, we provided evidence of specific roles for these novel BMP signaling modes, with BMP-Smad3 facilitating cancer cell invasion and BMP-Smad2 mediating dorsoventral axis specification during zebrafish embryonic development. Finally, we report data supporting that this novel mode of signaling occurs in human cancers.
How might BMPs induce Smad2/3 phosphorylation? Structural studies have shown the existence of heterooligomeric signaling complexes of 2 BMPRII receptors with only 1 BMP type I receptor (44), leaving potential space for a type I receptor of another ligand class (i.e., ALK5/7) in the complex. Moreover, the extracellular domains of BMP type I and II receptors in heterocomplexes do not contact each other directly (13, 37), allowing these BMP receptors to form complexes with other receptors. In this study, we provided evidence that BMPRII forms BMP-stimulated complexes with ALK5 and ALK7, ALK3 forms complexes with ALK5, and ALK6 forms complexes with ALK7. In addition, specific roles were identified for type II TGF-β superfamily receptors, with TβRII mediating BMP2-induced Smad2 phosphorylation and BMPRII mediating BMP2-induced Smad3 phosphorylation. The specificity of signaling at the type II receptor level supports the formation of specific ligand–receptor complexes. In contrast, ALK5 and ALK3, as well as ALK7 and ALK6, assembled to conduct BMP2 signaling to Smad2 and Smad3, respectively (Fig. 8D). At the type I receptor level, the individual ALK receptors did not compensate for each other in mediating BMP2-induced Smad2/3 signaling, as disrupting the function of 1 ALK conferred robust effects on BMP-induced Smad2/3, but more modest effects on BMP-induced Smad1/5 signaling (Fig. 4). These findings are consistent with a model in which heterocomplex formation across ligand classes results in crosstalk between BMP and canonical TGF-β Smads, as well as with the embryonic lethal phenotype of nearly all ALK murine KO models. We demonstrated the important finding that in cells where BMP did not stimulate heterocomplex formation between BMP-binding ALKs and Smad2/3-phosphorylating ALKs, BMP did not stimulate this novel mode of signaling (Fig. 6), providing further support for the proposed mechanism described herein.
BMP2-induced Smad2/3 phosphorylation occurred preferentially in cancer and embryonic cell lines, but rarely in adult, nontransformed cells, suggesting that while noncanonical BMP signaling to Smad2/3 is active during early embryonic development (Fig. 7), further development provides a nonpermissive environment for this mode of signaling. In addition, consistent with a dedifferentiated phenotype and the coopting of embryonic pathways, including the EMT pathways, during cancer progression, this mode of signaling is also activated. Indeed, we observed a consistent increase in BMP2-induced Smad3 phosphorylation as cancer progression models progressed from replicating benign to metastatic disease in the murine breast cancer progression series. Furthermore, we showed that epithelial cells that cannot induce Smad2 or Smad3 phosphorylation in response to BMP2 stimulation can phosphorylate Smad2/3 after BMP2 treatment after undergoing EMT. These results further support EMT as a crucial part of cancer progression and provide a potential mechanism by which canonical BMP switches to promote cancer progression.
The current results are also reminiscent of those supporting preferential TGF-β-stimulated Smad1/5 phosphorylation in the context of cancer progression (10, 11). In these studies, TGF-β-induced Smad1/5 phosphorylation was dependent on ALK5 and regulated migration in a tumorigenic context (10). Moreover, it was demonstrated that TGF-β signaling through Smad1/5 mediates anchorage-independent growth, potentially via the ability of ALK5/3 or ALK2 to form complexes, resulting in mixed Smad1/2 complexes (11). Although we report specific roles of TβRII in mediating BMP2-induced Smad2; BMPRII in mediating BMP2-induced Smad3 signaling; and heterocomplex crosstalk between BMP-binding ALKs (ALK3/6) and Smad2/3-phosphorylating ALKs (ALK5/7), the alterations in TGF-β superfamily signaling that promote these crosstalk events during embryonic development and cancer progression remain under investigation.
BMP3, the classic BMP antagonist, has been demonstrated to promote mesenchymal stem cell proliferation through Smad2 phosphorylation (45). However, contrary to the mechanism delineated in the current study, BMP3 signals through ActRIIB and ALK4 in the context of mesenchymal stem cells. Furthermore, BMP9 has been demonstrated to induce Smad2 phosphorylation (46, 47) in pulmonary endothelial cells by signaling through ActRII (47). Taken together, these results suggest that members of the BMP family signal through diverse receptors and pathways, dictated perhaps by the specific affinity of BMP ligands for cell surface receptors. For example, BMP7 has a much higher affinity for ALK6 than for ALK3, whereas BMP2 has a higher affinity for ALK3 (44). In addition, numerous BMP family coreceptors, including TβRIII (48), endoglin (49), dragon (50), and other members of the repulsive guidance molecule (RGM) family (51) can regulate this signaling, enabling binding to lower affinity, lower abundance receptors.
The BMP signaling pathway, along with other morphogens, is responsible for patterning the dorsoventral axis during zebrafish embryonic development (5–7) through Smad5 activity (52). In our study, CA-Smad2 partially rescued dorsalization caused by BMP2b silencing, whereas DN-Smad2 partially replicated the BMP2b-knockdown phenotype. These data suggest that BMP signals through Smad2, in part, to regulate dorsoventral axis formation and establish a role for noncanonical BMP signaling during embryonic development. The partial rescue of CA-Smad2 and partial replication of DN-Smad2, suggest that BMP2 also signals through other pathways to mediate these effects. Indeed, BMP signaling through Smads1/5/8 has been demonstrated to have a role in mediating dorsoventral axis specification (52).
BMP signaling pathways increase cancer cell migration (40) and invasion via up-regulation of MMPs (40) and induction of EMT (40, 42). In our study, Smad3 phosphorylation facilitated invasion with BMP treatment, whereas Smad2 or Smad2/3 did not (Fig. 7A). Smad2 may suppress Smad3-mediated invasion thereby decreasing invasion when both Smad2 and Smad3 are expressed. The increased aggressiveness of MDA-MB-231-Smad3 cells is consistent with the in vivo mouse model, demonstrating that conditional deletion of both Smad1 and Smad5 in somatic gonad cells lead to metastatic tumor development with 100% penetrance (53). In addition, relative to normal tissue, Smad1/5/8- and Smad2/3-responsive genes were coexpressed to a greater degree in human breast and liver cancer specimens (Fig. 8A–C), consistent with our findings that BMPs signaled via Smad1/5 and Smad2/3 in the context of transformed cells. The distinct roles of the BMP2-induced Smad1/5 vs. Smad3 pathway, and increased BMP2-induced Smad3 signaling within the cancer progression pathway suggest a potential mechanism by which BMP switches from a tumor suppressor to a tumor promoter, with the noncanonical Smad2/3 pathways becoming activated during cancer progression and driving the tumor-promoting effects.
BMP signaling pathways play a role in several human diseases. Alterations resulting in either increased or decreased BMP signaling have been identified, including loss-of-function mutations in BMP receptors in primary pulmonary hypertension and in juvenile polyposis syndrome and gain-of-function mutations in BMP receptors in fibrodysplasia ossificans progressiva (54). The current finding that BMPs can signal through Smad2/3 suggests that increases or decreases in this novel mode of signaling are responsible, in part, for the pathophysiology of these diseases. Moreover, in Loeys-Dietz syndrome, where loss-of-function mutants in TβRI and TβRII result in paradoxical increases in Smad2/3 signaling, the current findings suggest that BMP-mediated signaling to Smad2/3 provides a mechanism for this finding. The precise contribution of BMP-mediated signaling to Smad2/3 to these human diseases is currently under investigation.
Although the TGF-β-signaling pathway suppresses cancer formation, once initiated, it promotes cancer progression through multiple mechanisms, including promoting EMT and invasion and migration of cancer cells, suppressing the immune system, and promoting angiogenesis. Accordingly, anti-TGF-β therapies are currently being investigated, including TGF-β-neutralizing antibodies (i.e., GC1008, ref. 54) and small-molecule ALK5 inhibitors (i.e., LY2157299, ref. 55). TGF-β neutralizing antibodies are expected to be specific to TGF-β-mediated signaling; however, as BMP can signal to Smad2/3, this novel mode of signaling provides a potential mechanism of resistance to TGF-β-neutralizing antibody therapy. In contrast, small-molecule inhibitors inhibit the kinase activity of ALK5, thereby inhibiting canonical TGF-β signaling and, as demonstrated in this work, also inhibiting BMP signaling through Smad2/3. BMP expression is associated with cancer progression and an increase in bone metastasis (43), making it a provocative target for anticancer treatment as well. Therefore, use of an ALK5 inhibitor to inhibit both BMP and TGF-β signaling may be a more beneficial anticancer therapy than inhibition of TGF-β alone. Studies are needed to investigate whether broader inhibition of TGF-β and BMP pathways will be tolerated and provide therapeutic benefit for cancer patients.
In summary, we established that BMPs induce not only canonical Smad1/5/8 phosphorylation, but noncanonical signaling to Smad2/3, as well. These signaling pathways are differentially regulated at the level of the type II receptors, where BMPRII facilitates BMP2-induced Smad1/5/8 and Smad3 phosphorylation, and TβRII mediates Smad2 phosphorylation. Moreover, heterodimeric receptor complexes of receptors from different ligand classes can assemble, facilitating crosstalk between BMP and TGF-β ligands. Furthermore, we showed biological relevance by demonstrating BMP's ability to facilitate invasion through Smad3 and pattern the dorsoventral axis via Smad2 signaling during embryonic development. These novel signaling pathways are also active in human cancers, suggesting that BMP signaling to Smad2/3 is a critical component of dedifferentiation and cancer progression.
Supplementary Material
Acknowledgments
The authors thank Drs. Paul Yu (Massachusetts General Hospital, Boston, MA, USA), Fred Miller (Wayne State University, Detroit, MI, USA), and Harold Moses (Vanderbilt University, Nashville, TN, USA) for reagents, Kyu Seo Kim (Duke University) for help with cloning and in vitro transcription, and Brad Gersh and Alok Tewari (Duke University) for data processing and analysis.
This work was supported in part by U.S. National Institutes of Health grant R01-CA136786 (to G.C.B.), Komen for the Cure grant SAC100002 (to G.C.B.), American Heart Association grant AHA-11POST7160006 (to C.G.) and seed funding from the Center of Human Disease Modeling. N.K. is a Distinguished Brumley Professor.
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ActRII
- type II activin receptor
- ALK
- activin receptor-like kinase
- BMP
- bone morphogenetic protein
- BMPRII
- type II bone morphogenetic receptor
- CA
- constitutively active
- co-IP
- coimmunoprecipitation
- DN
- dominant negative
- EMT
- epithelial–mesenchymal transition
- FGF
- fibroblast growth factor
- GDF
- growth and differentiation factor
- HMEC
- human mammary endothelial cell
- IP
- immunoprecipitation
- KO
- knockout
- MO
- morpholino
- PLA
- proximity ligation assay
- TβRII
- type II transforming growth factor receptor
- TβRIII
- type III transforming growth factor receptor
- TGF-β
- transforming growth factor β
REFERENCES
- 1. Massague J. (1998) TGF-beta signal transduction. Annu. Rev. Biochem. 67, 753–791 [DOI] [PubMed] [Google Scholar]
- 2. Miyazono K., Kamiya Y., Morikawa M. (2010) Bone morphogenetic protein receptors and signal transduction. J. Biochem. 147, 35–51 [DOI] [PubMed] [Google Scholar]
- 3. Ebner R., Chen R. H., Lawler S., Zioncheck T., Derynck R. (1993) Determination of type I receptor specificity by the type II receptors for TGF-beta or activin. Science 262, 900–902 [DOI] [PubMed] [Google Scholar]
- 4. Wrana J. L., Attisano L., Wieser R., Ventura F., Massague J. (1994) Mechanism of activation of the TGF-beta receptor. Nature 370, 341–347 [DOI] [PubMed] [Google Scholar]
- 5. Dick A., Hild M., Bauer H., Imai Y., Maifeld H., Schier A. F., Talbot W. S., Bouwmeester T., Hammerschmidt M. (2000) Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development 127, 343–354 [DOI] [PubMed] [Google Scholar]
- 6. Kishimoto Y., Lee K. H., Zon L., Hammerschmidt M., Schulte-Merker S. (1997) The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124, 4457–4466 [DOI] [PubMed] [Google Scholar]
- 7. Schmid B., Furthauer M., Connors S. A., Trout J., Thisse B., Thisse C., Mullins M. C. (2000) Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development 127, 957–967 [DOI] [PubMed] [Google Scholar]
- 8. Wieser R., Wrana J. L., Massague J. (1995) GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 14, 2199–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu F., Ventura F., Doody J., Massague J. (1995) Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol. 15, 3479–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu I. M., Schilling S. H., Knouse K. A., Choy L., Derynck R., Wang X. F. (2009) TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO J. 28, 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daly A. C., Randall R. A., Hill C. S. (2008) Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol. Cell. Biol. 28, 6889–6902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirsch T., Sebald W., Dreyer M. K. (2000) Crystal structure of the BMP-2-BRIA ectodomain complex. Nat. Struct. Biol. 7, 492–496 [DOI] [PubMed] [Google Scholar]
- 13. Allendorph G. P., Vale W. W., Choe S. (2006) Structure of the ternary signaling complex of a TGF-beta superfamily member. Proc. Natl. Acad. Sci. U. S. A. 103, 7643–7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massague J., Wotton D. (2000) Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 19, 1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massague J., Seoane J., Wotton D. (2005) Smad transcription factors. Genes Dev. 19, 2783–2810 [DOI] [PubMed] [Google Scholar]
- 16. Derynck R., Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 17. Heldin C. H., Miyazono K., ten Dijke P. (1997) TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390, 465–471 [DOI] [PubMed] [Google Scholar]
- 18. Cheifetz S., Hernandez H., Laiho M., ten Dijke P., Iwata K. K., Massague J. (1990) Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J. Biol. Chem. 265, 20533–20538 [PubMed] [Google Scholar]
- 19. Lopez-Casillas F., Wrana J. L., Massague J. (1993) Betaglycan presents ligand to the TGF beta signaling receptor. Cell 73, 1435–1444 [DOI] [PubMed] [Google Scholar]
- 20. Westerfield M. (1995) The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 3rd Ed., University of Oregon Press, Eugene, OR, USA [Google Scholar]
- 21. Imai Y., Talbot W. S. (2001) Morpholino phenocopies of the bmp2b/swirl and bmp7/snailhouse mutations. Genesis 30, 160–163 [DOI] [PubMed] [Google Scholar]
- 22. Uemura M., Swenson E. S., Gaca M. D., Giordano F. J., Reiss M., Wells R. G. (2005) Smad2 and Smad3 play different roles in rat hepatic stellate cell function and alpha-smooth muscle actin organization. Mol. Biol. Cell 16, 4214–4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang B., Vu M., Booker T., Santner S. J., Miller F. R., Anver M. R., Wakefield L. M. (2003) TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J. Clin. Invest. 112, 1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aslakson C. J., Miller F. R. (1992) Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 52, 1399–1405 [PubMed] [Google Scholar]
- 25. Kalluri R., Weinberg R. A. (2009) The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kropf J., Schurek J. O., Wollner A., Gressner A. M. (1997) Immunological measurement of transforming growth factor-beta 1 (TGF-beta1) in blood: assay development and comparison. Clin. Chem. 43, 1965–1974 [PubMed] [Google Scholar]
- 27. Grainger D. J., Mosedale D. E., Metcalfe J. C., Weissberg P. L., Kemp P. R. (1995) Active and acid-activatable TGF-beta in human sera, platelets and plasma. Clin. Chim. Acta 235, 11–31 [DOI] [PubMed] [Google Scholar]
- 28. Koenig B. B., Cook J. S., Wolsing D. H., Ting J., Tiesman J. P., Correa P. E., Olson C. A., Pecquet A. L., Ventura F., Grant R. A. (1994) Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol. Cell. Biol. 14, 5961–5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sebald W., Nickel J., Zhang J. L., Mueller T. D. (2004) Molecular recognition in bone morphogenetic protein (BMP)/receptor interaction. Biol. Chem. 385, 697–710 [DOI] [PubMed] [Google Scholar]
- 30. Sugimoto H., Yang C., LeBleu V. S., Soubasakos M. A., Giraldo M., Zeisberg M., Kalluri R. (2007) BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J. 21, 256–264 [DOI] [PubMed] [Google Scholar]
- 31. Herrera B., Inman G. J. (2009) A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins: identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daluiski A., Engstrand T., Bahamonde M. E., Gamer L. W., Agius E., Stevenson S. L., Cox K., Rosen V., Lyons K. M. (2001) Bone morphogenetic protein-3 is a negative regulator of bone density. Nat. Genet. 27, 84–88 [DOI] [PubMed] [Google Scholar]
- 33. Bierie B., Stover D. G., Abel T. W., Chytil A., Gorska A. E., Aakre M., Forrester E., Yang L., Wagner K. U., Moses H. L. (2008) Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 68, 1809–1819 [DOI] [PubMed] [Google Scholar]
- 34. Cheng N., Bhowmick N. A., Chytil A., Gorksa A. E., Brown K. A., Muraoka R., Arteaga C. L., Neilson E. G., Hayward S. W., Moses H. L. (2005) Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene 24, 5053–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laiho M., Weis M. B., Massague J. (1990) Concomitant loss of transforming growth factor (TGF)-beta receptor types I and II in TGF-beta-resistant cell mutants implicates both receptor types in signal transduction. J. Biol. Chem. 265, 18518–18524 [PubMed] [Google Scholar]
- 36. Yu P. B., Beppu H., Kawai N., Li E., Bloch K. D. (2005) Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J. Biol. Chem. 280, 24443–24450 [DOI] [PubMed] [Google Scholar]
- 37. Ehrlich M., Gutman O., Knaus P., Henis Y. I. (2012) Oligomeric interactions of TGF-beta and BMP receptors. FEBS Lett. 586, 1885–1896 [DOI] [PubMed] [Google Scholar]
- 38. Inman G. J., Nicolas F. J., Callahan J. F., Harling J. D., Gaster L. M., Reith A. D., Laping N. J., Hill C. S. (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62, 65–74 [DOI] [PubMed] [Google Scholar]
- 39. Boergermann J. H., Kopf J., Yu P. B., Knaus P. (2010) Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int. J. Biochem. Cell Biol. 42, 1802–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Theriault B. L., Shepherd T. G., Mujoomdar M. L., Nachtigal M. W. (2007) BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis 28, 1153–1162 [DOI] [PubMed] [Google Scholar]
- 41. Gordon K. J., Kirkbride K. C., How T., Blobe G. C. (2009) Bone morphogenetic proteins induce pancreatic cancer cell invasiveness through a Smad1-dependent mechanism that involves matrix metalloproteinase-2. Carcinogenesis 30, 238–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deng H., Ravikumar T. S., Yang W. L. (2009) Overexpression of bone morphogenetic protein 4 enhances the invasiveness of Smad4-deficient human colorectal cancer cells. Cancer Lett. 281, 220–231 [DOI] [PubMed] [Google Scholar]
- 43. Hsu M. Y., Rovinsky S. A., Lai C. Y., Qasem S., Liu X., How J., Engelhardt J. F., Murphy G. F. (2008) Aggressive melanoma cells escape from BMP7-mediated autocrine growth inhibition through coordinated Noggin upregulation. Lab. Invest. 88, 842–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heinecke K., Seher A., Schmitz W., Mueller T. D., Sebald W., Nickel J. (2009) Receptor oligomerization and beyond: a case study in bone morphogenetic proteins. BMC Biol. 7, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stewart A., Guan H., Yang K. (2010) BMP-3 promotes mesenchymal stem cell proliferation through the TGF-beta/activin signaling pathway. J. Cell. Physiol. 223, 658–666 [DOI] [PubMed] [Google Scholar]
- 46. Star G. P., Giovinazzo M., Langleben D. (2010) Bone morphogenic protein-9 stimulates endothelin-1 release from human pulmonary microvascular endothelial cells: a potential mechanism for elevated ET-1 levels in pulmonary arterial hypertension. Microvasc. Res. 80, 349–354 [DOI] [PubMed] [Google Scholar]
- 47. Upton P. D., Davies R. J., Trembath R. C., Morrell N. W. (2009) Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J. Biol. Chem. 284, 15794–15804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kirkbride K. C., Townsend T. A., Bruinsma M. W., Barnett J. V., Blobe G. C. (2008) Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J. Biol. Chem. 283, 7628–7637 [DOI] [PubMed] [Google Scholar]
- 49. Ishibashi O., Ikegame M., Takizawa F., Yoshizawa T., Moksed M. A., Iizawa F., Mera H., Matsuda A., Kawashima H. (2010) Endoglin is involved in BMP-2-induced osteogenic differentiation of periodontal ligament cells through a pathway independent of Smad-1/5/8 phosphorylation. J. Cell. Physiol. 222, 465–473 [DOI] [PubMed] [Google Scholar]
- 50. Xia Y., Babitt J. L., Bouley R., Zhang Y., Da Silva N., Chen S., Zhuang Z., Samad T. A., Brenner G. J., Anderson J. L., Hong C. C., Schneyer A. L., Brown D., Lin H. Y. (2010) Dragon enhances BMP signaling and increases transepithelial resistance in kidney epithelial cells. J Am. Soc. Nephrol. 21, 666–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Corradini E., Babitt J. L., Lin H. Y. (2009) The RGM/DRAGON family of BMP co-receptors. Cytokine Growth Factor Rev. 20, 389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hild M., Dick A., Rauch G. J., Meier A., Bouwmeester T., Haffter P., Hammerschmidt M. (1999) The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development 126, 2149–2159 [DOI] [PubMed] [Google Scholar]
- 53. Pangas S. A., Li X., Umans L., Zwijsen A., Huylebroeck D., Gutierrez C., Wang D., Martin J. F., Jamin S. P., Behringer R. R., Robertson E. J., Matzuk M. M. (2008) Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol. Cell. Biol. 28, 248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elliott R. L., Blobe G. C. (2005) Role of transforming growth factor Beta in human cancer J. Clin. Oncol. 23, 2078–2093 [DOI] [PubMed] [Google Scholar]
- 55. Bueno L., de Alwis D. P., Pitou C., Yingling J., Lahn M., Glatt S., Troconiz I. F. (2008) Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur. J. Cancer 44, 142–150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.