Abstract
Sirtuins have been widely reported to be involved in multiple biological processes; however, their function in oocyte meiosis has not been. Here, by confocal scanning and quantitative analysis, we show that specific depletion of Sirt2 in mouse oocytes results in spindle defects and chromosome disorganization (35.5±8.7 vs. 9.6±3.8% control; P<0.05), with impaired microtubule-kinetochore interaction. Moreover, knockdown and overexpression experiments reveal that Sirt2 modulates the acetylation status of histone H4K16 and α-tubulin in oocytes, which may in part mediate the defective phenotypes described above by influencing microtubule dynamics and kinetochore function. Finally, we find lower Sirt2 protein level in oocytes from aged mice by immunoblotting and that maternal age-associated meiotic defects can be ameliorated through overexpression of Sirt2 (33.2±5.1% old vs.12.7±5.2% old+Sirt2; P<0.05), providing support for the hypothesis that decreased Sirt2 is one of a number of factors contributing to oocyte age-dependent deficits. In summary, our data indicate a role for Sirt2 during oocyte meiosis and uncover a striking beneficial effect of increased Sirt2 expression on aged oocytes.—Zhang, L., Hou, X., Ma, R., Moley, K., Schedl, T., Wang, Q. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis.
Keywords: sirtuin, infertility, acetylation
A common cause of female infertility is poor oocyte quality. In mammals, oocytes are arrested within ovarian follicles at the diplotene stage of the first meiotic prophase, which is also termed germinal vesicle (GV) stage. Following stimulation by pituitary luteinizing hormone (LH), fully grown oocytes reinitiate meiosis, as indicated by GV breakdown (GVBD). Microtubules then organize into the specialized barrel-shaped bipolar spindle, with all the chromosomes aligned at the spindle equator, and proceed through the reductional meiosis I (MI) division, extruding the first polar body (Pb1), followed by formation of the meiosis II (MII) spindle, with arrest at metaphase of MII until fertilization (1, 2). During meiotic maturation, accurate control of spindle assembly and chromosome organization is necessary to produce a healthy oocyte. Errors at any number of steps in this process can lead to the generation of aneuploid eggs. Fertilization of aneuploid eggs in humans is a main cause of pregnancy loss and, if survival occurs to term, will result in developmental disabilities (3). One of the most well-known causes is chromosomal and spindle defects, which become much more prevalent with advancing maternal age and are considered the major factors responsible for the increased incidence of miscarriage and birth defects in women over 35 yr of age (4–6). Although various molecules and pathways have been proposed to contribute to age-associated deficits in oocyte meiosis, mechanisms that modulate the meiotic apparatus remain to be discovered, and management of fertility issues associated with advancing maternal age continues to be a challenge.
Sirtuins are NAD-dependent deacetylases that are highly conserved from bacteria to humans. In mammals the sirtuin family comprises 7 proteins (Sirt1–Sirt7), which vary in tissue specificity, subcellular localization, enzymatic activity and targets (7). The founding member Sirt1 has been the most extensively investigated. Lines of studies showed that Sirt1 is involved in transcriptional regulation, chromatin modification, energy metabolism and aging through deacetylating histone H3/H4 or nonhistone substrates, such as PGC-1α and LXRα (8–10). To date, SIRT2 has been linked to the regulation of mitotic progression (11), oxidative stress response (12), microtubule dynamics (13), chromatin condensation (14) and cell migration (15). Sirt3, Sirt4 and Sirt5 are found in the mitochondrial matrix and may directly control the activity of metabolic enzymes; they have crucial roles in the metabolic adaptation to dietary conditions, such as calorie restriction and fasting (16–18). More recently, it became apparent that Sirt3 also affects oxidative stress defense by protecting cells from reactive oxygen species (ROS) (19–21). Compared with Sirt1-Sirt5, not much is known about the physiology of Sirt6 and Sirt7. They were reported to have a role in genome maintenance and transcriptional activation (22–25). All the sirtuins are expressed in mouse and porcine oocytes (21, 26), thus potentially having important roles in oocyte development and function. In addition, resveratrol, initially found as an activator of yeast Sir2, was shown to protect against age-associated infertility in mice (27). Overall, sirtuins are implicated in multiple critical biological processes, yet their role during oocyte meiosis remains unknown. While investigating the role of each of the sirtuins in mouse oocyte development employing a morpholino (MO) knockdown (KD) screen, we discovered the involvement of Sirt2 in the regulation of spindle assembly and chromosome organization during meiosis, and report our findings here.
MATERIALS AND METHODS
All chemicals and culture media were purchased from Sigma (St. Louis, MO, USA) unless stated otherwise.
Mice
ICR mice were used in all experiments. To generate a natural aging mouse model, 42–45 wk old female mice (near the end of their reproductive life span) were selected. All experiments were approved by the Animal Care and Use Committee of Nanjing Medical University and were performed in accordance with institutional guidelines.
Antibodies
Rabbit polyclonal anti-Sirt2, mouse monoclonal anti-α-tubulin-FITC, and anti-acetyl-tubulin (Lys-40) antibodies were purchased from Sigma; rabbit polyclonal anti-β-actin, rabbit polyclonal anti-Myc, and rabbit monoclonal H4K16ac antibodies were purchased from Abcam (Cambridge, MA, USA); Human anti-centromere CREST antibody was purchased from Fitzgerald Industries International (Concord, MA, USA); rabbit polyclonal H3K9ac and H4K12ac antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA); and rabbit polyclonal H3K14ac antibody was obtained from Epigentek Group Inc. (Brooklyn, NY, USA). FITC-conjugated goat anti-rabbit IgG and TRITC-conjugated goat anti-rabbit IgG were purchased from Thermo Fisher Scientific (Rockford, IL, USA). Cy5-conjugated goat anti-human IgG and Cy5-conjugated goat anti-rabbit IgG were purchased from Jackson ImmunoResearch Laboratory (West Grove, PA, USA).
Oocyte collection and culture
Female mice (young 6–8 wk; old 42–45 wk) were used for oocyte collection. To collect fully grown GV oocytes, mice were superovulated with 5 IU pregnant mare serum gonadotropin (PMSG) by intraperitoneal injection, and 48 h later, cumulus-enclosed oocytes were obtained by manual rupturing of antral ovarian follicles. Cumulus cells were removed by repeatedly pipetting. For in vitro maturation, GV oocytes were cultured in M2 medium under mineral oil at 37°C in a 5% CO2 incubator.
To collect ovulated MII oocytes, mice received an injection of 5 IU human chorionic gonadotropin (hCG) 2 d after PMSG priming. Oocytes were recovered from oviduct ampullae 13.5 h after hCG treatment, and cumulus cells were removed by incubating briefly in 1 mg/ml hyaluronidase.
Plasmid construction and mRNA synthesis
Total RNA was extracted from 100 mouse oocytes using the Arcturus PicoPure RNA Isolation Kit (Applied Biosystems, Foster City, CA, USA), and the cDNA was generated with QIAquick PCR Purification Kit (Qiagen, Düsseldorf, Germany). The following primers were used to amplify the CDS sequence of Sirt2: forward primer, 5′-GGGGGCCGGCCG TCTCGGCCTCTTCTTGT-3′, and reverse primer, 5′-GGGGGCGCGCCGCCTGTTGTCTGGGAAT-3′. PCR products were purified, digested with FseI and AscI (New England Biolabs, Beverly, MA, USA), then cloned into the pCS2+ vector with 6 Myc tags.
For the synthesis of Myc-Sirt2 mRNA, the Sirt2-pCS2+ plasmids were linearized by NotI. Capped cRNAs were made using in vitro transcription with SP6 mMessage mMachine (Ambion, Austin, TX, USA) according to the manufacturer's instruction, and then purified by RNeasy Micro Kit (Qiagen). Synthesized RNA was portioned into aliquots and stored at −80°C.
Sirt2 KD and overexpression
Microinjections of morpholino or mRNA, with a Narishige (Tokyo, Japan) microinjector, were used to knock down or overexpress Sirt2 in mouse oocytes, respectively.
For overexpression experiments, 10 pl Myc–Sirt2 mRNA solution (10 ng/μl) was injected into cytoplasm of GV oocytes. The same amount of RNase-free PBS was injected as control.
For KD experiments, Sirt2 MO targeting initiation of translation 5′-TCGGGACTGTCACCG ACTGCTCTGT-3′ (Gene Tools, Philomath, OR, USA) was diluted with water to give a stock concentration of 1 mM, and then 2.5 nl MO solution was injected into oocytes. An MO standard control was injected as control.
After injections, oocytes were arrested at the GV stage in M2 medium supplemented with 2.5μM milrinone for 20 h to facilitate either KD of Sirt2 mRNA translation or permit Sirt2 overexpression, then washed 3 times in milrinone-free M2 medium, and cultured for 3 h to evaluate meiotic resumption (GVBD) or 14 h to determine the maturation status (Pb1 extrusion).
Western blotting
A pool of ∼150 oocytes was lysed in Laemmli sample buffer containing protease inhibitor and then subjected to 10% SDS-PAGE. The separated proteins were transferred to a PVDF membrane. Membranes were blocked in TBS containing 0.1% Tween 20 and 5% low-fat dry milk for 1 h and then incubated with primary antibodies as follows: rabbit anti-Sirt2 antibody (1:800) and rabbit anti-Myc antibody (1:1000). After multiple washes in TBS containing 0.1% Tween 20 and incubation with HRP-conjugated secondary antibodies. The protein bands were visualized using an ECL Plus Western Blotting Detection System (GE Healthcare, Little Chalfont, UK). The membrane was then washed and reblotted with anti-β-actin antibody (1:10,000) for loading control.
Immunofluorescence
Oocytes were fixed with 4% paraformaldehyde for 30 min and then permeabilized with 0.5% Triton X-100 for 20 min. Following blocking in 1% BSA-supplemented PBS for 1 h, samples were incubated overnight at 4°C with primary antibodies as follows: anti-H3K9ac antibody, anti-H3K14ac antibody, anti-H4K12ac antibody, anti-H4K16ac antibody, FITC-conjugated anti-tubulin antibody. To detect kinetochores, oocytes were colabeled with CREST (1:500) according to the previous protocol (28). Chromosomes were evaluated by staining with propidium iodide (PI; red) or Hoechst 33342 (blue) for 10 min. As PI is a DNA intercalater, and labels all double-stranded nucleic acids; so DNase free RNase from Boehringer (Ingelheim, Germany) at 25 mg/ml was added to the PI solution to get rid of the RNA. After 3 washes in PBS, oocyte samples were mounted on antifade medium (Vectashield; Vector Laboratories, Burlingame, CA, USA) and then examined under a laser scanning confocal microscope (LSM 710; Carl Zeiss, Oberkochen, Germany) equipped with the ×40 or ×63 oil objectives. Hoechst 33342 was visualized using a 405-nm laser (λEm 461 nm), FITC was visualized using a 488-nm laser (λEm 519 nm), TRITC and PI were visualized using a 561-nm laser (λEm 617 nm), and Cy5 was visualized using a 639-nm laser (λEm 670 nm). ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA) was used to quantify the intensity of fluorescence, as described previously (29).
Chromosome spread
Chromosome preparations for MII oocytes were as described previously (30). In brief, oocytes were treated with 1% sodium citrate for 20 min, individually transferred to a glass slide, and then fixed with several drops of 3 parts methanol to 1 part acetic acid. After air drying and nuclear staining, the chromosomes were observed by fluorescence microscopy.
Statistical analysis
Data are presented as means ± sd, unless otherwise indicated. Statistical comparisons were made with Student's t test and ANOVA when appropriate. Quantitative data were analyzed with Prism 5 software (GraphPad, San Diego, CA, USA). Values of P < 0.05 were considered to be significant.
RESULTS
Sirt2 KD adversely affects meiotic progression
To explore the function of Sirt2, fully grown oocytes were microinjected with specifically designed MO (Sirt2 MO); a sham MO standard was injected as control. After injections, oocytes were arrested at the GV stage in medium supplemented with milrinone for 20 h, during which the MO blocked endogenous Sirt2 mRNA translation. Then oocytes were washed in milrinone-free medium and cultured until the appropriate time points to analyze meiotic progression. Immunoblotting confirmed that Sirt2 protein level was knocked down (Fig. 1A). After 3 h culture, both control and Sirt2-KD groups resume meiosis normally, indicated by the similar GVBD rate (91.9±5.2 vs. 93.3±6.6% control; P>0.05; Fig. 1C). However, only 45.8% of Sirt2-KD oocytes (n=138) extruded Pb1 at 14 h, which was significantly reduced compared to control MO-injected oocytes (89.6%, n=162; Fig. 1B, D). Thus, more than half of Sirt2-KD oocytes failed to complete MI and form Pb1 (Fig. 1B, red arrowheads). In a small number of cases where a meiotic division appears to have been completed, a symmetrical “2-cell-like” egg was observed (Fig. 1B, black arrowhead), a phenotype that was ∼3 times more prevalent than in control oocytes. Together, these results suggest that Sirt2 is essential for oocyte maturation and meiotic divisions.
Figure 1.
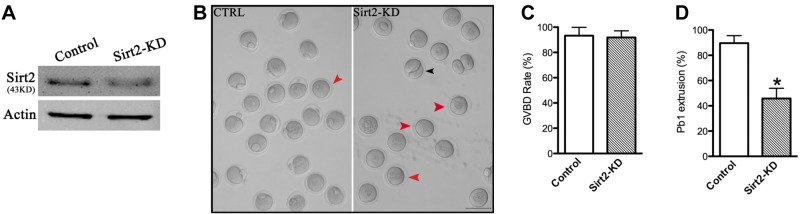
Effects of Sirt2 KD on oocyte maturation. Fully grown oocytes microinjected with Sirt2-MO were arrested at GV stage with milrinone for 20 h to block mRNA translation, washed in milrinone-free medium, and then cultured for 3 and 14 h to evaluate the rate of GVBD and Pb1 extrusion. A sham-MO standard was injected as control. A) KD of endogenous Sirt2 protein expression after Sirt2-MO injection was verified by Western blot analysis. B) Phase-contrast images of control MO-injected and Sirt2-KD oocytes. Red arrowheads indicate oocytes that fail to extrude a polar body; black arrowheads indicate oocytes with apparent symmetrical division. Scale bar = 100 μm. C, D) Quantitative analysis of GVBD rate (C) and Pb1 extrusion rate (D) in control (n=162) and Sirt2-KD (n=138) oocytes. Graph shows means ± sd of results obtained in 3 independent experiments. *P < 0.05 vs. control.
Localization of Sirt2 on the meiotic spindle
To further define the cellular events in which Sirt2 is involved during meiotic maturation, we examined its localization at different stages of mouse oocyte development using immunofluorescence microscopy. As shown in Fig. 2A, at GV stage, Sirt2 uniformly resides in the cytoplasm and nucleus of GV oocytes. Remarkably, as the oocytes enter into metaphase, Sirt2 becomes concentrated on the spindle region. During anaphase and telophase, Sirt2 continues to associate with the meiotic spindle region, especially with the midbody (arrows), a structure formed by the bundled microtubules originally from polar microtubules after metaphase. To confirm the above observations, we performed the double staining to evaluate the spatial relationship between Sirt2 and spindle. Metaphase oocytes labeled with Sirt2 and α-tubulin antibodies clearly showed the overlapping fluorescence signals, indicating that Sirt2 is enriched on the meiotic spindle (Fig. 2B).
Figure 2.
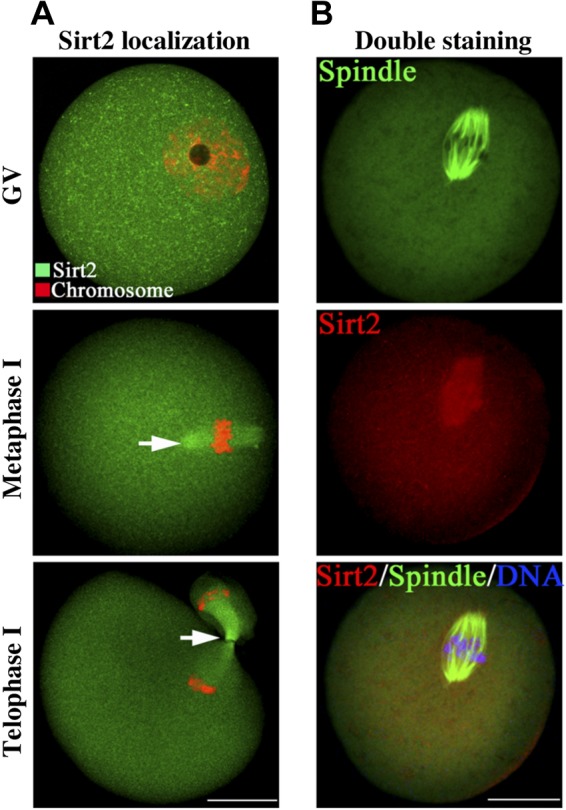
Confocal imaging analysis of the Sirt2 localization during oocyte meiosis. A) Oocytes at GV, metaphase, and telophase stages were immunolabeled with Sirt2 antibody (green) and counterstained with PI to visualize DNA (red). Arrows indicate Sirt2 signal. B) Double labeling of metaphase oocytes with Sirt2 antibody (red) and α-tubulin antibody (green), and counterstaining of DNA with Hoechst 33342 (blue), confirming the Sirt2 localization on the meiotic spindle. Scale bars = 30 μm.
Sirt2 functions in spindle formation and chromosome organization
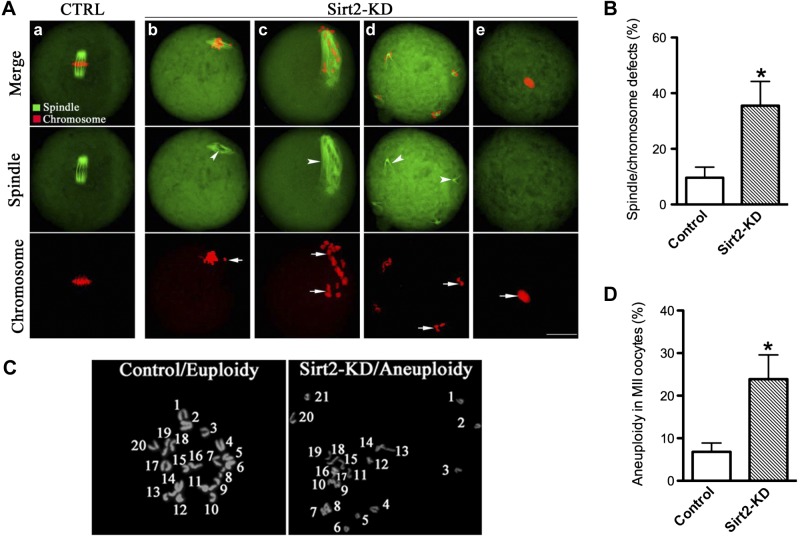
The specific positioning of Sirt2 to the spindle led us to hypothesize that Sirt2 plays a regulatory role in the meiotic apparatus and thus explore the consequences of Sirt2 ablation in oocytes. For this purpose, Sirt2 was knocked down by MO microinjection, and then oocytes were immunolabeled with anti-tubulin antibody to visualize the spindle and costained with PI for chromosomes. By performing confocal scanning and quantitative analysis, we found that most control MO-injected oocytes at metaphase presented with a typical barrel-shape spindle and well-aligned chromosomes on the metaphase plate (Fig. 3Aa). Only 9.6 ± 3.8% of control oocytes displayed the abnormal spindle and chromosomes (n=142; Fig. 3B). In striking contrast, a high frequency of spindle defects and chromosome disorganization was readily observed in Sirt2-KD oocytes (35.5±8.7%, n=128; Fig. 3B), showing diverse malformed spindles (Fig. 3Ab, c; arrowheads) and multipolar spindles (Fig. 3Ad, arrowheads), with the displacement of one or several chromosomes from equator (Fig. 3A, arrows). Notably, another severe phenotype of Sirt2-KD oocytes is that the spindle was not even formed, with the presence of irregular chromatin lump (Fig. 3Ae, arrows). These findings suggest that, in many cases, Sirt2-depleted oocytes cannot properly organize the meiotic spindle and align the meiotic chromosomes.
Figure 3.
Sirt2 knockdown causes spindle defects and chromosome misalignment in oocyte meiosis. A) Control MO-injected and Sirt2-KD oocytes were stained with α-tubulin antibody to visualize the spindle (green) and counterstained with PI to visualize chromosomes (red). a) Control MII oocytes present a typical barrel-shape spindle and well-aligned chromosomes on the metaphase plate. b–e) Spindle defects (arrowheads) and chromosome misalignment (arrows) were readily observed in Sirt2-KD MII oocytes. Representative confocal sections are shown. B) Quantification of control and Sirt2-KD oocytes with spindle defects or chromosome misalignment. Data are expressed as mean ± sd percentage from 3 independent experiments in which ≥120 oocytes were analyzed. C) Chromosome spread of control and Sirt2-KD MII oocytes. Representative negative fluorescence micrographs of euploid control oocytes and aneuploid Sirt2-KD oocytes. D) Incidence of aneuploidy in control and Sirt2-KD oocytes: 30 control oocytes and 38 Sirt2-KD oocytes were analyzed. Scale bars = 20 μm. *P < 0.05 vs. controls.
In addition, to further confirm whether the spindle defects and chromosome misalignment in Sirt2-KD oocytes would act to generate aneuploid eggs, we analyzed the karyotype of MII oocytes by chromosome spreading. As shown in Fig. 3C, D, aneuploidy was observed in 23.9% of Sirt2-KD oocytes, which is significantly higher than control cells (6.8%).
Sirt2 KD impairs kinetochore-microtubule interaction
Coordination between spindle maintenance and chromosome movement largely relies on kinetochore microtubule dynamics, which in turn is responsible for the generation of tension across sister kinetochores in mammalian mitosis (31). We, therefore, asked whether interkinetochore tension was defective in Sirt2-KD oocytes by measuring the distance between kinetochores in each bivalent. To test this, we immunolabeled MI oocytes with CREST to detect kinetochores, with anti-tubulin antibody to visualize the spindle and costained with Hoechst 33342 for chromosomes, as shown in Fig. 4A. We found that interkinetochore spacing was reduced in Sirt2-KD oocytes compared with control cells (average=1.21±0.56 μm compared with 1.85±0.32 μm in control cells; Fig. 4B).
Figure 4.
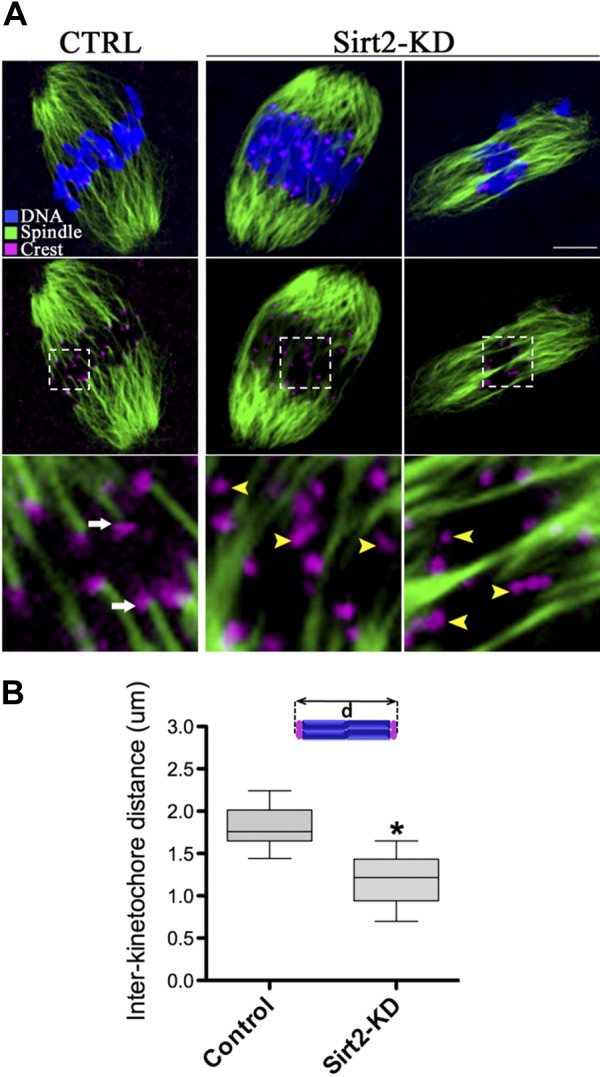
Sirt2-KD oocytes display impaired microtubule-kinetochore interaction. A) Control and Sirt2-KD MI oocytes were labeled with CREST to detect kinetochore (purple) and α-tubulin antibody to visualize spindle (green) and counterstained with Hoechst 33342 for chromosomes (blue). Representative confocal sections are shown. Images (top and middle panels) and enlargements (bottom panels) correspond to the merge of selected focal planes. Arrows indicate bioriented and attached kinetochores; arrowheads show defective and unattached kinetochores. Bottom panels show enlarged view of boxed areas in middle panels. B) Box plot of interkinetochore distance (in micrometers) measured in oocytes as indicated. Horizontal lines are the median; 12 control oocytes and 10 Sirt2-KD oocytes were analyzed respectively. Quantitative data were analyzed with Prism 5 software (GraphPad). *P < 0.05 vs. controls.
Simultaneously, we investigated the stability of kinetochore microtubules in the Sirt2-KD oocytes. Oocytes were briefly chilled at 4°C to induce depolymerization of microtubules that were not stably attached to kinetochores. We noticed that kinetochores were invariably attached to the microtubules in control oocyte (Fig. 4A; arrows); conversely, usually 3–5 unattached kinetochores can be detected in Sirt2-KD oocyte (Fig. 4A; arrowheads). Collectively, these findings are indicative of, in Sirt2-KD oocytes, reduction in the pulling forces across kinetochores and the stability of kinetochore microtubules, which could, at least in part, contribute to the chromosome alignment failure observed in our experiments.
Sirt2 KD leads to the hyperacetylation of H4K16 and α-tubulin
The effects of Sirt2 KD on spindle formation and chromosome alignment prompted us to consider the potential deacetylation targets in oocytes that might mediate this process. To date, a few substrates have been reported in different tissue and cell types. Cytosolic Sirt2 can function as an α-tubulin deacetylase and is suggested to have a role in oligodendroglial differentiation (13, 32). In the nucleus, Sirt2 acts as a histone deacetylase to regulate chromatin compaction and cell cycle (10, 33). Sirt2 also can deacetylate partitioning defective 3 homologue (PAR3) in Schwann cells to control myelin formation (34). In addition, Sirt2 may have roles in metabolic homeostasis by deacetylating phosphoenolpyruvate carboxykinase (PEPCK) and FOXO1 (35, 36).
Histone and α-tubulin appear to be the potential targets that could account for the phenotype of Sirt2-KD oocytes, because of the following properties: the acetylation status of histones is associated with various aspects of chromosome structure; and α-tubulin is a major component of the spindle. We thus first examined whether global levels of acetylation on specific histone residues were affected in Sirt2-KD oocytes. Unexpectedly, knockdown of Sirt2 resulted in a specific and drastic increase of H4K16 acetylation (mean fluorescence intensity 41.8±10.2 pixels compared with 9.6±1.6 pixels in control cells; Fig. 5G, H), whereas H3K9, H3K14 and H4K12 acetylation levels remained unchanged (Fig. 5A–F). Of note, hypoacetylation of H4K16 is critical to maintain kinetochore function in both mitotic cells and mammalian oocytes (37, 38).
Figure 5.
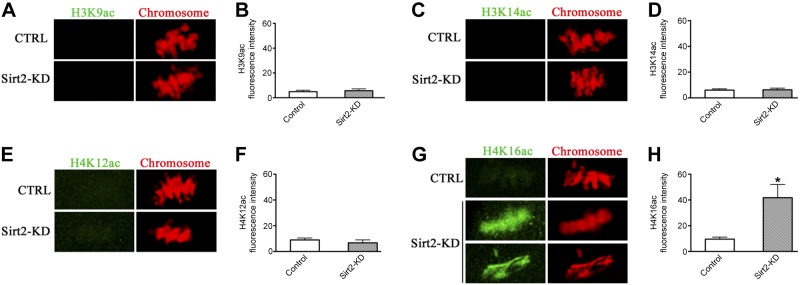
Sirt2 KD induces H4K16 hyperacetylation in oocytes. Metaphase II oocytes were immunolabeled with a panel of antibodies against different acetylated histones (green), and counterstained with PI to visualize chromosomes (red). A) Representative images of acetylated H3K9 (H3K9ac) in control and Sirt2-KD oocytes. B) Quantification of the data shown in A. C) Representative images of acetylated H3K14 (H3K14ac) in control and Sirt2-KD oocytes. D) Quantification of the data shown in C. E) Representative images of acetylated H4K12 (H4K12ac) in control and Sirt2-KD oocytes. F) Quantification of the data shown in E. G) Representative images of acetylated H4K16 (H4K16ac) in control and Sirt2-KD oocytes. H) Quantification of the data shown in G. For each histone variant, ≥30 oocytes for each group were analyzed, and the experiments were conducted 3 times. Bars represent means ± sd. *P < 0.05 vs. controls.
Tubulin is one of the most abundant nonhistone proteins that is subjected to acetylation, which occurs on lysine-40 of the α-tubulin subunit (39, 40). α-Tubulin acetylation serves as a marker for the presence of stable microtubules, may affect the activity of microtubule-associated proteins and microtubule-based motors (40–43). Since Sirt2 appears to be spatially correlated with spindle microtubules during oocyte meiosis, it is possible that Sirt2 regulates spindle function by directly deacetylating α-tubulin. To test this, we next evaluated the effects of Sirt2 depletion on the state of tubulin acetylation by staining oocytes with anti-acetylated α-tubulin antibody. We found that the acetylation levels of α-tubulin were significantly increased in Sirt2-KD oocytes in comparison with controls (mean fluorescence intensity 48.2±13.7 pixels compared with 27.4±6.9 pixels in control cells; Fig. 6A, B). Note that high levels of acetylated α-tubulin and H4K16 are correlated with abnormal spindle morphology and chromosome alignment, in more than 70% of affected oocytes, while the fluorescence intensity measurements shown were determined in all Sirt3-KD oocytes (Figs. 5G and 6B); thus, the levels of acetylated α-tubulin and H4K16 in affected oocytes are likely to be even larger. Altogether, KD of Sirt2 induced hyperacetylation of both H4K16 and α-tubulin in mouse oocytes, which could consequently result in spindle disorganization and chromosome congress failure via disturbing microtubule network and kinetochore function.
Figure 6.
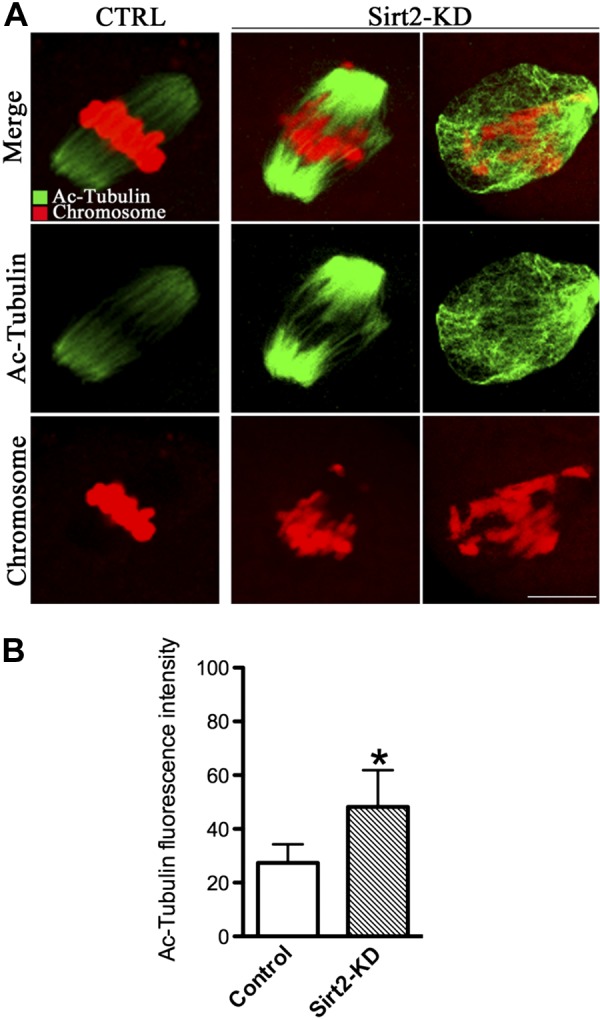
Increased acetylation of α-tubulin in Sirt2-KD oocytes. Metaphase II oocyte were immunolabeled with acetylated α-tubulin antibody (green), and chromosomes were counterstained with PI (red). A) Representative images of acetylated α-tubulin in control MO-injected and Sirt2-KD oocytes. B) Quantification of the data shown in A. Experiments were conducted 3 times, and ≥30 oocytes for each group were analyzed. Bars represent means ± sd. Scale bars = 15 μm. *P < 0.05 vs. control.
Sirt2 overexpression ameliorates maternal age-associated oocyte meiotic defects
It is well documented that female fertility decreases with advanced maternal age due to chromosomal and spindle abnormality in oocytes (6). To explore whether Sirt2 is involved in the maternal age-associated meiotic defects, the following three experiments were conducted.
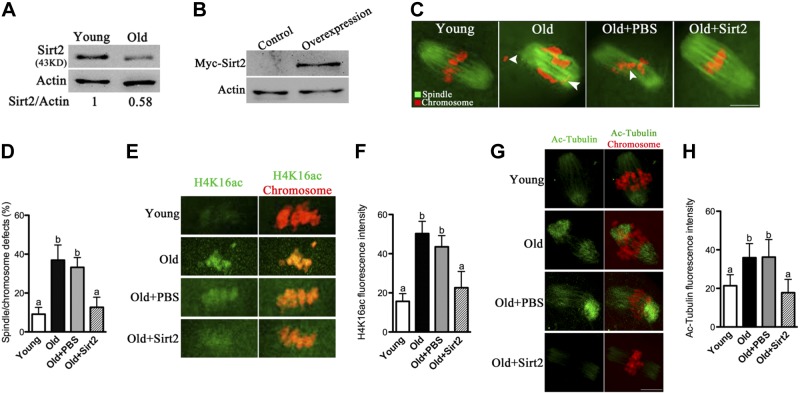
First, we performed Western blot analysis of oocytes isolated from young (6–8 wk) and aged (45–58 wk) mice, respectively. For brevity, these oocytes are called “young oocytes” and “old oocytes” here. Interestingly, we found that Sirt2 levels decrease in old oocytes compared to their young controls (Fig. 7A), suggesting that such a reduction may contribute to the penetrance of observed meiotic defects in old oocytes.
Figure 7.
Maternal age-associated oocyte spindle and chromosome abnormalities suppressed by Sirt2 overexpression. A) Western blot analysis revealed the reduced Sirt2 expression in ovulated MII oocytes from aged females compared to young controls. Actin served as a loading control throughout. Band intensity was calculated using ImageJ software; ratio of Sirt2/Actin expression was normalized, and values are indicated. B) PBS (control group) or exogenous Myc-Sirt2 mRNA (overexpression group) was microinjected into old GV oocytes, which were arrested for 20 h with milrinone to allow synthesis of new Myc-Sirt2 protein. Then the oocytes were collected for Western blot, detecting with anti-Myc Tag antibody. Results indicated that exogenous Myc-Sirt2 protein was efficiently overexpressed. C) Representative examples of meiotic spindle and chromosomes at MII stage in young oocytes, old oocytes, and old oocytes injected with PBS or Myc-Sirt2 mRNA. Arrowheads indicate misaligned chromosomes. D) Incidence of spindle/chromosome defects in indicated oocytes. Data are expressed as mean ± sd percentage from 3 independent experiments in which ≥100 oocytes were analyzed. E) Representative images of acetylated H4K16 (H4K16ac) at MII stage in indicated oocytes. F) Quantification of the data shown in E. G) Representative images of acetylated α-tubulin at MII stage in indicated oocytes. H) Quantification of the data shown in G. Experiments were conducted 3 times, and ≥30 oocytes for each group were analyzed. Different superscript letters indicate significant differences (P<0.05). Scale bars = 15 μm.
Next, we performed overexpression experiments to test whether enhancing Sirt2 expression in old oocytes could rescue their meiotic phenotypes. Exogenous Myc-Sirt2 mRNA was injected into old GV oocytes, which were arrested for 20 h with milrinone to allow synthesis of new Sirt2 protein. The oocytes were then washed and matured in normal medium to check their spindle and chromosome organization. Immunoblotting with anti-Myc Tag antibody confirmed that exogenous Myc-Sirt2 protein was efficiently overexpressed (Fig. 7B). Confocal analysis revealed that 90.9 ± 3.5% (n=118) of ovulated MII oocytes obtained from young mice exhibited normal spindle formation and chromosome alignment (with only 9.1% abnormal); however, 36.9 ± 7.8% (n=108) of MII oocytes retrieved from aged mice showed spindle defects or misaligned chromosomes. Importantly, these abnormalities were only detected in 12.7 ± 5.2% (n=102) of old oocytes with Sirt2 overexpression, which is significantly decreased as compared to those old oocytes injected with PBS (33.2±5.1%, n=110) (Fig. 7C, D).
Finally, we also assessed the acetylation status of H4K16 and α-tubulin in young oocytes and old oocytes, as well as old oocytes overexpressing Sirt2. Our data demonstrated that, compared to young oocytes, both H4K16 and tubulin acetylation were markedly increased in old oocytes; however, Sirt2 overexpression was able to restore their acetylation to approximately normal levels, as shown in Fig. 7E–H. Taken together, these results suggested that Sirt2 overexpression lowers H4K16 and α-tubulin acetylation levels in old oocytes and reduces the penetrance of maternal age-associated meiotic defects.
DISCUSSION
The studies presented here were designed to investigate the functions of Sirt2 during oocyte meiosis. We found spindle defects and chromosome misalignment, with deficient microtubule-kinetochore interaction, on specific KD of Sirt2 in mouse oocytes. Moreover, we discovered that Sirt2 controls the acetylation state of both α-tubulin and histone H4K16 in oocytes, which are critical for maintaining spindle morphology and kinetochore function. Finally, we provide strong evidence that Sirt2 is a key factor impacting oocyte aging.
Sirt2, H4K16/α-tubulin deacetylation, and spindle/chromosome organization
Although all sirtuins are expressed in mouse oocytes (21), their function during mammalian oocyte meiosis was not known. Here we demonstrated that Sirt2 is important for proper spindle formation and chromosome organization (Fig. 3). In support of this conclusion, similar to findings with mitotic cells (13, 44), we observe enrichment of Sirt2 protein on the meiotic apparatus—the spindle and midbody during oocyte maturation— presumably for its function in the meiotic divisions (Fig. 2). Furthermore, our data indicate that Sirt2 action in spindle/chromosome organization is likely mediated through histone H4K16 and α-tubulin deacetylation.
As revealed by our KD and overexpression experiments (Figs. 5–7), Sirt2 controls the level of H4K16 and α-tubulin acetylation in mouse oocytes. Both H4K16 and α-tubulin have been shown to be preferred substrates of Sirt2 during mitosis in somatic cells (10, 13). Studies suggest that H4K16ac inhibits folding of the chromatin fiber and, therefore, Sirt2 deactetylase activity facilitates the formation of high-order chromatin organization (45, 46). Furthermore, histone hyperacetylation can interfere with kinetochore assembly by disrupting pericentromeric heterochromatin in somatic cells (47). In budding yeast, which has 125-bp point centromeres (in contrast to 0.1- to 5-Mbp regional centromeres in fission yeast and humans), hypoacetylation of H4K16 is crucial for maintaining kinetochore function (37, 38). Of note, pulling forces across kinetochores are apparently defective, and the stability of kinetochore-microtubule is reduced in Sirt2-KD oocytes (Fig. 4). Thus, these findings support the notion that Sirt2 depletion-induced H4K16 hyperacetylation may compromise kinetochore function, thereby contribute to the misaligned chromosome phenotype we observed in oocytes.
The microtubule network is formed by the polymerization of α- and β-tubulin heterodimers (48). Regulation of the microtubule dynamics and organization is essential for bipolar spindle assembly and function. Tubulin subunits are subjected to numerous post-translational modifications, including tyrosination, phosphorylation, polyglycylation and acetylation (49). Tubulin acetylation occurs on Lys-40 of the α-tubulin subunit (40). Acetylated α-tubulin is abundant in stable microtubules but is absent in dynamic subcellular structures, such as the leading edges of the fibroblasts and neuronal growth cones (40, 42). Recent studies showed that tubulin acetylation restricts the number of protofilaments in nematode touch receptor neurons, which suggests that tubulin acetylation is involved in microtubule organization (50, 51). Tubulin acetylation has also been reported to influence the ability of microtubules to bind microtubule-associated proteins (MAPs) and motor proteins and, hence, may regulate microtubule stability and function (52–54). Acetylated microtubules have been observed in mouse oocytes (55, 56); however, their function(s) in meiosis are not clear. Here, we revealed that Sirt2 modulates the acetylation levels of α-tubulin in mouse oocytes and, correspondingly, spindle disorganization was detected in Sirt2-KD oocytes (Fig. 3, 6, and 7). It remains unknown how α-tubulin hyperacetylation, induced by Sirt2 KD, is related to the observed oocyte spindle defects. A plausible hypothesis is that hyperacetylation increases microtubule stability, disrupting spindle microtubule dynamics necessary for chromosome segregation and/or alters binding of tubulin to associated proteins.
We propose that KD of Sirt2 induces hyperacetylation of α-tubulin and H4K16, which in turn impairs microtubule stability and kinetochore function and results in oocyte meiotic spindle defects and chromosome misalignment. Our data do not distinguish whether α-tubulin, H4K16, or both mediate the meiotic phenotypes. We also cannot rule out that Sirt2 may act on other substrates, for example cohesins, in its function during oocyte maturation. Additional experiments will be required to understand Sirt2 function in these processes.
Sirt2 and oocyte aging
A hallmark of animal development is an age-related decrease in fertility. In mammals, this condition is largely attributed to females producing eggs of reduced developmental competence, particularly with chromosomal and spindle abnormalities (57, 58). Multiple factors appear to contribute to the maternal age effect, for example inefficient spindle assembly checkpoint control and loss of sister chromatid cohesion (59). However, additional factors are likely to contribute and their identification will be important for the clinical management of fertility issues associated with maternal age. In the present study, we observed that Sirt2 protein levels are reduced in old mouse oocytes, which display increased frequency of MII spindle defects and misaligned chromosomes. Through overexpression of Sirt2 the oocyte meiotic defects where ameliorated, with the tubulin and histone acetylation levels restored (Fig. 7). Both weakened kinetochore function and compromised histone deacetylation have been proposed to contribute to the meiotic defects observed with oocyte aging (60–62), consistent with our findings. Recently, Selesniemi et al. (63) have reported that caloric restriction or loss of metabolic regulator PGC-1α improves oocyte quality in aged female mice. Dissection in greater depth of the molecular pathways mediating the effects of Sirt2 on oocyte aging, such as the possible interaction between Sirt2 and PGC-1α, deserves further investigation.
In addition, genetic studies revealed that Sirt2−/−mice displayed normal embryonic and postnatal development (33, 64, 65), whereas their fertility issues have not been fully characterized and reported. It is also worthy of note that, despite that Sirt3-knockout mice were suggested to be viable and fertile (66), embryos derived from Sirt3−/− eggs showed the significantly decreased fertilization and blastocyst formation rates, regardless of the paternal genotype (21). Hence, our ongoing research is to systematically analyze oocyte quality and the related reproductive phenotypes using Sirt2 knockout and transgenic mice model.
In summary, our data indicate a role for Sirt2 during oocyte maturation and uncover the striking beneficial effects of Sirt2 overexpression on aged oocytes, which opens a new area for understanding mechanisms as well as assessing oocyte quality. Furthermore, additional studies are warranted to evaluate the therapeutic utility of sirtuin manipulation for fertility issues associated with maternal aging.
Acknowledgments
The authors thank Professor Dong Zhang (Nanjing Medical University) for technical assistance, particularly with the kinetochore detection.
This work was supported by the National Natural Science Foundation of China (31271541 and 31301181), Nanjing Medical University Startup Funding (2012RC04), and the U.S. National Institutes of Health (GM100756; T.S.).
The authors declare no conflicts of interest.
Footnotes
- GV
- germinal vesicle
- GVBD
- germial vesicle breakdown
- hCG
- human chorionic gonadotropin
- KD
- knockdown
- LH
- luteinizing hormone
- MI
- meiosis I
- MII
- meiosis II
- MO
- morpholino
- Pb1
- first polar body
- PI
- propidium iodide
- PMSG
- pregnant mare serum gonadotropin
REFERENCES
- 1. Wang Q., Sun Q. Y. (2007) Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod. Fertil. Dev. 19, 1–12 [DOI] [PubMed] [Google Scholar]
- 2. Eppig J. J., O'Brien M., Wigglesworth K. (1996) Mammalian oocyte growth and development in vitro. Mol. Reprod. Dev. 44, 260–273 [DOI] [PubMed] [Google Scholar]
- 3. Hassold T., Hunt P. (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280–291 [DOI] [PubMed] [Google Scholar]
- 4. Battaglia D. E., Goodwin P., Klein N. A., Soules M. R. (1996) Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum. Reprod. 11, 2217–2222 [DOI] [PubMed] [Google Scholar]
- 5. Henderson S. A., Edwards R. G. (1968) Chiasma frequency and maternal age in mammals. Nature 218, 22–28 [DOI] [PubMed] [Google Scholar]
- 6. Hunt P. A., Hassold T. J. (2008) Human female meiosis: what makes a good egg go bad? Trends Genet. 24, 86–93 [DOI] [PubMed] [Google Scholar]
- 7. Houtkooper R. H., Pirinen E., Auwerx J. (2012) Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imai S., Armstrong C. M., Kaeberlein M., Guarente L. (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 [DOI] [PubMed] [Google Scholar]
- 9. Vaquero A., Scher M., Erdjument-Bromage H., Tempst P., Serrano L., Reinberg D. (2007) SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450, 440–444 [DOI] [PubMed] [Google Scholar]
- 10. Vaquero A., Scher M. B., Lee D. H., Sutton A., Cheng H. L., Alt F. W., Serrano L., Sternglanz R., Reinberg D. (2006) SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 20, 1256–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dryden S. C., Nahhas F. A., Nowak J. E., Goustin A. S., Tainsky M. A. (2003) Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol. Cell. Biol. 23, 3173–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang F., Nguyen M., Qin F. X., Tong Q. (2007) SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6, 505–514 [DOI] [PubMed] [Google Scholar]
- 13. North B. J., Marshall B. L., Borra M. T., Denu J. M., Verdin E. (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11, 437–444 [DOI] [PubMed] [Google Scholar]
- 14. Inoue T., Hiratsuka M., Osaki M., Yamada H., Kishimoto I., Yamaguchi S., Nakano S., Katoh M., Ito H., Oshimura M. (2007) SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene 26, 945–957 [DOI] [PubMed] [Google Scholar]
- 15. Pandithage R., Lilischkis R., Harting K., Wolf A., Jedamzik B., Luscher-Firzlaff J., Vervoorts J., Lasonder E., Kremmer E., Knoll B., Luscher B. (2008) The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J. Cell. Biol. 180, 915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirschey M. D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D. B., Grueter C. A., Harris C., Biddinger S., Ilkayeva O. R., Stevens R. D., Li Y., Saha A. K., Ruderman N. B., Bain J. R., Newgard C. B., Farese R. V., Jr., Alt F. W., Kahn C. R., Verdin E. (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakagawa T., Lomb D. J., Haigis M. C., Guarente L. (2009) SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137, 560–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakagawa T., Guarente L. (2011) Sirtuins at a glance. J. Cell Sci. 124, 833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Someya S., Yu W., Hallows W. C., Xu J., Vann J. M., Leeuwenburgh C., Tanokura M., Denu J. M., Prolla T. A. (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143, 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jing E., Emanuelli B., Hirschey M. D., Boucher J., Lee K. Y., Lombard D., Verdin E. M., Kahn C. R. (2011) Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. U. S. A. 108, 14608–14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawamura Y., Uchijima Y., Horike N., Tonami K., Nishiyama K., Amano T., Asano T., Kurihara Y., Kurihara H. (2010) Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J. Clin. Invest. 120, 2817–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mostoslavsky R., Chua K. F., Lombard D. B., Pang W. W., Fischer M. R., Gellon L., Liu P., Mostoslavsky G., Franco S., Murphy M. M., Mills K. D., Patel P., Hsu J. T., Hong A. L., Ford E., Cheng H. L., Kennedy C., Nunez N., Bronson R., Frendewey D., Auerbach W., Valenzuela D., Karow M., Hottiger M. O., Hursting S., Barrett J. C., Guarente L., Mulligan R., Demple B., Yancopoulos G. D., Alt F. W. (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 [DOI] [PubMed] [Google Scholar]
- 23. Zhong L., D'Urso A., Toiber D., Sebastian C., Henry R. E., Vadysirisack D. D., Guimaraes A., Marinelli B., Wikstrom J. D., Nir T., Clish C. B., Vaitheesvaran B., Iliopoulos O., Kurland I., Dor Y., Weissleder R., Shirihai O. S., Ellisen L. W., Espinosa J. M., Mostoslavsky R. (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ford E., Voit R., Liszt G., Magin C., Grummt I., Guarente L. (2006) Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 20, 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vakhrusheva O., Smolka C., Gajawada P., Kostin S., Boettger T., Kubin T., Braun T., Bober E. (2008) Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 102, 703–710 [DOI] [PubMed] [Google Scholar]
- 26. Kwak S. S., Cheong S. A., Yoon J. D., Jeon Y., Hyun S. H. (2012) Expression patterns of sirtuin genes in porcine preimplantation embryos and effects of sirtuin inhibitors on in vitro embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology 78, 1597–1610 [DOI] [PubMed] [Google Scholar]
- 27. Liu M., Yin Y., Ye X., Zeng M., Zhao Q., Keefe D. L., Liu L. (2013) Resveratrol protects against age-associated infertility in mice. Hum. Reprod. 28, 707–717 [DOI] [PubMed] [Google Scholar]
- 28. Zhang D., Ma W., Li Y. H., Hou Y., Li S. W., Meng X. Q., Sun X. F., Sun Q. Y., Wang W. H. (2004) Intra-oocyte localization of MAD2 and its relationship with kinetochores, microtubules, and chromosomes in rat oocytes during meiosis. Biol. Reprod. 71, 740–748 [DOI] [PubMed] [Google Scholar]
- 29. Wang Q., Chi M. M., Moley K. H. (2012) Live imaging reveals the link between decreased glucose uptake in ovarian cumulus cells and impaired oocyte quality in female diabetic mice. Endocrinology 153, 1984–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hunt P. A., Koehler K. E., Susiarjo M., Hodges C. A., Ilagan A., Voigt R. C., Thomas S., Thomas B. F., Hassold T. J. (2003) Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 13, 546–553 [DOI] [PubMed] [Google Scholar]
- 31. Nezi L., Musacchio A. (2009) Sister chromatid tension and the spindle assembly checkpoint. Curr. Opin. Cell Biol. 21, 785–795 [DOI] [PubMed] [Google Scholar]
- 32. Li W., Zhang B., Tang J., Cao Q., Wu Y., Wu C., Guo J., Ling E. A., Liang F. (2007) Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J. Neurosci. 27, 2606–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Serrano L., Martinez-Redondo P., Marazuela-Duque A., Vazquez B. N., Dooley S. J., Voigt P., Beck D. B., Kane-Goldsmith N., Tong Q., Rabanal R. M., Fondevila D., Munoz P., Kruger M., Tischfield J. A., Vaquero A. (2013) The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev. 27, 639–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beirowski B., Gustin J., Armour S. M., Yamamoto H., Viader A., North B. J., Michan S., Baloh R. H., Golden J. P., Schmidt R. E., Sinclair D. A., Auwerx J., Milbrandt J. (2011) Sir-two-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling. Proc. Natl. Acad. Sci. U. S. A. 108, E952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang W., Wang S., Xiao M., Lin Y., Zhou L., Lei Q., Xiong Y., Guan K. L., Zhao S. (2011) Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol. Cell 43, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jing E., Gesta S., Kahn C. R. (2007) SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 6, 105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choy J. S., Acuna R., Au W. C., Basrai M. A. (2011) A role for histone H4K16 hypoacetylation in Saccharomyces cerevisiae kinetochore function. Genetics 189, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma P., Schultz R. M. (2013) Histone deacetylase 2 (HDAC2) regulates chromosome segregation and kinetochore function via H4K16 deacetylation during oocyte maturation in mouse. PLoS Genet. 9, e1003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nogales E., Whittaker M., Milligan R. A., Downing K. H. (1999) High-resolution model of the microtubule. Cell 96, 79–88 [DOI] [PubMed] [Google Scholar]
- 40. Piperno G., LeDizet M., Chang X. J. (1987) Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell. Biol. 104, 289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inoue T., Hiratsuka M., Osaki M., Oshimura M. (2007) The molecular biology of mammalian SIRT proteins: SIRT2 in cell cycle regulation. Cell Cycle 6, 1011–1018 [DOI] [PubMed] [Google Scholar]
- 42. Robson S. J., Burgoyne R. D. (1989) Differential localisation of tyrosinated, detyrosinated, and acetylated alpha-tubulins in neurites and growth cones of dorsal root ganglion neurons. Cell Motil. Cytoskel. 12, 273–282 [DOI] [PubMed] [Google Scholar]
- 43. Zilberman Y., Ballestrem C., Carramusa L., Mazitschek R., Khochbin S., Bershadsky A. (2009) Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J. Cell Sci. 122, 3531–3541 [DOI] [PubMed] [Google Scholar]
- 44. North B. J., Verdin E. (2007) Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PloS One 2, e784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shogren-Knaak M., Ishii H., Sun J. M., Pazin M. J., Davie J. R., Peterson C. L. (2006) Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311, 844–847 [DOI] [PubMed] [Google Scholar]
- 46. Robinson P. J., An W., Routh A., Martino F., Chapman L., Roeder R. G., Rhodes D. (2008) 30 nm chromatin fibre decompaction requires both H4-K16 acetylation and linker histone eviction. J. Mol. Biol. 381, 816–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Robbins A. R., Jablonski S. A., Yen T. J., Yoda K., Robey R., Bates S. E., Sackett D. L. (2005) Inhibitors of histone deacetylases alter kinetochore assembly by disrupting pericentromeric heterochromatin. Cell Cycle 4, 717–726 [DOI] [PubMed] [Google Scholar]
- 48. Nogales E. (2000) Structural insights into microtubule function. Annual. Rev. Biochem. 69, 277–302 [DOI] [PubMed] [Google Scholar]
- 49. MacRae T. H. (1997) Tubulin post-translational modifications–enzymes and their mechanisms of action. Eur. J. Biochem. 244 265–278 [DOI] [PubMed] [Google Scholar]
- 50. Cueva J. G., Hsin J., Huang K. C., Goodman M. B. (2012) Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Curr. Biol. 22, 1066–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Topalidou I., Keller C., Kalebic N., Nguyen K. C., Somhegyi H., Politi K. A., Heppenstall P., Hall D. H., Chalfie M. (2012) Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr. Biol. 22, 1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dompierre J. P., Godin J. D., Charrin B. C., Cordelieres F. P., King S. J., Humbert S., Saudou F. (2007) Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J. Neurosci. 27, 3571–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reed N. A., Cai D., Blasius T. L., Jih G. T., Meyhofer E., Gaertig J., Verhey K. J. (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 16, 2166–2172 [DOI] [PubMed] [Google Scholar]
- 54. Sudo H., Baas P. W. (2010) Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J. Neurosci. 30, 7215–7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Pennart H., Houliston E., Maro B. (1988) Post-translational modifications of tubulin and the dynamics of microtubules in mouse oocytes and zygotes. Biol. Cell 64, 375–378 [DOI] [PubMed] [Google Scholar]
- 56. Schatten G., Simerly C., Asai D. J., Szoke E., Cooke P., Schatten H. (1988) Acetylated alpha-tubulin in microtubules during mouse fertilization and early development. Dev. Biol. 130, 74–86 [DOI] [PubMed] [Google Scholar]
- 57. Luke B., Brown M. B., Wantman E., Lederman A., Gibbons W., Schattman G. L., Lobo R. A., Leach R. E., Stern J. E. (2012) Cumulative birth rates with linked assisted reproductive technology cycles. N. Engl. J. Med. 366, 2483–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Navot D., Drews M. R., Bergh P. A., Guzman I., Karstaedt A., Scott R. T., Jr., Garrisi G. J., Hofmann G. E. (1994) Age-related decline in female fertility is not due to diminished capacity of the uterus to sustain embryo implantation. Fertil. Steril. 61, 97–101 [DOI] [PubMed] [Google Scholar]
- 59. Nagaoka S. I., Hassold T. J., Hunt P. A. (2012) Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13, 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chiang T., Duncan F. E., Schindler K., Schultz R. M., Lampson M. A. (2010) Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr. Biol. 20, 1522–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Akiyama T., Nagata M., Aoki F. (2006) Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proc. Natl. Acad. Sci. U. S. A. 103, 7339–7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van den Berg I. M., Eleveld C., van der Hoeven M., Birnie E., Steegers E. A., Galjaard R. J., Laven J. S., van Doorninck J. H. (2011) Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Hum. Reprod. 26, 1181–1190 [DOI] [PubMed] [Google Scholar]
- 63. Selesniemi K., Lee H. J., Muhlhauser A., Tilly J. L. (2011) Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl. Acad. Sci. U. S. A. 108, 12319–12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim H. S., Vassilopoulos A., Wang R. H., Lahusen T., Xiao Z., Xu X., Li C., Veenstra T. D., Li B., Yu H., Ji J., Wang X. W., Park S. H., Cha Y. I., Gius D., Deng C. X. (2011) SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 20, 487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bobrowska A., Donmez G., Weiss A., Guarente L., Bates G. (2012) SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington's disease phenotypes in vivo. PloS One 7, e34805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lombard D. B., Alt F. W., Cheng H. L., Bunkenborg J., Streeper R. S., Mostoslavsky R., Kim J., Yancopoulos G., Valenzuela D., Murphy A., Yang Y., Chen Y., Hirschey M. D., Bronson R. T., Haigis M., Guarente L. P., Farese R. V., Jr., Weissman S., Verdin E., Schwer B. (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27, 8807–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]