Abstract
Teneurin-4 (Ten-4), a transmembrane protein, is highly expressed in the central nervous system; however, its cellular and molecular function in neuronal differentiation remains unknown. In this study, we aimed to elucidate the function of Ten-4 in neurite outgrowth. Ten-4 expression was induced during neurite outgrowth of the neuroblastoma cell line Neuro-2a. Ten-4 protein was localized at the neurite growth cones. Knockdown of Ten-4 expression in Neuro-2a cells decreased the formation of the filopodia-like protrusions and the length of individual neurites. Conversely, overexpression of Ten-4 promoted filopodia-like protrusion formation. In addition, knockdown and overexpression of Ten-4 reduced and elevated the activation of focal adhesion kinase (FAK) and Rho-family small GTPases, Cdc42 and Rac1, key molecules for the membranous protrusion formation downstream of FAK, respectively. Inhibition of the activation of FAK and neural Wiskott-Aldrich syndrome protein (N-WASP), which is a downstream regulator of FAK and Cdc42, blocked protrusion formation by Ten-4 overexpression. Further, Ten-4 colocalized with phosphorylated FAK in the filopodia-like protrusion regions. Together, our findings show that Ten-4 is a novel positive regulator of cellular protrusion formation and neurite outgrowth through the FAK signaling pathway.—Suzuki, N., Numakawa, T., Chou, J., de Vega, S., Mizuniwa, C., Sekimoto, K., Adachi, N., Kunugi, H., Arikawa-Hirasawa, E., Yamada, Y., Akazawa, C. Teneurin-4 promotes cellular protrusion formation and neurite outgrowth through focal adhesion kinase signaling.
Keywords: neuronal differentiation, cytoskeleton
Teneurin (Ten-m/Odz) is a family of type II transmembrane proteins that are highly conserved from invertebrates to mammals. Teneurins consist of an N-terminal intracellular domain and a large C-terminal extracellular domain (1). Teneurins are primarily identified as one of the pair-rule genes in Drosophila. Drosophila teneurins, ten-a and ten-m, are expressed in various tissues, including the nervous system, and are critical for axon path-finding and target recognition in synaptic regions and in the neuromuscular junction of the central and peripheral nervous systems, respectively (2–5). Caenorhabditis elegans teneurin, ten-1, is required for normal axon guidance in pharyngeal neurons (6). In vertebrates, there are 4 isoforms, Ten-1–4. All teneurin members are highly expressed in subpopulations of neurons in the central nervous system (CNS), but they are also observed in nonneural tissues (1). In the brain, the expression patterns of the teneurins largely do not overlap. For instance, in chick embryos, expression of Ten-1 and Ten-2 is found in the nuclei of the tectofugal and thalamofugal pathways, respectively, where neuronal differentiation occurs (7). During development of the mouse cerebral cortex, all the teneurin members, Ten-1–4, are expressed in differentiating neurons, and both overlapping and complementary expression patterns of the 4 members are observed (8). In vivo and in vitro studies have revealed that Ten-1–3 are required for neuronal differentiation steps, such as filopodia formation, neurite outgrowth, and formation of the neural circuit (9–12). We recently demonstrated the essential role of Ten-4 in oligodendrocyte differentiation and myelination of small-diameter axons in the CNS (13). A single-nucleotide polymorphism (SNP) mutation is identified in the Ten-4 gene (ODZ4) in human bipolar disease (14). However, the cellular and molecular functions of Ten-4 during neuronal differentiation have not been elucidated.
Neurite outgrowth is a critical event in neuronal differentiation. During neurite outgrowth, small cell membranous protrusions called filopodia are formed and function as antennae for detecting extracellular cues for extension or retraction of neurites (15). Lamellipodia, another type of cell membrane protrusion, are also observed in the tips of neurites, which are called growth cones. Filopodia and lamellipodia synergistically regulate migration of the growth cones (15). A properly coordinated balance of these cell membranous structures is essential for normal neurite outgrowth. In addition, various molecules are involved in regulating these membranous structures. Rho-family GTPase Cdc42 and Rac1 are key molecules for filopodia and lamellipodia formation, respectively. The GTP-bound form of Cdc42 interacts with neural Wiskott-Aldrich syndrome protein (N-WASP) and activates the actin-related protein-2/3 (ARP2/3) complex, which induces formation of the branched actin network (15). The GTP-bound form of Rac1 promotes the branched actin network formation for lamellipodia (15). Focal adhesion kinase (FAK) is one of the critical upstream regulators of the Rho-family GTPases and N-WASP for neurite outgrowth and is activated by extracellular matrix proteins or growth factors through integrins or receptors for the growth factors, respectively (16–18). The signaling of these molecules orchestrates crucial pathways for filopodia/lamellipodia formation and neurite outgrowth.
In this study, we focused on the biological function of Ten-4 in neurite outgrowth. We found that Ten-4 was required for formation of filopodia-like cell protrusions and neurite extension in the neuroblastoma cell line Neuro-2a. Ten-4 was colocalized with FAK and activated FAK, Cdc42, and Rac1. In addition, we found that the inhibition of FAK and N-WASP abolished the Ten-4 activity, suggesting that Ten-4 is an upstream molecule of the FAK pathway. To our knowledge, our results showed, for the first time, the function of Ten-4 in neurite outgrowth and demonstrated that Ten-4 is a novel positive regulator of cellular protrusion formation via the FAK signaling.
MATERIALS AND METHODS
Cell culture
Mouse neuroblastoma cell line Neuro-2a and rat pheochromocytoma cell line PC12 were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA, or Life Technologies), as well as 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies). For differentiation, Neuro-2a cells and PC12 cells were cultured in different media [Neuro-2a, N2a medium: DMEM with N2 supplement (Life Technologies) or insulin/transferrin/selenium (Roche Applied Science, Penzberg, Germany); PC12 medium: DMEM with insulin/transferrin/selenium and 5 ng/ml of nerve growth factor (Alomone Laboratories, Jerusalem, Israel)].
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was prepared from cell cultures using TRI reagent (Sigma-Aldrich, St. Louis, MO, USA). After 1 μg of total RNA digestion with DNase I (Sigma-Aldrich), the RNA samples were prepared for RT using SuperScript III Reverse Transcriptase (Life Technologies) and Oligo dT Primer (Life Technologies). For quantitative RT-PCR, cDNA was amplified for an initial denaturation at 95°C for 15 min, and then 45 PCR cycles of 94°C for 10 s, 58.5°C for 30 s, and 72°C for 30 s, using IQ SYBR green supermix (Bio-Rad Laboratories, Hercules, CA, USA) and gene-specific primers, as follows: Ten-4, 5′-GTGGACAAGTTTGGGCTCATTTAC-3′ (forward), 5′-GGGTTGATGGCTAAGTCTGTGG-3′ (reverse); MAP2, 5′-GCAACGCCAATGGATTTCC-3′ (forward), 5′-CTCTTGTTCACCTTTCAGGACTGC-3′ (reverse); NeuN, 5′-TCTCTTGTCCGTTTGCTTCCAG-3′ (forward), 5′-TCCGATGCTGTAGGTTGCTGTG-3′ (reverse); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-CCACTAACATCAAATGGGGTGAGG-3′ (forward), 5′-TACTTGGCAGGTTTCTCCAGGC-3′ (reverse).
For the semiquantitative RT-PCR, cDNA was amplified with 30 PCR cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s using the Ex-TaqDNA polymerase (Takara Bio Inc., Otsu, Japan) with the following primers; Ten-4, 5′-GTGGACAAGTTTGGGCTCATTTAC-3′ (forward), 5′-GGGTTGATGGCTAAGTCTGTGG-3′ (reverse); and β-actin, 5′-ATTGCTGACAGGATGCAGAA-3′ (forward), 5′-TAGAGCCACCAATCCACACAG-3′ (reverse).
Western blot analysis
Protein samples from cell cultures were dissolved in lithium dodecyl sulfate (LDS) sample buffer (Life Technologies) or sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 10 mM dithiothreitol (Life Technologies). After sonicating 3 times for 10 s each, the samples were denatured at 70°C with the LDS sample buffer and at 95°C with the SDS-PAGE sample buffer, for 10 min. The denatured samples were analyzed using a 4–12% polyacrylamide Bis-Tris gel (Life Technologies) for Ten-4 and FAK and a 12% polyacrylamide gel for Cdc42 and Rac1. After electrophoresis, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Life Technologies or GE Healthcare, Little Chalfont, UK). The membrane was blocked with 5% skim milk in Tris-buffered saline. Primary and secondary antibodies and SuperSignal West Dura chemiluminescent substrate (Thermo Fisher Scientific) were used to detect proteins. The following antibodies were used in Western blot analysis: Ten-4 (13); α-tubulin (Sigma-Aldrich); phosphorylated FAK (p-FAK; Tyr397; Life Technologies); FAK (Merck Millipore, Billerica, MA, USA); Cdc42 (Merck Millipore); Rac1 (Merck Millipore); rabbit immunoglobulin G conjugated with horseradish peroxidase (IgG-HRP; Cell Signaling Technology, Danvers, MA, USA); and mouse IgG-HRP (Cell Signaling Technology).
Immunostaining
To immunostain the cells in culture, the cells were fixed with 4% paraformaldehyde (PFA; Wako, Osaka, Japan) and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich). After permeabilization, the samples were blocked with Power Block Universal Blocking Reagent (BioGenex Laboratories, Fremont, CA, USA). Proteins were detected by primary and secondary antibodies, as follows: Ten-4 (13); interleukin-2 receptor (IL2R; Merck Millipore); V5 (Life Technologies); p-FAK (Tyr397; Life Technologies); rabbit IgG-Cy3 and IgG-Cy5 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA); rabbit and mouse IgG-Alexa Fluor 488, IgG-Alexa Fluor 594, and IgG-Alexa Fluor 647 (Life Technologies); and phalloidin-rhodamine and phalloidin-Alexa Fluor 488 (Life Technologies).
Confocal microscopy images were obtained using a Zeiss LSM 510 NLO META confocal microscope equipped with A-Plan-Apochromat ×63 (1.4 NA) or Plan-Neofluar ×40 (1.3 NA) objectives (Zeiss, Thornwood, NY, USA). Argon (488 nm) HeNe1 (543 nm), and HeNe2 (633 nm) gas lasers were used to excite Alexa Fluor 488, rhodamine and Cy3, and Cy5 and Alexa Fluor 647 fluorophores, respectively. The pinholes for each laser line were aligned. The 2-photon laser was tuned to 750 nm and used at ∼6% power. All confocal settings were set to the same parameters for each experiment.
Knockdown of Ten-4
For knockdown of Ten-4, On-Target Plus small interfering RNA (siRNA), which contains the targeting sequence of Ten-4 (Thermo Fisher Scientific), was used. On-Target Plus nontargeting siRNA (Thermo Fisher Scientific) was used as control. The siRNA and the green fluorescent protein (GFP)-expression plasmid (Lonza, Basel, Switzerland) were cotransfected into Neuro-2a cells using the Nucleofector 96-well Shuttle System (Lonza) or Neon Transfection System (Life Technologies). The knockdown efficiency was assessed by quantitative RT-PCR, Western blot analysis, and immunostaining. GFP-positive cells were chosen for the morphological analyses as siRNA-transfected cells.
Overexpression of Ten-4
The expression plasmid of Ten-4 was prepared using the pEF1B-V5/His vector (Life Technologies). For the full Ten-4 coding sequence, the 5′ fragment (fragment A) and the 3′ fragment (fragment B) of the Ten-4 coding sequence were amplified by KOD Hot Start DNA Polymerase (Merck Millipore), using the cDNA mixture from the wild-type mouse spinal cord. The PCR primers with restriction enzyme sites (forward primer for fragment A containing an SpeI site at 5′; reverse primer for fragment B containing a NotI site at 3′) were as follows: fragment A, 5′-GACTAGTCCACCATGGACGTGAAGGAGAGGAAG-3′ (forward) and 5′-CCGGGAATTCGGATGTTTCCCAGGTCG-3′ (reverse); fragment B, 5′-CCGGGAATTCGATTTATCCGG-3′ (forward) and 5′-GATAAGAATGCGGCCGCCCCTTCGGCCCATCTCGCTTTG-3′ (reverse). Fragments A and B were cut by SpeI/EcoRI and NotI/EcoRI, respectively. The EcoRI site was the internal site in the Ten-4 coding sequence. These fragments were then cloned into the SpeI and NotI sites of pEF1B-V5/His. The Ten-4 expression plasmid (pTen-4) was transfected into cells with Lipofectamine LTX (Life Technologies). Ten-4 protein was detected by both Western blot analysis and immunostaining using the anti-Ten-4 or anti-V5 antibody, as described above.
Analysis of cell morphology
Confocal images were taken of phalloidin staining in Neuro-2a cells to determine the length of the neurites and the density of the filopodia-like protrusions. Cell processes that were longer than the diameters of their cell bodies were considered to be neurites for purposes of analysis, in accordance with a previous report (19). Neurite lengths were measured individually and scored using ImageJ software [U.S. National Institutes of Health (NIH), Bethesda, MD, USA] as follows. Eight-bit grayscale images were created, and the signal from phalloidin staining was thresholded. The software measured neurite length and the filopodia-like protrusions were counted manually using the thresholded images. Densities of the protrusions were calculated using the length of the neurites and the number of protrusions. At least 20 neurites were analyzed in each experimental condition. Three independent experiments were carried out.
Cdc42 and Rac1 activation assay
For the pull-down assay of the GTP-bound form of Cdc42 and Rac1, the Cdc42 activation assay kit (Cytoskeleton, Denver, CO, USA or Merck Millipore), including the Cdc42/Rac interactive binding (CRIB) domain of PAK-conjugated beads, was used, following the manufacturer's instructions.
Constructs of dominant-negative FAK (DN-FAK)
The plasmid of DN-FAK, which consists of the extracellular and transmembrane domains of human IL2R and mouse mutant FAK with a phenylalanine substitution in the tyrosine 397 residue, and the mock plasmid, which expresses only the extracellular and transmembrane domains of human IL2R, were kindly provided by Dr. Kenneth M. Yamada (Laboratory of Cellular and Developmental Biology, National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD, USA; ref. 20). These plasmids were cotransfected with the pTen-4 into Neuro-2a cells using Lipofectamine LTX.
N-WASP inhibition assay
To analyze the effect of the N-WASP inhibitor, Wiskostatin (Merck Millipore) was added to the culture medium of Ten-4-overexpressing Neuro-2a cells, and the cells were incubated for 15 min. Wiskostatin was dissolved in dimethyl sulfoxide (DMSO), and its final concentration in the culture was 10 μM. DMSO was used as a control. After incubation, the cells were fixed with 4% PFA and immunostained as described above.
RESULTS
Induction of Ten-4 expression in Neuro-2a and its localization at the neurite growth cones
Neurite formation and extension were observed in both mouse neuroblastoma cell line Neuro-2a and rat pheochromocytoma cell line PC12 cells after 3 d of differentiation with N2a medium and PC12 medium, respectively (Fig. 1A and refs. 21, 22). We first analyzed the expression levels of Ten-4 mRNA in Neuro-2a and PC12 cells during neurite outgrowth by quantitative RT-PCR. We found that Ten-4 mRNA expression levels were induced in both cell lines 3 d after induction of neurite outgrowth (Fig. 1B). However, the increased level of Ten-4 mRNA expression was higher in the Neuro-2a cells than in the PC12 cells (Fig. 1B). Semiquantitative RT-PCR analyses also showed higher Ten-4 expression in Neuro-2a cells than in PC12 cells with both N2a and PC12 media (Fig. 1C). Therefore, we used Neuro-2a for further analysis of the Ten-4 function in neurite outgrowth.
Figure 1.
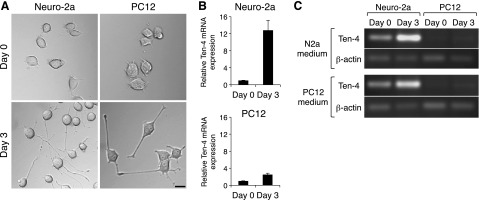
Ten-4 expression in neurite outgrowth of Neuro-2a and PC12 cells. A) Morphology of Neuro-2a and PC12 cells during neurite outgrowth. Both Neuro-2a and PC12 cells formed and extended neurites 3 d after induction of neurite outgrowth in N2a medium and PC12 medium, respectively. Scale bar = 20 μm. B) Quantitative RT-PCR of Ten-4 in Neuro-2a and PC12 cells during neurite outgrowth in N2a medium and PC12 medium, respectively. Relative increase of Ten-4 mRNA expression in the Neuro-2a neurite outgrowth on d 3 was higher than that in PC12 cells. Ten-4 mRNA expression was normalized using GAPDH mRNA expression. Normalized Ten-4 expression in each cell on d 0 was set as 1.0. Error bars = se. C) Semiquantitative RT-PCR of Ten-4 in Neuro-2a and PC12 cells during neurite outgrowth with both N2a and PC12 media. Neuro-2a expressed Ten-4 higher than PC12 on d 0 and 3 in both N2a and PC12 media. The induction of Ten-4 expression on d 3 was observed in either N2a or PC12 medium, while the expression level of the control β-actin was not changed.
We observed that the expression levels of both Ten-4 mRNA and protein were increased during neurite outgrowth in a time-dependent manner (Fig. 2A, B). Before induction of neurite outgrowth, no neurites were observed, but small filopodia-like protrusions were seen (Fig. 2Ca, c; arrow). The Neuro-2a cells began to form neurites and filopodia-like protrusions on d 1 (Fig. 2Cd, f: arrows), and they extended the neurites and developed the filopodia-like protrusions in the neurites on d 3 (Fig. 2Cg, i; arrows). Immunostaining of Ten-4 revealed that the Ten-4 protein was localized in the outgrowing tips of the neurites on d 1 (Fig. 2Cd, e; arrowhead), while before induction of neurite outgrowth, the Ten-4 protein was weakly detected, and signal of Ten-4 protein expression was mostly detected throughout the cell bodies (Fig. 2Ca, b; arrowhead). We further found that on d 3, the expression level of the Ten-4 protein was strong along the neurites, and Ten-4 accumulation was observed in the growth cone regions (Fig. 2Cg, h; arrowheads). In addition, 79% of differentiated Neuro-2a cells were positive for Ten-4 at the neurite growth cones in this culture, as shown in Fig. 2C.
Figure 2.
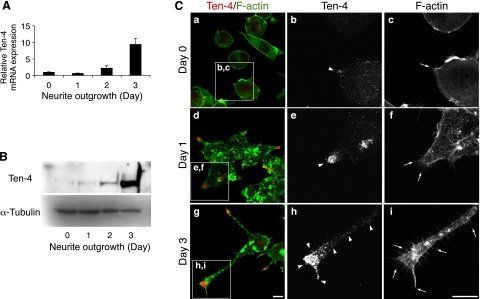
Analysis of Ten-4 expression in Neuro-2a neurite outgrowth. A) Quantitative RT-PCR of Ten-4 in Neuro-2a cells during neurite outgrowth. mRNA expression of Ten-4 increased with time after induction of neurite outgrowth. Ten-4 mRNA expression was normalized using GAPDH mRNA expression. Normalized expression of Ten-4 on d 0 was set as 1.0. Error bars = se. B) Western blotting of Ten-4 in Neuro-2a cells during neurite outgrowth. Highest expression of Ten-4 was observed 3 d after induction of neurite outgrowth. Expression of α-tubulin was used as a control. C) Immunostaining of Ten-4 in Neuro-2a cells before induction of differentiation (a–c), 1 d (d–f), and 3 d (g–i) after induction of neurite outgrowth. Ten-4 protein was localized and accumulated at the tips of the neurites. Panels b, c; e, f; and h, i show enlarged images of boxed areas in panels a, d, and g, respectively. Arrows indicate filopodia-like membranous protrusions; arrowheads indicate Ten-4 expression at a growth cone or along a neurite. Scale bar = 10 μm.
Inhibitory effect of Ten-4 knockdown on neurite outgrowth and filopodia-like protrusion formation in Neuro-2a cells
We next examined the effect of Ten-4 knockdown on neuronal differentiation and neurite outgrowth of Neuro-2a by using Ten-4 siRNA (siTen-4). Quantitative RT-PCR, Western blotting, and immunostaining showed that the expression levels of Ten-4 mRNA and protein in siTen-4-transfected Neuro-2a (siTen-4-Neuro-2a) cells were significantly lower 3 d after induction of differentiation, compared with control siRNA (siCont)-transfected Neuro-2a (siCont-Neuro-2a) cells (mRNA: 51.9±4.4%; protein by Western blot analysis: 32.0±9.7%; protein by immunostaining: 37.1±5.0%, in siTen-4-Neuro-2a relative to 100% for siCont-Neuro-2a; Fig. 3A, B and Supplemental Fig. S1). We measured the expression of MAP2 and NeuN, markers for neuronal differentiation, in siTen-4-Neuro-2a, and we found no significant differences in mRNA expression level of either gene in siTen-4-Neuro-2a, compared to those in siCont-Neuro-2a (Fig. 3A). This indicated that siTen-4 specifically decreased Ten-4 expression and that the reduced Ten-4 expression did not affect the expression of the neuronal differentiation markers in Neuro-2a cells. However, we found that the neurites were significantly shorter in siTen-4-Neuro-2a after the induction of differentiation (75.0±9.8% in siTen-4-Neuro-2a relative to 100% for siCont-Neuro-2a; Fig. 3Ca, d, g). In addition, the filopodia-like protrusions in the neurites were reduced in number in siTen-4-Neuro-2a compared with siCont-Neuro-2a cells (75.7±6.0% in siTen-4-Neuro-2a relative to 100% for siCont-Neuro-2a; Fig. 3Cc, f, arrows; h). These results suggested that Ten-4 is required for neurite outgrowth and filopodia-like protrusion formation in Neuro-2a cells.
Figure 3.
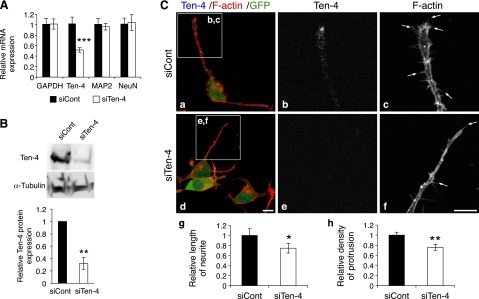
Reduced neurite outgrowth and filopodia-like protrusion formation by Ten-4 knockdown in Neuro-2a cells. A) Quantitative RT-PCR of Ten-4, MAP2, and NeuN in control siRNA-transfected Neuro-2a (siCont-Neuro-2a) or Ten-4 siRNA-transfected Neuro-2a (siTen-4-Neuro-2a) cells, 3 d after induction of neurite outgrowth. Ten-4 mRNA expression in siTen-4-Neuro-2a cells was 51.9 ± 4.4% of Ten-4 mRNA expression in siCont-Neuro-2a cells. No differences in MAP2 and NeuN mRNA expression were observed. mRNA expression of each gene was normalized using GAPDH mRNA expression. Normalized expression of each gene in siCont-Neuro-2a cells was set as 1.0. Error bars = se. ***P < 0.005; Student's t test. B) Western blot analysis of Ten-4 in siCont- and siTen-4-Neuro-2a cells, 3 d after neurite outgrowth was induced. Ten-4 expression significantly decreased in siTen-4-Neuro-2a cells (32.0±9.7% of that in siCont-Neuro-2a). Intensity of the Ten-4 bands was normalized using the intensity of the α-tubulin bands. **P < 0.01; Student's t test. C) Immunostaining of siCont- and siTen-4-Neuro-2a cells, 3 d after induction of differentiation. In siTen-4-Neuro-2a cells (d–f), the length of neurites and the density of filopodia-like protrusions were reduced, compared with those in siCont-Neuro-2a cells (a–c). F-actin staining was used to assess neurite outgrowth and protrusion formation. The GFP expression vector was cotransfected with siRNA to label transfected cells. Panels b, c and e, f show enlarged images of boxed areas in panels a and d, respectively; panels g and h denote quantitation of the length of neurites and the density of protrusions, respectively. Averaged values in siCont-Neuro-2a cells were set as 1.0. Arrows indicate filopodia-like protrusions. Scale bar = 10 μm. Error bars = se. *P < 0.05; **P < 0.01; Student's t test.
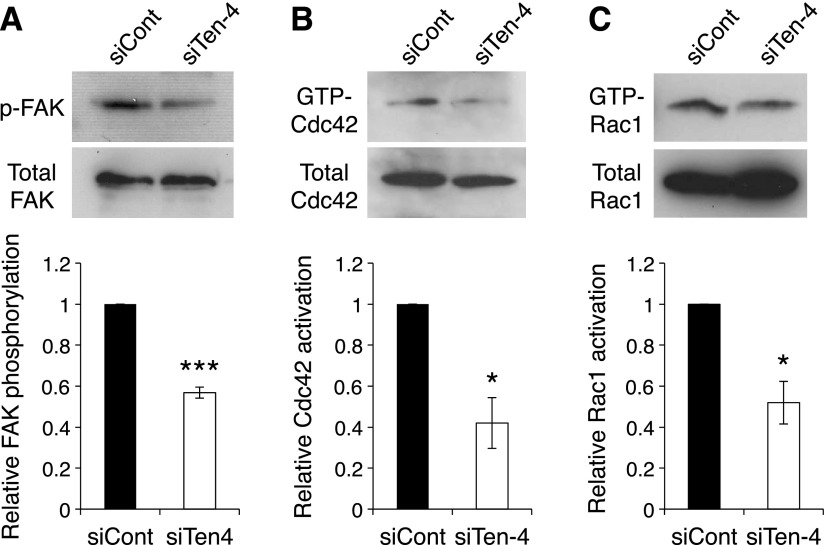
Because FAK and the Rho-family small GTPases, Cdc42 and Rac1, regulate neurite outgrowth in the growing tips of neurites and filopodia formation (15–17), we next examined the activation of FAK, Cdc42, and Rac1 in siTen-4-Neuro-2a cells. After the induction of neurite outgrowth, the activation of FAK, Cdc42, and Rac1 were reduced in siTen-4-Neuro-2a cells, compared to that in siCont-Neuro-2a cells (FAK: 56.8±2.7%; Cdc42: 42.0±12.4%; Rac1: 52.0±10.4%, in siTen-4-Neuro-2a relative to 100% for siCont-Neuro-2a; Fig. 4). These results indicated that Ten-4 is necessary for the activation of FAK, Cdc42, and Rac1 during neurite outgrowth.
Figure 4.
Reduced activation of FAK, Cdc42, and Rac1 by Ten-4 knockdown in Neuro-2a cells. A) Western blot analysis of p-FAK (Tyr397) and total FAK in differentiating siCont- or siTen-4-transfected Neuro-2a cells after induction of differentiation. Phosphorylation level of FAK was reduced in siTen-4-Neuro-2a cells. Intensity of the p-FAK bands was normalized using the intensity of the total FAK bands. B, C) Pull-down assay of the GTP-bound form of Cdc42 (B) and Rac1 (C) using a recombinant protein of the CRIB domain of PAK in differentiating siCont- and siTen-4-Neuro-2a cells. Active GTP-bound form of both Cdc42 and Rac1 was reduced in siTen-4-Neuro-2a cells. Intensity of bands of GTP-Cdc42 and GTP-Rac1 was normalized using the intensity of total Cdc42 and Rac1 bands, respectively. Error bars = se. *P < 0.05, ***P < 0.005; Student's t test.
Increase of filopodia-like protrusions by overexpression of Ten-4 in Neuro-2a cells
To analyze further the function of Ten-4 in neurite outgrowth, we examined the effect of overexpression of Ten-4 on Neuro-2a cell neurite outgrowth using the Ten-4 expression plasmid (pTen-4). When pTen-4-transfected Neuro-2a (pTen-4-Neuro-2a) cells were incubated for 3 d in the differentiation medium, a high level of Ten-4 expression was observed in both the mRNA and protein levels, compared with the mock plasmid-transfected Neuro-2a (Mock-Neuro-2a) cells (mRNA: 309±25 fold in pTen-4-Neuro-2a relative to 100% for Mock-Neuro-2a; Fig. 5A, B). In pTen-4-Neuro-2a cells, neurite length was normal; however, the filopodia-like membrane protrusions in the neurites significantly increased (length of neurites: 1.08±0.12-fold; density of protrusions: 1.27±0.06-fold, in pTen-4-Neuro-2a relative to 100% for Mock-Neuro-2a; Fig. 5Ca, c, d, f, arrows; g, h). Interestingly, some F-actin-free protrusions that were positive for Ten-4 were found in pTen-4-Neuro-2a cells (Fig. 5Ce, f; arrowhead). These observations suggested that Ten-4 overexpression promotes the formation of the filopodia-like protrusions in neurites. A similar Ten-4 overexpression was observed in COS-7 cells. Ten-4 overexpression promoted the filopodia-like protrusions (Supplemental Fig. S2E–H), while mock-transfected COS-7 cells did not express Ten-4 and possessed smooth cell membranes (Supplemental Fig. S2A–D).
Figure 5.

Increased filopodia-like protrusions by Ten-4 overexpression in Neuro-2a cells. A) Quantitative RT-PCR of Ten-4 in Neuro-2a cells transfected with the plasmid for the control (Mock-Neuro-2a) or Ten-4 overexpression (pTen-4-Neuro-2a), 3 d after induction of differentiation. Expression level of Ten-4 mRNA in pTen-4-Neuro-2a cells was even higher than that in Mock-Neuro-2a cells. mRNA expression of Ten-4 was normalized using GAPDH mRNA expression. Normalized expression of Ten-4 in Mock-Neuro-2a cells was set as 1.0. Error bars = se. ***P < 0.001; Student's t test. B) Western blotting of Ten-4 in Mock- and pTen-4-Neuro-2a cells, 3 d after induction of differentiation. Ten-4 expression significantly increased in pTen-4-Neuro-2a cells. Expression of α-tubulin was used as a control. Image of a Western blot of Ten-4 was prepared with a short exposure in the development step, since the Ten-4 band in pTen-4-Neuro-2a cells was intense. A band of Ten-4 protein in Mock-Neuro-2a cells was detected with normal exposure, similar to Figs. 2B and 3B. C) Immunostaining of Mock-Neuro-2a (a–c) and pTen-4-Neuro-2a (d–f) cells, 3 d after differentiation induction. In pTen-4-Neuro-2a cells, more numerous filopodia-like protrusions were formed, as compared with Mock-Neuro-2a cells. F-actin staining was used to assess morphology. Panels b, c and e, f show enlarged images of boxed areas in panels a and d, respectively; panels g and h denote quantitation of the length of neurites and the density of protrusions, respectively. Averaged values in Mock-Neuro-2a cells were set as 1.0. Arrows indicate filopodia-like protrusions; arrowheads indicate Ten-4 expression in protrusions without F-actin. Scale bar = 10 μm. Error bars = se. ***P < 0.001; Student's t test.
As the activation of FAK, Cdc42, and Rac1 was attenuated by Ten-4 knockdown, we evaluated the activation of these proteins in pTen-4-Neuro-2a cells. After induction of neurite outgrowth, the activation of FAK, Cdc42, and Rac1 was elevated in pTen-4-Neuro-2a cells, compared to that in Mock-Neuro-2a cells (FAK: 1.97±0.29-fold; Cdc42: 3.06±0.66-fold; Rac1: 1.98±0.28-fold, in pTen-4-Neuro-2a relative to 100% for Mock-Neuro-2a; Fig. 6). These results suggested that the activation of FAK, Cdc42, and Rac1 is promoted by Ten-4 overexpression. These data are consistent with the Ten-4-knockdown results in which the opposite findings were observed (Fig. 4).
Figure 6.
Increased FAK, Cdc42, and Rac1 activation by Ten-4 overexpression in Neuro-2a cells. A) Western blotting of p-FAK (Tyr397) and total FAK differentiating Neuro-2a cells transfected with the plasmid for the control (Mock-Neuro-2a) or Ten-4 overexpression (pTen-4-Neuro-2a). Phosphorylation level of FAK was increased in pTen-4-Neuro-2a cells. Intensity of the p-FAK bands was normalized using the intensity of the total FAK bands. B, C) Pull-down assay of the GTP-bound form of Cdc42 (B) and Rac1 (C) using a recombinant protein of the CRIB domain of PAK in differentiating Mock-Neuro-2a or pTen-4-Neuro-2a. Active GTP-bound forms of Cdc42 and Rac1 were elevated in pTen-4-Neuro-2a cells. Intensity of bands of GTP-Cdc42 and GTP-Rac1 was normalized using the intensities of total Cdc42 and Rac1 bands, respectively. Error bars = se. *P < 0.05; Student's t test.
Inhibitory effect of DN-FAK and the N-WASP inhibitor on the filopodia-like protrusion formation by Ten-4 overexpression
We further confirmed the Ten-4-FAK signaling for filopodia-like protrusion formation by transfection of DN-FAK into pTen-4-Neuro-2a cells (Fig. 7). DN-FAK was expressed as a fusion protein of the mutant FAK, which possesses a phenylalanine substitution in the tyrosine 397 residue, with the extracellular and transmembrane domains of IL2R. The mock plasmid encoded only the IL2R region. Therefore, protrusion formation of Ten-4 and IL2R dual-positive cells was analyzed. Filopodia-like process formation in Ten-4- and DN-FAK-cotransfected cells was decreased (Fig. 7D–G), in comparison with that in Ten-4- and mock-cotransfected cells (Fig. 7A–C). We then performed the inhibition assay of N-WASP, a critical regulator of filopodia downstream of FAK and Cdc42 (15, 17). The effect of Wiskostatin, a selective inhibitor of N-WASP, on filopodia-like protrusion formation by Ten-4 overexpression was examined. In the presence of Wiskostatin, the formation of the filopodia-like protrusions was substantially inhibited in pTen-4-Neuro-2a cells (Fig. 8D–G), compared with the control (DMSO; Fig. 8A–C). These results indicated that the filopodia-like protrusion formation by Ten-4 is mediated by activated FAK and N-WASP.
Figure 7.
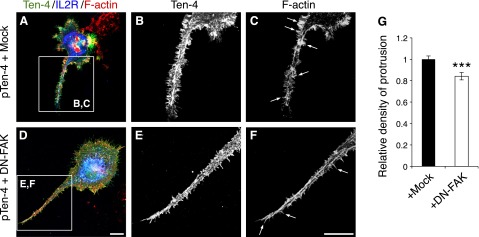
Inhibitory effect of DN-FAK on the filopodia-like protrusion formation by Ten-4 overexpression in Neuro-2a cells. A–F) Transfection of pIL2R-DN-FAK inhibited protrusion formation in Ten-4-overexpressing Neuro-2a (pTen-4 Neuro-2a) cells (A–C), compared with the mock pIL2R plasmid (D–F). Panels B, C and E, F show enlarged images of boxed areas in panels A and D, respectively. G) Quantitation of the density of protrusions. Averaged values in mock-transfected pTen-4-Neuro-2a cells were set as 1.0. Arrows indicate filopodia-like protrusions. Scale bar = 10 μm. Error bars = se. ***P < 0.005; Student's t test.
Figure 8.

Effect of the N-WASP inhibitor on the filopodia-like formation in Ten-4-overexpressing Neuro-2a cells. A–F) Immunostaining of the Ten-4 expression plasmid-transfected Neuro-2a (pTen-4-Neuro-2a) cells in the absence (A–C) or presence (D–F) of the N-WASP inhibitor, Wiskostatin. In the presence of Wiskostatin, filopodia-like process formation by Ten-4 overexpression was significantly inhibited (D–F). DMSO was used as a control (A–C). Panels B, C and E, F show enlarged images of boxed areas in panels A and D, respectively. G) Quantitation of the density of protrusions. Averaged values in the control were set as 1.0. Arrows indicate filopodia-like protrusions. Scale bar = 10 μm. Error bars = se. ***P < 0.001; Student's t test.
Colocalization of Ten-4 with p-FAK
FAK is activated when it is recruited to the microdomain regions of the plasma membrane (23). To examine whether the transmembrane protein Ten-4 colocalizes with FAK, we performed dual-immunostaining of Ten-4 and p-FAK in pTen-4-Neuro-2a cells. Confocal images of the immunostaining showed that Ten-4 colocalized with p-FAK in the plasma membrane regions of neurites, especially at the tips of the filopodia-like protrusions, in pTen-4-Neuro-2a (Fig. 9D, arrows). The intensity of p-FAK in pTen-4-Neuro-2a cells was stronger than that in nontransfected Neuro-2a cells (Fig. 9B, C), which is consistent with the result of the biochemical assay (Fig. 6A). Without transfection with pTen-4, p-FAK was localized in neurites, including growth cones, and in cell bodies (Supplemental Fig. S3A, B). The staining pattern of p-FAK was similar to that of endogenous Ten-4, although localization of p-FAK in neurites was broader than that of Ten-4 (Supplemental Fig. S3A, C; arrows and arrowheads). These results indicate that most phosphorylation of FAK occurs spatially close to the Ten-4 protein. Taken together, our results suggest that Ten-4 may function as a regulator for signaling molecules, including FAK, for the filopodia-like protrusion formation in Neuro-2a neurite outgrowth.
Figure 9.

Colocalization of Ten-4 with FAK. A, B) Dual-immunostaining of Ten-4 (V5; A) with p-FAK at Tyr397 (B) in Ten-4-overexpressing Neuro-2a (pTen-4-Neuro-2a) cells. C) Ten-4 colocalized with p-FAK in the filopodia-like protrusions of pTen-4-Neuro-2a cells. D) Higher-magnification image of the boxed area in panel C shows tip regions of the processes. Arrows indicate colocalization of Ten-4 with p-FAK at a tip area. Scale bars = 10 μm.
DISCUSSION
In the present study, we show that Ten-4 is a positive regulator of the filopodia-like protrusion formation and neurite outgrowth in Neuro-2a cells. We found that Ten-4 was localized and accumulated in the growth cones of Neuro-2a neurites (Fig. 2C). Knocking down the Ten-4 expression inhibited neurite outgrowth, filopodia-like protrusion formation, and the activation of the signaling molecules for the protrusion formation, such as FAK, Cdc42, and Rac1 (Figs. 3 and 4). From these results, we propose the following mechanism for Ten-4 action in neuronal differentiation: Ten-4 activates the signaling pathways of these molecules, promotes the filopodia-like protrusion formation, and results in neurite outgrowth in Neuro-2a cells. Overexpression of Ten-4 increased protrusion formation and activation of FAK, Cdc42, and Rac1 (Figs. 5 and 6), which is consistent with the knockdown results. The promoting effect of protrusion formation by Ten-4 overexpression was moderate, compared with the Ten-4 expression levels. In addition, Ten-4 overexpression did not affect neurite length (Fig. 5C). This may be due to the shortage of other endogenous proteins, which function together with Ten-4 for neurite outgrowth, or to an impairment of the proper signaling for neurite extension because of abnormal activation of Ten-4 signaling in multiple regions. Overexpression of Ten-4 abnormally activated FAK in neurite and cell body regions (Fig. 9). Proper balance of amount, activation, and distribution of critical proteins should be required for neurite outgrowth by Ten-4. We also observed Ten-4 expression in F-actin-free protrusions in Ten-4-overexpressing Neuro-2a cells (Fig. 5C). Similar F-actin-deficient protrusions are found in the insulin receptor substrate protein 53 kDa (IRSp53)-overexpressing motor neuron-neuroblastoma hybrid cell line NSC34, presumably because of the unbalanced expression level between IRSp53 and its cooperative factor (24). In the F-actin-free protrusions of Ten-4-overexpressing Neuro-2a cells, coeffectors for actin filament formation by Ten-4 may be insufficient.
A number of studies have been carried out to elucidate the molecular mechanism of neurite outgrowth. Cytoskeletal organization is one of the critical steps in this mechanism. FAK and Rho-family GTPases, Cdc42 and Rac1, as well as N-WASP, play roles in actin cytoskeletal organization in neuronal cells. The Cdc42-N-WASP and Rac1 pathways are activated downstream of the signaling of ephrin–ephrin receptor and netrin-1-deleted in colorectal cancer (DCC) for filopodia formation and neurite outgrowth (25–27). Upstream of Cdc42 and N-WASP, FAK positively regulates neurite extension and/or filopodia formation on laminin (17, 18). In the present study, we found that Ten-4 activated FAK, Cdc42, and Rac1, and the activation of FAK and N-WASP was required for Ten-4 function (Figs. 4 and 6–8). In our previous study, we found that Ten-4-FAK signaling is required for cellular process formation of the oligodendrocyte cell line CG-4 (13). Those and present studies indicate that FAK is a key molecule in Ten-4 signaling and that Cdc42-N-WASP and Rac1 pathways downstream of FAK are likely activated by Ten-4 to induce the membranous protrusion formation. Furthermore, Ten-4 colocalized with phosphorylated FAK in the filopodia-like protrusions (Fig. 9). FAK activation is induced when FAK is recruited to the microdomains in the plasma membrane (23). Therefore, Ten-4 may function as a platform for the molecular complex that initiates the activation of FAK and its downstream pathways for neurite outgrowth. The transmembrane proteins, Ten-4, ephrin receptors, DCC, and integrins, commonly activate Cdc42-N-WASP and Rac1 pathways. Their extracellular ligands (e.g., ephrins for ephrin receptors, netrin-1 for DCC, laminins for integrins) presumably determine their specificity. The extracellular ligands for Ten-4 are not yet known; therefore, the identification of the ligands for Ten-4 would provide relevant information for understanding this mechanism. It is also possible that Ten-4 is a critical regulator in the ephrin receptor, DCC, or integrin pathway, activating its signaling for neurite outgrowth.
Teneurins are expressed in various tissues, but predominantly in the nervous system. Invertebrate teneurins are expressed in neurons and are required for axon pathfinding in C. elegans and Drosophila, respectively (2, 3, 6). Further, Drosophila teneurins, ten-a and ten-m, form heterophilic and/or homophilic interactions between projecting and targeted neurons and between neurons and muscle cells in the neuromuscular junction, in order to choose proper synaptic partners (4, 5). In addition, the Drosophila ten-m protein physically associates with filamin and α-spectrin, which regulate the actin cytoskeleton (3, 4). These studies show the critical functions of invertebrate teneurins for neurite formation and axon guidance via cytoskeletal organization. In vertebrates, the teneurin family comprises 4 isoforms, Ten-1–4, and all of the vertebrate teneurins are expressed in subpopulations of differentiating neurons. Ten-1 has been shown to promote neurite outgrowth through its heparin-binding activity (10). Rubin et al. (11) demonstrate that overexpression of Ten-2 promotes filopodia formation and enlargement of growth cones in Neuro-2a cells. Ten-2 and Ten-3 are required for visual circuit formation (9, 12). In this work, we demonstrate for the first time the promoting function of Ten-4 for filopodia-like protrusion formation and neurite outgrowth. From these observations, we conclude that the teneurin family proteins have common biological activities in neuronal differentiation. In the mouse brain cortex, a complementary expression pattern of the four teneurins is observed; their expressions overlap in some regions. Among the family members, Ten-4 is specifically expressed in the differentiating layer 5 neurons, which exist in the rostral part of the cerebral cortex in the early postnatal stage (8). Ten-4 might exert critical functions in these neurons. The layer 5 neurons extend long axons to the spinal cord and form the corticospinal tract. A severe myelination defect is observed in the corticospinal tract of Ten-4-deficient (fur/fur) mice, because of the dysfunction of oligodendrocytes (13). This defect may be due not only to impaired oligodendrocyte function, but also to the inhibition of some neuronal functions. In Neuro-2a cells, Ten-1, Ten-3, and Ten-4 were expressed, while Ten-2 was hardly detected by RT-PCR (data not shown), which is consistent with the previously shown Western blotting data (11). Latrophilin-1, a receptor of α-latrotoxin, conducts the positive activity in synapse functions through specific binding to Ten-2, but not to Ten-1, Ten-3, or Ten-4 (28). This evidence suggests that the expression patterns and ligands for the teneurin members may determine their functions in specific tissues.
An SNP mutation has recently been identified in the Ten-4 gene in patients with bipolar disorder (14). Previous genetic and molecular analyses showed that dedicator of cytokinesis 9 (DOCK9), an activator of Cdc42 for dendrite outgrowth, and oligodendroglial genes, such as myelin proteins, are related to bipolar disorder (29–31). Because Ten-4 is an upstream regulator of Cdc42 and regulates the expression levels of myelin proteins (Figs. 4 and 6 and ref. 13), it is reasonable to argue that Ten-4's functions in neurons and/or oligodendrocytes may be involved in the disorder. Filopodia is an initial structure during the dendritic spine formation, and various genes participating in synaptogenesis are implicated in bipolar disorder (32, 33). The Ten-4 function in the filopodia-like protrusion formation may be crucial for development or plasticity of synapses in the pathology of this disorder.
In summary, our results using Neuro-2a cells provide evidence that Ten-4 positively regulates the formation of filopodia-like protrusion and neurite outgrowth. This is the first report, to our knowledge, that demonstrates the cellular function and molecular signaling of Ten-4 in neuronal differentiation. Our findings will facilitate a better understanding of the molecular mechanism of neuronal differentiation.
Supplementary Material
Acknowledgments
The authors thank Dr. Yukiko Goda (RIKEN BSI, Wako, Japan) for her valuable suggestion.
This work was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, U.S. National Institutes of Health (Y.Y.), a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (24300139; T.N.), the MEXT Program for Strategic Research Foundation at Private Universities 2011–2015 (S.V. and E.A-H.), a Grant-in-Aid for Young Scientists from MEXT (25860701; N.S.), and a Grant-in-Aid from the Ministry of Health, Labor, and Welfare of Japan (23040101; C.A.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- CNS
- central nervous system
- CRIB
- Cdc42/Rac interactive binding
- DCC
- deleted in colorectal cancer
- DMEM
- Dulbecco's modified Eagle's medium
- DMSO
- dimethyl sulfoxide
- DN-FAK
- dominant-negative focal adhesion kinase
- FAK
- focal adhesion kinase
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GFP
- green fluorescent protein
- IgG
- immunoglobulin G
- IL2R
- interleukin-2 receptor
- Mock-Neuro-2a
- mock plasmid-transfected Neuro-2a
- N-WASP
- neural Wiskott-Aldrich syndrome protein
- PFA
- paraformaldehyde
- p-FAK
- phosphorylated focal adhesion kinase
- pTen-4
- Ten-4 expression plasmid
- pTen-4-Neuro-2a
- pTen-4-transfected Neuro-2a
- RT-PCR
- reverse transcription-polymerase chain reaction
- siCont-Neuro-2a
- control siRNA-transfected Neuro-2a
- siRNA
- small interfering RNA
- siTen-4
- Ten-4 siRNA
- siTen-4-Neuro-2a
- Ten-4 siRNA-transfected Neuro-2a
- SNP
- single-nucleotide polymorphism
- Ten-1–4
- teneurin 1–4
REFERENCES
- 1. Tucker R. P., Chiquet-Ehrismann R. (2006) Teneurins: a conserved family of transmembrane proteins involved in intercellular signaling during development. Dev. Biol. 290, 237–245 [DOI] [PubMed] [Google Scholar]
- 2. Levine A., Bashan-Ahrend A., Budai-Hadrian O., Gartenberg D., Menasherow S., Wides R. (1994) Odd Oz: a novel Drosophila pair rule gene. Cell 77, 587–598 [DOI] [PubMed] [Google Scholar]
- 3. Zheng L., Michelson Y., Freger V., Avraham Z., Venken K. J., Bellen H. J., Justice M. J., Wides R. (2011) Drosophila Ten-m and filamin affect motor neuron growth cone guidance. PLoS One 6, e22956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mosca T. J., Hong W., Dani V. S., Favaloro V., Luo L. (2012) Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature 484, 237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hong W., Mosca T. J., Luo L. (2012) Teneurins instruct synaptic partner matching in an olfactory map. Nature 484, 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morck C., Vivekanand V., Jafari G., Pilon M. (2010) C. elegans ten-1 is synthetic lethal with mutations in cytoskeleton regulators, and enhances many axon guidance defective mutants. BMC Dev. Biol. 10, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubin B. P., Tucker R. P., Brown-Luedi M., Martin D., Chiquet-Ehrismann R. (2002) Teneurin 2 is expressed by the neurons of the thalamofugal visual system in situ and promotes homophilic cell-cell adhesion in vitro. Development 129, 4697–4705 [DOI] [PubMed] [Google Scholar]
- 8. Li H., Bishop K. M., O'Leary D. D. (2006) Potential target genes of EMX2 include Odz/Ten-M and other gene families with implications for cortical patterning. Mol. Cell. Neurosci. 33, 136–149 [DOI] [PubMed] [Google Scholar]
- 9. Leamey C. A., Merlin S., Lattouf P., Sawatari A., Zhou X., Demel N., Glendining K. A., Oohashi T., Sur M., Fassler R. (2007) Ten_m3 regulates eye-specific patterning in the mammalian visual pathway and is required for binocular vision. PLoS Biol. 5, e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minet A. D., Rubin B. P., Tucker R. P., Baumgartner S., Chiquet-Ehrismann R. (1999) Teneurin-1, a vertebrate homologue of the Drosophila pair-rule gene ten-m, is a neuronal protein with a novel type of heparin-binding domain. J. Cell Sci. 112, 2019–2032 [DOI] [PubMed] [Google Scholar]
- 11. Rubin B. P., Tucker R. P., Martin D., Chiquet-Ehrismann R. (1999) Teneurins: a novel family of neuronal cell surface proteins in vertebrates, homologous to the Drosophila pair-rule gene product Ten-m. Dev. Biol. 216, 195–209 [DOI] [PubMed] [Google Scholar]
- 12. Young T. R., Bourke M., Zhou X., Oohashi T., Sawatari A., Fassler R., Leamey C. A. (2013) Ten-m2 is required for the generation of binocular visual circuits. J. Neurosci. 33, 12490–12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki N., Fukushi M., Kosaki K., Doyle A. D., de Vega S., Yoshizaki K., Akazawa C., Arikawa-Hirasawa E., Yamada Y. (2012) Teneurin-4 is a novel regulator of oligodendrocyte differentiation and myelination of small-diameter axons in the CNS. J. Neurosci. 32, 11586–11599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sklar P., Ripke S., Scott L. J., Andreassen O. A., Cichon S., Craddock N., Edenberg H. J., Nurnberger J. I., Jr., Rietschel M., Blackwood D., Corvin A., Flickinger M., Guan W., Mattingsdal M., McQuillin A., Kwan P., Wienker T. F., Daly M., Dudbridge F., Holmans P. A., Lin D., Burmeister M., Greenwood T. A., Hamshere M. L., Muglia P., Smith E. N., Zandi P. P., Nievergelt C. M., McKinney R., Shilling P. D., Schork N. J., Bloss C. S., Foroud T., Koller D. L., Gershon E. S., Liu C., Badner J. A., Scheftner W. A., Lawson W. B., Nwulia E. A., Hipolito M., Coryell W., Rice J., Byerley W., McMahon F. J., Schulze T. G., Berrettini W., Lohoff F. W., Potash J. B., Mahon P. B., McInnis M. G., Zollner S., Zhang P., Craig D. W., Szelinger S., Barrett T. B., Breuer R., Meier S., Strohmaier J., Witt S. H., Tozzi F., Farmer A., McGuffin P., Strauss J., Xu W., Kennedy J. L., Vincent J. B., Matthews K., Day R., Ferreira M. A., O'Dushlaine C., Perlis R., Raychaudhuri S., Ruderfer D., Hyoun P. L., Smoller J. W., Li J., Absher D., Thompson R. C., Meng F. G., Schatzberg A. F., Bunney W. E., Barchas J. D., Jones E. G., Watson S. J., Myers R. M., Akil H., Boehnke M., Chambert K., Moran J., Scolnick E., Djurovic S., Melle I., Morken G., Gill M., Morris D., Quinn E., Muhleisen T. W., Degenhardt F. A., Mattheisen M., Schumacher J., Maier W., Steffens M., Propping P., Nothen M. M., Anjorin A., Bass N., Gurling H., Kandaswamy R., Lawrence J., McGhee K., McIntosh A., McLean A. W., Muir W. J., Pickard B. S., Breen G., St Clair D., Caesar S., Gordon-Smith K., Jones L., Fraser C., Green E. K., Grozeva D., Jones I. R., Kirov G., Moskvina V., Nikolov I., O'Donovan M. C., Owen M. J., Collier D. A., Elkin A., Williamson R., Young A. H., Ferrier I. N., Stefansson K., Stefansson H., Thornorgeirsson T., Steinberg S., Gustafsson O., Bergen S. E., Nimgaonkar V., Hultman C., Landen M., Lichtenstein P., Sullivan P., Schalling M., Osby U., Backlund L., Frisen L., Langstrom N., Jamain S., Leboyer M., Etain B., Bellivier F., Petursson H., Sigur Sson E., Muller-Mysok B., Lucae S., Schwarz M., Schofield P. R., Martin N., Montgomery G. W., Lathrop M., Oskarsson H., Bauer M., Wright A., Mitchell P. B., Hautzinger M., Reif A., Kelsoe J. R., Purcell S. M. (2011) Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 43, 977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mattila P. K., Lappalainen P. (2008) Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454 [DOI] [PubMed] [Google Scholar]
- 16. Chacon M. R., Fazzari P. (2011) FAK: dynamic integration of guidance signals at the growth cone. Cell Adh. Migr. 5, 52–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chacon M. R., Navarro A. I., Cuesto G., del Pino I., Scott R., Morales M., Rico B. (2012) Focal adhesion kinase regulates actin nucleation and neuronal filopodia formation during axonal growth. Development 139, 3200–3210 [DOI] [PubMed] [Google Scholar]
- 18. Myers J. P., Robles E., Ducharme-Smith A., Gomez T. M. (2012) Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J. Cell Sci. 125, 2918–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyazaki H., Oyama F., Wong H. K., Kaneko K., Sakurai T., Tamaoka A., Nukina N. (2007) BACE1 modulates filopodia-like protrusions induced by sodium channel beta4 subunit. Biochem. Biophys. Res. Commun. 361, 43–48 [DOI] [PubMed] [Google Scholar]
- 20. Katz B. Z., Miyamoto S., Teramoto H., Zohar M., Krylov D., Vinson C., Gutkind J. S., Yamada K. M. (2002) Direct transmembrane clustering and cytoplasmic dimerization of focal adhesion kinase initiates its tyrosine phosphorylation. Biochim. Biophys. Acta 1592, 141–152 [DOI] [PubMed] [Google Scholar]
- 21. Greene L. A., Tischler A. S. (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. U. S. A. 73, 2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu G., Fang Y., Lu Z. H., Ledeen R. W. (1998) Induction of axon-like and dendrite-like processes in neuroblastoma cells. J. Neurocytol. 27, 1–14 [DOI] [PubMed] [Google Scholar]
- 23. Seong J., Ouyang M., Kim T., Sun J., Wen P. C., Lu S., Zhuo Y., Llewellyn N. M., Schlaepfer D. D., Guan J. L., Chien S., Wang Y. (2011) Detection of focal adhesion kinase activation at membrane microdomains by fluorescence resonance energy transfer. Nat. Commun. 2, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crespi A., Ferrari I., Lonati P., Disanza A., Fornasari D., Scita G., Padovano V., Pietrini G. (2012) LIN7 regulates the filopodium- and neurite-promoting activity of IRSp53. J. Cell Sci. 125, 4543–4554 [DOI] [PubMed] [Google Scholar]
- 25. Shekarabi M., Moore S. W., Tritsch N. X., Morris S. J., Bouchard J. F., Kennedy T. E. (2005) Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J. Neurosci. 25, 3132–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C., Wirth A., Ponimaskin E. (2012) Cdc42: an important regulator of neuronal morphology. Int. J. Biochem. Cell Biol. 44, 447–451 [DOI] [PubMed] [Google Scholar]
- 27. Tanaka M., Ohashi R., Nakamura R., Shinmura K., Kamo T., Sakai R., Sugimura H. (2004) Tiam1 mediates neurite outgrowth induced by ephrin-B1 and EphA2. EMBO J. 23, 1075–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silva J. P., Lelianova V. G., Ermolyuk Y. S., Vysokov N., Hitchen P. G., Berninghausen O., Rahman M. A., Zangrandi A., Fidalgo S., Tonevitsky A. G., Dell A., Volynski K. E., Ushkaryov Y. A. (2011) Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc. Natl. Acad. Sci. U. S. A. 108, 12113–12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahon K., Burdick K. E., Szeszko P. R. (2010) A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci. Biobehav. Rev. 34, 533–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuramoto K., Negishi M., Katoh H. (2009) Regulation of dendrite growth by the Cdc42 activator Zizimin1/Dock9 in hippocampal neurons. J. Neurosci. Res. 87, 1794–1805 [DOI] [PubMed] [Google Scholar]
- 31. Detera-Wadleigh S. D., Liu C. Y., Maheshwari M., Cardona I., Corona W., Akula N., Steele C. J., Badner J. A., Kundu M., Kassem L., Potash J. B., Gibbs R., Gershon E. S., McMahon F. J. (2007) Sequence variation in DOCK9 and heterogeneity in bipolar disorder. Psychiatr. Genet. 17, 274–286 [DOI] [PubMed] [Google Scholar]
- 32. Ginsberg S. D., Hemby S. E., Smiley J. F. (2012) Expression profiling in neuropsychiatric disorders: emphasis on glutamate receptors in bipolar disorder. Pharmacol. Biochem. Behav. 100, 705–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Menna E., Fossati G., Scita G., Matteoli M. (2011) From filopodia to synapses: the role of actin-capping and anti-capping proteins. Eur. J. Neurosci. 34, 1655–1662 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.