Abstract
Siglecs are sialic acid-binding Ig-like lectins that recognize sialoglycans via amino-terminal V-set domains. CD33-related Siglecs (CD33rSiglecs) on innate immune cells recognize endogenous sialoglycans as “self-associated molecular patterns” (SAMPs), dampening immune responses via cytosolic immunoreceptor tyrosine-based inhibition motifs that recruit tyrosine phosphatases. However, sialic acid-expressing pathogens subvert this mechanism through molecular mimicry. Meanwhile, endogenous host SAMPs must continually evolve to evade other pathogens that exploit sialic acids as invasion targets. We hypothesized that these opposing selection forces have accelerated CD33rSiglec evolution. We address this by comparative analysis of major CD33rSiglec (Siglec-3, Siglec-5, and Siglec-9) orthologs in humans, chimpanzees, and baboons. Recombinant soluble molecules displaying ligand-binding domains show marked quantitative and qualitative interspecies differences in interactions with strains of the sialylated pathogen, group B Streptococcus, and with sialoglycans presented as gangliosides or in the form of sialoglycan microarrays, including variations such as N-glycolyl and O-acetyl groups. Primate Siglecs also show quantitative and qualitative intra- and interspecies variations in expression patterns on leukocytes, both in circulation and in tissues. Taken together our data explain why the CD33rSiglec-encoding gene cluster is undergoing rapid evolution via multiple mechanisms, driven by the need to maintain self-recognition by innate immune cells, while escaping 2 distinct mechanisms of pathogen subversion.—Padler-Karavani, V., Hurtado-Ziola, N., Chang, Y.-C., Sonnenburg, J. L., Ronaghy, A., Yu, H., Verhagen, A., Nizet, V., Chen, X., Varki, N., Varki, A., Angata, T. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates.
Keywords: sialic acids, N-acetylneuraminic acid, N-glycolylneuraminic acid, innate immunity
Sialic acids (Sias) are a diverse family of 9-carbon backbone acidic sugars that cap many glycan chains on vertebrate cell surfaces (1, 2). Siglecs are a family of Sia-binding Ig-like lectins that can mediate cis or trans interactions with endogenous Sia-containing ligands (3). These type 1 transmembrane proteins are composed of an extracellular N-terminal Ig-like V-set domain followed by 1−16 Ig-like C2-set domains, a transmembrane region, and a cytosolic tail. The V-set domain contains a canonical arginine residue important for Sia binding (3, 4). These “I-type” lectins (5, 6) can be divided into 2 main groups: the evolutionary conserved Siglecs (Siglec-1, -2, -4, and -15); and the rapidly evolving group of CD33-related Siglecs (CD33rSiglecs) comprising Siglec-3 (CD33), Siglec-5 through Siglec-14 and Siglec-16 to Siglec-17 in primates (3, 7–9).
CD33rSiglecs are expressed on leukocytes and modulate cellular signaling via regulatory motifs in their cytoplasmic domains. Most CD33rSiglecs have a cytoplasmic immunoreceptor tyrosine-based inhibitory motif [ITIM; in Siglec-5 through Siglec-12 in primates (Table 1 and refs. 3, 7)], which on phosphorylation leads to recruitment of tyrosine phosphatases (e.g., SHP-1 and SHP-2; refs. 10–12), which dephosphorylate downstream kinases and dampen immune responses (13, 14). Some primate CD33rSiglecs lack ITIMs (Siglec-13, -14, -16, and -17) but instead have a positively charged amino acid in their transmembrane domain that can engage adapter molecules (e.g., DAP12) with an immunoreceptor tyrosine-based activating motif, which in turn recruit tyrosine kinase (e.g., Syk), thereby leading to either cellular activation or inhibition (8, 9, 15, 16). Given their expression patterns and functional characteristics, CD33rSiglecs are suggested to play a role in recognizing endogenous sialoglycans as a signature of “self,” thus, dampening inappropriate immune responses (17–19) against host cells. This behavior has raised the concept of CD33rSiglec ligands as “self-associated molecular patterns” (SAMPs; ref. 17), in contrast to pathogen- or danger-associated molecular patterns (20–22).
Table 1.
ITIM-containing CD33rSiglec orthologs in primate species
| Inhibitory CD33-related Siglecs in primates | ||
|---|---|---|
| Human | Chimpanzee | Baboon |
| Siglec-3 | Siglec-3 | Siglec-3 |
| Siglec-5 | Siglec-Va | Siglec-5 |
| Siglec-6 | Siglec-6 | Siglec-VIa |
| Siglec-7 | Siglec-7 | NF |
| Siglec-8 | Siglec-8 | Siglec-8 |
| Siglec-9 | Siglec-9 | Siglec-9 |
| Siglec-10 | Siglec-10 | Siglec-10 |
| Siglec-11b | Siglec-11 | Siglec-11 |
| Siglec-XIIa | Siglec-12 | NFb |
NF, not found in the genome.
Siglecs missing the arginine residue involved in Sia recognition.
Species-specific gene conversion by SIGLEC16P.
Several pathogenic microorganisms, such as group B Streptococcus, Escherichia coli K1, Neisseria meningitidis, Neisseria gonorrhoeae, Haemophilus influenzae, and Trypanosoma cruzi, have evolved convergent mechanisms to express surface sialoglycans remarkably similar to those of vertebrate hosts. This “molecular mimicry” of the host probably provides a strong selective advantage to the pathogen, because there are several different independently evolved pathways to this type of mimicry (23–25). A sialylated “cloak” could allow pathogens to masquerade as self, thus, evading host immune clearance responses that respond normally to nonsialylated microbes. Indeed, binding of sialylated bacteria to CD33rSiglecs has been demonstrated to dampen immune responses via ITIM signaling (14, 26). Thus, there is strong evolutionary pressure for the Sia-binding properties of CD33rSiglecs to evolve away from such pathogen mimicry. However, this means of escape cannot occur at the cost of losing endogenous host SAMP recognition.
At the same time, another strong evolutionary force shaping the host sialome is the fact that many major pathogens such as influenza viruses and malarial parasites recognize and bind to specific host Sia motifs as part of their invasion mechanisms (27). Thus, the host sialome must also continually evolve to minimize such recognition. This process would, in turn, require host CD33rSiglecs to “keep up” with these changes or risk losing their SAMP recognition ability. As an example of species-specific sialome changes, a major difference between humans and other primates is the loss of the CMAH gene, which eliminated ability of humans to produce the Sia N-glycolylneuraminic acid (Neu5Gc), leaving an excess of its precursor N-acetylneuraminic acid (Neu5Ac; ref. 28). This loss of Neu5Gc expression in humans may have been selected to avoid the Neu5Gc-preferring Plasmodium reichenowi (29–31) and eventually contributed to the emergence of a human-specific malaria pathogen, Plasmodium falciparum (refs. 29, 30), which preferentially binds Neu5Ac. Loss of Neu5Gc was even suggested to have driven speciation of the genus Homo as a result of anti-Neu5Gc antibody reactions against Neu5Gc-positive sperm in the female genital tract (32).
Multispecies comparisons of the CD33rSiglec gene cluster showed extensive differences between humans, chimpanzees, baboons, and murine species, involving rapid evolution through multiple mechanisms that range from expansions of gene subsets, gene deletions, pseudogenization, gene conversion events, and exon shuffling to higher rates of nonsynonymous substitutions (amino acid changes) in the V-set domain (7, 15, 33, 34). This is in contrast to the adjacent and very conserved kallikrein-like genes (33). There has also been some evidence of convergent evolution in the binding specificities of nonorthologous Siglecs, such as Siglec-8 in humans with Siglec-F in mice (35, 36). Taken together, such data have been used to suggest that multiple evolutionary “Red Queen” effects have been driving an extraordinarily rapid evolution of this gene family (7). However, despite abundant circumstantial evidence and plausible evolutionary reasoning, there is very limited experimental evidence for the rapid evolution of CD33rSiglecs at a functional level. Here we use recombinant soluble versions of 3 different CD33rSiglecs from 3 related primate species in a comprehensive analysis of binding affinities, including recognition of pathogenic bacteria, in vitro binding to unique sialylated glycoconjugates, and state-of-the-art glycan microarrays. In addition, we explore the native expression of these 3 CD33rSiglecs on leukocytes from the 3 hominid species, in circulation and in tissues. Taken together, our data strongly support the suggestion that multiple forces have shaped the rapid evolution of the gene cluster encoding CD33rSiglecs, driven by the need to maintain immune cell recognition of self while simultaneously escaping pathogen subversion of this mechanism.
MATERIALS AND METHODS
Human blood samples
Normal human blood samples were obtained from healthy adult male and female blood donors of various ethnic backgrounds who were students and personnel at the University of California at San Diego School of Medicine (La Jolla, CA, USA), with approval from the institutional review board. Written informed consent was obtained in advance from the volunteers, and the samples were deidentified.
Nonhuman primate blood samples
Blood samples were obtained from 20 humans, 20 chimpanzees, and 4 gorillas for use in flow cytometry experiments. Chimpanzee (Pan troglodytes) blood samples were drawn at Yerkes Primate Research Center (Atlanta, GA, USA) or the Primate Foundation of Arizona (Mesa, AZ, USA). Gorilla (Gorilla gorilla) samples were from the San Diego Zoo (San Diego, CA, USA), the Lincoln Park Zoo (Chicago, IL, USA), or Zoo Atlanta (Atlanta, GA, USA). All samples were obtained under local institutional animal care and use committee approvals and were collected only as additional tubes during routine blood draws for health checks. All these collections preceded the recent Institute of Medicine recommendations on the use of chimpanzee in research (37) and the U.S. National Institutes of Health Council of Councils' Announcement of Agency Decision: Recommendations on the Use of Chimpanzees in NIH-Supported Research (final report on June 26, 2013) but are still consistent with these recommendations.
Antibodies
Purified human IgG and Cy3-conjugated goat anti-human IgG (H+L) were from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Anti-CD33/Siglec-3 (clone HIM3-4) was purchased from BD Pharmingen (San Diego, CA, USA), and anti-Siglec-9 (E10-286) was made by BD Pharmingen and characterized in our laboratory via a contractual agreement with University of California at San Diego (La Jolla, CA, USA). Mouse anti-human Siglec-5 (clone 1A5) was a kind gift from Paul R. Crocker (University of Dundee, Dundee, UK). The negative control antibody, an IgG isotype, X63 (hybridoma P3-X63-Ag8; American Type Culture Collection, Manassas, VA, USA), was produced in our laboratory. Goat anti-mouse IgGs (H+L) conjugated to fluorescein isothiocyanate (FITC), phycoerythrin, or alkaline phosphatase were purchased from Jackson ImmunoResearch Laboratories. We used the following antibodies for immunohistochemical analysis: mouse anti-human CD68 clone PG-M1 (Dako North America Inc., Carpenteria, CA, USA), mouse anti-Siglec-5/14 clone 194128, mouse anti-Siglec-9 clone 191240 (R&D, Minneapolis, MN, USA), mouse anti-CD3, mouse anti-CD19, rabbit antineutrophil elastase, rabbit anti-CD19, rabbit anti-CD68 (Abcam, Cambridge, MA, USA), mouse anti-neutrophil elastase (GeneTex, Irvine, CA, USA), rabbit anti-Siglec-3 (CD33; LifeSpan Biosciences, Seattle, WA, USA), biotinylated goat anti-rabbit IgG, biotinylated donkey anti-mouse IgG, Cy3-conjugated donkey anti-mouse IgG, Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories), and streptavidin Alexa Fluor 488 (Invitrogen, Eugene, OR, USA).
Gangliosides
Monosialoganglioside GM3 (GM3) derivatives were obtained as follows. Neu5Ac GM3 was purchased from Matreya, Inc. (1503; Pleasant Gap, PA, USA). Gangliosides 4-O-acetyl-Neu5Gc GM3 and Neu5Gc GM3 were isolated from horse erythrocytes using a process reported previously (38). De-N-acetyl (DeNAc) GM3 was produced by treating Neu5Ac GM3 with tetramethylammonium hydroxide and heat as described (39). 2-Keto-3-deoxy-d-glycero-d-galacto-nononic acid (Kdn) GM3, isolated from rainbow trout as described previously (40), was a kind gift from the late Yasuo Inoue.
Anti-human CD33rSiglec antibody cross-reactivity with chimpanzee and baboon CD33rSiglecs
An enzyme-linked immunosorbent assay (ELISA) was used to determine the cross-reactivity of mouse anti-human CD33rSiglec antibodies with recombinant chimpanzee and baboon CD33rSiglec proteins. Incubations were done at 4°C, and each sample was analyzed in triplicate. In brief, the CD33rSiglec-Fc chimeric protein (2 μg/ml, 100 μl/well) was immobilized in a 96-well plate overnight in 50 mM carbonate bicarbonate buffer (18.3 mM Na2CO3 and 84 mM NaHCO2, pH 9.5). The wells were washed 3 times and blocked with ELISA buffer (20 mM HEPES, 125 mM NaCl, and 0.5% BSA) for 1 h. The appropriate mouse anti-human CD33rSiglec antibody was added to each well, incubated for a minimum of 3 h to overnight, and washed. Goat anti-mouse IgG conjugated to alkaline phosphatase (1:2000−1:5000 dilution) was added for 1 h, and the wells were washed once more. Each well was incubated with 10 mM p-nitrophenyl phosphate at room temperature, allowed to develop, and read at 405 nm on a plate reader.
Construction of recombinant Siglec-Fc chimeras
Siglec-Fc chimeras were constructed as described previously (41) with some modifications (see Results). Genomic DNA segments containing the first 4 exons encoding the signal peptide and first 2 Ig-like domains (2D) were amplified from appropriate BAC clones (or 3 exons in the case of Siglec-9 that lacks an intron between the signal peptide and the first Ig-like domain) and subcloned into EcoRV-XbaI (or NheI in the cases when XbaI is present in the amplified segments) sites of EK-Fc/pcDNA. The BAC clones used as templates were the same as those used for sequencing of the primate Siglec gene cluster (33), allowing us to eliminate PCR-generated artificial mutant clones or fusion products between irrelevant segments. See Tables 2 and 3 for the template and primers used. The PCR-amplified fragments were cloned into the expression vector EK-Fc/pcDNA (41) to yield a chimeric protein of the first 2 extracellular domains of the Siglecs with a human IgG Fc tail and a FLAG epitope tag/enterokinase cleavage site (DYKDDDDK) in between.
Table 2.
Template and primers used for Siglec cloning
| Siglec | Template (BAC) | Forward primer | Reverse primer | Restriction digestion |
|---|---|---|---|---|
| Hsa Siglec-3 | CTD-3187F8 | HsaSig3-F1 | HsaSig3-R1 | NheI |
| Hsa Siglec-5 | CTC-470E3 | HsaSig5-F1 | HsaSig5-R1 | XbaI |
| Hsa Siglec-9 | CTD-3187F8 | HsaSig9-F1 | HsaSig9-R1 | XbaI |
| Ptr Siglec-3 | CH251-126O24 | HsaSig3-F1 | HsaSig3-R1 | NheI |
| Ptr Siglec-5 | CH251-426A12 | HsaSig5-F1 | HsaSig5-R1 | XbaI |
| Ptr Siglec-9 | CH251-126O24 | HsaSig9-F1 | HsaSig9-R1 | XbaI |
| Pcy Siglec-3 | RP41-94H15 | PcySig3-F1 | PcySig3-R1 | XbaI |
| Pcy Siglec-5 | RP41-126M5 | HsaSig5-F1 | HsaSig5-R1 | XbaI |
| Pcy Siglec-9 | RP41-94H15 | HsaSig9-F1 | HsaSig9-R1 | XbaI |
Has, human (for Homo sapiens); Ptr, chimpanzee (for Pan troglodytes); Pcy, baboon (for Papio cynocephalus anubis). See Table 3 for primer sequences.
Table 3.
Primer sequences
| Primer | Sequence |
|---|---|
| HsaSig3-F1 | CCCGCTAGCGCCACCATGCCGCTGCTGCTACTGCTGCCC |
| HsaSig3-R1 | ATCTCCTGGAAAGATACCAGTTG |
| HsaSig5-F1 | CCCTCTAGAGCCACCATGCTGCCCCTGCTGCTGCTGCCC |
| HsaSig5-R1 | ATCCCTGAAGATGGTGATGGTCTGTG |
| HsaSig9-F1 | CCCTCTAGAGCCACCATGCTGCTGCTGCTGCTGCTGCTGCCC |
| HsaSig9-R1 | ATCTCCTTGGAAGACAGTCAYGG |
| PcySig3-F1 | CCCTCTAGAGCCACCATGCCGCTGCTGCTGCTGCTGCTGCTGCC |
| PcySig3-R1 | ATCTCCTAGAAAGATATCAGTTC |
Production of recombinant soluble Siglec-Fc chimeras
Siglec-Fc chimeras were produced as described previously (41). In brief, CHO-TAg cells were maintained in minimal essential medium-α with 10% fetal calf serum (FCS) supplemented with G418 (1 mg/ml). Confluent cells were spilt and grown overnight to near-confluence. Cells were then transfected with filter-sterilized Siglec-Fc plasmid by Lipofectamine in Opti-MEM (0.1 μg of DNA+0.5 μl of Lipofectamine/1 cm2) and incubated at 37°C under 5% CO2 for 5 h; then fresh medium (Opti-MEM with 10% FCS) was added 1:1 and cells were incubated overnight as described above. Medium was replaced by Opti-MEM with 2% low IgG FCS, and cells were cultured for 3 d. Subsequently, the medium supernatant containing the secreted Siglec-Fc was collected, fresh medium was added (Opti-MEM with 2% low IgG FCS), cells were cultured for an additional 3 d, and supernatant was collected. Medium supernatant (collected at d 3 and d 6) was centrifuged to remove cell debris, and then cleared medium was filter-sterilized (0.22 μm) and kept at 4°C until Siglec-Fc purification.
For Siglec-Fc purification, 1 M Tris-HCl buffer, pH 8.0 (final concentration 20 mM), and 10% sodium azide solution was added (final concentration 0.02%) and then protein A-Sepharose suspension (GE Healthcare, Chalfont St. Giles, UK) was added at 1:500 (∼100 μl as a packed volume per volume of media, up to 50 ml) and incubated while rolling at 4°C for 2 d to maximize binding. Subsequently, medium was loaded over a disposable column assembly at 4°C, and all medium was drained. The column was washed with 20 column volumes of Tris-buffered saline [TBS; 20 mM Tris (pH 8.2) and 150 mM NaCl] and then twice with 10 column volumes of 0.1 M citrate-NaOH buffer (pH 5.8), to remove the bovine IgG.
To remove Sias, protein A-bound Siglec-Fcs were treated with sialidase. In brief, column-washed protein A-Sepharose was transferred into Eppendorf tubes through suspending in 500 μl of 20 mM HEPES-NaOH buffer (pH 7.0). Then 25 mU of Arthrobacter ureafaciens sialidase (AUS) was added, and the tube was incubated at room temperature for 1 h on a rocking shaker. AUS-treated protein A-bound Siglec-Fc was transferred to a disposable column assembly at 4°C, drained, and washed 5 times each with 4 column volumes of TBS to remove the AUS. The desialylated Siglec-Fcs were eluted 3 times with 1 ml (4 column volumes) of 0.1 M glycine-HCl buffer (pH 3.0), with immediate addition of 0.3 ml of 1 M Tris-HCl buffer (pH 8.0) to neutralize. Buffer was then exchanged to phosphate-buffered saline (PBS) by ultrafiltration (10,000 Da cutoff).
Siglec-Fc binding to FITC-labeled bacteria
Binding assays were performed as described previously (42) with some modifications. Costar 96-well ELISA plates (Corning Life Sciences, New York, NY, USA) were coated with protein A (Pierce, Rockford, IL, USA) in 50 mM sodium carbonate/bicarbonate (pH 9.5) at 1 μg/well and incubated at 4°C overnight. Coating buffer was removed, and plates were washed 3 times with assay buffer (TBS with 1% ovalbumin) and then blocked with the same buffer for 1 h at room temperature. Buffer was removed, and Siglec-Fc chimeras or control human IgG (Jackson ImmunoResearch Laboratories) diluted in assay buffer were added at 0.5 μg/well in 6 replicates and allowed to adhere for 3 h at room temperature. At the same time, FITC-labeled bacteria were prepared. Group B Streptococcus (GBS) wild-type (WT) strains of serotypes Ia (A909), Ib (M709), and III (COH1) are isolates from human neonates with invasive infections. GBS isolates grown in Todd-Hewitt broth (pH 7.5) to early exponential phase at 37°C without shaking were washed once with pyrogen-free PBS, aspirated, and resuspended in 50 mM sodium acetate (pH 5.5) to 1.2 OD600/ml (6×108 colony-forming units (CFU)/ml). For enzymatic removal of Sias from capsular polysaccharide (CPS), GBS isolates in 50 mM sodium acetate (pH 5.5) were incubated with 100 mU/ml AUS with rotating for 3 h at 37°C and then were washed twice with PBS (control bacteria were treated the same way only without AUS). Subsequently, GBS isolates were labeled with FITC (Fluka, Buchs, Switzerland) at 3 × 108 CFU/100 μl in filtered (0.22 μm) FITC solution (1 mg/ml in PBS) and incubated for 1 h at 37°C. FITC-labeled GBS cells were washed 4–5 times in PBS to remove trace amounts of free FITC and then were resuspended in PBS to 2.0 OD600 (108 CFU/ml). To test Siglec-Fc binding to bacteria, Siglec-Fc-coated plates were washed 3 times with assay buffer, wells were drained, and FITC-labeled GBS cells (with or without AUS treatment) was added in triplicate at 1 × 107 CFU/100 μl/well. The plate was centrifuged at 100 g for 10 min, and bacteria were allowed to adhere for 30 min at 37°C. The initial fluorescence intensity was verified, wells were gently washed 3 times with assay buffer to remove unbound bacteria, and the residual fluorescence intensity (excitation, 488 nm; emission, 530 nm) was measured using a SpectraMax M3 fluorescent plate reader. Final binding was determined by subtracting the residual background bacterial binding to human IgG.
Siglec-Fc binding to gangliosides by ELISA
The ganglioside-binding assay was modified from a conventional ELISA and performed as follows. All assays were done in triplicate in a 96-well flat-bottom plate (269620; Nalge Nunc International, Penfield, NY, USA). GM3 gangliosides were diluted in cold methanol and immobilized onto a 96-well plate (30−50 pmol/well) by evaporation at room temperature. Wells were washed 3 times and blocked for 1 h at room temperature with ELISA buffer (20 mM HEPES, 125 mM NaCl, and 0.5% BSA). Recombinant CD33rSiglec-Fc was added to each well (0.25 μg/well) and incubated for 1–2 h at room temperature. The wells were washed as above. A 1:1000 dilution of alkaline phosphatase-conjugated goat anti-human IgG (Bio-Rad Laboratories, Hercules, CA, USA) was added to each well and incubated for ≥30 min at room temperature, followed by 3 washes as before. The reaction was developed with p-nitrophenyl phosphate and read at 405 nm on a plate reader, and the results were analyzed.
Siglec-Fc binding to sialoglycan microarray
Binding assays were performed as described previously (43). In brief, arrays were fabricated by Kamtek Inc. (Gaithersburg, MD, USA) on epoxide-derivatized slides (Corning; Thermo Fisher Scientific, Waltham, MA, USA) with 8 full subarrays/slide as described previously (array 1; ref. 43). Siglec-Fc binding was tested by sialoglycan microarray (43) at 200 μl/subarray in 3 concentrations (10, 20, and 40 μg/ml) and detected with 1.5 μg/ml Cy3-conjugated goat anti-human IgG. In each Siglec-Fc array tested and at each concentration, binding of relative fluorescence units (RFU; F532−B532) was ranked as the percentage of the maximal signal in each array [rank=100×(glycan RFU/RFU maximum at each concentration)], and the relative rankings were then averaged to obtain the average rank of each glycan for each Siglec-Fc (43). To demonstrate Sia-specific binding, arrays were pretreated with mild periodate, as described previously (43).
Siglec detection on cells by flow cytometry
Whole blood was collected into K3EDTA Vacutainers (BD Biosciences, Franklin Lakes, NJ, USA) from all nonhuman primate subjects during routine health examinations, bubble-wrapped, placed on ice packs, and shipped overnight. Human blood was collected from volunteers at approximately the same time and stored overnight on ice packs to mimic transportation conditions. Leukocytes were isolated by hypotonic lysis of erythrocytes (lysis buffer: 150 mM NH4Cl, 10.0 mM KHCO3, and 0.1 mM EDTA) for 5 min at room temperature for 1−3 rounds of lysis. Cells were immediately put into a solution of 1% BSA/PBS and counted, cell quality was determined, and cells were placed on ice until needed. In all cases, the isolated cells were used within 3 h. About 1 × 106 cells were incubated with anti-Siglec antibody (1 μg/ml for purified antibodies or a dilution of 1:10 to 1:100 for hybridoma supernatant or isotype control X63) for 1 h on ice, washed, incubated with goat anti-mouse IgG-PE for 30 min, and washed once again. The cells were then double-stained for CD3, CD4, CD13, CD14, CD16, CD19, CD22, CD45, or CD56 for 30 min and washed. Cells were resuspended in 0.4 ml of 1% BSA in PBS. About 10,000 events per treatment were examined on a FACSCalibur flow cytometer (BD Biosciences) and recorded as data files. Forward and side scatter and CD marker staining were used to determine the quality of the processed leukocytes. Only those samples with characteristic flow cytometry patterns were used for further analysis. FlowJo software (Tree Star, Ashland, OR, USA) was used to produce histograms. Statistical analysis was done with FlowJo and Prism6 (GraphPad Software, Inc., San Diego, CA, USA).
Tissue expression of Siglecs using immunohistochemistry
The tissue expression of the 3 Siglecs was analyzed by immunostaining of spleen sections using anti-Siglec-3, anti-Siglec-5, anti-Siglec-9 and double staining with antineutrophil elastase or anti-CD68. Frozen sections of multiple different samples of human spleen from the U.S. National Cancer Institute-funded cooperative human tissue network and from chimp or gorilla frozen tissues from the Yerkes Primate Research Center were first treated to remove endogenous biotin, followed by fixation in 10% neutral buffered formalin. Three washes in washing buffer (TBS and 0.1% Tween 20) followed each subsequent step of the immunostaining protocol. The primary antibodies (diluted in 1% BSA in washing buffer) were either incubated 30–60 min at room temperature or occasionally overnight at 4°C in a humidified chamber. After blocking of endogenous nonspecific binding sites, the sections were overlaid with monoclonal rabbit anti-Siglec-3 (CD33) at 5.3 μg/ml (1:200), mouse anti-Siglec-5/14 at 2.5 μg/ml (1:200), or mouse anti-Siglec-9 at 2.5 μg/ml (1:200). The binding of the primary anti-Siglec antibodies was detected using a biotinylated secondary IgG (1:100), followed by alkaline phosphatase streptavidin, developed using a substrate kit (Vector Blue; Vector Laboratories Inc., Burlingame, CA, USA). Nuclei were counterstained with nuclear fast red for 5 min at room temperature, and the slides were washed and aqueous mounted for viewing and digital photomicrography. For double-staining studies, the binding of anti-CD68 (a pan-macrophage marker) was detected using a Cy3-conjugated secondary IgG (1:500) followed by overlaying with anti-Siglec-3 (1:100), anti-Siglec-5/14, or Siglec-9, the binding of which was detected using biotinylated anti-rabbit or anti-mouse IgG and Alexa Fluor 488-conjugated streptavidin (1:400). Double stains with rabbit anti-human neutrophil elastase (1:50) were detected using Cy3-conjugated anti-rabbit IgG (1:500), and then the slides were overlaid with anti-Siglec-5 (1:200), biotinylated anti-mouse IgG (1:100), and finally Alexa Fluor 488-conjugated streptavidin (1:400). The slides were aqueous mounted for viewing and digital photomicrography using either the BZ-9000 microscope for fluorescence labeled slides (Keyence, Itasca, IL, USA) or the Motic microscope (Motic Hong Kong, Hong Kong) and digital imaging for brightfield images. The Adobe Photoshop magic wand feature (Adobe Systems, Inc., San Jose, CA, USA) was used for pixel quantification of the expression of the 3 Siglecs on human and primate spleen frozen sections from multiple digital photomicrographs taken at ×40 view, following established procedures (44).
Statistical analysis
Data for statistical analysis are described in the figure legends.
RESULTS
Cloning and expression of recombinant soluble Siglec-Fc chimeras of the N-terminal extracellular domains of CD33rSiglecs from the genomic Siglec gene clusters of human, chimpanzee, and baboon
Table 1 summarizes current knowledge of ITIM-containing CD33rSiglec orthologs in human, chimpanzee, and baboon genomes. In some instances, orthologs have not been found (e.g., absence of Siglec-7 and Siglec-12 in baboon). In other cases, Siglec orthologs harbor mutations in an essential arginine residue of the binding pocket and therefore may not bind to Sias (i.e., human Siglec-XII, chimpanzee Siglec-V, and baboon Siglec-VI; use of the Roman numeral is an accepted convention in the field for such molecules). To investigate differences between humans and nonhuman primates, we chose to focus primarily on Siglec-3 and Siglec-9, because functional orthologs are found in all 3 species (Table 1), and each has relatively broad sialoglycan specificity (7, 43) and is expressed on circulating blood cells (7). To give further insight, we also compared Siglec-5 from human and baboon, including the arginine-mutated Siglec-V from chimpanzee where appropriate.
Whereas the N-terminal V-set domain of CD33rSiglecs contains the Sia-binding site, the following C2-set-like domain appears to stabilize it. To facilitate comparative investigation of primate CD33rSiglec binding properties, we generated chimeric proteins fused to human IgG-Fc region. To circumvent the necessity for cDNA cloning, the genomic DNA fragments encoding the signal peptide and first 2 Ig-like domains were amplified by PCR and cloned into human IgG Fc-containing expression vector (EK-Fc/pcDNA). These exons are typically found clustered within ∼1.5 kb genomic segment and intervened with short introns. Therefore, amplification of the genomic segments by PCR and direct in-frame cloning into the vector produced expression constructs for the recombinant proteins. The resulting constructs were transfected into CHO-TAg cells, and fusion proteins were purified from the culture supernatant with protein A. Subsequently, the binding characteristics of these Siglec-Fc chimeric proteins to sialylated bacteria, gangliosides, and sialoglycan microarray were extensively evaluated.
Binding of Siglec Fc-chimeras to GBS strains
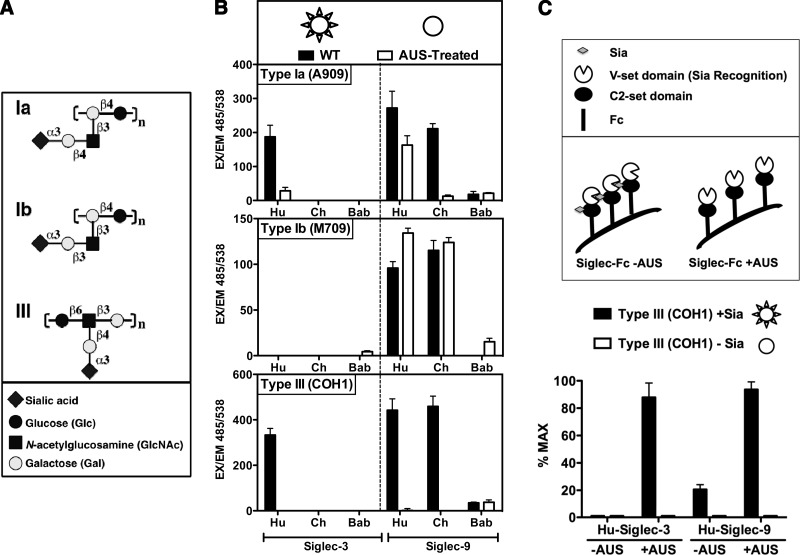
GBS is a Gram-positive human pathogen that is a leading cause of life-threatening infections such as sepsis and meningitis in newborns and infants (in fact, disease in adults now outnumber these pediatric cases). Human CD33rSiglecs were previously shown to bind to various GBS strains typically via their sialylated CPSs (14, 42), but sometimes even in a Sia-independent manner, i.e., human Siglec-5 (45). There are 9 serotypes of GBS characterized by their CPSs composed of repetitive sialoglycan building blocks [units of Neu5Acα2–3Galβ1–4Glc(NAc)], linked to varied underlying polysaccharide backbones. We evaluated binding of human, chimpanzee, and baboon Siglec-Fcs to GBS strains of CPS serotypes Ia, Ib, and III, among the most common strains associated with newborn infection (Fig. 1A). Sia-dependent binding was assessed on sialylated bacteria compared with bacteria pretreated with the AUS that removes the Sia from CPS (Fig. 1B). Because human Siglec-5 strongly binds to GBS bacteria via the surface-anchored β-protein in a Sia-independent manner (45) and chimpanzee Siglec-V is mutated in the Sia-binding domain (7), we focused on comparing binding of Siglec-3-Fc and Siglec-9-Fc among the 3 species. The Siglec-Fcs were also pretreated with sialidase to remove potentially interfering cis ligands carried by N-glycans on the same molecule. Although human Siglec-3-Fc binds to sialylated GBS serotypes Ia and III, neither the chimpanzee nor the baboon orthologs showed any binding (Fig. 1B). Moreover, although baboon Siglec-9-Fc did not bind to any of the GBS strains tested, both human and chimpanzee Siglec-9-Fc showed Sia-dependent binding to serotype III and Sia-independent binding to serotype Ib (Fig. 1B). Whereas Siglec-9-Fc binding to GBS serotype Ia was completely Sia-dependent for chimpanzee, the human ortholog also showed some Sia-independent binding. The Sia-dependent binding to GBS bacteria was dramatically reduced when the Siglec-Fc was not pretreated with AUS, probably due to Siglec-Siglec cis binding (Fig. 1C). This result suggests that optimal engagement of sialylated bacteria in vivo involves a strong competition between in cis and in trans binding events. Overall, these assays revealed marked binding differences between the human, chimpanzee, and baboon Siglec orthologs to different strains of the sialylated GBS bacterium.
Figure 1.
Binding of Siglec-Fc chimeras to GBS are divergent among the 3 species. A) Diagram of various types of GBS CPS. B) Differences in binding patterns of human (Hu), chimpanzee (Ch) and baboon (Bab) Siglec-3-Fc and Siglec-9-Fc to GBS. Top panel: schematic diagram of WT vs. AUS-treated GBS. WT GBS CPS carries terminal Sias that are represented as spikes over a circle. When such bacteria are treated with AUS, the Sia are peeled off; hence, the desialylated bacteria are represented as circles without spikes. Left panel: human Siglec-3 showed binding to GBS type Ia and type III in a Sia-dependent manner, whereas neither chimpanzee nor baboon Siglec-3 showed significant GBS binding. Right panel: human and chimpanzee Siglec-9 showed Sia-independent (type Ib), partially Sia-dependent (type Ia), or Sia-dependent (type III) binding to GBS, whereas the dependence on Sia in the binding to type Ia differed between human and chimpanzee Siglec-9. Baboon Siglec-9 showed no significant binding to GBS. Ex, excitation; Em, emission. C) Effects of AUS pretreatment of Siglec-Fc chimeras on binding to GBS. Siglec-Fc chimeras carry sialylated glycans and can potentially bind to each other when found in proximity (cis binding). However, when the Siglec-Fc chimeras are pretreated with AUS, the terminal Sias are removed and their binding sites are free to bind other sialylated targets (trans binding).
GM3 ganglioside ligand-binding patterns of CD33rSiglecs are also divergent among the 3 species
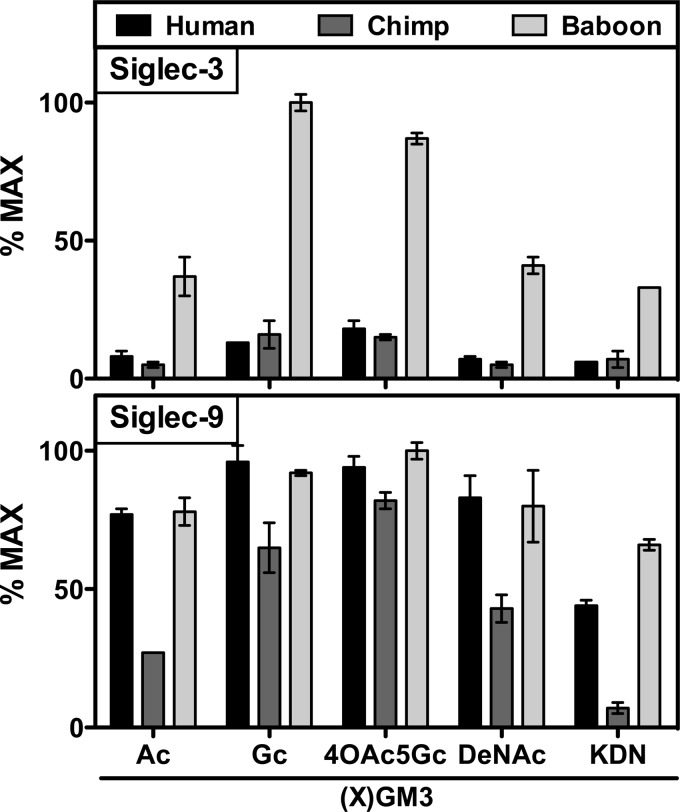
To test the potential species variability in ganglioside recognition, we used the common GM3 and some of its derivatives in our binding assay. GM3 gangliosides are monosialylated glycosphingolipids found in the outer leaflet of animal cell membranes, making them potential binding partners for CD33rSiglecs. Although they share the common structure Siaα2–3Galβ1–4Glcβ1–1′-ceramide, they can have variations in Sia types, including the 2 most common Sia types in vertebrates, Neu5Ac and its hydroxylated form, Neu5Gc, as well as the less common DeNAc and Kdn forms. We used an ELISA to determine binding patterns of Siglec-3-Fc and Siglec-9-Fc from human, chimpanzee, and baboon. We found that Siglec-9 demonstrated a relative preference for Neu5Gc over Neu5Ac in all 3 species (Fig. 2). Similarly, Siglec-9-Fc demonstrated broad binding to 4-O-acetyl-N-glycolylneuraminic acid (4OAc5Gc) across species. Although baboon Siglec-9 strongly recognized Kdn and DeNAc, the human Siglec-9 showed a strong DeNAc and moderate Kdn interaction, and chimp Siglec-9 demonstrated moderate to weak interactions with DeNAc, Neu5Ac, and Kdn. Although the Siglec-3-Fc/GM3 signal for human and chimpanzee was lower, a similar slight preference for Neu5Gc was evident. In general, baboon Siglec-3-Fc bound strongly to all GM3 molecules, with a robust Neu5Gc interaction. Overall, the 3 species show markedly differential binding patterns to different types of GM3 gangliosides, which only display subtle structural differences from each other, in the type of Sias presented.
Figure 2.
Ganglioside binding patterns of Siglec-Fc-chimeras are divergent between the 3 species. Binding patterns of Fc chimeras of CD33rSiglec (Siglec-3 and -9) from human, chimpanzee (chimp), and baboon were tested by ELISA using multiple types of GM3-based gangliosides presenting different types of Sias. Top panel: Siglec-3 of baboon showed robust binding to most GM3s tested, with strong preference for Neu5Gc. Human and chimpanzee Siglec-3 also showed some preference toward Neu5Gc, but not as marked as that of baboon Siglec-3. Bottom panel: Siglec-9 of human and baboon showed similar patterns of binding, whereas chimpanzee Siglec-9 showed weaker binding. Ac, Neu5Ac; Gc, Neu5Gc.
Sialoglycan microarray analysis confirms and extends the variable species-specific binding patterns
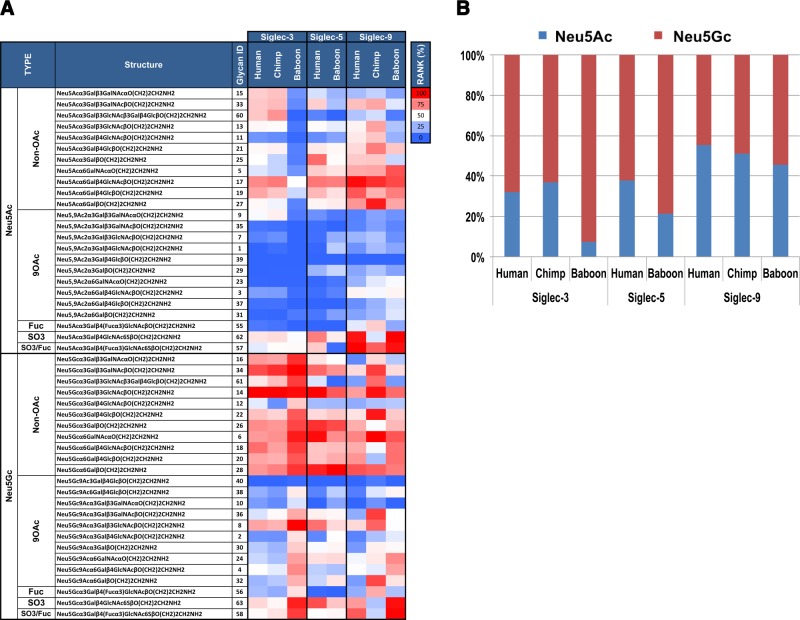
To gain further insight into the recognition properties of Fc chimeras of CD33rSiglecs from human, chimpanzee, and baboon, each was tested on a novel sialoglycan microarray representing the natural diversity of Sias in nature (43). The array was printed with monosialylated glycans containing Neu5Ac or Neu5Gc; in some instances, the terminal Sia was modified with an O-acetyl group at position 9 (9OAc). Fc chimeras of Siglec-3, Siglec-5, and Siglec-9 from all 3 species were tested at 3 concentrations each (Fig. 3). To directly compare the Sia specificity between the 3 species, the binding was ranked as a percentage of the maximal signal in each tested array, and the relative rankings were then averaged to obtain the average rank of each glycan. Each Siglec-Fc average rank was then ordered according to Sia type, and the summary data are presented in a heat map (Fig. 3A). Across species, all Siglecs showed preference to non-O-acetylated Sia (Sia>9OAc-Sia). The importance of the Sia side chain in recognition was further corroborated by the observation that mild periodate treatment of microarrays (which truncates the Sia side chain unless it is blocked by O-acetylation) dramatically reduced Siglec-Fc binding (data not shown). Baboon Siglec-3-Fc showed a clear preference for Neu5Gc-containing glycans with almost no binding to any of the Neu5Ac glycans. The chimpanzee and human Siglec-3-Fc also preferred Neu5Gc glycans but showed a more relaxed phenotype with additional binding to some Neu5Ac glycans, especially those attached to the underlying structures N-acetyl-d-lactosamine (Galβ1–4GlcNAc, glycan 17) or lactose (Galβ1–4Glc, glycan 19) and to some extent those with core-1 (Galβ1–3GalNAcα/β-linked; glycans 15 and 33). Baboon Siglec-3-Fc also showed a preference for non-O-acetylated Neu5Gc or for sulfated glycans (glycans 63 and 58) with much lower binding to the nonsulfated sialyl-Lewisx [Siaα2-3Galβ1–4(Fucα1–3)GlcNAc, glycan 56]. Across species, in Siglec-3-Fc, the Neu5Gcα2–3 linked to βcore-1 (glycan 34) and to βtype-1 (glycan 14) showed the strongest binding (Fig. 3). The binding patterns of human and baboon Siglec-5-Fc were similar (Fig. 3A; the naturally mutated chimpanzee Siglec-V-Fc did not bind the array, data not shown). In both human and baboon Siglec-5-Fc, Neu5Gc was preferred over Neu5Ac, with a further slight preference for the α2–6 linkage over the α2–3 linkage (α2–6>α2–3; Fig. 3A) and a strong preference for Neu5Gcα2–3/6Gal glycans (glycans 26, 28, and 14). However, there was a more relaxed phenotype in the human than in the baboon Siglec-5-Fc toward Neu5Ac glycans, similar to that of Siglec-3-Fc (Fig. 3A). With Siglec-9-Fc, across species, the Neu5Gc preference was not as strong as it was for Siglec-5-Fc or Siglec-3-Fc, and there was no binding to 9-O-acetylated-Neu5Ac, but with broader Neu5Ac-glycan recognition, including in baboon. In terms of binding patterns, those for the human and baboon were more similar than those for the human and chimpanzee, especially with regard to Neu5Gc glycan recognition (Fig. 3A). Although chimpanzee Siglec-9-Fc showed some preference for the Neu5Gcα2–3 linkage (glycans 34, 14, and 22 bind the best) over the Neu5Gcα2–6 linkage (although glycan 6 is also a strong binder), the opposite was the case with the human and baboon. For Neu5Ac glycans, in all species, the Neu5Acα2–6 linkage was preferred over the Neu5Acα2–3 linkage (Fig. 3A). To specifically focus the Neu5Ac vs. the Neu5Gc preferences across species, we combined and averaged the fluorescence values for all the non-O-acetylated-Neu5Ac glycans and the non-O-acetylated-Neu5Gc glycans and presented this result as 100% stacked columns (Fig. 3B). This result clearly showed the preference for Neu5Gc glycans in baboon and the relaxed phenotype toward Neu5Ac glycans in human and chimpanzee, which was especially pronounced in Siglec-3-Fc and Siglec-5-Fc, whereas in human Siglec-9-Fc, the Neu5Ac glycans are only slightly preferred over the Neu5Gc glycans, with the opposite being observed in baboon. Taken together, these data confirm and extend the notion that CD33rSiglecs are undergoing rapid evolution of their Sia-binding characteristics.
Figure 3.
Sialoglycan microarray analysis shows variable species-specific Siglec binding patterns. A) Binding of human, chimpanzee, and baboon Siglec-Fc chimeras was assayed at 10, 20, and 40 μg/ml and detected with 1.5 μg/ml Cy3-conjugated goat anti-human IgG. Binding was ranked (43) as percentage of maximal signal in each array [rank=100×(glycan RFU/RFU Max at each concentration)] and the relative ranking was then averaged to obtain the average rank of each glycan. Each Siglec-Fc average rank is presented as a heat map (red, white, and blue represent the maximum, 50th percentile, and minimum, respectively). Major findings include: all Siglecs showed preference toward non-9-O-acetylated sialic acids; all Siglecs showed preferences toward Neu5Gc over Neu5Ac to differing extents, with baboon Siglec-3 the most extreme; and binding preferences of human and baboon Siglec-9 were similar to each other, whereas chimpanzee (chimp) Siglec-9 showed unique properties, confirming the result shown in Fig. 2. B) Neu5Ac vs. Neu5Gc binding preferences of human, chimpanzee, and baboon Siglec-3-, Siglec-5-, and Siglec-9-Fcs were demonstrated after combining and averaging RFU values for all the non-O-acetylated-Neu5Ac glycans and the non-O-acetylated-Neu5Gc glycans and are presented as percentage stacked columns. Human Siglecs showed increased “tolerance” toward Neu5Ac.
Expression patterns and levels of CD33rSiglecs in peripheral blood leukocytes in human, chimpanzee, and gorilla
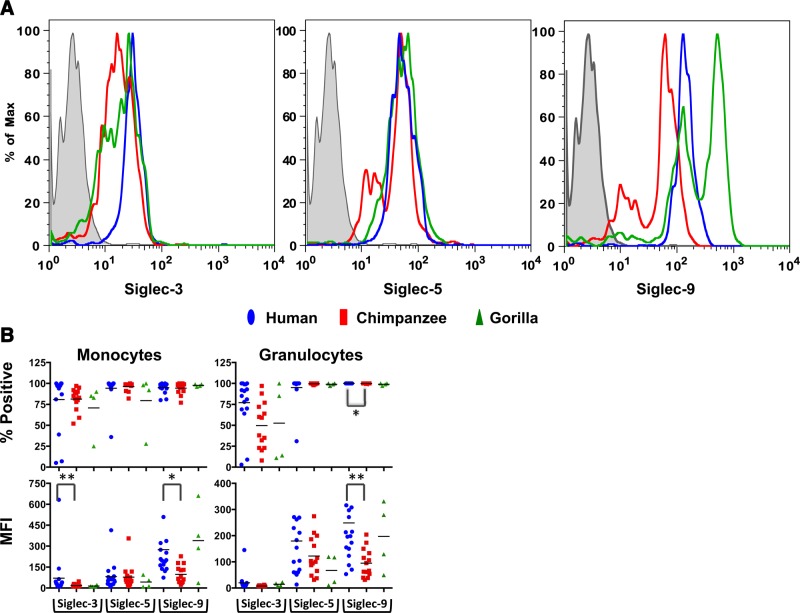
We had previously shown that humans express very low levels of CD33rSiglecs on their lymphocytes in contrast with those for other great apes (46). Here we sought to compare Siglec expression across species on other blood cells, i.e., monocytes and granulocytes. The available mouse anti-human Siglec-3, Siglec-5, and Siglec-9 antibodies cross-react with the corresponding Siglecs from chimpanzee and gorilla but were limited in their use in baboon (tested by ELISA and by flow cytometry; data not shown). Thus, we compared Siglec expression in blood samples of human, chimpanzee, and gorilla (note that the anti-human Siglec-5 antibody we used also recognizes Siglec-14). For the direct comparison of Siglec expression by flow cytometry, we measured the fraction of cells that demonstrate anti-Siglec antibody binding (percentage of positive cells), as well as the median fluorescence intensity (MFI) as a measure of expression strength across the positive cells.
Examination of CD14+ monocytes for Siglec-3 showed expression on more than 80% of the population (Fig. 4A, left panel, B); however, a Kruskal-Wallis test showed no major differences among the 3 species examined. Furthermore, the MFIs were different across the species (Fig. 4B, lower left) with a significant difference between human and chimpanzee expression. In addition, the Siglec-3 expression was uniform within the human monocyte population (Fig. 4A, left panel) compared with expression in the chimpanzee and gorilla, both of which showed another subpopulation of low-staining monocytes. Expression of Siglec-5 on monocytes was also fairly similar among the 3 species (Fig. 4A, center panel, B). Siglec-9 was expressed on >90% of all monocytes (Fig. 4B, top left panel), but with great variations in the amounts expressed, both among species and between individuals (Fig. 4B, bottom left panel). Again, although human monocytes had only a single population of Siglec-9-expressing monocytes, the chimpanzee and gorilla monocytes showed 2 subpopulations of Siglec-9-expressing monocytes (Fig. 4A, right panel). There was also a significantly lower expression level (as judged by the MFI) of Siglec-9 on chimpanzee monocytes and granulocytes compared with that of humans; there was no statistical difference between gorilla and chimpanzee or human, presumably because of the limited number of gorilla samples used in the study. Moreover, whereas 75–100% of the monocytes (CD14+) and 100% of the granulocytes (predominately neutrophils) expressed Siglec-5 and Siglec-9, the proportion of Siglec-3+ cells was highly variable among individuals (Fig. 4B). As before, there was a statistical difference between human and chimpanzee Siglec-9 MFIs. This pattern may suggest differential regulation of expression for Siglec-3 vs. Siglec-5 and Siglec-9 and is applicable to both monocytes and granulocytes across the species. Overall, these data suggest that although levels of Siglec-3, Siglec-5, and Siglec-9 in both monocytes and granulocytes are relatively similar across species, there are intra- and interspecies variations in their expression patterns.
Figure 4.
Intra- and interspecies variability in blood leukocyte cell type expression patterns and expression levels in flow cytometry analysis. A) Representative antibody staining patterns of monocytes. Gray-shaded area represents human monocytes stained with the negative control antibody. B) Overall summary of CD33-related Siglec (CDrSiglec) expressions on monocytes and granulocytes, represented as percentage of positive cells (top panels) and MFI (bottom panels) of each population. Each point represents an individual donor. We found a significant difference in MFI between human and chimpanzee monocytes for Siglec-3 (P=0.004) and for Siglec-9 (P=0.02). Furthermore, there was a significant difference in granulocyte MFI and percentage of positive cells between human and chimpanzee (P=0.008 and P=0.02, respectively; Kruskal-Wallis test).
Species variability in tissue expression patterns of CD33rSiglecs
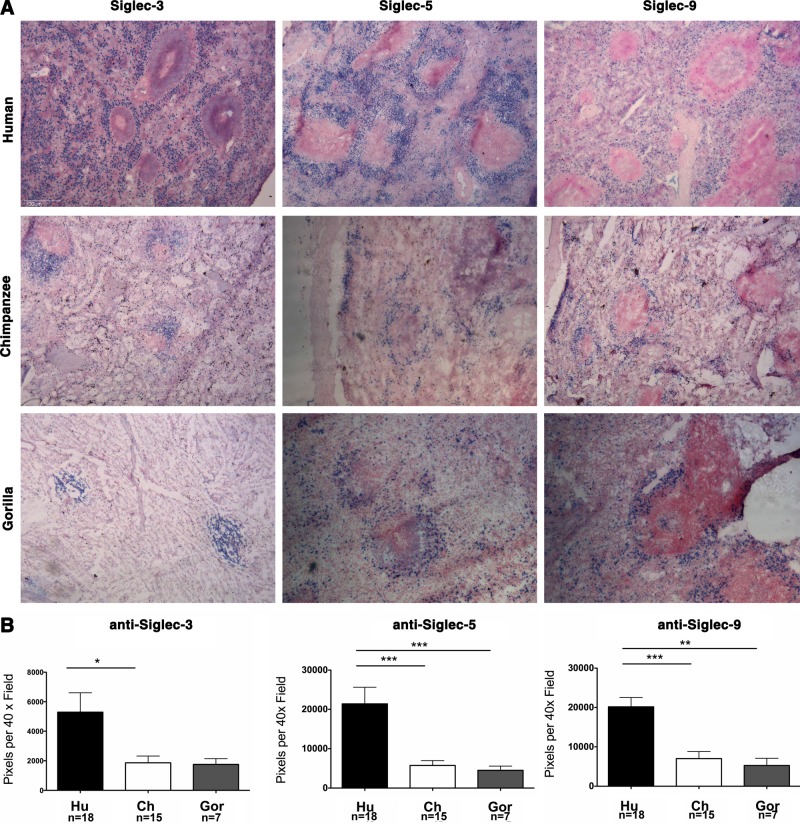
To investigate the tissue expression patterns of Siglec-3, Siglec-5, or Siglec-9 across species, we studied spleens from humans (n=18), chimpanzees (n=15), and gorillas (n=7) with anti-Siglec-3, anti-Siglec-5, or anti-Siglec-9, respectively. In the chimpanzee and gorilla spleens, Siglec-3-, Siglec-5-, or Siglec-9-positive cells (blue) were limited to the perifollicular zone, whereas those of the human spleen extended beyond the perifollicular zone (Fig. 5A). Quantitatively, human spleens contained more Siglec-positive cells than those of the other species (Fig. 5B; by pixel counting). The numbers of Siglec-5- or Siglec-9-positive pixels in the human spleen were greater than those in the chimpanzee (P<0.0001 and P=0.0006, respectively) or gorilla spleens (P=0.0002 and P=0.004, respectively). Whereas the number of Siglec-3-positive pixels in the human spleen was greater than that in the chimpanzee spleen (P=0.0484), it was not significantly different from that in the gorilla spleen (P=0.14; Fig. 5B) because there were lower numbers of gorilla spleens available for analysis.
Figure 5.
Variability in expression patterns of Siglecs between human, chimpanzee, and gorilla spleens. A) Frozen sections of human, chimpanzee, and gorilla spleen were overlaid with anti-Siglec-3, anti-Siglec-5, or anti-Siglec-9 followed by biotinylated anti-mouse or anti-rabbit IgG, alkaline phosphatase streptavidin, and developed using a substrate kit as described in the Materials and Methods. The blue color indicates binding of the antibody. Sections were counterstained using a nuclear stain (red) (×40). B) Comparison of the number of pixels per ×40 field of frozen human (Hu, black bars), chimpanzee (Ch, white bars), and gorilla (Gor, gray bars) spleen sections overlaid with anti-Siglec-3, anti-Siglec-5, or anti-Siglec-9 (blue). *P < 0.05; **P < 0.005; ***P < 0.0005.
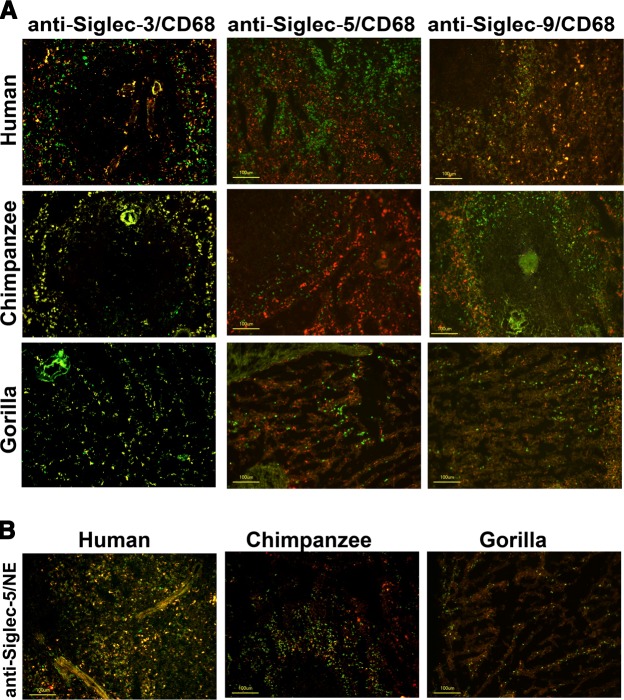
To further determine the expression pattern of Siglec on immune cells in those tissues, we tested the colocalization of Siglecs on cells positive for CD68 (macrophages) or neutrophil elastase (neutrophils) in human, chimpanzee, and gorilla spleens. Double staining showed that most splenic macrophages of all 3 species expressed Siglec-3, whereas Siglec-9 was only detected in human splenic macrophages (Fig. 6A). In addition, almost all elastase-positive neutrophils in human, chimpanzee, and gorilla spleens expressed Siglec-5 (Fig. 6B). There was no strong colocalization of B cells (CD19+) or T cells (CD3+) with Siglec-3, Siglec-5, or Siglec-9 expression (data not shown). In summary, in the chimpanzee and gorilla spleens, there were fewer numbers of Siglec-3-positive macrophages, Siglec-5-positive neutrophils, or Siglec-9-positive macrophages or neutrophils. Furthermore, these cells are limited to the perifollicular zones and only occasionally are found within the follicle. Cells of the human spleens are greater in number, extend beyond the perifollicular zone, and are not found within the splenic follicle. These clear differences further support the hypothesis that the expression pattern of CD33rSiglec leukocytes is rapidly evolving in closely related primates such as human, chimpanzee, and gorilla.
Figure 6.
Immune cell expression patterns of Siglecs between human, chimpanzee, and gorilla spleens. A) Siglec expression on spleen macrophages. Frozen spleen sections from human, chimpanzee, and gorilla were stained with anti-CD68 (a pan-macrophage marker; detected with Cy3-conjugated IgG; red fluorescence), and anti-Siglec-3, anti-Siglec-5, or anti-Siglec-9 (detected by biotinylated IgG and Alexa Fluor 488-conjugated streptavidin; green fluorescence). Siglec-3 was expressed on macrophages of all 3 species, whereas Siglec-5 was not expressed on macrophages. Siglec-9 was only detectable on a subset of human macrophages but not on chimpanzee or gorilla macrophages. ×200; scale bar = 100 μm. B) Siglec-5 expression on spleen neutrophils. Frozen spleen sections were stained with antineutrophil elastase (a neutrophil marker; detected by Cy3-conjugated IgG; red fluorescence) and anti-Siglec-5 (detected by biotinylated IgG and subsequently by Alexa Fluor 488-conjugated streptavidin; green fluorescence). Double positive cells are orange/yellow. ×200; scale bar = 100 μm.
DISCUSSION
The marked interspecies differences noted in the CDD33rSiglec encoding gene cluster imply that the encoded family of proteins might be rapidly evolving, and some examples and potential evolutionary reasons have been discussed (7, 15, 28, 33–36). Here we provide the first systematic comparative experimental evidence for such rapid evolution of CD33rSiglecs at the phenotypic level by comparing related primate species. To compare binding and recognition patterns of functional orthologs, we generated Siglec-Fc chimeric proteins from 3 primate species: human, chimpanzee, and baboon, which had a common ancestor only ∼25 million yr ago and which share >90% homology in genomic sequences. Using these tools, we investigated recognition of sialylated bacteria, monosialogangliosides, and sialoglycan microarrays by 3 major CD33rSiglec orthologs, which revealed striking interspecies differences. Sialoglycan microarray analysis of binding to multiple sialylated glycans also revealed an overall preference for Neu5Gc glycans. Whereas baboon Siglecs showed a strong preference for Neu5Gc glycans, human and chimpanzee orthologs showed a more relaxed phenotype, also recognizing Neu5Ac glycans. This was especially pronounced in Siglec-3 and Siglec-5 but also was evident in Siglec-9. Such a relaxation of specificity away from Neu5Gc presumably occurred in the common ancestor of humans and chimpanzees and may have allowed the human lineage to tolerate the later inactivation of the CMAH gene and loss of Neu5Gc expression (28). The loss of Neu5Gc may then have allowed establishment of a species-specific pathogen (29, 30) and potentially the speciation of the genus Homo (32).
Overall, multiple assays provide key support to the notion that CD33rSiglecs are undergoing rapid evolution of their Sia-binding characteristics. Furthermore, we explored the native expression of these 3 CD33rSiglecs on leukocytes from humans and nonhuman primates, in circulation (blood) and in tissues (spleen). Because of limitations on antibody cross-recognition, we replaced the baboon with the more closely related gorilla as an out group in these studies. Circulating monocyte and granulocyte expression patterns and levels of Siglec-3, Siglec-5, and Siglec-9 were somewhat similar across these 3 species. However, there were intraspecies variations in expression levels and multiple subpopulations of cells with differential Siglec expression characteristics among chimpanzee and gorilla monocytes. In addition, Siglec expression levels in spleens showed pronounced interspecies differences for macrophages and neutrophils and in the localization of Siglec-positive cells within the organ. These findings provide further experimental evidence that CD33rSiglecs are rapidly evolving in closely related primates.
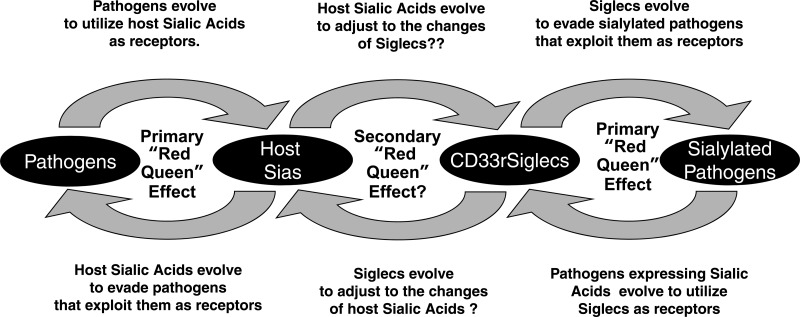
Taken together, these findings provide further support for multiple Red Queen effects involving Siglecs and Sias, a continuing evolutionary arms race between pathogens and hosts (Fig. 7 and refs. 3, 7, 28). Thus, the host must undergo evolutionary changes in Sia expression patterns to escape from pathogens that are rapidly evolving to recognize Sias. However, this in turn requires rapid evolution of host CD33rSiglecs to maintain self-recognition of the changing host sialome. Meanwhile, the CD33rSiglecs also need to rapidly evolve to escape sialylated pathogens that exploit them for infecting the host. These and other forces evidently shape the rapid evolution observed in the gene cluster encoding CD33rSiglecs.
Figure 7.
Probable evolutionary chain of Red Queen effects involving Sias and CD33rSiglecs. See text for discussion. [Based on Varki and Angata (7). Originally modified by T.A. for Glycoforum/Glycowords (http://www.glycoforum.gr.jp/science/word/evolution/ES-C04E.html), and further modified here.]
Acknowledgments
The authors thank Yerkes Primate Research Center (Atlanta, GA, USA), Zoo Atlanta (Atlanta, GA, USA), the San Diego Zoo (San Diego, CA, USA), the Lincoln Park Zoo (Chicago, IL, USA), the Primate Foundation of Arizona (Mesa, AZ), and the Southwest Primate Research Center (San Antonio, TX, USA), for providing nonhuman primate blood samples.
The laboratories of A.V. and V.N. are supported by the University of California at San Diego Program of Excellence in Glycosciences (U.S. National Institutes of Health grant P01HL107150). A.V. is also supported by the Mathers Foundation of New York. V.P.-K. was partially supported by an International Sepharadic Education Foundation postdoctoral fellowship.
The authors declare no conflicts of interest.
Footnotes
- 4OAc5Gc
- 4-O-acetyl-N-glycolylneuraminic acid
- AUS
- Arthrobacter ureafaciens sialidase
- BSA
- bovine serum albumin
- CFU
- colony-forming units
- CPS
- capsulated polysaccharide
- DeNAc
- de-N-acetyl
- ELISA
- enzyme-linked immunosorbent assay
- FCS
- fetal calf serum
- FITC
- fluorescein isothiocyanate
- GBS
- group B Streptococcus
- GM3
- monosialoganglioside GM3
- ITIM
- immunoreceptor tyrosine-based inhibitory motif
- KDN
- 2-keto-3-deoxy-d-glycero-d-galacto-nononic acid
- MFI
- mean fluorescence intensity
- Neu5Ac
- N-acetylneuraminic acid
- Neu5Gc
- N-glycolylneuraminic acid
- PBS
- phosphate-buffered saline
- RFU
- relative fluorescent units
- SAMP
- self-associated molecular patterns
- Sia
- sialic acid
- TBS
- Tris-buffered saline
- WT
- wild type
REFERENCES
- 1. Kelm S., Schauer R. (1997) Sialic acids in molecular and cellular interactions. Int. Rev. Cytol. 175, 137–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angata T., Varki A. (2002) Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem. Rev. 102, 439–469 [DOI] [PubMed] [Google Scholar]
- 3. Crocker P. R., Paulson J. C., Varki A. (2007) Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 [DOI] [PubMed] [Google Scholar]
- 4. Kelm S., Pelz A., Schauer R., Filbin M. T., Tang S., De Bellard M.-E., Schnaar R. L., Mahoney J. A., Hartnell A., Bradfield P., Crocker P. R. (1994) Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 4, 965–972 [DOI] [PubMed] [Google Scholar]
- 5. Powell L. D., Varki A. (1995) I-type lectins. J. Biol. Chem. 270, 14243–14246 [DOI] [PubMed] [Google Scholar]
- 6. Angata T., Brinkman-Van der Linden E. (2002) I-type lectins. Biochim. Biophys. Acta 1572, 294–316 [DOI] [PubMed] [Google Scholar]
- 7. Varki A., Angata T. (2006) Siglecs—the major subfamily of I-type lectins. Glycobiology 16, 1R−27R [DOI] [PubMed] [Google Scholar]
- 8. Cao H., Lakner U., de Bono B., Traherne J. A., Trowsdale J., Barrow A. D. (2008) SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur. J. Immunol. 38, 2303–2315 [DOI] [PubMed] [Google Scholar]
- 9. Wang X., Mitra N., Secundino I., Banda K., Cruz P., Padler-Karavani V., Verhagen A., Reid C., Lari M., Rizzi E., Balsamo C., Corti G., De Bellis G., Longo L., Beggs W., Caramelli D., Tishkoff S. A., Hayakawa T., Green E. D., Mullikin J. C., Nizet V., Bui J., Varki A. (2012) Specific inactivation of two immunomodulatory SIGLEC genes during human evolution. Proc. Natl. Acad. Sci. U. S. A. 109, 9935–9940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu Z. B., Maoui M., Wu L. T., Banville D., Shen S. H. (2001) mSiglec-E, a novel mouse CD33-related siglec (sialic acid-binding immunoglobulin-like lectin) that recruits Src homology 2 (SH2)-domain-containing protein tyrosine phosphatases SHP-1 and SHP-2. Biochem. J. 353, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Angata T., Kerr S. C., Greaves D. R., Varki N. M., Crocker P. R., Varki A. (2002) Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J. Biol. Chem. 277, 24466–24474 [DOI] [PubMed] [Google Scholar]
- 12. Avril T., Floyd H., Lopez F., Vivier E., Crocker P. R. (2004) The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related siglecs expressed on human monocytes and NK cells. J. Immunol. 173, 6841–6849 [DOI] [PubMed] [Google Scholar]
- 13. Liu Y., Chen G. Y., Zheng P. (2009) CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 30, 557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlin A. F., Uchiyama S., Chang Y. C., Lewis A. L., Nizet V., Varki A. (2009) Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113, 3333–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao H., Crocker P. R. (2011) Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation? Immunology 132, 18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Angata T., Hayakawa T., Yamanaka M., Varki A., Nakamura M. (2006) Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 20, 1964–1973 [DOI] [PubMed] [Google Scholar]
- 17. Varki A. (2011) Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology 21, 1121–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paulson J. C., Macauley M. S., Kawasaki N. (2012) Siglecs as sensors of self in innate and adaptive immune responses. Ann. N. Y. Acad. Sci. 1253, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crocker P. R., McMillan S. J., Richards H. E. (2012) CD33-related siglecs as potential modulators of inflammatory responses. Ann. N. Y. Acad. Sci. 1253, 102–111 [DOI] [PubMed] [Google Scholar]
- 20. Chen G. Y., Nunez G. (2010) Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medzhitov R., Janeway C. A. J. (1997) Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 295–298 [DOI] [PubMed] [Google Scholar]
- 22. Matzinger P. (2002) The danger model: a renewed sense of self. Science 296, 301–305 [DOI] [PubMed] [Google Scholar]
- 23. Vimr E., Lichtensteiger C. (2002) To sialylate, or not to sialylate: that is the question. Trends Microbiol. 10, 254–257 [DOI] [PubMed] [Google Scholar]
- 24. Severi E., Hood D. W., Thomas G. H. (2007) Sialic acid utilization by bacterial pathogens. Microbiology 153, 2817–2822 [DOI] [PubMed] [Google Scholar]
- 25. Baker C. J. (2013) The spectrum of perinatal group B streptococcal disease. Vaccine 31 (Suppl. 4), D3–D6 [DOI] [PubMed] [Google Scholar]
- 26. Khatua B., Bhattacharya K., Mandal C. (2012) Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J. Leukoc. Biol. 91, 641–655 [DOI] [PubMed] [Google Scholar]
- 27. Esko J. D., Sharon N. (2009) Microbial lectins: hemagglutinins, adhesins, and toxins. In Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E., eds) pp. 489–500, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA: [PubMed] [Google Scholar]
- 28. Varki A. (2010) Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. U. S. A. 107(Suppl. 2), 8939–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin M. J., Rayner J. C., Gagneux P., Barnwell J. W., Varki A. (2005) Evolution of human-chimpanzee differences in malaria susceptibility: relationship to human genetic loss of N-glycolylneuraminic acid. Proc. Natl. Acad. Sci. U. S. A. 102, 12819–12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varki A., Gagneux P. (2009) Human-specific evolution of sialic acid targets: explaining the malignant malaria mystery? Proc. Natl. Acad. Sci. U. S. A. 106, 14739–14740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rich S. M., Leendertz F. H., Xu G., LeBreton M., Djoko C. F., Aminake M. N., Takang E. E., Diffo J. L., Pike B. L., Rosenthal B. M., Formenty P., Boesch C., Ayala F. J., Wolfe N. D. (2009) The origin of malignant malaria. Proc. Natl. Acad. Sci. U. S. A. 106, 14902–14907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghaderi D., Springer S. A., Ma F., Cohen M., Secrest P., Taylor R. E., Varki A., Gagneux P. (2011) Sexual selection by female immunity against paternal antigens can fix loss of function alleles. Proc. Natl. Acad. Sci. U. S. A. 108, 17743–17748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Angata T., Margulies E. H., Green E. D., Varki A. (2004) Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc. Natl. Acad. Sci. U. S. A. 101, 13251–13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sonnenburg J. L., Altheide T. K., Varki A. (2004) A uniquely human consequence of domain-specific functional adaptation in a sialic acid-binding receptor. Glycobiology 14, 339–346 [DOI] [PubMed] [Google Scholar]
- 35. Bochner B. S., Alvarez R. A., Mehta P., Bovin N. V., Blixt O., White J. R., Schnaar R. L. (2005) Glycan array screening reveals a candidate ligand for Siglec-8. J. Biol. Chem. 280, 4307–4312 [DOI] [PubMed] [Google Scholar]
- 36. Guo J. P., Brummet M. E., Myers A. C., Na H. J., Rowland E., Schnaar R. L., Zheng T., Zhu Z., Bochner B. S. (2011) Characterization of expression of glycan ligands for Siglec-F in normal mouse lungs. Am. J. Respir. Cell Mol. Biol. 44, 238–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Altevogt B. M., Pankevich D. E., Shelton-Davenport M. K., Kahn J. P. (2011) Chimpanzees in Biomedical and Behavioral Research: Assessing the Necessity, National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- 38. Stults C. L. M., Sweeley C. C., Macher B. A. (1989) Glycosphingolipids: structure, biological source, and properties. Methods Enzymol. 179, 167–214 [DOI] [PubMed] [Google Scholar]
- 39. Sjoberg E. R., Chammas R., Ozawa H., Kawashima I., Khoo K.-H., Morris H. R., Dell A., Tai T., Varki A. (1995) Expression of de-N-acetyl-gangliosides in human melanoma cells is induced by genistein or nocodazole. J. Biol. Chem. 270, 2921–2930 [DOI] [PubMed] [Google Scholar]
- 40. Song Y., Kitajima K., Inoue S., Khoo K.-H., Morris H. R., Dell A., Inoue Y. (1995) Expression of new KDN-gangliosides in rainbow trout testis during spermatogenesis and their structural identification. Glycobiology 5, 207–218 [DOI] [PubMed] [Google Scholar]
- 41. Angata T., Varki A. (2000) Cloning, characterization, and phylogenetic analysis of Siglec-9, a new member of the CD33-related group of Siglecs—evidence for co-evolution with sialic acid synthesis pathways. J. Biol. Chem. 275, 22127–22135 [DOI] [PubMed] [Google Scholar]
- 42. Carlin A. F., Lewis A. L., Varki A., Nizet V. (2007) Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 89, 1231–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Padler-Karavani V., Song X., Yu H., Hurtado-Ziola N., Huang S., Muthana S., Chokhawala H. A., Cheng J., Verhagen A., Langereis M. A., Kleene R., Schachner M., de Groot R. J., Lasanajak Y., Matsuda H., Schwab R., Chen X., Smith D. F., Cummings R. D., Varki A. (2012) Cross-comparison of protein recognition of sialic acid diversity on two novel sialoglycan microarrays. J. Biol. Chem. 287, 22593–22608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lehr H. A., Mankoff D. A., Corwin D., Santeusanio G., Gown A. M. (1997) Application of Photoshop-based image analysis to quantification of hormone receptor expression in breast cancer. J. Histochem. Cytochem. 45, 1559–1565 [DOI] [PubMed] [Google Scholar]
- 45. Carlin A. F., Chang Y. C., Areschoug T., Lindahl G., Hurtado-Ziola N., King C. C., Varki A., Nizet V. (2009) Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J. Exp. Med. 206, 1691–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen D. H., Hurtado-Ziola N., Gagneux P., Varki A. (2006) Loss of Siglec expression on T lymphocytes during human evolution. Proc. Natl. Acad. Sci. U. S. A. 103, 7765–7770 [DOI] [PMC free article] [PubMed] [Google Scholar]