Abstract
Skeletal muscle wasting attributed to inactivity has significant adverse functional consequences. Accumulating evidence suggests that peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and TNF-like weak inducer of apoptosis (TWEAK)-Fn14 system are key regulators of skeletal muscle mass in various catabolic states. While the activation of TWEAK-Fn14 signaling causes muscle wasting, PGC-1α preserves muscle mass in several conditions, including functional denervation and aging. However, it remains unknown whether there is any regulatory interaction between PGC-1α and TWEAK-Fn14 system during muscle atrophy. Here we demonstrate that TWEAK significantly reduces the levels of PGC-1α and mitochondrial content (∼50%) in skeletal muscle. Levels of PGC-1α are significantly increased in skeletal muscle of TWEAK-knockout (KO) and Fn14-KO mice compared to wild-type mice on denervation. Transgenic (Tg) overexpression of PGC-1α inhibited progressive muscle wasting in TWEAK-Tg mice. PGC-1α inhibited the TWEAK-induced activation of NF-κB (∼50%) and dramatically reduced (∼90%) the expression of atrogenes such as MAFbx and MuRF1. Intriguingly, muscle-specific overexpression of PGC-1α also prevented the inducible expression of Fn14 in denervated skeletal muscle. Collectively, our study demonstrates that TWEAK induces muscle atrophy through repressing the levels of PGC-1α. Overexpression of PGC-1α not only blocks the TWEAK-induced atrophy program but also diminishes the expression of Fn14 in denervated skeletal muscle.—Hindi, S. M., Mishra, V., Bhatnagar, S., Tajrishi, M. M., Ogura, Y., Yan, Z., Burkly, L. C., Zheng, T. S., Kumar, A. Regulatory circuitry of TWEAK-Fn14 system and PGC-1α in skeletal muscle atrophy program.
Keywords: mitochondria, NF-κB, denervation, ubiquitin-proteasome system, MuRF1, MAFBx, SP-1
Loss of skeletal muscle mass, commonly referred to as atrophy or wasting, is a debilitating consequence of aging, disuse (immobilization, denervation, unloading, etc.), starvation, and many chronic disease states (1, 2). While triggering events vary in different conditions, imbalance between protein synthesis and protein degradation is the common molecular representation of muscle atrophy (3, 4). Such disruption in protein homeostasis is mediated, in part, by dysregulated production of proinflammatory cytokines and impaired energy supply (1, 5). It has been consistently observed that in almost all atrophic conditions, the activation of the ubiquitin-proteasome system (UPS) is a common denominator for the loss of skeletal muscle mass (6). Rapid muscle atrophy stems from degradation of thick and thin filament proteins, some of which are targeted by muscle-specific E3 ubiquitin ligases MuRF1 and MAFBx (7–10). Furthermore, it is increasingly evident that the changes in the mitochondrial content, integrity, and function play a critical role in promoting muscle wasting (11, 12). Impairment in mitochondrial function is an important feature of aged skeletal muscle (13–16). However, it remains equivocal whether dysfunctional mitochondria are a cause or consequence of aged muscle (17–22). Nevertheless, triggering events that cause the loss of skeletal muscle mass and mitochondrial content in atrophic conditions remain less understood.
Tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) is a proinflammatory cytokine belonging to the TNF superfamily (23, 24). TWEAK acts on the target cells through binding to Fn14 receptor, a member of TNF receptor superfamily (23). Recently TWEAK has been identified as a key mediator of muscle wasting. Muscle-specific TWEAK transgenic (Tg) mice expressing high levels (>17-fold) of TWEAK died at perinatal or neonatal stages due to excessive muscle loss (25). TWEAK Tg mice expressing lower levels of TWEAK, yet a few (∼ 5–6) folds higher, were normal in size and did not display any phenotype in unchallenged conditions at early adulthood (26). However, on exposure to atrophy-triggering stimuli, such as denervation, TWEAK-Tg mice displayed a more profound loss of skeletal muscle mass. Moreover, TWEAK-knockout (KO) mice were resistant to denervation-induced muscle atrophy. On denervation, the expression of TWEAK receptor Fn14 is drastically increased, while the levels of TWEAK remain unchanged in denervated skeletal muscle (26). Although TWEAK has been found to activate UPS, autophagy, and caspases in cultured myotubes (27), UPS appears to be the major pathway by which TWEAK drives protein degradation in vivo (26).
In addition to fiber atrophy, a prominent characteristic of TWEAK-Tg mice is a noticeable shift toward a fast-contracting glycolytic fiber type, while slow-contracting oxidative muscle fibers dominate in TWEAK-KO mice (26). Moreover, TWEAK inhibits oxidative metabolism in skeletal muscle (28). Recent studies have suggested that peroxisome proliferator-activated receptor (PPAR) γ coactivator 1α (PGC-1α) is a key player in regulation of skeletal muscle fiber composition, mitochondrial content, and oxidative metabolism (29–31). PGC-1α Tg mice overexpressing PGC-1α in skeletal muscle show increased mitochondrial biogenesis and oxidative capacity, resistance to fatigue, and improved aerobic performance (32–35). PGC-1's also play a key role in preserving skeletal muscle mass in catabolic states (36, 37). Levels of PGC-1α are repressed in atrophying skeletal muscle in many atrophying conditions including denervation (36). Interestingly, repression in PGC-1α levels in response to denervation occurs very early (within 2–3 d) and persists for ≥2 wk, implying that the levels of PGC-1α are reduced much before a noticeable reduction in skeletal muscle mass (36). Although maintaining PGC-1α levels through treatment with 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) failed to show muscle sparing potentially due to other metabolic effects of AICAR (37), muscle-specific overexpression of PGC-1α was sufficient to block the inducible expression of MuRF1 and MAFBx and preserve skeletal muscle mass in response to denervation (36). PGC-1α Tg mice are also resilient to age-associated muscle wasting and display an extended lifespan (38).
While the TWEAK-Fn14 dyad has been found to be an important regulator of muscle atrophy, fiber-type composition, and oxidative metabolism, it remains unknown whether TWEAK induces muscle atrophy through regulating the levels of PGC-1α. Furthermore, the role of PGC-1α in inducible expression of Fn14 in atrophying skeletal muscle has not been yet investigated. In this study, using genetic mouse models, we demonstrate that a reciprocal interaction between the TWEAK-Fn14 system and PGC-1α regulates the skeletal muscle atrophy program. TWEAK represses the levels of PGC-1α in skeletal muscle through activation of the NF-κB pathway. Forced expression of PGC-1α inhibits TWEAK-induced atrophy program both in vitro and in vivo. Moreover, muscle-specific overexpression of PGC-1α inhibits the expression of Fn14 receptor in skeletal muscle of mice on denervation.
MATERIALS AND METHODS
Animals
Tg mice expressing full-length TWEAK cDNA under the control of muscle creatine kinase (MCK) promoter have been previously described (25, 26). TWEAK-KO mice as described previously (39) were provided by Dr. A. Ashkenazi (Genentech, South San Francisco, CA, USA). Muscle-specific PGC-1α-Tg mice as described previously (34) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). TWEAK-Tg mice were crossed with PGC-1α-Tg mice to generate TWEAK-PGC-1α double-Tg mice. Fn14-KO mice used for this study have been described previously (40). All mice were in the C57BL/6 background, and their genotype was determined by PCR from tail DNA.
Sciatic denervation was performed following a protocol as described previously (41). Mice were anesthetized with an intraperitoneal injection of 2,2,2,-tribromoethanol (Avertin), shaving the right hind quarters, making a 0.5-cm incision ∼0.5 cm proximal to the knee on the lateral side of the right leg, separating the muscles at the fascia and lifting out the sciatic nerve with a surgical hook or forceps, removing a 2–3-mm piece of sciatic nerve, and finally closing the incision with surgical sutures. At various time points postdenervation, muscle tissue was collected from euthanized mice for biochemical and histological studies. All experimental protocols with mice were approved in advance by the Institutional Animal Care and Use Committee at University of Louisville.
Cell culture
C2C12 (a mouse myoblastic cell line) cells were purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were grown in DMEM containing 10% FBS. For primary myoblast cultures, satellite cells/myoblasts were isolated from hind limb of 8-wk-old mice using a method as described previously (42). Briefly, mice were euthanized, and tibial anterior (TA), gastrocnemius, and extensor digitorum longus (EDL) muscles were isolated. Excess connective tissues and fat were cleaned in sterile PBS followed by mincing of skeletal muscle in DMEM and enzymatic dissociation with 0.1% Pronase. The digested slurry was spun, pelleted, and triturated several times and then passed through a 70-μm cell strainer (BD Falcon; BD Biosciences, San Jose, CA, USA). The filtrate was spun at 1000 g and resuspended in myoblast growth medium (Ham's F-10 medium with 20% FBS supplemented with 5 ng/ml basic fibroblast growth factor). Cells were first refed after 3 d of initial plating. During first few passages, cells were also enriched by preplating for selection of pure myoblast population. On selection, the cells were cultured in a 1:1 ratio of myoblast growth medium and growth medium (DMEM with 20% FBS) till reaching 80% confluence. To induce differentiation, the cells were incubated in differentiation medium (DM; 2% horse serum in DMEM) for 96 h.
For single-myofiber isolation, EDL muscle was dissected tendon to tendon from mice and enzymatically digested in DMEM medium supplemented with collagenase (400 IU/ml, LS004196; Worthington, Lakewood, NJ, USA) at 37°C for 45 min. Postdigestion, single myofibers were released by trituration in myofiber culture medium (DMEM with 10% FBS). Single myofibers were plated in 6-well tissue culture plates.
Cloning of Fn14 promoter and electroporation in TA muscle
The mouse Fn14 promoter fragment (up to −2 kb from first ATG in exon1 of mouse Fn14 gene) was isolated by performing PCR using mouse genomic DNA as a template and placed in front of the firefly luciferase reporter in pGL3 plasmid (Promega, Madison, WI, USA). Injection of plasmid DNA into the TA muscle of mice and electroporation were performed according to a protocol as described previously (26, 43) with minor modifications. In brief, 2 kb Fn14-Luciferase promoter construct and pRL-TK Renilla luciferase plasmids (Promega) were prepared using an endotoxin-free kit (Qiagen, Valencia, CA, USA) and suspended in sterile saline solution in a 10:1 ratio. Mice were anesthetized, and a small portion of TA muscle of both hind limbs was surgically exposed and injected with plasmid DNA (20 μg in 20 μl saline). After 1 min of plasmid DNA injection, a pair of platinum plate electrodes was placed against the closely shaved skin on either side of the small surgical incision, and electric pulses were delivered transcutaneously. Four 20-ms square-wave pulses of 1 Hz frequency at 75 V/cm were generated using a stimulator (model S88; Grass Technologies, West Warwick, RI, USA) and delivered to the muscle. The polarity was then reversed, and a further 4 pulses were delivered to the muscle. After electroporation, the wound was closed with surgical clips, and mice were returned to their cages and fed a standard diet. After 7 d of plasmid electroporation, the left hind limb was denervated, whereas sham surgery was performed on the right side. Finally, after 3 d of denervation, the mice were euthanized, TA muscle was isolated, and muscle extracts made were used for measurement of luciferase and Renilla activity using a Dual-luciferase reporter assay system (Promega).
Histology and morphometric analysis
Hind limb muscles from mice were isolated and frozen in isopentane cooled in liquid nitrogen and sectioned in a microtome cryostat. For the assessment of tissue morphology, 10-μm-thick transverse sections of muscles were stained with hematoxylin and eosin (H&E) and examined under a Nikon Eclipse TE 2000-U microscope (Nikon, Melville, NY, USA). Fiber cross-sectional area (CSA) was analyzed in H&E-stained muscle sections using Nikon NIS Elements BR 3.00 software (Nikon). For each muscle, the distribution of fiber CSA was calculated by analyzing 200 to 250 myofibers as described previously (41).
Indirect immunofluorescence and myotube diameter measurements
For immunohistochemistry study, paraformaldehyde-fixed cultured myotubes were blocked in 1% bovine serum albumin in PBS for 1 h and incubated with anti-MF20 (1:250; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) in blocking solution at 4°C overnight under humidified conditions. A brief PBS wash was applied before incubation with Alexa Fluor 488-conjugated secondary antibody (1:3000; Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature and then washed 3 times for 30 min with PBS followed by incubation with DAPI (1:5000) for 3 min and subsequent PBS washes. Immunostained myotubes were visualized at room temperature on Nikon Eclipse TE 2000-U microscope (Nikon) and a digital camera (Nikon Digital Sight DS-Fi1). Pictures of the myotubes were captured, and diameter of the myotubes was measured using NIS Elements BR 3.00 software (Nikon). Myotube diameter was quantified as follows: 10 fields were chosen randomly, and 10 myotubes were measured per field. The average diameter per myotube was calculated as the mean of the 5 measurements taken along the length of the myotube.
Transmission electron microscopy
Mitochondrial density in skeletal muscle was determined following a protocol as described previously (41). Briefly, soleus muscle from wild-type (WT) and TWEAK-Tg mice were fixed in 3% glutaraldehyde in 0.1 M cocodylate buffer overnight, followed by fixing in 1% cocodylate-buffered osmium tetroxide. The tissue was dehydrated through a series of graded alcohols and embedded in LX-112 plastic (Ladd Research Industries, Williston, VT, USA). Longitudinal sections (80 nm) were cut using an ultramicrotome (LKB, Bromma, Swededn) and stained with uranium acetate and lead citrate. Samples were analyzed using a transmission electron microscope (CM 12; Philips, Amsterdam, The Netherlands) operating at 60 kV. The pictures were captured at ×8800 using a 3.2-megapixel digital camera (Sia-7C; Kodak, Rochester, NY, USA) at room temperature. No imaging medium was used to visualize the pictures, and images were stored as JPEG files. Image levels were equally adjusted using Photoshop CS2 software (Adobe Systems, San Jose, CA, USA).
Transient transfection and reporter gene activity
C2C12 myoblasts plated in 12-well tissue culture plates were transfected with vector alone or IκBα super-repressor plasmid along with PGC-1α-Luc reporter plasmid (from Addgene, Cambridge, MA, USA) in 10:1 ratio using Lipofectamine transfection reagent following a protocol suggested by the manufacturer (Invitrogen). Transfection efficiency was controlled by cotransfection of myoblasts with Renilla luciferase encoding plasmid, pRL-TK (Promega). When >90% confluent, the cells were differentiated into myotubes by changing medium to DM and incubation for addition 96 h. After TWEAK treatment, specimens were processed for luciferase expression using a dual luciferase assay system (Promega) with reporter lysis buffer per the manufacturer's instructions. Luciferase measurements were made using a luminometer (model 3010; Analytic Scientific Instruments, Richmond, CA, USA).
RNA isolation and quantitative real-time PCR (QRT-PCR)
RNA isolation and QRT-PCR were performed using a method as described previously (26, 41, 43).
Western blot
Quantitative estimation of specific protein was performed by a Western blot test using a method as described previously (26, 41). In brief, skeletal muscle tissues or cells were washed with PBS and homogenized in lysis buffer (50 mM Tris-Cl, pH 8.0; 200 mM NaCl; 50 mM NaF; 1 mM DTT; 1 mM sodium orthovanadate; 0.3% IGEPAL; and protease inhibitors). Approximately 100 μg protein was resolved on each lane on 10 or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrotransferred onto nitrocellulose or polyvinylidene difluoride membrane, and probed using anti-PGC-1α (1:500, cat. no. ST1203; Calbiochem, La Jolla, CA, USA), Anti-total oxphos cocktail (1:500, ab110413; Abcam, Cambridge, MA, USA), anti-phospho-p65 (1:1000, 3033; Cell Signaling Technology, Danvers, MA, USA), anti-p65 (1:1000, 8242; Cell Signaling Technology), anti-p100/p52 (1:1000, 4882; Cell Signaling Technology), and anti-Fn14 (1:500, 4403; Cell Signaling Technology) and detected by enhanced chemiluminescence. For loading controls, the membranes were stripped and reprobed with anti-α-tubulin (1:3000, 2125; Cell Signaling Technology).
Electrophoretic mobility shift assay (EMSA)
DNA binding of NF-κB and specificity protein 1 (SP1) transcription factors was measured by performing EMSA as described previously (41). In brief, 20 μg of nuclear extracts prepared from myotubes was incubated with 16 fmol of 32P end-labeled NF-κB consensus oligonucleotide (Promega) at 37°C for 30 min, and the DNA-protein complex was resolved on a 7.5% native polyacrylamide gel. For measuring binding of SP1 to Fn14 promoter, nuclear extracts were prepared from undenervated and denervated skeletal muscle of mice. We used 5′-AAGGCGGGGGCGGGGGCGGAGC-3′ oligonucleotides from mouse Fn14 promoter, which contain a conserved SP1 site. The radioactive bands from the dried gel were visualized and quantified by PhosphorImager (GE Healthcare, Piscataway, NJ, USA) using ImageQuant TL software (GE Healthcare).
Statistical analysis
Results are expressed as means ± sd. Statistical analyses used a 2-tailed t test to compare quantitative data populations with normal distribution and equal variance. A value of P < 0.05 was considered statistically significant unless otherwise specified.
RESULTS
TWEAK inhibits expression of PGC-1α and mitochondrial genes in cultured myotubes
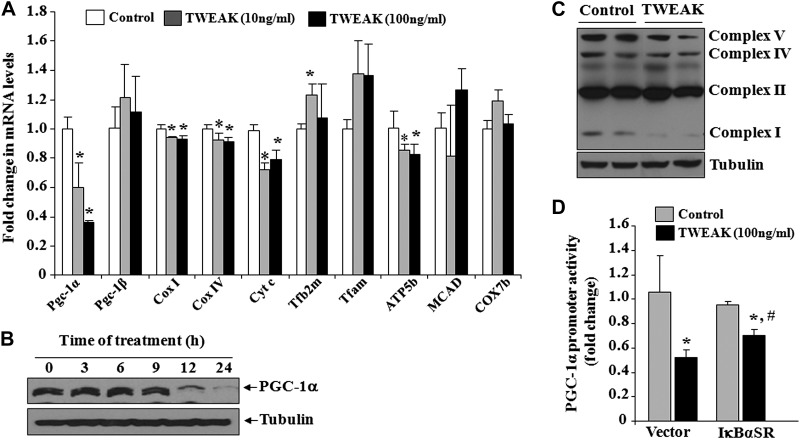
We first investigated whether TWEAK affects the transcript levels of PGC-1α and mitochondria-related genes in cultured muscle cells. C2C12 myotubes were treated with different concentrations of soluble TWEAK protein for 24 h followed by quantification of relative mRNA levels by performing a QRT-PCR assay. Results showed that mRNA levels of PGC-1α were significantly reduced in myotubes in response to TWEAK treatment (Fig. 1A). Furthermore, the mRNA levels of several mitochondrial genes such as cytochrome c oxidase subunit I (Cox I), Cox IV, cytochrome c (Cyt c), and ATP synthase subunit β (ATP5b) were significantly decreased on treatment with TWEAK. By contrast, mRNA levels of PGC-1β, transcription factor A mitochondrial (Tfam), medium-chain acyl-coenzyme A dehydrogenase (MCAD), and COX7b remained comparable between control and TWEAK-treated myotubes (Fig. 1A). We also performed a Western blot test to measure protein levels of PGC-1α in TWEAK-treated myotubes. As shown in Fig. 1B, TWEAK reduced the protein levels of PGC-1α in a time-dependent manner. Furthermore, the protein levels of mitochondrial complex I, IV, and V were reduced in TWEAK-treated myotubes compared to untreated controls (Fig. 1C).
Figure 1.
TWEAK inhibits the expression of PGC-1α in cultured myotubes. A) C2C12 myotubes were treated with indicated amounts of soluble TWEAK protein for 24 h, and mRNA levels of PGC-1α, PGC-1β, Cox I, Cox IV, Cyt c, Tfb2m, Tfam, ATP5b, MCAD, and COX7b were measured by QRT-PCR. B) C2C12 myotubes were treated with TWEAK (100 ng/ml) for indicated periods, and protein extracts prepared were probed for PGC-1α by performing Western blot tests. C) C2C12 myotubes were treated with 100 ng/ml TWEAK for 72 h, and levels of various mitochondrial complexes were measured by Western blot tests. D) C2C12 myoblasts were transfected with pcDNA3 (vector) or IκBαSR plasmid along with PGC-1α-Luc plasmid and Renilla plasmid in a 10:1 ratio. The myoblasts were differentiated into myotubes by incubation in DM for 72 h, followed by treatment with 100 ng/ml TWEAK for 24 h. Graph shows relative luciferase activity in control and TWEAK-treated myotubes; n = 3/group. Error bars = sd. *P < 0.05 vs. TWEAK-untreated C2C12 myotubes; #P < 0.05 vs. TWEAK-treated C2C12 myotubes transfected with vector alone.
TWEAK has been found to activate a number of signaling pathways including NF-κB in myotubes (24). We investigated whether NF-κB is involved in TWEAK-induced repression of PGC-1α in cultured myotubes. To understand the role of NF-κB, C2C12 myoblasts were transfected with vector alone or IκBα super-repressor mutant (IκBαSR; a dominant negative inhibitor of NF-κB) plasmid along with PGC-1α promoter construct and differentiated into myotubes. The cells were then treated with TWEAK for 24 h, and relative amount of luciferase activity was measured. Results showed that TWEAK significantly reduced the transactivation of PGC-1α promoter in C2C12 myotubes. However, the PGC-1α promoter activity was significantly higher in IκBαSR-expressing myotubes (Fig. 1D). These data indicate that TWEAK inhibits PGC-1α activity, at least in part, through the activation of NF-κB signaling pathway.
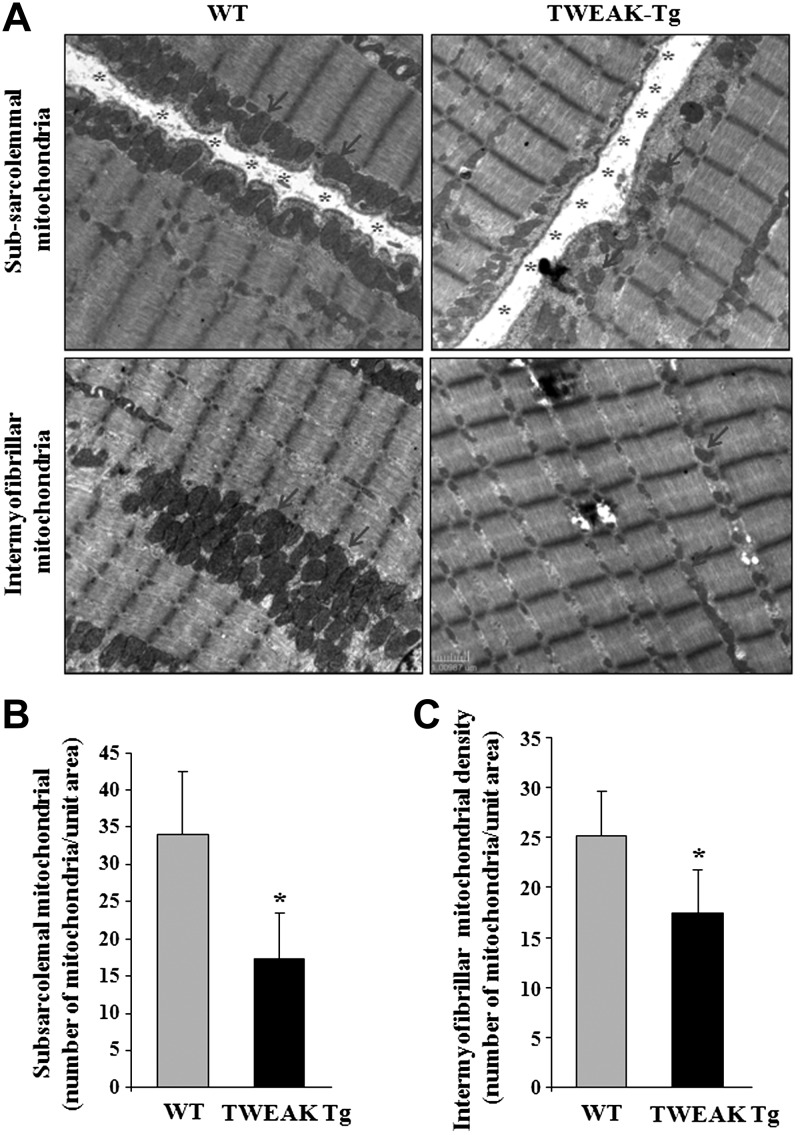
We have previously shown that muscle-specific TWEAK-Tg mice overexpressing TWEAK (∼5-fold higher than WT) show early onset (around 5–6 mo) of muscle atrophy (26). However, it remains unknown whether TWEAK also reduces mitochondrial content in skeletal muscle in vivo. To address this issue, we performed electron microscopy analysis of skeletal muscle of 6-mo-old TWEAK-Tg mice and their age-matched WT littermates. Interestingly, our results showed that the levels of both subsarcolemmal and intermyofibrillar mitochondria content are significantly reduced in skeletal muscle of TWEAK-Tg mice compared to WT littermates (Fig. 2). Taken together, these results are suggestive that TWEAK represses the expression of PGC-1α and mitochondrial content in skeletal muscle.
Figure 2.
Reduced mitochondrial content in skeletal muscle of TWEAK Tg mice. Soleus muscle of 6-mo-old TWEAK-Tg mice and WT littermates were isolated; longitudinal sections were made and processed for transmission electron microscopy analysis. A) Representative images of longitudinal soleus muscle. Abundance and size of mitochondria were reduced in TWEAK-Tg mice compared to WT mice. Arrows indicate mitochondria in muscle sections. Asterisks indicate subsarcolemmal space in longitudinal sections. Scale bar = 1 μm. B) Quantification of the number of subsarcolemmal mitochondria in soleus muscle of WT and TWEAK-Tg mice. C) Quantification of the number of intermyofibrillar mitochondria in soleus muscle sections of WT and TWEAK-Tg mice; n = 3/group. Error bars = sd. *P < 0.05 vs. WT mice.
TWEAK suppresses the levels of PGC-1α in denervated skeletal muscle of mice
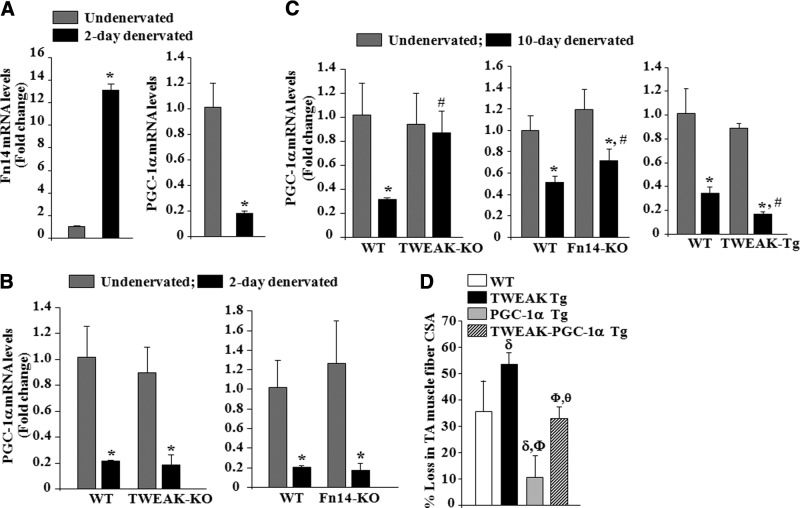
We have previously reported that the expression of TWEAK receptor Fn14 is dramatically increased in 4 d denervated skeletal muscle of mice (26). An earlier study has also shown that the levels of PGC-1α in skeletal muscle fall within 2 d of denervation (36). We next sought to determine whether there is any correlation between repression of PGC-1α and inducible expression of Fn14 in denervated muscle. Consistent with published report (36), the levels of PGC-1α were found to be drastically reduced within 2 d of denervation. By contrast, the levels of Fn14 were dramatically increased (∼14 fold) in skeletal muscle measured 2 d postdenervation (Fig. 3A). To evaluate whether TWEAK-Fn14 signaling is responsible for the repression of PGC-1α in denervated skeletal muscle, we employed TWEAK-KO and Fn14-KO mice. WT, TWEAK-KO, and Fn14-KO mice were denervated (meaning transection of sciatic nerve), and mRNA levels of PGC-1α were studied in TA muscle at 2 and 10 d postdenervation. Denervation significantly reduced mRNA levels of PGC-1α in 2 d denervated TA muscle of WT, TWEAK-KO, and Fn14-KO mice. However, there was no significant difference in the transcript levels of PGC-1α in TA muscle of WT, TWEAK-KO, and Fn14-KO mice 2 d postdenervation (Fig. 3B). By contrast, transcript levels of PGC-1α were significantly higher in TA muscle of TWEAK-KO and Fn14-KO mice compared to WT mice 10 d postdenervation (Fig. 3C). We also measured mRNA levels of PGC-1α in denervated skeletal muscle of TWEAK-Tg mice. As shown in Fig. 3C, mRNA levels of PGC-1α were further reduced in skeletal muscle of TWEAK-Tg mice compared to WT mice 10 d postdenervation (Fig. 3C). Moreover, our analysis showed that transcript levels of mitochondria-related genes were significantly higher in 10 d denervated muscle of TWEAK-KO and significantly reduced in TWEAK-Tg mice compared to their corresponding control mice (Supplemental Fig. S1). These results imply that while TWEAK-Fn14 signaling is not involved in the initial fall in PGC-1α, it maintains PGC-1α in a repressed state in denervated muscle.
Figure 3.
TWEAK represses PGC-1α levels in denervated skeletal muscle of mice. Quantitative estimation of mRNA levels of different genes in TA muscle by QRT-PCR assay. A) Relative mRNA levels of TWEAK receptor Fn14 and PGC-1α in undenervated and 2 d denervated muscle of 3-mo-old WT mice; n = 3/group. *P < 0.01 vs. contralateral undenervated muscle. B) Relative mRNA levels of PGC-1α in undenervated and 2 d denervated TA muscle of WT, TWEAK-KO, and Fn14-KO mice. C) Relative mRNA levels of PGC-1α in undenervated and 10 d denervated TA muscle of WT, TWEAK-KO, Fn14-KO, and TWEAK-Tg mice; n = 6/group. *P < 0.01 vs. contralateral undenervated muscle; #P < 0.05 vs. denervated muscle of WT mice. D) Percentage loss in fiber CSA in 3-mo-old WT, TWEAK-Tg, PGC-1α Tg, and TWEAK-PGC-1α double-Tg mice at 12 d postdenervation; n = 4/group. Error bars = sd. δP < 0.05 vs. denervated muscle of WT mice. ΦP < 0.05 vs. TWEAK Tg mice; θP < 0.05 vs. PGC-1α Tg mice.
TWEAK-Tg mice show more pronounced atrophy compared to WT mice on denervation (26). Since TWEAK represses PGC-1α levels in denervated muscle, we next investigated whether overexpression of PGC-1α can attenuate denervation-induced muscle atrophy in TWEAK-Tg mice. TWEAK Tg mice were crossed with PGC-1α Tg mice to generate littermate WT, TWEAK-Tg, PGC-1α-Tg, and TWEAK-PGC-1α double-Tg mice. At the age of 3 mo, left hind limb muscles of these mice were denervated by transecting the sciatic nerve, whereas sham surgery was performed on the right hind limb. After 12 d of denervation, TA muscle was isolated and processed for H&E staining and quantification of fiber CSA. In agreement with previously published reports (26, 36), denervation-induced loss in TA muscle fiber CSA was significantly higher in TWEAK-Tg mice and significantly reduced in PGC-1α Tg mice compared to littermate WT mice. Notably, denervation-induced loss in fiber CSA in TWEAK-PGC-1α double-Tg mice was significantly less compared to TWEAK Tg mice (Fig. 3D). These results further suggest that TWEAK causes atrophy in denervated muscle through repressing PGC-1α.
Tg overexpression of PGC-1α inhibits progressive muscle atrophy in TWEAK-Tg mice
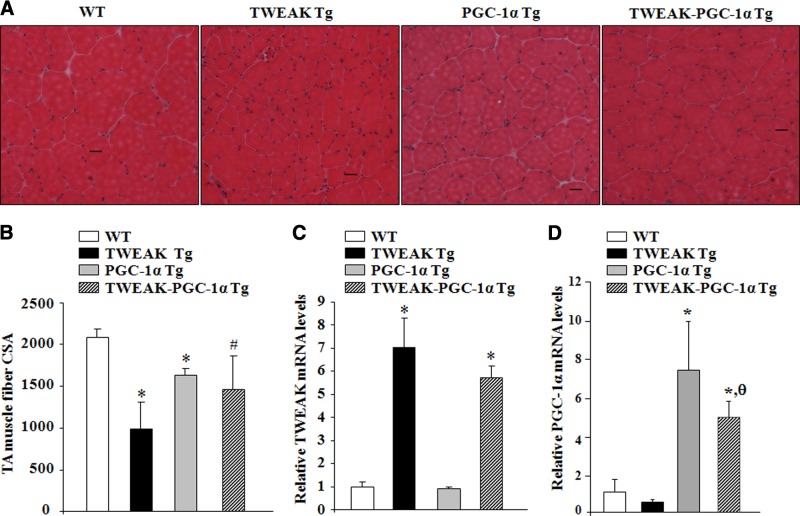
Skeletal muscle-specific Tg overexpression of high levels of TWEAK cause perinatal and neonatal lethality in mice (25). By contrast, TWEAK-Tg mice, expressing 5- to 6-fold higher levels of TWEAK, do not show any noticeable muscle atrophy at early age (up to 3–4 mo), potentially due to neutralization of low levels of TWEAK in skeletal muscle by other factors present during development. However, by the age of 6 mo, these mice start showing considerable muscle atrophy, evident by a significant reduction in fiber CSA compared to WT littermates (26). To investigate whether TWEAK-induced progressive muscle atrophy in vivo can be rescued through overexpression of PGC-1α, TA muscle of 15-mo-old littermate WT, TWEAK-Tg, PGC-1α-Tg, and TWEAK-PGC-1α double-Tg mice was isolated and analyzed by performing H&E staining. TWEAK-Tg mice showed a significant reduction in fiber CSA compared to WT mice (Fig. 4A, B). TWEAK-PGC-1α double-Tg mice showed no significant reduction in fiber CSA compared to PGC-1α-Tg mice. Furthermore, TA muscle fiber CSA of TWEAK-PGC-1α double-Tg mice was significantly higher compared to TWEAK-Tg mice (Fig. 4A, B). The levels of TWEAK were comparable between TWEAK-Tg and TWEAK-PGC-1α double-Tg mice indicating that overexpression of PGC-1α does not affect the levels of TWEAK in skeletal muscle of TWEAK-Tg mice (Fig. 4C). However, we observed a significant decrease in mRNA levels of PGC-1α in skeletal muscle of TWEAK-PGC-1α double-Tg compared to PGC-1α-Tg littermates, which could be attributed to the repression of endogenous PGC-1α levels due to overexpression of TWEAK (Fig. 4D). Indeed, mRNA levels of PGC-1α were also found to be reduced in 15-mo-old TWEAK-Tg mice compared to littermate WT mice (Fig. 4D). These results suggest that TWEAK represses PGC-1α levels, and overexpression of PGC-1α is sufficient to inhibit TWEAK-induced muscle atrophy in vivo.
Figure 4.
Tg overexpression of PGC-1α inhibits the progressive muscle atrophy in TWEAK-Tg mice. A) TA muscle isolated from 15-mo-old littermate WT, TWEAK-Tg, PGC-1α-Tg, and TWEAK-PGC-1α double-Tg mice were processed for H&E staining. Representative photomicrographs demonstrate that TWEAK-induced fiber atrophy is rescued in TWEAK-PGC-1α double-Tg mice. Scale bar = 20 μm. B) Quantitative estimation of fiber CSA in TA muscle of 15-mo-old WT, TWEAK-Tg, PGC-1α-Tg, and TWEAK-PGC-1α double-Tg mice; n = 5/group. C, D) Relative mRNA levels of TWEAK (C) and PGC-1α (D) in TA muscle of 15-mo-old WT, TWEAK-Tg, PGC-1α-Tg, and TWEAK-PGC-1α double-Tg mice; n = 3/group. Error bars = sd. *P < 0.05 vs. WT mice; #P < 0.05 vs. TWEAK-Tg mice; θP < 0.05 vs. PGC-1α-Tg mice.
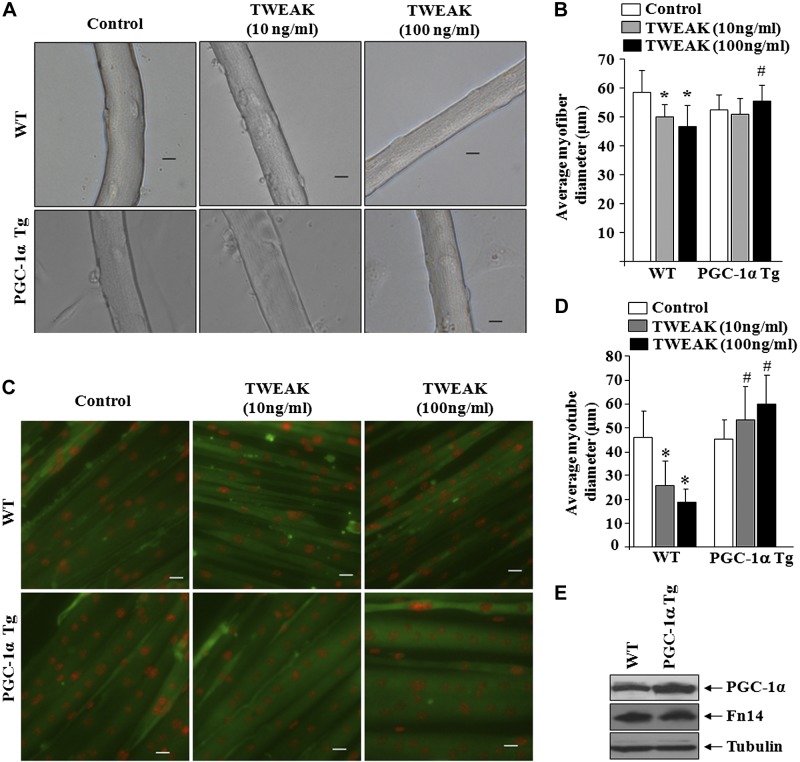
Overexpression of PGC-1α inhibits atrophy in cultured myofibers and primary myotubes
Skeletal muscle-specific Tg overexpression of PGC-1α inhibits fiber atrophy in response to denervation (36). Our results demonstrate that Tg overexpression of PGC-1α inhibits progressive fiber atrophy in TWEAK-Tg mice. However, it is not clear whether PGC-1α rescues TWEAK-induced muscle atrophy in vivo through direct or indirect mechanisms. To address this issue, we next sought to determine whether TWEAK-induced atrophy can be rescued by overexpression of PGC-1α in cultured muscle cells. For this analysis, we first established single myofiber cultures from EDL muscle of littermate WT and PGC-1α-Tg mice. These myofibers were treated with different concentration of TWEAK protein for 72 h, and their diameter was quantified. As shown in Fig. 5A, treatment with TWEAK led to significant atrophy in cultured myofibers. Interestingly, the TWEAK-induced atrophy was significantly rescued in myofibers from PGC-1α-Tg mice (Fig. 5A, B). In a separate experiment, we also prepared primary myoblasts from WT and PGC-1α-Tg mice and differentiated them into myotubes. Myotubes were then treated with TWEAK protein for 72 h followed by immunostaining for myosin heavy chain and measuring their diameter. Results showed that average myotube diameter was significantly higher in primary myotubes from PGC-1α-Tg mice compared to WT mice on treatment with TWEAK (Fig. 5C, D). By performing a Western blot test, we confirmed that EDL muscle of PGC-1α-Tg mice expressed increased amounts of PGC-1α compared to littermate WTs (Supplemental Fig. S2). Similarly, myotubes from PGC-1α-Tg mice contained increased amounts of PGC-1α protein compared to those from WT mice, and the levels of TWEAK receptor Fn14 were comparable between WT and PGC-1α-Tg myotubes (Fig. 5E).
Figure 5.
Overexpression of PGC-1α inhibits TWEAK-induced atrophy in cultured muscle cells. Myofiber cultures established from EDL muscle of WT and PGC-1α Tg mice were treated with indicated concentration of soluble TWEAK protein for 72 h. A) Representative photomicrographs of untreated and TWEAK-treated cultured myofibers. Scale bar = 20 μm. B) Quantitative estimation of average myofiber diameter after 72 h of TWEAK treatment. C) Primary myoblasts prepared from littermate WT and PGC-1α Tg mice were differentiated into myotubes, followed by treatment with indicated concentrations of soluble TWEAK protein for 72 h and immunostaining for MyHC protein (green). Nuclei were counterstained with DAPI (red). Representative photomicrographs of myotubes are shown. Scale bar = 20 μm. D) Quantitative analysis of myotube diameter in untreated and TWEAK-treated myotubes. E) Protein levels of PGC-1α and Fn14 in myotubes prepared from WT and PGC-1α Tg mice. Error bars = sd. *P < 0.05 vs. untreated primary myotubes or myofibers of WT mice; #P < 0.05 vs. corresponding TWEAK-treated myotubes or myofibers of WT mice.
PGC-1α inhibits the TWEAK-induced activation of NF-κB in cultured myotubes
We next investigated the mechanisms by which PGC-1α prevents TWEAK-induced atrophy. NF-κB is a major nuclear transcription factor that causes muscle atrophy through augmenting the expression of several components of UPS (1). It has been reported that TWEAK can activate both canonical and noncanonical NF-κB signaling in cultured myotubes and in denervated skeletal muscle of mice (25, 26). We examined whether PGC-1α affects the TWEAK-induced activation of NF-κB. Primary myotubes prepared from WT and PGC-1α Tg mice were treated with TWEAK for different time intervals, and DNA-binding activity of NF-κB was measured by performing EMSA. As shown in Fig. 6A, TWEAK-induced activation of NF-κB was inhibited in myotubes prepared from PGC-1α-Tg mice compared to those from WT mice. To determine whether PGC-1α inhibits the canonical or noncanonical NF-κB pathways, we performed a Western blot test for phosphorylated IκBα and p65 protein (markers of canonical pathways) and p100/p52 (markers of noncanonical pathway) (1). As shown in Fig. 6B, TWEAK-induced phosphorylation of IκBα and p65 was considerably reduced in PGC-1α expressing myotubes compared with WT myotubes. Intriguingly, total p65 protein level was also found to be diminished in PGC-1α-expressing myotubes (Fig. 6B). Furthermore, the proteolytic degradation of p100 into p52 occurred at a slower rate in PGC-1α-Tg myotubes compared to WT myotubes on treatment with TWEAK (Fig. 6B). These results suggest that overexpression of PGC-1α inhibits the TWEAK-induced activation of both canonical and noncanonical NF-κB signaling in cultured myotubes.
Figure 6.
PGC-1α inhibits the TWEAK-induced activation of NF-κB in myotubes. Primary myotubes prepared from WT and PGC-1α Tg mice were treated with 100 ng/ml TWEAK protein for indicated time period and analyzed for the activation of NF-κB. A) Representative EMSA gels demonstrate that TWEAK-induced increase in DNA-binding activity of NF-κB is inhibited in PGC-1α-overexpressing myotubes compared with WT myotubes. B) Representative immunoblots demonstrate that PGC-1α inhibits the TWEAK-induced phosphorylation of IκBα and p65 proteins and attenuates the degradation of p100 into p52 subunit of NF-κB. Levels of an unrelated protein, β-actin, remained unaffected by TWEAK treatment.
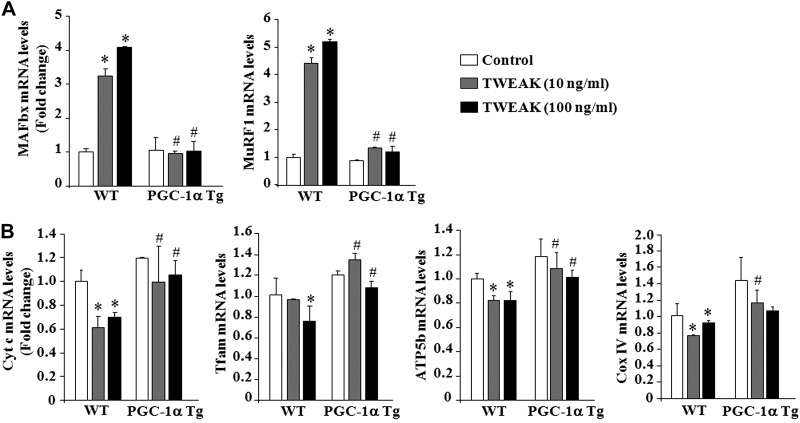
Overexpression of PGC-1α inhibits the expression of atrogenes and augments the levels of mitochondria-related molecules in TWEAK-treated myotubes
The UPS is one of the major pathways that cause degradation of bulk of muscle proteins in various atrophying conditions (44). Among several markers of UPS, 2 E3 ubiquitin ligases, MAFbx/Atrogin-1 and MuRF1 (also known as atrogenes), have been found to be highly expressed in atrophying skeletal muscles (9, 10). We next sought to determine whether PGC-1α can block the TWEAK-induced expression of MAFbx and MuRF1. As shown in Fig. 7A, TWEAK increased the transcript levels of both MAFbx and MuRF1 in WT myotubes. However, TWEAK failed to increase the expression of these atrogenes in myotubes prepared from PGC-1α-Tg mice (Fig. 7A). We also investigated whether overexpression of PGC-1α rescues mitochondria content in TWEAK-treated myotubes. As shown in Fig. 7B, mRNA levels of several mitochondrial genes Cyt c, Tfam, ATP5b, and CoxIV were significantly higher in myotubes prepared from PGC-1α-Tg compared to those from WT mice on treatment with TWEAK. Taken together, these results demonstrate that PGC-1α inhibits the TWEAK-induced atrophy program in cultured myotubes.
Figure 7.
Overexpression of PGC-1α blunts the expression of atrogenes and augments the levels of mitochondria-related genes in TWEAK-treated myotubes. Primary myoblasts prepared from littermate WT and PGC-1α Tg mice were differentiated into myotubes, followed by treatment with indicated concentrations of soluble TWEAK protein for 24 h. Total RNA from myotubes was extracted and processed to measure mRNA levels of different genes by performing QRT-PCR. A) Relative mRNA levels of MAFbx and MuRF1 in control and TWEAK-treated myotubes of WT and PGC-1α Tg mice. B) Relative mRNA levels of Cyt c, Tfam, ATP5b, and Cox IV in control and TWEAK-treated myotubes of WT and PGC-1α Tg mice. Error bars = sd. *P < 0.05 vs. untreated myotubes of WT mice; #P < 0.05 vs. corresponding TWEAK-treated myotubes of WT mice.
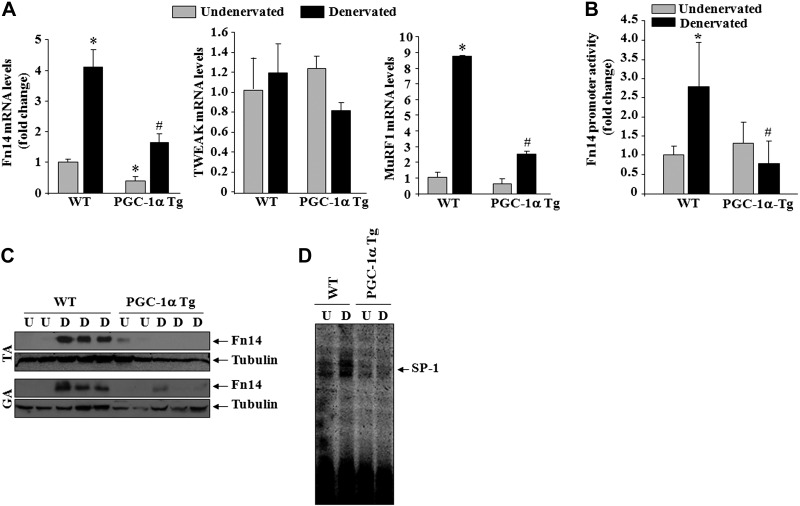
Overexpression of PGC-1α inhibits the expression of Fn14 in denervated muscle
The expression of Fn14 is increased in a number of atrophic conditions including denervation (41, 43). Previous studies have shown that PGC-1α can affect the expression of several genes by modulating the activity of various transcription factors (29, 30, 45). Since the expression of Fn14 is dramatically induced in denervated skeletal muscle, we investigated whether overexpression of PGC-1α can also affect the expression of Fn14 in skeletal muscle in response to denervation. WT and PGC-1α-Tg mice were subjected to denervation followed by measuring the mRNA levels of Fn14 in skeletal muscle. Interestingly, denervation-induced expression of Fn14 was significantly inhibited in skeletal muscle of PGC-1α-Tg mice compared to WT littermates (Fig. 8A). There was no significant difference in mRNA levels of TWEAK between undenervated or denervated skeletal muscle of WT and PGC-1α-Tg mice. Consistent with previously published report (36), mRNA levels of MuRF1 were also significantly reduced in PGC-1α-Tg mice compared to WT mice on denervation (Fig. 8A). Furthermore, by electroporating TA muscle with a ∼2-kb Fn14 promoter reporter construct, we validated that transactivation of Fn14 promoter is significantly inhibited in denervated skeletal muscle of PGC-1α-Tg mice compared to WT mice (Fig. 8B). To further validate that overexpression of PGC-1α inhibits the expression of Fn14 in denervated muscle, we also performed a Western blot test. As shown in Fig. 8C, there was almost no increase in the levels of Fn14 protein in TA and GA muscle of PGC-1α-Tg mice on denervation.
Figure 8.
Overexpression of PGC-1α inhibits the expression of Fn14 in denervated muscle of mice. A) Relative mRNA levels of Fn14, TWEAK, and MuRF1 in undenervated and denervated TA muscle of WT and PGC-1α-Tg mice, measured by performing QRT-PCR assay; n = 6/group. B) Fold change in Fn14 promoter reporter gene activity (normalized using Renilla luciferase) in TA muscle of WT and PGC-1α Tg mice after denervation; n = 3. #P < 0.05 vs. denervated muscle of WT mice. C) Representative immunoblots demonstrating levels of Fn14 protein in undenervated and denervated TA and GA muscle of littermate WT and PGC-1α Tg mice. D) Representative EMSA gel demonstrates that denervation-induced DNA-binding activity of SP-1 is inhibited in PGC-1α Tg mice compared to WT mice. U, undenervated; D, denervated. *P < 0.05 vs. undenervated muscle of WT mice; #P < 0.05 vs. denervated muscle of WT mice.
Previous studies have shown that human and mouse Fn14 promoters lack the typical TATA box sequence important for mammalian transcription initiation but instead contain a consensus SP1 transcription factor binding sequence close to the transcription start site (46). While the biological significance of TATA-less promoters remains debatable, it has been argued that SP1-mediated TATA-less mechanism causes preferential responsiveness to specific activators (47). By performing EMSA, we investigated whether the binding of SP1 to its consensus sequence in Fn14 promoter is increased in skeletal muscle on denervation and whether PGC-1α affects this binding. As shown in Fig. 8D, denervation increased DNA-binding activity of SP1 in WT mice. However, DNA-binding activity of SP1 did not show any increase in skeletal muscle of PGC-1α-Tg mice on denervation. These data suggest that PGC-1α inhibits the expression of Fn14 potentially through diminishing the binding of SP1 transcription factor to Fn14 promoter in denervated skeletal muscle.
DISCUSSION
Skeletal muscle wasting has become a major health burden with an increasingly sedentary lifestyle (3). Although significant progress has now been made toward understanding the molecular mechanisms of muscle wasting, there is still no approved drug for the treatment of muscle wasting. Our previous findings have established the role of TWEAK-Fn14 dyad in initiation of cascades of protein degradation in skeletal muscle in many conditions, including denervation (24–27). TWEAK stimulates the activation of NF-κB, which subsequently increases the expression of MuRF1, a major component of UPS, in denervated skeletal muscle (25, 26). By contrast, PGC-1α protects skeletal muscle fibers from undergoing atrophy in diverse conditions (36). As opposed to TWEAK, PGC-1α blunts the activation of proteolytic pathways that are known to cause the loss of skeletal muscle mass (36, 37). In the present study, we tested the hypothesis that a coordinated interaction between TWEAK-Fn14 and PGC-1α regulates skeletal muscle atrophy program.
A fall in the levels of PGC-1α precedes the activation of proteolytic pathways in many atrophic conditions leading to the premise that deregulation in PGC-1α is the molecular switch that triggers protein breakdown (36). Indeed, skeletal muscle overexpressing PGC-1α displayed resistance to atrophy stimuli through blunting FoxO3- and NF-κB-mediated up-regulation of MAFbx and MuRF1 (36, 37). By contrast, TWEAK-induced muscle atrophy takes place through activating these mechanisms. In denervated TWEAK-KO mice, skeletal muscle mass was rescued in a mechanism similar to that observed as a result of PGC-1α overexpression, suggesting that TWEAK-induced muscle atrophy may take place in a PGC-1α -regulated manner (26). Our results in this study have established this link by demonstrating that addition of TWEAK to cultured myotubes represses the mRNA and protein levels of PGC-1α and reduces mitochondrial content (Figs. 1 and 2), whereas overexpression of PGC-1α rescues muscle atrophy and mitochondria content (Figs. 5 and 7). These findings are consistent with our recent report that skeletal muscle of TWEAK-KO mice show enhanced mitochondrial content and increased oxidative phosphorylation (28). While TWEAK reduces the levels of PGC-1α in cultured myotubes, our results demonstrate that TWEAK-Fn14 signaling is not the stimulus for initial repression of PGC-1α in denervated skeletal muscle, evident by comparable levels of PGC-1α transcripts in skeletal muscle of WT, TWEAK-KO, and Fn14-KO mice 2 d postdenervation (Fig. 3B). By contrast, 10 d postdenervation, the levels of PGC-1α were significantly higher in skeletal muscle of TWEAK-KO and Fn14-KO mice (Fig. 3C). These findings suggest that initial drop in PGC-1α levels occurs independent of TWEAK-Fn14 signaling. However, the activation of TWEAK-Fn14 signaling, consequent of increased expression of Fn14, maintains PGC-1α levels in a repressed state in denervated skeletal muscle.
The physiological significance of PGC-1α in TWEAK-induced muscle atrophy in vivo is evident by the findings that progressive muscle atrophy in TWEAK-Tg mice is significantly rescued through the overexpression of PGC-1α (Fig. 4). On similar lines, overexpression of PGC-1α inhibits rapid muscle atrophy that occurs in TWEAK-Tg mice on denervation (Fig. 3D). Our previous study has also shown that denervation-induced muscle atrophy is inhibited in TWEAK-KO mice. Moreover, we have recently reported that transcript levels of PGC-1α are significantly increased in 5- to 6-mo-old TWEAK-KO mice and at this age, TWEAK-KO mice show improved fiber size, exercise tolerance, and oxidative phosphorylation capacity in skeletal muscle (28). While TWEAK receptor Fn14 is expressed in skeletal muscle of neonatal and young mice, its expression is dramatically reduced in adults, which provides some explanation to why a small increase (5- to 6-fold) in expression of TWEAK in skeletal muscle does not cause any noticeable muscle atrophy in mice. However, many catabolic conditions, such as denervation, immobilization, and starvation, dramatically increase the levels of Fn14 in skeletal muscle, leading to a more rapid onset and severe muscle atrophy in TWEAK-Tg mice. We have also evidence that the expression of Fn14 in skeletal muscle increases with age, which may account for late onset of muscle atrophy in TWEAK-Tg mice (unpublished observation). Whether the inhibition of TWEAK-Fn14 signaling attenuates age-associated muscle atrophy is an area of future research. Collectively, these results suggest that the activity of TWEAK-Fn14 system in skeletal muscle is increased in various atrophy conditions leading to repression of PGC-1α and eventually loss of skeletal muscle mass.
The mechanisms by which TWEAK reduces levels of PGC-1α in skeletal muscle remain less understood. However, a recent study has demonstrated that TWEAK increases membrane translocation of adaptor protein TNF receptor-associated factor 2 (TRAF2), in an Fn14-dependent manner, in cardiomyocytes (48). Knockdown of TRAF2 using shRNA or blocking activation of IκB kinase β (IKKβ), an upstream activator of canonical NF-κB signaling (1), prevents the TWEAK-mediated repression of PGC-1α in cardiomyocytes, highlighting a pivotal role of Fn14-TRAF2-IKKβ-NF-κB signaling cascade in this process (48). Our results also demonstrate that one of the mechanisms by which TWEAK represses PGC-1α levels in skeletal muscle cells is through the activation of NF-κB signaling pathway (Fig. 1D). We speculate that TWEAK-induced NF-κB is a key mechanism for maintaining PGC-1α in a repressed state in denervated skeletal muscle. While the fall in PGC-1α levels occurs within 2 d of denervation, activation of NF-κB is observed 5–6 d postdenervation in skeletal muscle. Notably, the activation of NF-κB is significantly reduced in denervated muscle of TWEAK-KO and increased in TWEAK-Tg mice (26). Therefore, the increased levels of PGC-1α observed in 10 d denervated muscle of TWEAK-KO and Fn14-KO mice (Fig. 3C) could be a result of diminished activation of NF-κB.
It is notable that the activation of NF-κB is a critical event in skeletal muscle wasting in response to diverse stimuli (1). Constitutive activation of NF-κB causes severe muscle wasting in mice, whereas its inhibition blocks muscle wasting in many conditions, including denervation, cancer cachexia, and in response to proinflammatory cytokines, including TWEAK (25, 49–51). While TWEAK inhibits the expression of PGC-1α through the activation of NF-κB, our results also demonstrate that overexpression of PGC-1α attenuates the TWEAK-induced activation of NF-κB in cultured myotubes (Fig. 6). These findings are in agreement with recently published reports also demonstrating that the forced expression of PGC-1α inhibits the activation of NF-κB in skeletal muscle cells in response to TNF-α and other wasting conditions (37, 52). Moreover, it has been found that PGC-1α inhibits TNF-α-induced activation of NF-κB through directly repressing the phosphorylation of p65 subunit of NF-κB (52). Consistently, we have also found that PGC-1α inhibits the TWEAK-induced phosphorylation of p65 in cultured myotubes (Fig. 6B). However, we also observed reduced phosphorylation of IκBα in PGC-1α-overexpressing myotubes in response to TWEAK treatment (Fig. 6B), suggesting that PGC-1α might be inhibiting NF-κB activation through other mechanisms as well. Indeed, in addition to canonical pathway, we found that overexpression of PGC-1α attenuates the activation of noncanonical arm of NF-κB signaling (Fig. 6B), further suggesting that PGC-1α acts upstream of IKKs in NF-κB pathway.
While the reciprocal feedback mechanism between NF-κB and PGC-α is clearly evident, and our study demonstrates that TWEAK-Fn14 signaling plays a regulatory role in this process, it remains enigmatic how such interaction between PGC-1α and TWEAK regulates skeletal muscle mass in vivo in atrophic conditions. It is possible that the high levels of PGC-1α prevent the activation of NF-κB in response to catabolic stimuli. However, it is also likely that continuous presence of catabolic stimuli eventually overrides the inhibitory effect of PGC-1α on NF-κB activation, leading to repression of PGC-1α and muscle atrophy. Strategies that increase the expression or stability of PGC-1α can be a potential approach to preserve skeletal muscle in atrophic conditions. Alternatively, the inhibition of NF-κB signaling can be a potential approach to maintain PGC-1α levels and skeletal muscle mass.
Another interesting observation of the present study is that PGC-1α represses the expression of Fn14 in denervated skeletal muscle. Fn14 is expressed at very low levels in unchallenged skeletal muscle; however, the expression of Fn14 is drastically increased on denervation (Fig. 8 and ref, 26). Our findings indicate that PGC-1α keeps Fn14 in repressed state in normal muscle. A fall in PGC-1α levels on denervation causes derepression of Fn14 promoter, leading to the activation of TWEAK-Fn14 pathway, which subsequently causes muscle proteolysis through the activation of NF-κB and augmenting the expression of the components of UPS. The mechanisms by which PGC-1α inhibit Fn14 expression in skeletal muscle remain enigmatic. Being a cofactor, binding of PGC-1α to specific transcription factors generally leads to transcriptional activation of genes. However, in the settings of muscle atrophy, PGC-1α has been found to physically interact and block the activity of FoxO3 transcription factor to suppress the expression of MAFbx and MuRF1 (36). Our bioinformatics analysis showed that Fn14 promoter does not contain consensus binding sites for FoxO family transcription factors (data not shown). However, Fn14 is a TATA-less promoter, and it contains a conserved SP1 binding site close to the transcription initiation site (46). Our results showed that denervation causes increased binding of SP1 to consensus SP1 site in Fn14 promoter, and this binding was abolished in denervated muscle of PGC-1α Tg mice, implying that PGC-1α interferes with the activation or binding of SP1 to Fn14 promoter in denervated muscle (Fig. 8D). Moreover, Fn14 promoter also contains consensus sequences for many other transcription factors, which may also be involved in the up-regulation of Fn14 expression in denervated muscle (46, 53).
Our recent analysis has also revealed that Fn14 promoter contains a cytosine-guanine dinucleotide (CpG) island close to transcription start site suggesting that DNA methylation may also regulate the expression of Fn14 in skeletal muscle. Indeed, we have found that DNA methyltransferases (Dnmts) interact with CpG island in Fn14 promoter, and many of the CpG sites are highly methylated in naive skeletal muscle of mice. By contrast, denervation causes the repression of Dnmt3 and hypomethylation of CpG sites in Fn14 promoter in skeletal muscle (unpublished results). Interestingly, the CpG sites in Fn14 promoter are constitutively hypomethylated in cultured myoblasts, indicating that the epigenetic regulation of Fn14 promoter by Dnmts is active only in differentiated skeletal muscle of adult mice but not in muscle progenitor cells, and therefore myoblasts express high levels of Fn14. Since PGC-1α is involved in many epigenetic mechanisms, it is possible that overexpression of PGC-1α prevents the expression of Fn14 in denervated muscle by maintaining Fn14 promoter in hypermethylated state through indirect mechanisms. When Fn14 promoter is hypomethylated, as has been the case in cultured myoblasts, the overexpression of PGC-1α does not affect the expression of Fn14. This might explain why Fn14 levels are comparable in primary myotubes of WT and PGC-1α-Tg mice that are formed on differentiation of myoblasts (Fig. 5E) It is also notable that MCK promoter used to generate PGC-1α-Tg mice is active only after myoblasts differentiate into myotubes. Future studies, focusing on characterization of Fn14 promoter in atrophying muscle, will identify additional mechanisms by which PGC-1α inhibits the expression of Fn14 in denervated skeletal muscle.
In summary, the present study provides genetic evidence that interaction between TWEAK-Fn14 signaling and PGC-1α regulates skeletal muscle atrophy program. TWEAK-Fn14 signaling represses the expression of PGC-1α in skeletal muscle. Conversely, PGC-1α prevents the expression of Fn14 in denervated skeletal muscle. Thus, a regulatory circuitry of PGC-1α and TWEAK-Fn14 system dictates skeletal muscle mass in catabolic conditions, and blocking TWEAK-Fn14 signaling appears to be an important therapeutic approach to maintaining PGC-1α levels and preserving skeletal muscle mass in atrophic conditions.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Avi Ashkenazi (Genentech, South San Francisco, CA, USA) for providing TWEAK-KO mice.
This work was supported by U.S. National Institutes of Health grants R01AR059810 and RO1AG029623 to A.K.
The authors declare no conflicts of interest. L.C.B. and T.S.Z are currently employees and stockholders in Biogen Idec. However, this does not alter the authors' adherence to all of the FASEB Journal policies on sharing data and materials.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ATP5b
- ATP synthase subunit β
- Cox I/IV
- cytochrome c oxidase subunit I/IV
- CpG
- cytosine-guanine dinucleotide
- CSA
- cross-sectional area
- Cyt c
- cytochrome c
- DM
- differentiation medium
- Dnmt
- DNA methyltransferase
- EDL
- extensor digitorum longus
- EMSA
- electrophoretic mobility shift assay
- H&E
- hematoxylin and eosin
- IKKβ
- IκB kinase β
- KO
- knockout
- MCAD
- medium-chain acyl-coenzyme A dehydrogenase
- MCK
- muscle creatine kinase
- PGC-1α
- PPAR γ coactivator 1α
- PPAR
- peroxisome proliferator-activated receptor
- QRT-PCR
- quantitative real-time PCR
- SP1
- specificity protein 1
- TA
- tibial anterior
- Tfam
- transcription factor A mitochondrial
- Tg
- transgenic
- TNF
- tumor necrosis factor
- TRAF2
- TNF receptor-associated factor 2
- TWEAK
- TNF-like weak inducer of apoptosis
- UPS
- ubiquitin-proteasome system
- WT
- wild-type
REFERENCES
- 1. Li H., Malhotra S., Kumar A. (2008) Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. 86, 1113–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonaldo P., Sandri M. (2013) Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 6, 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackman R. W., Kandarian S. C. (2004) The molecular basis of skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 287, C834–C843 [DOI] [PubMed] [Google Scholar]
- 4. Kandarian S. C., Stevenson E. J. (2002) Molecular events in skeletal muscle during disuse atrophy. Exerc. Sport Sci. Rev. 30, 111–116 [DOI] [PubMed] [Google Scholar]
- 5. Spate U., Schulze P. C. (2004) Proinflammatory cytokines and skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 7, 265–269 [DOI] [PubMed] [Google Scholar]
- 6. Lecker S. H., Goldberg A. L., Mitch W. E. (2006) Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 17, 1807–1819 [DOI] [PubMed] [Google Scholar]
- 7. Clarke B. A., Drujan D., Willis M. S., Murphy L. O., Corpina R. A., Burova E., Rakhilin S. V., Stitt T. N., Patterson C., Latres E., Glass D. J. (2007) The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 6, 376–385 [DOI] [PubMed] [Google Scholar]
- 8. Cohen S., Brault J. J., Gygi S. P., Glass D. J., Valenzuela D. M., Gartner C., Latres E., Goldberg A. L. (2009) During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J. Cell Biol. 185, 1083–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bodine S. C., Latres E., Baumhueter S., Lai V. K., Nunez L., Clarke B. A., Poueymirou W. T., Panaro F. J., Na E., Dharmarajan K., Pan Z. Q., Valenzuela D. M., DeChiara T. M., Stitt T. N., Yancopoulos G. D., Glass D. J. (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708 [DOI] [PubMed] [Google Scholar]
- 10. Gomes M. D., Lecker S. H., Jagoe R. T., Navon A., Goldberg A. L. (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. U. S. A. 98, 14440–14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Romanello V., Guadagnin E., Gomes L., Roder I., Sandri C., Petersen Y., Milan G., Masiero E., Del Piccolo P., Foretz M., Scorrano L., Rudolf R., Sandri M. (2010) Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 29, 1774–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lokireddy S., Wijesoma I. W., Teng S., Bonala S., Gluckman P. D., McFarlane C., Sharma M., Kambadur R. (2012) The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metab. 16, 613–624 [DOI] [PubMed] [Google Scholar]
- 13. Coggan A. R., Spina R. J., King D. S., Rogers M. A., Brown M., Nemeth P. M., Holloszy J. O. (1992) Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J. Gerontol. 47, B71–B76 [DOI] [PubMed] [Google Scholar]
- 14. Hagen J. L., Krause D. J., Baker D. J., Fu M. H., Tarnopolsky M. A., Hepple R. T. (2004) Skeletal muscle aging in F344BN F1-hybrid rats: I. Mitochondrial dysfunction contributes to the age-associated reduction in VO2max. J. Gerontol. A Biol. Sci. Med. Sci. 59, 1099–1110 [DOI] [PubMed] [Google Scholar]
- 15. Pastoris O., Boschi F., Verri M., Baiardi P., Felzani G., Vecchiet J., Dossena M., Catapano M. (2000) The effects of aging on enzyme activities and metabolite concentrations in skeletal muscle from sedentary male and female subjects. Exp. Gerontol. 35, 95–104 [DOI] [PubMed] [Google Scholar]
- 16. Kerner J., Turkaly P. J., Minkler P. E., Hoppel C. L. (2001) Aging skeletal muscle mitochondria in the rat: decreased uncoupling protein-3 content. Am. J. Physiol. Endocrinol. Metab. 281, E1054–E1062 [DOI] [PubMed] [Google Scholar]
- 17. Aiken J., Bua E., Cao Z., Lopez M., Wanagat J., McKenzie D., McKiernan S. (2002) Mitochondrial DNA deletion mutations and sarcopenia. Ann. N. Y. Acad. Sci. 959, 412–423 [DOI] [PubMed] [Google Scholar]
- 18. McKenzie D., Bua E., McKiernan S., Cao Z., Aiken J. M. (2002) Mitochondrial DNA deletion mutations: a causal role in sarcopenia. Eur. J. Biochem. 269, 2010–2015 [DOI] [PubMed] [Google Scholar]
- 19. Wanagat J., Cao Z., Pathare P., Aiken J. M. (2001) Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 15, 322–332 [DOI] [PubMed] [Google Scholar]
- 20. Peterson C. M., Johannsen D. L., Ravussin E. (2012) Skeletal muscle mitochondria and aging: a review. J. Age Res. 2012, 194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bua E., Johnson J., Herbst A., Delong B., McKenzie D., Salamat S., Aiken J. M. (2006) Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am. J. Hum. Genet. 79, 469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rowan S. L., Purves-Smith F. M., Solbak N. M., Hepple R. T. (2011) Accumulation of severely atrophic myofibers marks the acceleration of sarcopenia in slow and fast twitch muscles. Exp. Gerontol. 46, 660–669 [DOI] [PubMed] [Google Scholar]
- 23. Winkles J. A. (2008) The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat. Rev. Drug Discov. 7, 411–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhatnagar S., Kumar A. (2012) The TWEAK-Fn14 system: breaking the silence of cytokine-induced skeletal muscle wasting. Curr. Mol. Med. 12, 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dogra C., Changotra H., Wedhas N., Qin X., Wergedal J. E., Kumar A. (2007) TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 21, 1857–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mittal A., Bhatnagar S., Kumar A., Lach-Trifilieff E., Wauters S., Li H., Makonchuk D. Y., Glass D. J., Kumar A. (2010) The TWEAK–Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J. Cell Biol. 188, 833–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhatnagar S., Mittal A., Gupta S. K., Kumar A. (2012) TWEAK causes myotube atrophy through coordinated activation of ubiquitin-proteasome system, autophagy, and caspases. J. Cell. Physiol. 227, 1042–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sato S., Ogura Y., Mishra V., Shin J., Bhatnagar S., Hill B. G., Kumar A. (2013) TWEAK promotes exercise intolerance by decreasing skeletal muscle oxidative phosphorylation capacity. Skelet. Muscle 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arany Z. (2008) PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr. Opin. Genet. Dev. 18, 426–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Finck B. N., Kelly D. P. (2006) PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 116, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uldry M., Yang W., St-Pierre J., Lin J., Seale P., Spiegelman B. M. (2006) Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 3, 333–341 [DOI] [PubMed] [Google Scholar]
- 32. Arany Z., Lebrasseur N., Morris C., Smith E., Yang W., Ma Y., Chin S., Spiegelman B. M. (2007) The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 5, 35–46 [DOI] [PubMed] [Google Scholar]
- 33. Calvo J. A., Daniels T. G., Wang X., Paul A., Lin J., Spiegelman B. M., Stevenson S. C., Rangwala S. M. (2008) Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J. Appl. Physiol. 104, 1304–1312 [DOI] [PubMed] [Google Scholar]
- 34. Lin J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N., Lowell B. B., Bassel-Duby R., Spiegelman B. M. (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 [DOI] [PubMed] [Google Scholar]
- 35. Wende A. R., Schaeffer P. J., Parker G. J., Zechner C., Han D. H., Chen M. M., Hancock C. R., Lehman J. J., Huss J. M., McClain D. A., Holloszy J. O., Kelly D. P. (2007) A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J. Biol. Chem. 282, 36642–36651 [DOI] [PubMed] [Google Scholar]
- 36. Sandri M., Lin J., Handschin C., Yang W., Arany Z. P., Lecker S. H., Goldberg A. L., Spiegelman B. M. (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. U. S. A. 103, 16260–16265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brault J. J., Jespersen J. G., Goldberg A. L. (2011) Peroxisome proliferator-activated receptor gamma coactivator 1alpha or 1beta overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J. Biol. Chem. 285, 19460–19471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wenz T., Rossi S. G., Rotundo R. L., Spiegelman B. M., Moraes C. T. (2009) Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. U. S. A. 106, 20405–20410 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Maecker H., Varfolomeev E., Kischkel F., Lawrence D., LeBlanc H., Lee W., Hurst S., Danilenko D., Li J., Filvaroff E., Yang B., Daniel D., Ashkenazi A. (2005) TWEAK attenuates the transition from innate to adaptive immunity. Cell 123, 931–944 [DOI] [PubMed] [Google Scholar]
- 40. Girgenrath M., Weng S., Kostek C. A., Browning B., Wang M., Brown S. A., Winkles J. A., Michaelson J. S., Allaire N., Schneider P., Scott M. L., Hsu Y. M., Yagita H., Flavell R. A., Miller J. B., Burkly L. C., Zheng T. S. (2006) TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J. 25, 5826–5839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paul P. K., Gupta S. K., Bhatnagar S., Panguluri S. K., Darnay B. G., Choi Y., Kumar A. (2010) Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J. Cell Biol. 191, 1395–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hindi S. M., Paul P. K., Dahiya S., Mishra V., Bhatnagar S., Kuang S., Choi Y., Kumar A. (2012) Reciprocal interaction between TRAF6 and notch signaling regulates adult myofiber regeneration upon injury. Mol. Cell. Biol. 32, 4833–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paul P. K., Bhatnagar S., Mishra V., Srivastava S., Darnay B. G., Choi Y., Kumar A. (2012) The E3 ubiquitin ligase TRAF6 intercedes in starvation-induced skeletal muscle atrophy through multiple mechanisms. Mol. Cell. Biol. 32, 1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Glass D. J. (2010) Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 13, 225–229 [DOI] [PubMed] [Google Scholar]
- 45. Lin J., Handschin C., Spiegelman B. M. (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1, 361–370 [DOI] [PubMed] [Google Scholar]
- 46. Zheng T. S., Burkly L. C. (2008) No end in site: TWEAK/Fn14 activation- and autoimmunity-associated end-organ pathologies. J. Leukoc. Biol. 84, 338–347 [DOI] [PubMed] [Google Scholar]
- 47. Smale S. T. (1997) Transcription initiation from TATA-less promoters within eukaryotic protein-coding genes. Biochim. Biophys. Acta 1351, 73–88 [DOI] [PubMed] [Google Scholar]
- 48. Shi J., Jiang B., Qiu Y., Guan J., Jain M., Cao X., Bauer M., Su L., Burkly L. C., Leone T. C., Kelly D. P., Liao R. (2013) PGC1alpha plays a critical role in TWEAK-induced cardiac dysfunction. PloS ONE 8, e54054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cai D., Frantz J. D., Tawa N. E., Jr., Melendez P. A., Oh B. C., Lidov H. G., Hasselgren P. O., Frontera W. R., Lee J., Glass D. J., Shoelson S. E. (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119, 285–298 [DOI] [PubMed] [Google Scholar]
- 50. Mourkioti F., Kratsios P., Luedde T., Song Y. H., Delafontaine P., Adami R., Parente V., Bottinelli R., Pasparakis M., Rosenthal N. (2006) Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Invest. 116, 2945–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Y. P., Schwartz R. J., Waddell I. D., Holloway B. R., Reid M. B. (1998) Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 12, 871–880 [DOI] [PubMed] [Google Scholar]
- 52. Eisele P. S., Salatino S., Sobek J., Hottiger M. O., Handschin C. (2013) The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (PGC-1) coactivators repress the transcriptional activity of NF-kappaB in skeletal muscle cells. J. Biol. Chem. 288, 2246–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu C. L., Kandarian S. C., Jackman R. W. (2011) Identification of genes that elicit disuse muscle atrophy via the transcription factors p50 and Bcl-3. PloS ONE 6, e16171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.