Abstract
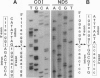
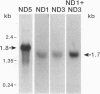
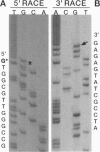
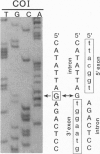
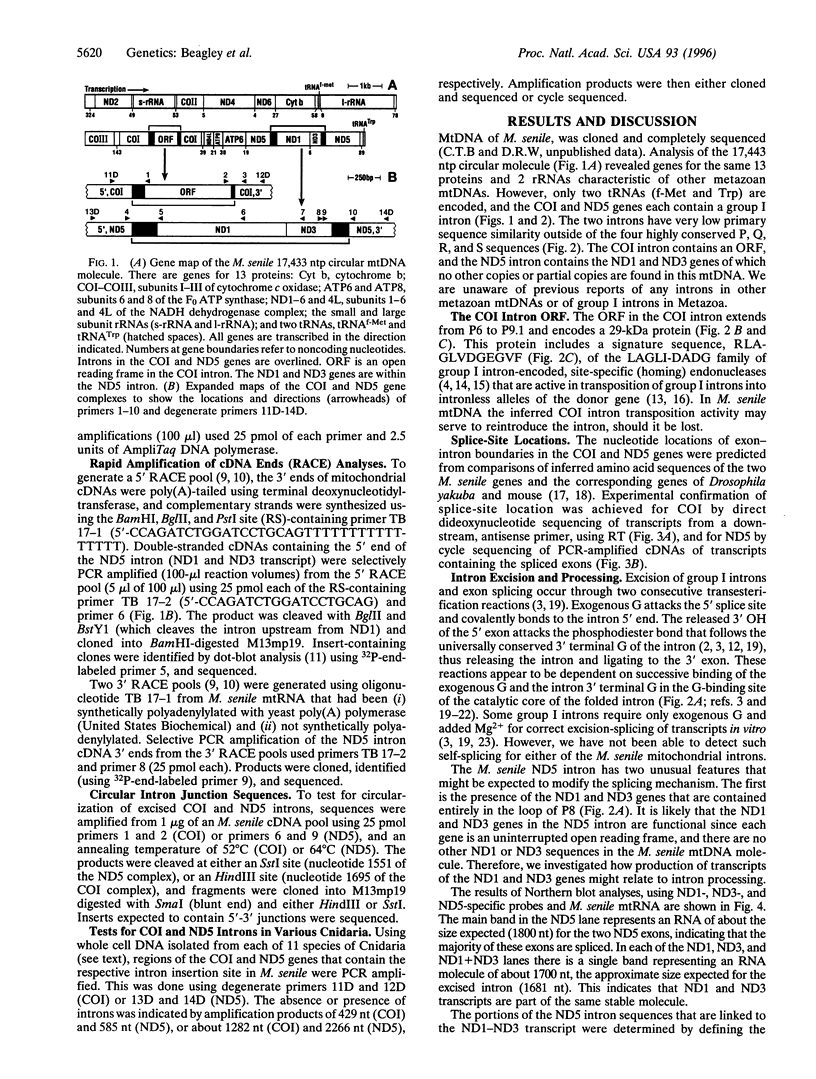
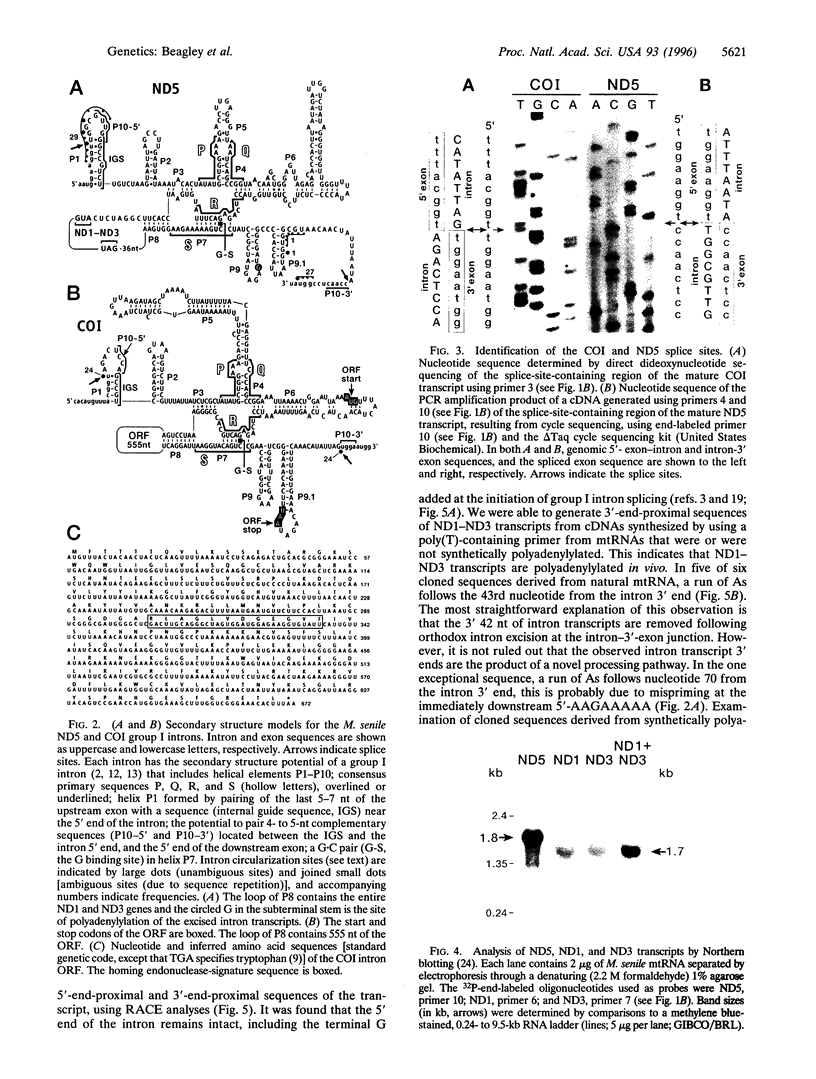
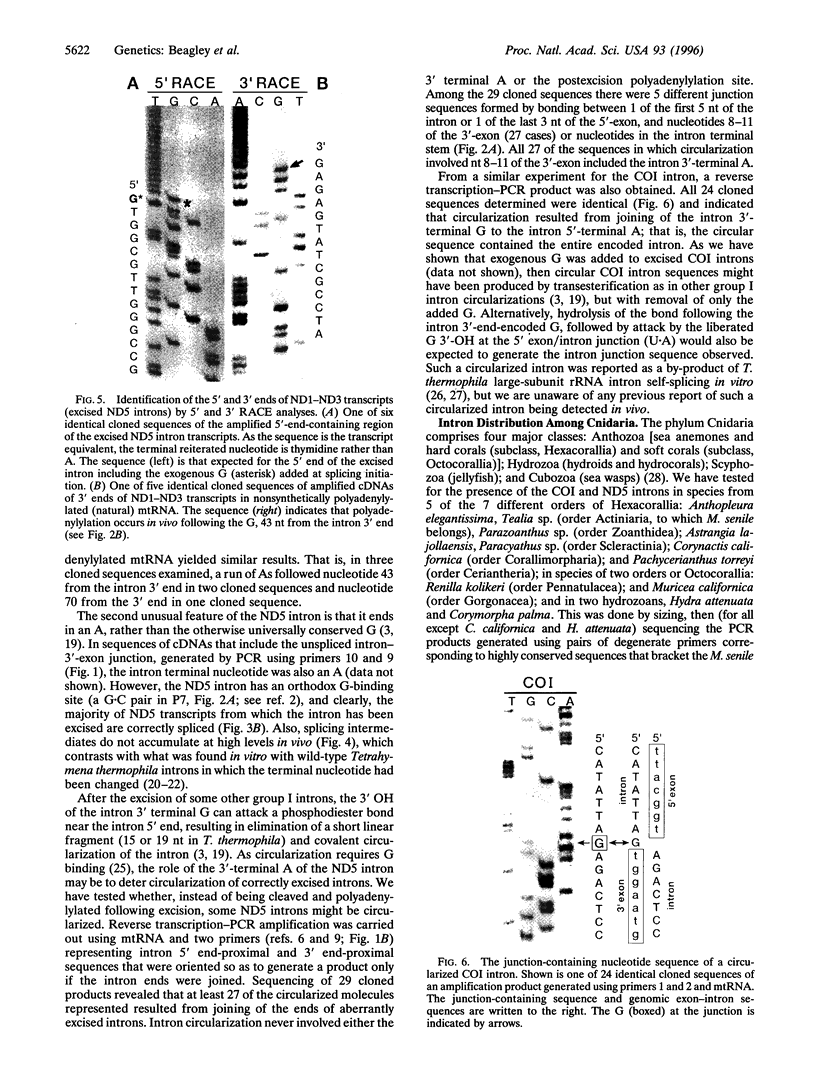
Mitochondrial genes for cytochrome c oxidase subunit I (COI) and NADH dehydrogenase subunit 5 (ND5) of the sea anemone Metridium senile (phylum Cnidaria) each contain a group I intron. This is in contrast to the reported absence of introns in all other metazoan mtDNAs so far examined. The ND5 intron is unusual in that it ends with A and contains two genes (ND1 and ND3) encoding additional subunits of NADH dehydrogenase. Correctly excised ND5 introns are not circularized but are precisely cleaved near their 3' ends and polyadenylylated to provide bicistronic transcripts of ND1 and ND3. COI introns, which encode a putative homing endonuclease, circularize, but in a way that retains the entire genome-encoded intron sequence (other group I introns are circularized with loss of a short segment of the intron 5' end). Introns were detected in the COI and ND5 genes of other sea anemones, but not in the COI and ND5 genes of other cnidarians. This suggests that the sea anemone mitochondrial introns may have been acquired relatively recently.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G. Animal mitochondrial DNA: an extreme example of genetic economy. Int Rev Cytol. 1985;93:93–145. doi: 10.1016/s0074-7696(08)61373-x. [DOI] [PubMed] [Google Scholar]
- Been M. D., Cech T. R. Sites of circularization of the Tetrahymena rRNA IVS are determined by sequence and influenced by position and secondary structure. Nucleic Acids Res. 1985 Dec 9;13(23):8389–8408. doi: 10.1093/nar/13.23.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been M. D., Perrotta A. T. Group I intron self-splicing with adenosine: evidence for a single nucleoside-binding site. Science. 1991 Apr 19;252(5004):434–437. doi: 10.1126/science.2017681. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Biniszkiewicz D., Cesnaviciene E., Shub D. A. Self-splicing group I intron in cyanobacterial initiator methionine tRNA: evidence for lateral transfer of introns in bacteria. EMBO J. 1994 Oct 3;13(19):4629–4635. doi: 10.1002/j.1460-2075.1994.tb06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- Boore J. L., Brown W. M. Complete sequence of the mitochondrial DNA of the annelid worm Lumbricus terrestris. Genetics. 1995 Sep;141(1):305–319. doi: 10.1093/genetics/141.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. M., RajBhandary U. L. Intron within the large rRNA gene of N. crassa mitochondria: a long open reading frame and a consensus sequence possibly important in splicing. Cell. 1982 Dec;31(3 Pt 2):509–520. doi: 10.1016/0092-8674(82)90307-5. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Conserved sequences and structures of group I introns: building an active site for RNA catalysis--a review. Gene. 1988 Dec 20;73(2):259–271. doi: 10.1016/0378-1119(88)90492-1. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing of group I introns. Annu Rev Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Domenico J. M., Michel F. DNA sequence and organization of the mitochondrial ND1 gene from Podospora anserina: analysis of alternate splice sites. Curr Genet. 1988 Sep;14(3):253–264. doi: 10.1007/BF00376746. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., McNally K. L., Domenico J. M., Matsuura E. T. The complete DNA sequence of the mitochondrial genome of Podospora anserina. Curr Genet. 1990 May;17(5):375–402. doi: 10.1007/BF00334517. [DOI] [PubMed] [Google Scholar]
- Dalgaard J. Z., Garrett R. A., Belfort M. A site-specific endonuclease encoded by a typical archaeal intron. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5414–5417. doi: 10.1073/pnas.90.12.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. The comings and goings of homing endonucleases and mobile introns. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5379–5381. doi: 10.1073/pnas.90.12.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Sullivan F. X., Cech T. R. New reactions of the ribosomal RNA precursor of Tetrahymena and the mechanism of self-splicing. J Mol Biol. 1986 May 5;189(1):143–165. doi: 10.1016/0022-2836(86)90387-6. [DOI] [PubMed] [Google Scholar]
- Kruger K., Grabowski P. J., Zaug A. J., Sands J., Gottschling D. E., Cech T. R. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982 Nov;31(1):147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Belfort M. Introns as mobile genetic elements. Annu Rev Biochem. 1993;62:587–622. doi: 10.1146/annurev.bi.62.070193.003103. [DOI] [PubMed] [Google Scholar]
- Michel F., Hanna M., Green R., Bartel D. P., Szostak J. W. The guanosine binding site of the Tetrahymena ribozyme. Nature. 1989 Nov 23;342(6248):391–395. doi: 10.1038/342391a0. [DOI] [PubMed] [Google Scholar]
- Michel F., Umesono K., Ozeki H. Comparative and functional anatomy of group II catalytic introns--a review. Gene. 1989 Oct 15;82(1):5–30. doi: 10.1016/0378-1119(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Michel F., Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990 Dec 5;216(3):585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- Nelson M. A., Macino G. Structure and expression of the overlapping ND4L and ND5 genes of Neurospora crassa mitochondria. Mol Gen Genet. 1987 Feb;206(2):307–317. doi: 10.1007/BF00333589. [DOI] [PubMed] [Google Scholar]
- Oda K., Yamato K., Ohta E., Nakamura Y., Takemura M., Nozato N., Akashi K., Kanegae T., Ogura Y., Kohchi T. Gene organization deduced from the complete sequence of liverwort Marchantia polymorpha mitochondrial DNA. A primitive form of plant mitochondrial genome. J Mol Biol. 1992 Jan 5;223(1):1–7. doi: 10.1016/0022-2836(92)90708-r. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Butow R. A. Mobile introns and intron-encoded proteins. Science. 1989 Dec 1;246(4934):1106–1109. doi: 10.1126/science.2479980. [DOI] [PubMed] [Google Scholar]
- Pont-Kingdon G. A., Beagley C. T., Okimoto R., Wolstenholme D. R. Mitochondrial DNA of the sea anemone, Metridium senile (Cnidaria): prokaryote-like genes for tRNA(f-Met) and small-subunit ribosomal RNA, and standard genetic code specificities for AGR and ATA codons. J Mol Evol. 1994 Oct;39(4):387–399. doi: 10.1007/BF00160271. [DOI] [PubMed] [Google Scholar]
- Price J. V., Cech T. R. Determinants of the 3' splice site for self-splicing of the Tetrahymena pre-rRNA. Genes Dev. 1988 Nov;2(11):1439–1447. doi: 10.1101/gad.2.11.1439. [DOI] [PubMed] [Google Scholar]
- Tanner N. K., Cech T. R. Guanosine binding required for cyclization of the self-splicing intervening sequence ribonucleic acid from Tetrahymena thermophila. Biochemistry. 1987 Jun 16;26(12):3330–3340. doi: 10.1021/bi00386a013. [DOI] [PubMed] [Google Scholar]
- Thomson M. C., Macfarlane J. L., Beagley C. T., Wolstenholme D. R. RNA editing of mat-r transcripts in maize and soybean increases similarity of the encoded protein to fungal and bryophyte group II intron maturases: evidence that mat-r encodes a functional protein. Nucleic Acids Res. 1994 Dec 25;22(25):5745–5752. doi: 10.1093/nar/22.25.5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M., Côté V., Otis C., Mercier J. P., Gray M. W., Lonergan K. M., Lemieux C. Evolutionary transfer of ORF-containing group I introns between different subcellular compartments (chloroplast and mitochondrion). Mol Biol Evol. 1995 Jul;12(4):533–545. doi: 10.1093/oxfordjournals.molbev.a040234. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R. Animal mitochondrial DNA: structure and evolution. Int Rev Cytol. 1992;141:173–216. doi: 10.1016/s0074-7696(08)62066-5. [DOI] [PubMed] [Google Scholar]