Abstract
The β-strands of GFP form a rigid barrel that protects the chromophore from external influence. Herein, we identified specific mutations in β-strand 7 that render the chromophore sensitive to interactions of GFP with another protein domain. In the process of converting the FRET-based protein kinase A (PKA) sensor AKAR2 into a single-wavelength PKA sensor containing a GFP and a quencher, we discovered that the quencher was not required and that the sensor response relied on changes in GFP intrinsic fluorescence. The identified mutations in β-strand 7 render GFP fluorescence intensity and lifetime sensitive to conformational changes of the PKA-sensing domain. In addition, sensors engineered from the GCaMP2 calcium indicator to incorporate a conformation-sensitive GFP (csGFP) exhibited calcium-dependent fluorescence changes. We further demonstrate that single GFP sensors report PKA dynamics in dendritic spines of neurons from brain slices on 2-photon imaging with a high signal-to-baseline ratio and minimal photobleaching. The susceptibility of GFP variants to dynamic interactions with other protein domains provides a new approach to generate single wavelength biosensors for high-resolution imaging.—Bonnot, A., Guiot, E., Hepp, R., Cavellini, L., Tricoire, L., Lambolez, B. Single-fluorophore biosensors based on conformation-sensitive GFP variants.
Keywords: green fluorescent protein, chromophore, genetically encoded indicators, intracellular second messengers, fluorescence lifetime imaging, high-resolution microscopy
The chromophore of the green fluorescent protein (GFP) from Aequorea victoria essentially results from autocyclization of the SYG[65–67] residues buried inside the rigid barrel-shaped structure of the protein (1). Nonetheless, other amino acid residues of the protein contribute to its optical properties. Accordingly, spectral variants of GFP have been obtained through amino acid substitutions in several regions of the protein, such as the popular blue-shifted cyan fluorescent protein (CFP; ref. 2) and yellow-shifted yellow fluorescent protein (YFP; ref. 3) variants, as well as a “dark YFP” (4) endowed with YFP absorption properties but minimal emission intensity.
A large variety of genetically encoded biosensors use GFP and its variants to report conformational changes of specific sensing domains elicited by biological signals (5, 6). Engineering of these sensors has been mainly based on two strategies. One relies on changes of fluorescence resonance energy transfer (FRET) between two spectrally different fluorescent proteins (7). A similar paradigm uses the dark-YFP mutant to quench the emission of a fluorescent protein on conformational changes of the sensor (4, 8). In principle, this latter paradigm circumvents the need for spectral separation and allows the collection of photons in the entire emission spectrum. The other strategy is based on the modulation of optical properties of a single, circularly permuted, fluorescent protein (5). This approach has yielded single-wavelength sensors with high signal-to-baseline responses but whose low baseline fluorescence often hampers visualization of sensor-expressing cells in tissue slices (9–11). Here, we used a dark-YFP-based quenching approach to engineer a sensor aimed at reporting protein kinase A (PKA) activity in brain slices using 2-photon microscopy.
The ubiquitous cyclic AMP/PKA pathway is a key intracellular signal pathway. Cyclic adenosine monophosphate (cAMP) is produced by adenylate cyclase and activates PKA, which exerts pleiotropic effects in the cell. In the brain, the cAMP/PKA pathway modulates neuronal excitability and synaptic transmission and mediates the effects of multiple neurotransmitters. The PKA FRET sensor A-kinase activity reporter 2 (AKAR2; ref. 12) contains a sensing domain, formed with a phosphothreonine binding domain and a PKA substrate peptide, flanked by CFP and YFP (see Fig. 1). When phosphorylated by PKA, the substrate peptide folds into the phosphothreonine binding pocket, which causes an increase of the YFP/CFP emission ratio.
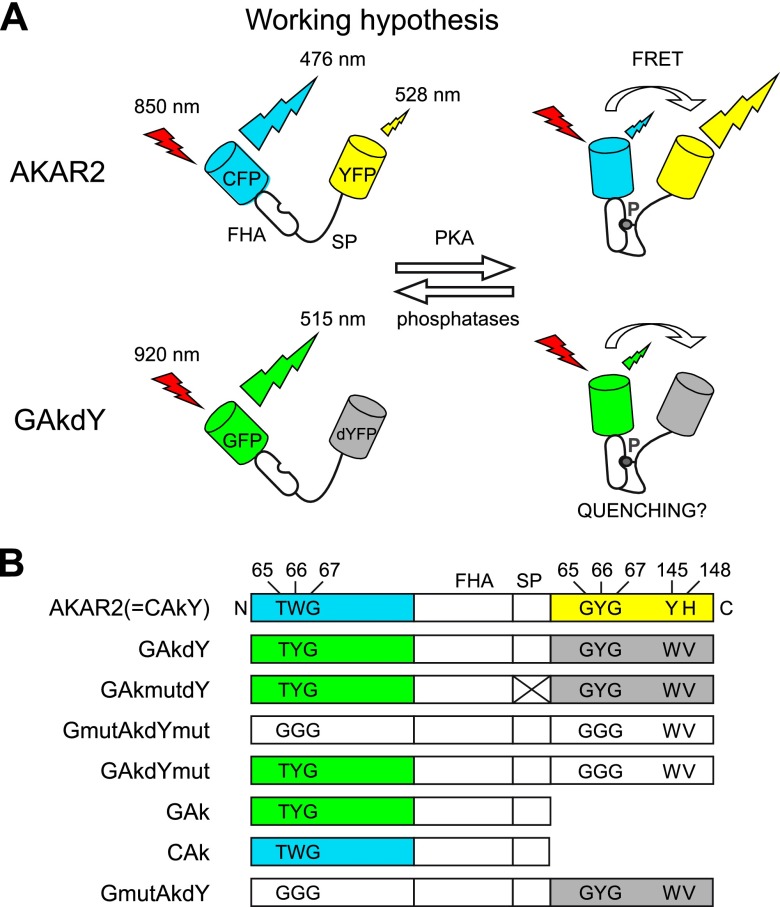
Figure 1.
Constructs derived from the FRET sensor AKAR2. A) Mechanisms of fluorescence changes reported for AKAR2 and anticipated for the newly generated GAKdY sensor following folding of the substrate peptide (SP) into the Forkhead-associated domain (FHA) on phosphorylation. B) Domain structure of most constructs used in this study, named after their constituent parts (see Abbreviations). Color boxes, gray boxes, and white boxes represent active fluorophores, fluorophores converted into quenchers, and inactivated fluorophores, respectively. Mutations at positions 65–67 (chromophore) and dark-YFP mutations are indicated. Inactivation of the sensing domain has been achieved via mutagenesis in SP (crossed box).
Here, we mutated the CFP and YFP domains of AKAR2 into GFP and the dark-YFP quencher, respectively. The new sensor reported PKA activation in living cells by an increase in fluorescence intensity. To explore the mechanisms of these fluorescence changes, additional sensors were engineered by inactivation, deletion, or substitution of the chromophores. We found that the interaction between two fluorophore domains was not required to generate a responsive sensor and that specific amino acid substitutions inside β-strand 7 of GFP were instead critical. We confirmed this property of β-strand 7 mutant of GFP on other sensors engineered from an existing calcium indicator. Finally, we found that PKA sensors incorporating this specific GFP mutant as single fluorophore exhibited a redistribution of fluorescence lifetimes on PKA activation and allowed the imaging of PKA dynamics in brain slices with high spatial resolution.
MATERIALS AND METHODS
PKA and calcium sensors
The PKA activity reporter AKAR2.1 (12) comprising the enhanced CFP (2) and the citrine version of YFP (13) was subcloned into the pcDNA3.1 plasmid (Invitrogen, Carlsbad, CA, USA). Constructs were derived from AKAR2.1 by site-directed mutagenesis (Quikchange XL kit, Stratagene, La Jolla, CA, USA) or fluorophore deletion and substitution as described in Fig. 1 and Table 1. A subset of these constructs (see Table 1) was subcloned into the pSinRep5 plasmid (Invitrogen) for production of recombinant Sindbis pseudovirions as described previously (14). The nucleotide sequences of GFP-A-kinase-sensing dark YFP (GAkdY), GFP-A-kinase-sensing dark YFP mutant (GAkdYmut), and GFP-A-kinase (GAk) have been submitted to the GenBank/European Bioinformatics Institute (EBI) Data Bank with accession numbers HM990101, HM990102, and HM990103, respectively. The conformation-sensitive GFP calmodulin peptide M13 (csGCaMP) constructs were obtained by amplifying the conformation-sensitive GFP (csGFP) sequence from GAk by PCR with oligonucleotides containing 0 to 2 linker sequences (see Fig. 3) and inserting the PCR products in pN1-GCaMP2 in the place of the cpGFP (15). The I146N mutation was introduced by site-directed mutagenesis. For bacterial expression, csGCaMP constructs and their I146N derivatives were subcloned into the pRSETc plasmid (Invitrogen).
Table 1.
Summary of point mutations in constructs derived from AKAR2 and of responses to PKA activation
| Probe | CFP-based fluorescent domain |
T391a | Citrine-based fluorescent domain |
FSK response, ΔF/F (%; N, n) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K26 | F64 | S65 | Y66 | N146 | M153 | V163 | N164 | A206 | S65 | Y66 | V68 | Q69 | S72 | Y145 | H148 | T203 | H231 | BHK | Neurons | ||
| AKAR2.1 | R | L | T | W | I | T | A | H | G | L | M | A | Y | I | NT | NT | |||||
| GAkdY | R | L | T | I | T | A | H | G | L | M | A | W | V | Y | I | 32 ± 12; 8, 53 | 44 ± 10; 3, 20 | ||||
| GAkmutdY | R | L | T | I | T | A | H | A | G | L | M | A | W | V | Y | I | NR; 3, 27 | NT | |||
| GmutAkdYmut | R | L | G | G | I | T | A | H | G | G | L | M | A | W | V | Y | I | NF; 5, 0 | NT | ||
| GAkdYmut | R | L | T | I | T | A | H | G | G | L | M | A | W | V | Y | I | 40 ± 16; 10, 59 | 74 ± 18; 7, 19 | |||
| GAk | R | L | T | I | T | A | H | 26 ± 10; 8, 52 | 47 ± 9; 6, 15 | ||||||||||||
| GA206KAk | R | L | T | I | T | A | H | K | 31 ± 11; 5, 27 | NT | |||||||||||
| eGAk | L | T | A | NR; 5, 34 | NT | ||||||||||||||||
| GI146NAk | R | L | T | T | A | H | NR; 5, 46 | NT | |||||||||||||
| GR26KAk | L | T | I | T | A | H | 28 ± 12; 5, 24 | NT | |||||||||||||
| GT153MAk | R | L | T | I | A | H | 28 ± 12; 7, 29 | NT | |||||||||||||
| GH164NAk | R | L | T | I | T | A | 27 ± 9; 5, 25 | NT | |||||||||||||
| CAk | R | L | T | W | I | T | A | H | −20 ± 6; 7, 51 | −16 ± 5; 7, 21 | |||||||||||
| GmutAkdY | R | L | G | G | I | T | A | H | G | L | M | A | W | V | Y | I | −17 ± 8; 7, 50 | −19 ± 7; 8, 31 | |||
| GmutAkY | R | L | G | G | I | T | A | H | G | L | M | A | Y | I | NR; 7, 63 | NT | |||||
Mutations refer to the sequence of wild-type GFP (position 47; accession no. M62653). Numbering begins with the start methionine of each fluorescent domain except for the mutation in the PKA-sensing domain. Citrine-based fluorescent domain is absent where all columns are blank. ΔF/F values are means ± sd. N, total number of experiments n, total number of cells; NF, not fluorescent; NR, not responsive; NT, not tested.
PKA sensor domain.
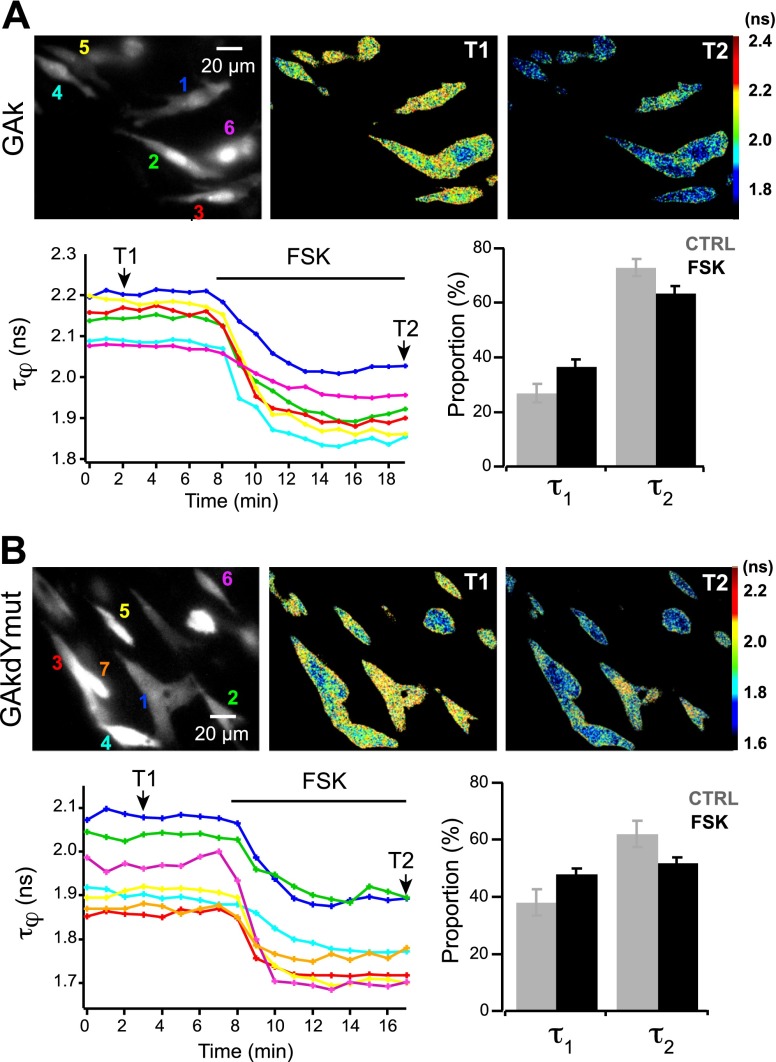
Figure 3.
Characterization of single-fluorophore sensors using FLIM. Grayscale fluorescence images show BHK cells expressing GAk (A) and GAkdYmut (B) sensors. Pseudocolor images of the same fields before (T1) and during (T2) application of FSK (13 μM) illustrate the decrease in fluorescence lifetime τφ on PKA activation. Traces show the variations of τφ measured in individual cells indicated in the grayscale images; colors correspond to numbers in top left panels. Bar graphs show variations of the proportion of the short-lifetime (τ1) and long-lifetime (τ2) components on FSK application.
Expression in baby hamster kidney (BHK) cells, human embryonic kidney (HEK) cells, and brain slices
BHK cells [CCL-10; American Type Culture Collection (ATCC), Manassas, VA, USA] and HEK-293 cells (CRL-1573; ATCC) grown at 37°C, 5% CO2 in Dulbecco's modified Eagle medium (DMEM) containing 5% (BHK)/10% (HEK) fetal calf serum were plated on glass coverslips the day before plasmid transfection was performed, using Lipofectamine 2000 (Invitrogen). Optical recordings were performed after overnight expression of plasmid constructs. Parasagittal slices (300 μm thick) of somatosensory cortex were obtained as described previously (16) from male Wistar rats (11–15 d old, Janvier Labs, St. Berthevin, France) in accordance with the guidelines of the French Ministry of Agriculture and the European Community. Slices were transferred onto a Millicell CM membrane (Millipore, Bedford, MA, USA) preequilibrated with culture medium [50% minimum essential medium (MEM), 50% Hank's balanced salt solution (HBSS), 6.5 mg/ml glucose, and 100 U/ml penicillin-streptomycin] and transduced with Sindbis pseudovirions by adding 5 μl of viral solution (∼105 infectious particles) onto the slice as described previously (22). Following overnight incubation at 35°C, 5% CO2, slices were allowed to equilibrate for 30 min in artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 2.5 KCl, 20 d-glucose, and 5 sodium pyruvate and saturated with 5% CO2/95% O2. BHK cells, HEK cells, and brain slices were then transferred to a recording chamber and perfused at 6 ml/min at 25°C (BHK and HEK) or 32°C (slices) with ACSF (BHK and slices) or with a solution containing (in mM) 135 NaCl, 2 CaCl2, 1 MgCl2, 5.4 KCl, 10 d-glucose, and 5 HEPES (pH 7.4) for HEK. Forskolin (FSK), ionomycin, carbachol (Sigma, St. Louis, MO, USA) and corticotrophin-releasing factor (CRF; Bachem, Bubendorf, Switzerland) were applied through the bath perfusion.
Spectrofluorometry
Single colonies of BL21(DE3)pLysS bacteria transformed with pRSET-csGCaMP plasmid were grown overnight at 37°C in 10 ml Luria-Bertani broth supplemented with ampicillin (100 μg/ml). The bacterial suspension (4 ml) was used to seed 50 ml of terrific broth supplemented with ampicillin. After 3 h growth at 37°C, protein expression was induced for 4 h at 37°C in the presence of 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). Then, cells were collected by centrifugation at 4°C and resuspended on ice in 5 ml Tris (50 mM, pH 8) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA), according to the manufacturer's instructions. After incubation with lysozyme (1 mg/ml) on ice, cells were lysed by freezing in liquid nitrogen and thawing at 42°C 3 times. After addition of KCl to a final concentration of 100 mM, the cell lysate was centrifuged, and the supernatant was used for spectral analysis. Emission spectra (excitation at 485 nm) were acquired at room temperature in opaque 96-well plate with a Spectromax M2 apparatus (Molecular Devices, Eugene, OR, USA). For each replicate, 200 μl of lysate was mixed with an equal volume of a solution containing 50 mM Tris (pH8), 100 mM KCl, and either 1 mM ethylenediaminetetraacetic acid (EDTA; calcium-free condition) or 2 mM CaCl2.
Optical recordings
Two-photon images were obtained with a custom-built 2-photon laser scanning microscope based on an Olympus BX51WI upright microscope (Olympus, Tokyo, Japan) with ×40 (0.8 NA) or ×60 (0.9 NA) water-immersion objectives and a titanium:sapphire laser (MaiTai HP; Spectra Physics, Ellicot City, MD, USA). Two-photon excitation was performed at 850 nm for CFP and at 920 nm for GFP, dark YFP, and YFP. Galvanometric mirrors (model 6210; Cambridge Technology, Cambridge, MA, USA) were used for bidirectional linescanning at 1.4 frame/s, and a piezo-driven objective scanner (P-721 PIFOC; Physik Instrumente GmbH, Auburn, MA, USA) was used for z-stack image acquisition. The system was controlled by the MPscope software (17). Nondescanned fluorescence was collected on a photomultiplier (H9305-03; Hamamatsu, Middlesex, NJ, USA). The 512- × 512-pixel images were exported using MATLAB (The Mathworks, Natick, MA, USA) and analyzed with ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA; http://rsbweb.nih.gov/ij/). Occasional x, y, and z drifts were corrected using custom macros developed from ImageJ plugins TurboReg, StackReg (18), MultiStackReg, and Image CorrelationJ (19). Fluorescence intensity of regions of interest (ROIs) was calculated for each time point from a single frame or average intensity projection of 3–5 frames by averaging pixel intensity. Variations of fluorescence intensity in a given ROI were expressed as the ratio ΔF/F0 and calculated according to the formula (F − F0)/F0. F corresponds to the fluorescence intensity in the ROI at a given time point, and F0 corresponds to the fluorescence intensity in the same ROI averaged over ≥6 time points during control baseline prior to drug application. Pseudocolor hue saturation value (HSV) encoding of fluorescence intensity was performed using IGOR Pro (WaveMetrics, Lake Oswego, OR, USA) custom procedures. Pseudocolor images were obtained by dividing, pixel-by-pixel. a raw fluorescence image F by the F0 image averaged over ≥6 time points prior to drug application. Color coding displays the ratio F/F0 (in hue) and the fluorescence F (in value). Statistical significance was determined using a 1-way analysis of variance (ANOVA) with a Tukey-Kramer multiple comparisons test. A value of P < 0.05 was considered significant. Throughout this work, N denotes the number of experiments and n the number of analyzed cells, unless otherwise stated. Data are expressed as means ± sd.
Spectral analyses using 1-photon or 2-photon microscopy
Excitation and emission spectra of PKA sensors were acquired at 25°C on BHK cells using 2-photon and 1-photon confocal microscopy, respectively. For excitation spectra, BHK cells were cultured, transfected, and recorded as described above. The laser wavelength was changed by 5-nm steps in the range 800–1040 nm. For each wavelength, a fluorescence image was recorded under fixed detection conditions. The fluorescence intensity was measured as a function of the wavelength. The spectrum was then obtained by correcting the fluorescence intensity by the square laser power that was previously measured at the focus of the objective for each wavelength. For emission spectra, BHK cells were cultured and transfected as described above, but in 8-well μ-Slides (Ibidi, Planegg/Martinsried, Germany). Prior to the recording, culture medium was replaced by 200 μl HEPES buffered saline (containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose, and 10 mM HEPES; pH 7.3). Images were taken using an inverted Leica TCS-SP5 confocal pointscanning microscope (Leica Microsystems, Mannheim, Germany) equipped with a ×40 (1.25 NA) oil-immersion objective. Excitation was performed with the 458-nm Ar laser. Spectral emission scans were acquired every 2 min in xyλt mode, scanning between 480 and 600 nm with a 4-nm step size and a 5-nm spectral window. After obtaining a stable baseline, 200 μl of HEPES buffered saline containing forskolin (12.5 μM final) was added into the well.
RESULTS
GFP fluorescence reports activation of the PKA-sensing domain
The CFP and YFP fluorophores of AKAR2 were converted into GFP and dark YFP using site-directed mutagenesis to generate the GAkdY sensor (Fig. 1 and Table 1). GAkdY was expressed in BHK cells, and its ability to report PKA activation was tested using 2-photon microscopy. Based on properties of the dark-YFP quencher and of the parental FRET probe AKAR2, we expected GAkdY to report PKA activation by a decrease in fluorescence intensity as a result of energy transfer from GFP to dark YFP (Fig. 1). Surprisingly, application of a maximally effective concentration of the adenylate cyclase activator FSK (13 μM; ref. 20) increased the fluorescence signal (32±12% of baseline; N=8, n=53; Fig. 2). No fluorescence change was observed when the same protocol was applied to BHK cells expressing GAkmutdY (N=3, n=27), a control construct bearing a mutation of the PKA phosphorylation site that prevents responses of the AKAR2 sensor (12, 20). Hence, GAkdY responses to FSK were dependent on phosphorylation of the sensing domain by PKA.
Figure 2.
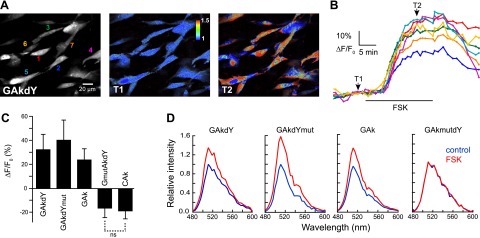
Characterization of PKA sensors in BHK cells. A) Grayscale fluorescence image shows the distribution of the GAkdY sensor in the cytoplasm and nucleus of BHK cells. Colored numbers (left panel) correspond to traces in panel B. Pseudocolor images of the same field before (T1) and during (T2) application of FSK (13 μM) illustrate the large increase in fluorescence of the GAkdY sensor on PKA activation. B) Traces show the time course and amplitude of fluorescence increases measured in the individual cells indicated in panel A. C) Mean responses of selected PKA sensors. Unless indicated (ns, nonsignificant), means are significantly different. D) Representative examples of emission spectra determined in individual cells illustrate the fluorescence increase of indicated sensors on PKA activation. No variation was observed with the inactive GAkmutdY construct. Each spectrum is the mean of 2 consecutive spectra acquired on the same cell in the presence or absence of FSK. Data were obtained using 2-photon (A–C) or 1-photon confocal microscopy (D).
GFP fluorescence is sensitive to intramolecular conformational changes of the PKA sensor
Because the above results suggest that fluorescence energy transfer was not responsible for GAkdY fluorescence changes, we next inactivated dark YFP to generate the GAkdYmut sensor (Fig. 1 and Table 1). This step was achieved by amino acid substitution to yield a glycine triplet at positions 65–67, which prevents chromophore formation of GFP and its spectral variants (21). Inactivation of both chromophores resulted in a nonfluorescent sensor GmutAkdYmut, attesting to the efficacy of the amino acid replacement (N=5). GAkdYmut reported PKA activation by FSK with a 40 ± 16% increase of its fluorescence (N=10, n=59; Fig. 2), confirming that sensors responses did not depend on fluorescence energy transfer.
It is known that the proximity between two GFP molecules can induce fluorescence changes that are resistant to inactivation of one of the two GFP chromophores (22). The possibility of GAkdY responses due to homotypic GFP interactions was tested by removing the dark-YFP sequence to generate the GAk sensor (Fig. 1B) or by introducing in the GFP moiety of GAk the A206K mutation that suppresses the formation of GFP dimers (23). Both GAk and GA206KAk reported PKA activation by FSK through a fluorescence increase (GAk: 26±10%; N=8, n=52; Fig. 2; GA206KAk: 31±11%; N=5, n=27; Table 1), thus excluding homotypic GFP interactions within the sensor or between sensor molecules, as a main cause of fluorescence changes. These results suggest that the fluorescence of our CFP-derived GFP is sensitive to conformational changes of the neighboring PKA-sensing domain.
The properties of GAkdY, GAkdYmut, GAk, and GAkmutdY were also tested using 1-photon confocal microscopy of BHK cells expressing these constructs. Emission spectra acquired in individual cells (N≥2 and n≥10 for each construct) showed that application of FSK increased the fluorescence of GAkdY, GAkdYmut, and GAk sensors, whereas no fluorescence change was observed with the inactive GAkmutdY construct (Fig. 2). These results confirm those obtained using 2-photon microscopy and show that responses of these sensors consist in variations of fluorescence intensity but not in conspicuous changes of spectral emission properties.
Responsive sensors depend on a flexible GFP domain and exhibit a redistribution of fluorescence lifetimes
We examined whether the widely used enhanced GFP (EGFP) is also sensitive to conformational changes of the PKA-sensing domain. Replacing the GFP moiety of GAk with EGFP virtually abolished responses of the resulting eGAK molecule to PKA activation by FSK (N=5, n=34; Table 1). EGFP differs from our CFP-derived GFP by 4 amino acid substitutions (Table 1), whose individual effects on GAk responses were next tested. The I146N substitution introduced in GAk abolished the response to FSK (N=5, n=46), while none of the other 3 decreased these responses (Table 1). The CFP mutation N146I lies in the [144–149] region at the N-terminal border of GFP β-strand 7, which is stable in EGFP but rapidly switches between two conformations in CFP. It was postulated that this flexibility participates in the complexity of CFP fluorescence lifetimes (24).
We thus characterized GAk and GAkdYmut responses in BHK cells using frequency-domain fluorescence lifetime imaging microscopy (FLIM; see Supplemental Material). Application of FSK elicited a shortening of the phase fluorescence lifetime τj of the GAk sensor (from 2.10±0.08 to 1.89±0.09 ns; N=4, n=22) and of the GAkdYmut sensor (from 1.87±0.10 to 1.70±0.10 ns; N=3, n=22; Fig. 3 and Supplemental Table S1). In contrast, FSK induced only minimal τφ variation of unresponsive constructs eGAk (from 2.28±0.05 to 2.24±0.05 ns; N=2, n=22; Supplemental Table S1) and GAkmutdY (N=2, n=8). The modulation fluorescence lifetime τM of these constructs exhibited similar tendencies (Supplemental Table S1). Comparison of τj and τM values provides indication on the complexity of fluorescence decay (25), close values implying a nearly monoexponential decay as for eGAk (0.02 ns), and a marked difference suggesting the existence of multiple lifetime components as for GAk and GAkdYmut (∼0.4 ns, Supplemental Table S1). Indeed, extraction of the lifetime components of GAk and GAkdYmut fluorescence (see Supplemental Material) revealed the presence of a fast and a slow component with similar values for the two sensors (GAk: τ1=0.77±0.12 ns, τ2=2.70±0.09 ns; GAkdYmut: τ1=0.90±0.13 ns, τ2=2.70±0.30 ns; Supplemental Table S1). No significant change of lifetime values was observed for either sensor following FSK application. However, an increase of the fast component proportion (α1) and a decrease of the slow component proportion (α2) occurred (Fig. 3 and Supplemental Table S1). Indeed, α1 increased from 27 ± 3% in control to 36 ± 3% in FSK for GAk and from 37 ± 5 to 48 ± 2% for GAkdYmut. In parallel, α2 decreased from 73 ± 3% in control to 64 ± 3% in FSK conditions for GAk (N=4, n=22) and from 62 ± 5 to 52 ± 2% for GAkdYmut (N=3, n=16).
In summary, we found that both sensors exhibited two fluorescence lifetime components and that application of FSK elicited an increase of the fast component proportion and a decrease of the slow component proportion. These results indicate that responses of the present sensors depend on conformational changes of the [144–149] region of GFP and are characterized by a redistribution of fluorescence lifetimes.
GFP spectral variants differentially respond to activation of the PKA-sensing domain
The CAk sensor incorporating CFP as a single chromophore was also investigated because it similarly bears the N146I mutation. CAk only differs from GAk by the chromophore mutation Y66W (Table 1). Nonetheless, CAk reported PKA activation by FSK through a decrease in fluorescence (20±6%; N=7, n=51; Fig. 2C), instead of an increase as observed for GAk. Dark-YFP mutations Y145W and H148V (4) also lie within the [144–149] GFP region.
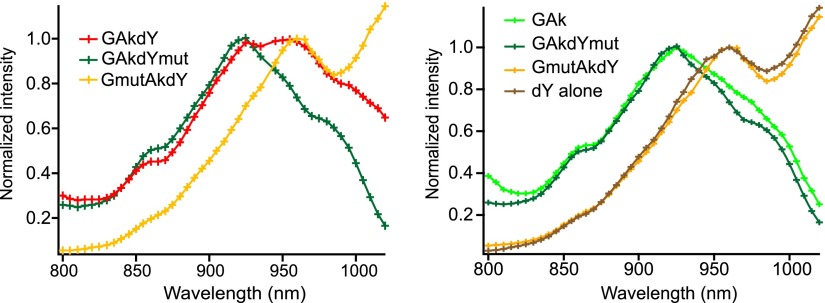
Inspection of GAkdY and GAkmutdY emission spectra revealed a main peak at 511 nm and a minor peak at 522 nm (see Fig. 2D). This minor peak was absent from GAkdYmut and GAk emission spectra, suggesting it was due to dark-YFP residual fluorescence. Given that the residual fluorescence of dark YFP is influenced by additional mutations (8) and that our dark YFP differed from that originally reported (4) by several mutations (see Table 1), we further examined the fluorescence properties of the GAkdY sensor. We found that the 2-photon excitation spectrum of GAkdY exhibited 2 discrete peaks at 920 and 960 nm (Fig. 4). In contrast, both GAkdYmut and GAk excitation spectra exhibited a single peak at 920 nm (Fig. 4). This finding suggests that dark-YFP fluorescence was responsible for the 960-nm peak of the GAkdY excitation spectrum. Indeed, both the GmutAkdY sensor (Fig. 1B and Table 1), which contains the chromophore-inactivating glycine [65–67] triplet in its GFP moiety, and dark YFP alone exhibited a single fluorescence peak at 960 nm (Fig. 4), while no fluorescence was detected in the double-chromophore mutant GmutAkdYmut.
Figure 4.
Two-photon excitation spectra. Superimposed excitation spectra obtained in BHK cells expressing constructs that incorporate either 2 active chromophores (GAkdY), an active and an inactivated chromophore (GAkdYmut and GmutAkdY), or a single active chromophore (GAk and dY). The dY spectrum was obtained from a construct expressing only the dark-YFP domain of GAkdY. Note that the GAkdY spectrum exhibited 2 discrete peaks at 920 and 960 nm. The latter was due to fluorescence emission by its dark-YFP moiety since both the GmutAkdY sensor, which contains the chromophore-inactivating glycine triplet (positions 65–67) in its GFP moiety, and dark-YFP alone showed a single fluorescence peak at 960 nm. Conversely, GAkdYmut and GAk exhibited almost identical spectra with a single peak at 920 nm.
We thus investigated responses of the GmutAkdY construct, which contains dark-YFP as single fluorophore. GmutAkdY reported PKA activation by FSK through a decrease in fluorescence (17±8%;, N=7, n=50; Fig. 2C). The dependence of these fluorescence changes on the [144–149] GFP region was assessed using the GmutAkY construct containing wild-type Y145 and H148 residues in its YFP moiety, which exhibited no fluorescence change on PKA activation (N=7, n=63; Table 1). These results indicate that several GFP variants are sensitive to intramolecular conformational changes of the PKA sensor through rearrangements of the [144–149] GFP region, and that the direction of fluorescence changes is spectral variant-dependent.
Conformation-sensitive GFP reports activation of a calcium-sensing domain
We next tested the ability of our CFP-derived GFP to report conformational changes of other sensing domains. The calcium sensor GCaMP2 is a fusion protein comprising a circular permuted GFP flanked by the M13 peptide and calmodulin that bind to each other in the presence of calcium, thereby increasing fluorescence intensity (9, 15). We modified GCaMP2 by replacing the circular permuted GFP by our CFP-derived GFP and introducing spacers of variable length on each side of the chromophore to explore opportunities of favorable intramolecular interactions with the sensing domain (Fig. 5A). The 9 resulting constructs, named csGCaMPs (csGCaMP0.0 to csGCaMP2.2), were expressed in HEK cells, and their responsiveness to calcium was tested by applying the calcium ionophore ionomycin (4 μM). Among 6 responsive constructs, csGCaMP0.1 showed the most robust fluorescence increase (12±6%; N=4, n=18; Fig. 5B) and was further analyzed. Application of carbachol (300 μM), which elicits intracellular calcium increase in HEK cells through activation of endogenous muscarinic acetylcholine receptors (26), also increased csGCaMP0.1 fluorescence (11±5%; N=4, n=14; Fig. 5C). Conversely, responses of this construct to ionomycin or carbachol application were abolished by the I146N substitution, suggesting that fluorescence changes of csGCaMP0.1 relied on β-strand 7 flexibility, as observed for the GAk PKA sensor. Finally, the calcium-dependence of csGCaMP0.1 fluorescence changes was assessed in a cell-free assay following bacterial expression. The fluorescence at the peak of csGCaMP0.1 emission in the presence of 1 mM calcium was increased by 31 ± 3% (n=2 samples tested in triplicate) as compared to calcium-free conditions (n=2 samples tested in triplicate, Fig. 5D). No fluorescence increase of the csGCaMP0.1 I146N mutant was observed in the same conditions. These results indicate that our CFP-derived GFP variant can be used to report activation of diverse sensing domains.
Figure 5.
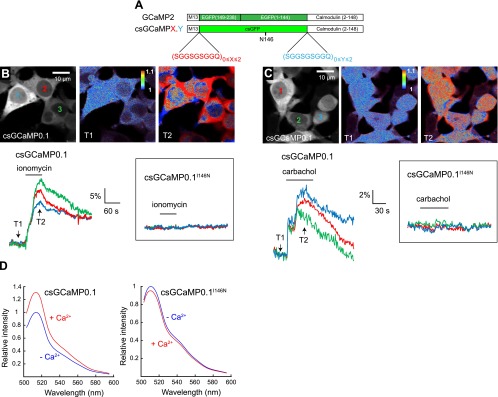
csGFP reports activation of a calcium-sensing domain. A) Domain structure of constructs derived from the GCaMP2 calcium sensor and incorporating a CFP-derived csGFP, as well as variable numbers of a nonapeptide spacer. B, C) Fluorescence images show the distribution of the csGCaMP0.1 sensor in the cytoplasm and nucleus of HEK cells (grayscale), and fluorescence increase of this sensor (pseudocolor) elicited by application of ionomycin (4 μM; B) or carbachol (300 μM; C). Traces illustrate csGCaMP0.1 responses measured in indicated cells (colored numbers in top left panels) and the absence of detectable response of the csGCaMP0.1 I146N mutant on application of these drugs (insets). D) Emission spectra of csGCaMP0.1 and csGCaMP0.1 I146N in the presence or absence of calcium (1 mM), measured in a cell-free assay following bacterial expression of the constructs.
Single fluorescent protein sensors report PKA activity in neurons of brain slices
We used recombinant Sindbis viruses to express the above PKA sensors in neurons of neocortical slices. Expression of the sensors was essentially observed in pyramidal neurons exhibiting a prominent apical dendrite extending toward the pial surface (see Fig. 6), consistent with previous reports (27, 28). Sensors were present in both the cytosol and the nucleus of neurons, in agreement with earlier observations with the AKAR2 sensor (20). Pyramidal neurons express receptors for the neuropeptide CRF, which is a potent activator of the cAMP/PKA pathway (16, 29). We thus examined responses of the sensors to PKA activation induced by stimulation of a plasma membrane receptor (CRF, 250 nM) or cytosolic adenylate cyclase (FSK, 13 μM).
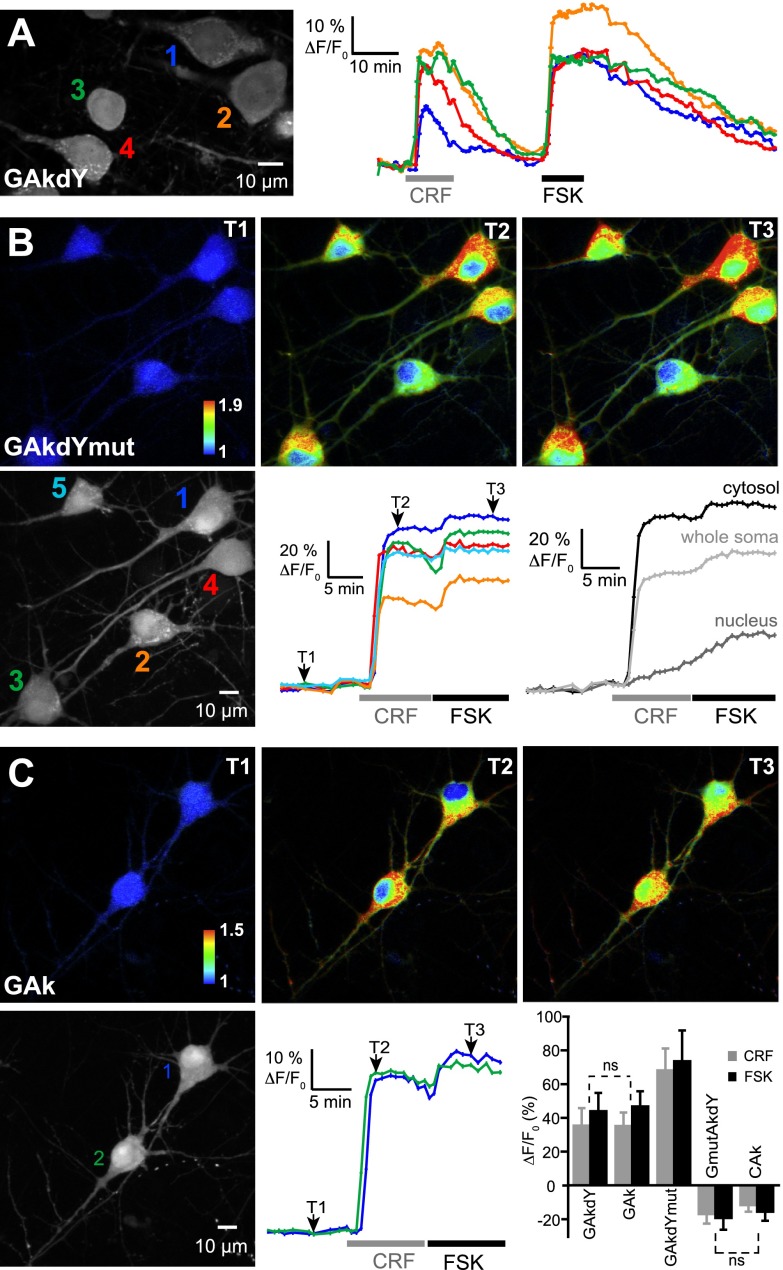
Figure 6.
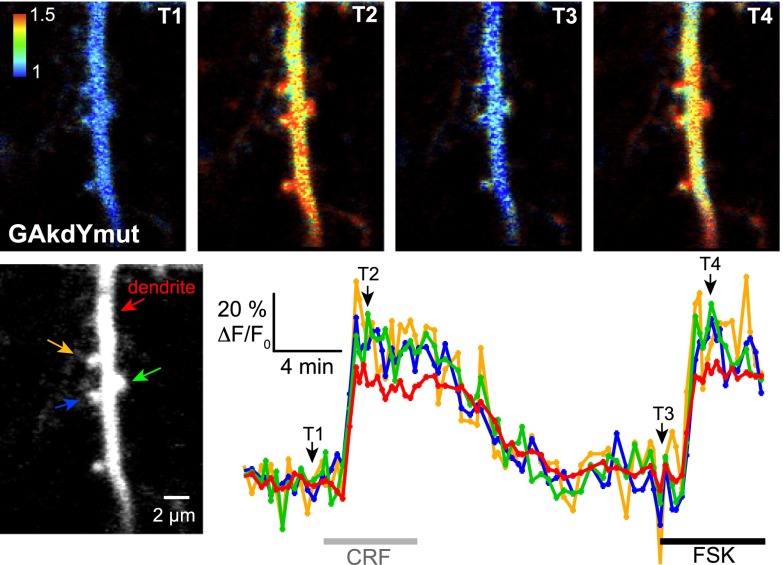
Single-fluorophore sensors as PKA reporters in brain slice. Grayscale fluorescence images show cytoplasmic and nuclear expression of indicated sensors following viral transfer in neocortical pyramidal neurons. A) Traces illustrate the reversibility of sensors responses to PKA activation by CRF (250 nM) and FSK (13 μM) on washout of the drugs and the virtual absence of photobleaching in our recording conditions. B, C) Pseudocolor images show GAkdYmut (B) and GAk (C) fluorescence intensity in control (T1), CRF (T2), and FSK (T3) conditions. Color traces show the variations of fluorescence intensity measured in the somatic cytosol of individual cells indicated in the corresponding grayscale image. Grayscale traces in panel B were obtained from cell 1 and illustrate the different time course and amplitude of cytosolic and nuclear responses of the sensors. Bar graph in panel C shows mean responses of PKA sensors measured in the somatic cytosol. Unless indicated (ns, nonsignificant), mean responses significantly differed between probes.
A first set of experiments was performed in wide-field microscopy under 1-photon excitation (excitation/emission filters: 480/525 nm). We found that GAkdY responded to FSK application by an increase in fluorescence (17±4%; N=7, n=59), consistent with results obtained in BHK cells. We next examined the suitability of single GFP sensors to long-lasting and stable 2-photon imaging of PKA activity in brain slices. Figure 6A displays an example of long-duration recordings (90 min) of pyramidal neurons, where no photobleaching of GAkdY fluorescence was observed. Following the response to CRF, the somatic fluorescence signal returned to baseline value on washout of the neuropeptide. The subsequent response to FSK also returned to values close to baseline on washout of the drug. Similar observations were made with GAkdYmut and GAk sensors. Hence, single GFP sensors allow stable 2-photon imaging of PKA activity for extended periods of time.
As exemplified in Fig. 6B, C, single chromophore sensors GAkdYmut and GAk responded to CRF application by large somatic fluorescence increases. Subsequent FSK application induced an additional fluorescence increase, consistent with earlier observations (22). Responses had a rapid onset at cytosolic locations, but slowly developed in nuclei, in agreement with previous reports (20, 30). Cytosolic responses of GAkdYmut to CRF and FSK (68±13 and 74±18%, respectively; N=7, n=19) were larger than those of GAkdY (36±10 and 44±10%, respectively; N=3, n=20) and GAk (35±8 and 47±9%, respectively; N=6, n=15), consistent with results obtained in BHK cells.
In contrast with GFP-based sensors, the single-fluorophore sensors GmutAkdY and CAk reported PKA activation in pyramidal neurons by a decrease in fluorescence (Fig. 6 graph and Supplemental Fig. S1), as observed in BHK cells. Application of CRF and FSK decreased GmutAkdY fluorescence by 17 ± 5 and 19 ± 7%, respectively (N=8, n=31) and CAk fluorescence by 12 ± 4 and 16 ± 5%, respectively (N=7, n=21). These results confirm, now in a neuronal intracellular environment, that the fluorescence of some GFP variants is sensitive to conformational changes of the neighboring PKA sensing domain, and that the direction of fluorescence changes is GFP variant dependent.
Single GFP sensors allow 2-photon imaging of PKA dynamics in thin neuronal processes
We next examined the suitability of our single GFP sensors to high-resolution imaging of PKA activity in fine processes, such as dendrites and spines. The dendritic tree of neocortical pyramidal neurons is covered with spines that are membrane protrusions with diameter in the micrometer range. Dendritic spines are the target of excitatory synapses, which are modulated by the cAMP/PKA pathway. In pyramidal neurons expressing the GAkdYmut sensor, PKA activation by CRF elicited large fluorescence increases in both dendritic shafts and spines (Fig. 7; 43±14 and 36±7% fluorescence increase, respectively; N=5 experiments, n=19 spines, 8 shafts), albeit of smaller amplitude than in neuronal somata (see above). Similar differences between dendritic and somatic responses to β-adrenergic receptor stimulation have been shown to result from different basal cAMP/PKA activity in these two subcellular compartments (31). Subsequent application of FSK elicited comparable responses (44±6 and 40±7% fluorescence increase for shafts and spines, respectively; N=3 experiments, n=9 spines, 5 shafts), suggesting that CRF induced almost maximal PKA activation in dendrites. These results show that the present single-GFP sensors report PKA dynamics in thin neuronal processes on 2-photon imaging in brain slices with a high signal-to-baseline ratio. The robust responses we observed in spine heads and their parent dendritic shafts with GAkdYmut further indicate that this sensor is suitable for the imaging of subtle physiological variations of PKA activity at individual synapses.
Figure 7.
Imaging PKA activity in neuronal processes. Grayscale fluorescence image shows a segment of the apical dendrite and associated spines of a pyramidal neuron expressing the GAkdYmut sensor. Traces show the variations of GAkdYmut fluorescence intensity elicited by CRF (250 nM) and FSK (13 μM) in RPIs including the dendritic shaft and 3 spines (indicated by arrows on the grayscale image). Pseudocolor images of the same field at indicated time points illustrate GAkdYmut responses on drug application.
DISCUSSION
We found that sensors comprising GFP variants as single fluorophores report conformational changes of PKA- or calcium-sensing domains via changes in fluorescence intensity. Fluorescence changes relied on specific amino acid substitutions in the [144–149] GFP region and were associated with redistribution of fluorescence lifetimes. Single GFP sensors allowed the imaging of PKA dynamics in brain slices with high resolution.
csGFP variants
The experiments we performed using 1- and 2-photon illumination revealed an increase of GAkdY fluorescence on PKA activation. Inactivation or deletion of the dark-YFP quencher (GAkdYmut and GAk) and mutation of the GFP dimerization domain (GA206KAk) demonstrated that sensor responsiveness requires neither fluorescence energy transfer nor homotypic GFP interaction. Responses were observed with sensors containing a single fluorophore on either side of the PKA-sensing domain (GAk, CAk, and GmutAkdY) or fused to a calcium-sensing domain (csGCaMP0.1). These results indicate that the fluorescence of some GFP variants is sensitive to intramolecular rearrangements resulting from activation of the PKA- or the calcium-sensing domains.
GFP β-strand 7 acts as an interface between the chromophore and the sensing domain
The 11 β strands of GFP interact with each other to form a rigid barrel structure surrounding the chromophore, but the interaction between strands 7 and 8 is not as tight as that between the other β strands (1). We found that mutations in the N-terminal half of β-strand 7 are critical to the responsiveness of our sensors. Mutation N146I enhances the fluorescence of the blue-shifted CFP chromophore TWG[65–67], presumably allowing the GFP scaffold to accommodate the bulk W66 residue (2). Indeed, the critical effect of substitutions at position 146 on CFP fluorescence has been recently assessed (32). Mutation N146I increases the flexibility of the [144–149] region of β-strand 7, which switches in CFP between two conformations, with Y145 and H148 interacting either with the chromophore (as in GFP) or with the solvent (33, 34). The opposite responses we observed depending on residue 66 being either Y (e.g., in GAk) or W (CAk) suggest that PKA-induced conformational rearrangements of the sensors favor a “GFP-like” conformation of the [144–149] region, thereby increasing GAk fluorescence and decreasing CAk fluorescence. Mutations Y145W and H148V largely reduce the emission of the YFP chromophore (GYG[65–67]; ref. 4), as well as that of the GFP chromophore (TYG[65–67]; ref. 35). We found that, unlike GmutAkY, GmutAkdY was a responsive sensor, implying that these mutations also confer conformation sensitivity to the residual fluorescence of YFP. Hence, we identified 3 positions in the [144–149] region that enable conformation-dependent modulation of 3 different chromophores (CFP, GFP, YFP). Combinatorial mutagenesis at these positions may provide means to manipulate basal fluorescence levels and the direction of fluorescence changes.
The observation that antibody derivatives able to modulate GFP fluorescence bind the [144–149] region and modify its interactions with the chromophore (36) suggests a unique ability of this region to mediate the effects of heterotypic interactions on GFP fluorescence. GFP naturally interacts with the luciferase aequorin to permit bioluminescence energy transfer between the two molecules (37, 38) but interacts also with other unrelated proteins. These heterotypic interactions involve primarily aromatic residues, which differ from those involved in GFP dimerization and whose side chains are exposed at the surface of the GFP barrel (39). Among these residues, Y151 neighbors the critical [144–149] region in β-strand 7 and may thus participate in interactions between GFP and the PKA or the calcium-sensing domains that lead to fluorescence variations on activation of our sensors.
Conformational changes modulate chromophore optical properties
It has been postulated that the flexibility of the [144–149] region is responsible for the multiple lifetime components of CFP fluorescence, which have been associated with different conformational states of the TWG[65–67] chromophore itself (24, 40, 41). We found that, unlike eGAk, the GAk and GAkdYmut sensors exhibit multiple fluorescence lifetimes as described for CFP, which implies that this property depends on the N146I mutation of the [144–149] region but not on the chromophore incorporating either the W66 (CFP) or Y66 residue (GAk and GAkdYmut). The redistribution of the short and long lifetime components we observed on PKA activation of these sensors is indicative of dynamic equilibrium between different states of their TYG[65–67] chromophore. Indeed, this chromophore and that of wild-type GFP (SYG[65–67]) exist as an equilibrium between a protonated and 2 deprotonated states with absorption maxima at 396, 476, and 490 nm, respectively named A, B, and I (42, 43). It is unlikely that the protonated A state was excited in our experimental conditions (FLIM, λex=489 nm; 2-photon, λex=920 nm). Nonetheless, the H-bond network proposed to stabilize the B state involves N146 (42, 43), whose N146I mutation may facilitate interconversion between deprotonated states. This condition raises the possibility that the short and long fluorescence lifetimes of our sensors result from two deprotonated states of the TYG[65–67] chromophore, whose interconversion is modulated by PKA-dependent rearrangements of the [144–149] region in our sensors. In the examples of chromophore modulation reported so far, fluorescence increases involve an increase in the deprotonated/protonated ratio (22, 36, 44). Hence, changes in the relative abundance of the 3 protonated and deprotonated states of the chromophore may explain that activation of GAk and GAkdYmut could elicit both an increase in fluorescence intensity and a decrease in mean lifetime.
Sensors based on csGFP variants
We have identified a restricted region of GFP β-strand 7 in which mutations allow heterotypic interactions to modulate GFP fluorescence. This finding has wide implications for the design and engineering of GFP-based sensors. Regarding FRET sensors, the conformation sensitivity of CFP potentially contributes to CFP/YFP ratio changes but can also affect FRET efficiency. Interestingly, an improved version of the AKAR2 probe comprises the cerulean CFP variant, which incorporates Y145A and H148D mutations (45). These mutations stabilize the [144–149] region (24), thereby presumably reducing conformation-dependent fluorescence changes that can decrease FRET efficiency. Regarding sensors based on a single fluorescent protein, the majority of them incorporate a circularly permuted GFP variant, in which a split in β-strand 7 allows the modulation of chromophore fluorescence (5, 6) but can result in low baseline fluorescence (9–11). The present results, together with data obtained on genetically encoded voltage sensors (46), show that chromophore modulation can occur in sensors comprising single, nonsplit, fluorescent proteins exhibiting readily detectable baseline fluorescence. Our sensors based on csGFP variants exhibited large responses to PKA activation and allowed the imaging of PKA dynamics in thin neuronal processes with high resolution. We also used csGFP variants to generate responsive calcium sensors, thereby providing a proof of concept of their general ability to report activation of diverse sensing domains. Our results thus suggest that the use of csGFP variants provides a new approach to generate single fluorophore biosensors, which should maximize photon collection and facilitate the simultaneous imaging of different, spectrally distinct, indicators.
Supplementary Material
Acknowledgments
The authors thank Drs. Michael J. O'Donovan, J. Woodland Hastings, and Carl H. Johnson for helpful comments on the manuscript. The authors thank the Université Pierre et Marie Curie (UPMC) IFR83 Cell Imaging Facility for valuable assistance. The authors thank Mélanie Cohen and Valérie Pichon [Physicochimie des Electrolytes, Colloïdes et Sciences Analytiques (PECSA) laboratory, Unité Mixte de Recherche (UMR) 7195, Ecole Supérieure de Physique et de Chimie Industrielles (ESPCI)-ParisTech, Paris, France] for giving access to the spectrofluorometer.
This work was supported by Centre National de la Recherche Scientifique (CNRS), UPMC-P6, and by grants from Ecole des Neurosciences de Paris (Network for Viral Transfer), Fondation pour la Recherche sur le Cerveau/Rotary Club de France, and Région Ile de France. L.T. was a U.S. National Institutes of Health/CNRS European Career Transition Award Fellow.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ACSF
- artificial cerebrospinal fluid
- AKAR
- A-kinase activity reporter
- BHK
- baby hamster kidney
- cAMP
- cyclic adenosine monophosphate
- CFP
- cyan fluorescent protein
- CRF
- corticotropin-releasing factor
- csGCaMP
- conformation-sensitive green fluorescent protein calmodulin peptide M13
- csGFP
- conformation-sensitive GFP
- DMEM
- Dulbecco's modified Eagle medium
- EDTA
- ethylenediaminetetraacetic acid
- EGFP
- enhanced GFP
- FLIM
- fluorescence lifetime imaging microscopy
- FRET
- fluorescence resonance energy transfer
- FSK
- forskolin
- GAk
- GFP-A-kinase
- GAkdY
- GFP-A-kinase-sensing dark YFP
- GAkdYmut
- GFP-A-kinase-sensing dark-YFP mutant
- GCaMP
- green fluorescent protein calmodulin peptide M13
- GFP
- green fluorescent protein
- HBSS
- Hank's balanced salt solution
- HEK
- human embryonic kidney
- IPTG
- isopropyl β-d-1-thiogalactopyranoside
- MEM
- minimum essential medium
- PKA
- protein kinase A
- ROI
- region of interest
- YFP
- yellow fluorescent protein
REFERENCES
- 1. Tsien R. Y. (1998) The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 [DOI] [PubMed] [Google Scholar]
- 2. Heim R., Tsien R. Y. (1996) Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr. Biol. 6, 178–182 [DOI] [PubMed] [Google Scholar]
- 3. Ormo M., Cubitt A. B., Kallio K., Gross L. A., Tsien R. Y., Remington S. J. (1996) Crystal structure of the Aequorea victoria green fluorescent protein. Science 273, 1392–1395 [DOI] [PubMed] [Google Scholar]
- 4. Ganesan S., meer-Beg S. M., Ng T. T., Vojnovic B., Wouters F. S. (2006) A dark yellow fluorescent protein (YFP)-based resonance energy-accepting chromoprotein (REACh) for Forster resonance energy transfer with GFP. Proc. Natl. Acad. Sci. U. S. A. 103, 4089–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. VanEngelenburg S. B., Palmer A. E. (2008) Fluorescent biosensors of protein function. Curr. Opin. Chem. Biol. 12, 60–65 [DOI] [PubMed] [Google Scholar]
- 6. Baird G. S., Zacharias D. A., Tsien R. Y. (1999) Circular permutation and receptor insertion within green fluorescent proteins. Proc. Natl. Acad. Sci. U. S. A. 96, 11241–11246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang J., Allen M. D. (2007) FRET-based biosensors for protein kinases: illuminating the kinome. Mol. Biosyst. 3, 759–765 [DOI] [PubMed] [Google Scholar]
- 8. Murakoshi H., Lee S. J., Yasuda R. (2008) Highly sensitive and quantitative FRET-FLIM imaging in single dendritic spines using improved non-radiative YFP. Brain Cell Biol. 36, 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakai J., Ohkura M., Imoto K. (2001) A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat. Biotechnol. 19, 137–141 [DOI] [PubMed] [Google Scholar]
- 10. Tian L., Hires S. A., Mao T., Huber D., Chiappe M. E., Chalasani S. H., Petreanu L., Akerboom J., McKinney S. A., Schreiter E. R., Bargmann C. I., Jayaraman V., Svoboda K., Looger L. L. (2009) Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mao T., O'Connor D. H., Scheuss V., Nakai J., Svoboda K. (2008) Characterization and subcellular targeting of GCaMP-type genetically-encoded calcium indicators. PLoS One 3, e1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang J., Hupfeld C. J., Taylor S. S., Olefsky J. M., Tsien R. Y. (2005) Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature 437, 569–573 [DOI] [PubMed] [Google Scholar]
- 13. Griesbeck O., Baird G. S., Campbell R. E., Zacharias D. A., Tsien R. Y. (2001) Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J. Biol. Chem. 276, 29188–29194 [DOI] [PubMed] [Google Scholar]
- 14. Hepp R., Tricoire L., Hu E., Gervasi N., Paupardin-Tritsch D., Lambolez B., Vincent P. (2007) Phosphodiesterase type 2 and the homeostasis of cyclic GMP in living thalamic neurons. J. Neurochem. 102, 1875–1886 [DOI] [PubMed] [Google Scholar]
- 15. Tallini Y. N., Ohkura M., Choi B. R., Ji G., Imoto K., Doran R., Lee J., Plan P., Wilson J., Xin H. B., Sanbe A., Gulick J., Mathai J., Robbins J., Salama G., Nakai J., Kotlikoff M. I. (2006) Imaging cellular signals in the heart in vivo: cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc. Natl. Acad. Sci. U. S. A. 103, 4753–4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu E., Demmou L., Cauli B., Gallopin T., Geoffroy H., Harris-Warrick R. M., Paupardin-Tritsch D., Lambolez B., Vincent P., Hepp R. (2010) VIP, CRF, and PACAP act at distinct receptors to elicit different cAMP/PKA. Dyn. Neocort. Cereb. Cortex 21,708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen Q. T., Tsai P. S., Kleinfeld D. (2006) MPScope: a versatile software suite for multiphoton microscopy. J. Neurosci. Methods 156, 351–359 [DOI] [PubMed] [Google Scholar]
- 18. Thevenaz P., Ruttimann U. E., Unser M. (1998) A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 [DOI] [PubMed] [Google Scholar]
- 19. Chinga G., Syverud K. (2007) Quantification of paper mass distributions within local picking areas. Nordic Pulp Paper Res. J. 22, 441–446 [Google Scholar]
- 20. Gervasi N., Hepp R., Tricoire L., Zhang J., Lambolez B., Paupardin-Tritsch D., Vincent P. (2007) Dynamics of protein kinase A signaling at the membrane, in the cytosol, and in the nucleus of neurons in mouse brain slices. J. Neurosci. 27, 2744–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barondeau D. P., Putnam C. D., Kassmann C. J., Tainer J. A., Getzoff E. D. (2003) Mechanism and energetics of green fluorescent protein chromophore synthesis revealed by trapped intermediate structures. Proc. Natl. Acad. Sci. U. S. A. 100, 12111–12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Angelis D. A., Miesenbock G., Zemelman B. V., Rothman J. E. (1998) PRIM: proximity imaging of green fluorescent protein-tagged polypeptides. Proc. Natl. Acad. Sci. U. S. A. 95, 12312–12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. (2002) Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 [DOI] [PubMed] [Google Scholar]
- 24. Lelimousin M., Noirclerc-Savoye M., Lazareno-Saez C., Paetzold B., Le Vot S., Chazal R., Macheboeuf P., Field M. J., Bourgeois D., Royant A. (2009) Intrinsic dynamics in ECFP and Cerulean control fluorescence quantum yield. Biochemistry 48, 10038–10046 [DOI] [PubMed] [Google Scholar]
- 25. Leray A., Riquet F. B., Richard E., Spriet C., Trinel D., Heliot L. (2009) Optimized protocol of a frequency domain fluorescence lifetime imaging microscope for FRET measurements. Microsc. Res. Tech. 72, 371–379 [DOI] [PubMed] [Google Scholar]
- 26. Thomas P., Smart T. G. (2005) HEK293 cell line: a vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 51, 187–200 [DOI] [PubMed] [Google Scholar]
- 27. Lendvai B., Stern E. A., Chen B., Svoboda K. (2000) Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876–881 [DOI] [PubMed] [Google Scholar]
- 28. Drobac E., Tricoire L., Chaffotte A. F., Guiot E., Lambolez B. (2009) Calcium imaging in single neurons from brain slices using bioluminescent reporters. J. Neurosci. Res. 88, 695–711 [DOI] [PubMed] [Google Scholar]
- 29. Gallopin T., Geoffroy H., Rossier J., Lambolez B. (2006) Cortical sources of CRF, NKB, and CCK and their effects on pyramidal cells in the neocortex. Cereb. Cortex 16, 1440–1452 [DOI] [PubMed] [Google Scholar]
- 30. Zhang J., Ma Y., Taylor S. S., Tsien R. Y. (2001) Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc. Natl. Acad. Sci. U. S. A. 98, 14997–15002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Castro L. R., Gervasi N., Guiot E., Cavellini L., Nikolaev V. O., Paupardin-Tritsch D., Vincent P. (2010) Type 4 phosphodiesterase plays different integrating roles in different cellular domains in pyramidal cortical neurons. J. Neurosci. 30, 6143–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goedhart J., von Stetten D., Noirclerc-Savoye M., Lelimousin M., Joosen L., Hink M. A., van Weeren L., Gadella T. W, Jr., Royant A. (2012) Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93%. Nat. Commun. 3, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seifert M. H., Ksiazek D., Azim M. K., Smialowski P., Budisa N., Holak T. A. (2002) Slow exchange in the chromophore of a green fluorescent protein variant. J. Am. Chem. Soc. 124, 7932–7942 [DOI] [PubMed] [Google Scholar]
- 34. Bae J. H., Rubini M., Jung G., Wiegand G., Seifert M. H., Azim M. K., Kim J. S., Zumbusch A., Holak T. A., Moroder L., Huber R., Budisa N. (2003) Expansion of the genetic code enables design of a novel “gold” class of green fluorescent proteins. J. Mol. Biol. 328, 1071–1081 [DOI] [PubMed] [Google Scholar]
- 35. Kummer A. D., Kompa C., Lossau H., Pollinger-Dammer F., Michel-Beyerle M. E., Silva C. M., Bylina E. J., Coleman W. J., Yang M. M., Youvan D. C. (1998) Dramatic reduction in fluorescence quantum yield in mutants of green fluorescent protein due to fast internal conversion. Chem. Phys. 237, 183–193 [Google Scholar]
- 36. Kirchhofer A., Helma J., Schmidthals K., Frauer C., Cui S., Karcher A., Pellis M., Muyldermans S., Casas-Delucchi C. S., Cardoso M. C., Leonhardt H., Hopfner K. P., Rothbauer U. (2010) Modulation of protein properties in living cells using nanobodies. Nat. Struct. Mol. Biol. 17, 133–138 [DOI] [PubMed] [Google Scholar]
- 37. Morin J. G., Hastings J. W. (1971) Energy transfer in a bioluminescent system. J. Cell. Physiol. 77, 313–318 [DOI] [PubMed] [Google Scholar]
- 38. Wilson T., Hastings J. W. (1998) Bioluminescence Annu. Rev. Cell Dev. Biol. 14, 197–230 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki N., Hiraki M., Yamada Y., Matsugaki N., Igarashi N., Kato R., Dikic I., Drew D., Iwata S., Wakatsuki S., Kawasaki M. (2010) Crystallization of small proteins assisted by green fluorescent protein. Acta Crystallogr. D Biol. Crystallogr. 66, 1059–1066 [DOI] [PubMed] [Google Scholar]
- 40. Villoing A., Ridhoir M., Cinquin B., Erard M., Alvarez L., Vallverdu G., Pernot P., Grailhe R., Merola F., Pasquier H. (2008) Complex fluorescence of the cyan fluorescent protein: comparisons with the H148D variant and consequences for quantitative cell imaging. Biochemistry 47, 12483–12492 [DOI] [PubMed] [Google Scholar]
- 41. Rizzo M. A., Springer G. H., Granada B., Piston D. W. (2004) An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 22, 445–449 [DOI] [PubMed] [Google Scholar]
- 42. Brejc K., Sixma T. K., Kitts P. A., Kain S. R., Tsien R. Y., Ormo M., Remington S. J. (1997) Structural basis for dual excitation and photoisomerization of the Aequorea victoria green fluorescent protein. Proc. Natl. Acad. Sci. U. S. A. 94, 2306–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cotlet M., Hofkens J., Maus M., Gensch T., Van Der Auweraer M., Michiels J., Dirix G., Van Guyse M., Vanderleyden J., Visser A. J. W. G., De Schryver F. C. (2001) Excited-state dynamics in the enhanced green fluorescent protein mutant probed by picosecond time-resolved single photon counting spectroscopy. J. Phys. Chem. B 105, 4999–5006 [Google Scholar]
- 44. Akerboom J., Rivera J. D., Guilbe M. M., Malave E. C., Hernandez H. H., Tian L., Hires S. A., Marvin J. S., Looger L. L., Schreiter E. R. (2009) Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J. Biol. Chem. 284, 6455–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Depry C., Zhang J. (2011) Visualization of PKA activity in plasma membrane microdomains. Mol. BioSyst. 7, 52–58 [DOI] [PubMed] [Google Scholar]
- 46. Mutoh H., Perron A., Akemann W., Iwamoto Y., Knopfel T. (2011) Optogenetic monitoring of membrane potentials. Exp. Physiol. 96, 13–18 [DOI] [PubMed] [Google Scholar]
- 47. Prasher D. C., Eckenrode V. K., Ward W. W., Prendergast F. G., Cormier M. J. (1992) Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111, 229–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.