Abstract
We have previously demonstrated that rat islets express a high density of angiotensin type 2 receptors and that activation of this receptor evokes insulinotropic effect. In this study, we evaluated the protective effects of Compound 21 (C21), a nonpeptide angiotensin type 2 receptor agonist, on islets in streptozotocin (STZ)-induced diabetes. Rats were assigned to five groups: normal, STZ, and STZ plus C21 (0.24, 0.48, and 0.96 mg/kg·d). C21 was continually infused by a sc implanted osmotic minipump for 14 days, and STZ was bolus injected on day 7. Body weight, water intake, urine excretion, and blood glucose were monitored daily. On the last day, the rats received an oral glucose tolerance test, and the pancreata were saved to examine islet morphology and biochemical parameters of oxidative stress and apoptosis. We found that, compared with control STZ rats, C21-treated STZ rats displayed less water intake and urine excretion, lower blood glucose, higher serum insulin concentration, and improved glucose tolerance. These rats had more islets, larger islet mass, and up-regulated insulin protein and proinsulin 2 mRNA expressions in the pancreas. Their islets displayed lower superoxide, decreased gp91 expression, and increased superoxide dismutase 1 expression as well as less apoptosis and down-regulated caspase-3 expression. In the epididymal adipose tissue of these rats, we found a decreased adipocyte size and up-regulated adipocyte protein 2 expression. The protective effects of C21 on β-cells against the toxic effects of STZ were also confirmed in cultured INS-1E cells. These data suggest that C21 ameliorates STZ-induced diabetes by protecting pancreatic islets via antioxidative and antiapoptotic effects.
The renin-angiotensin system (RAS) is critically important in regulating cardiovascular and renal function (1). In the central nervous system, increasing evidence supports the notion that this system exerts various biological effects other than the classic, and more widely studied, blood pressure regulation and water-electrolyte balance (2). Indeed, it has been shown that the RAS also regulates the endocrine and exocrine function of the pancreas (3). As the major effector of the RAS, angiotensin II (Ang II) acts through two major G protein-coupled receptor subtypes, the angiotensin type 1 receptor (AT1R) and type 2 receptor (AT2R) (4), both of which were recently implicated in pancreatic function (5). In general, the functional significance of the AT1R is well documented (6, 7, whereas the AT2R function is still controversial and not fully understood.
A series of experiments from our laboratory repeatedly demonstrated higher AT2R protein expression in adult rats and mice as compared with fetal and neonatal animals (8–10), a phenomenon different from the prevailing concept (11). Our findings implied that the AT2R exerts important physiological functions in adulthood. Indeed, several lines of evidence have shown the participation of the AT2R in renal function and sympathetic regulation in mature animals (12, 13). In a recent study comparing the relative density of AT2R protein among various tissues of adult rat, we found that the pancreas (both islet and acinar) uniquely expressed the highest level AT2R protein (14). These findings are consistent with those of Chappell et al (15, 16), who demonstrated abundant AT2R expression in adult canine and primate pancreas and assumed that the AT2R functioned as the predominant angiotensin receptor subtype in this organ. Taken together, these results imply that the pancreas is a preferred target of the AT2R. Indeed, in a recent study, we found that activation of islet AT2Rs significantly increased plasma insulin and improved glucose tolerance in normal rats by up-regulating proinsulin 2 gene expression, insulin protein expression, and insulin secretion (14). This newly identified insulinotropic pathway may be partially responsible for the Ang II-induced elevation of plasma insulin in intact animals (17–20) and for the Ang II-evoked promotion of insulin secretion in cultured human islets (21) and in INS-1 cells (22). These positive influences of Ang II on pancreatic islets, we believe, occur by predominantly stimulating local AT2Rs.
Compound 21 (C21) is the first nonpeptide AT2R agonist, which has a high affinity for the AT2R and is effective orally (23). This compound is therapeutically attractive in many disease states (24). As indicated above, in a previous study, we have found that C21 exerted insulinotropic effects in normal rats by directly promoting insulin biosynthesis and secretion in pancreatic islets (14). Given the fact that C21 also displays antioxidant effects (25) and antiapoptotic effect (26) and that oxidative stress contributes to streptozotocin (STZ)-induced diabetes (27, 28), we hypothesized that pretreatment with C21 protects pancreatic islets and β-cells against STZ-induced toxicity by reducing oxidative stress and suppressing apoptosis, and therefore ameliorates the diabetic syndrome.
Materials and Methods
Animal experiments
Sixty male Sprague Dawley rats (280–320 g; Charles River Laboratories, Wilmington, MA) were used in the current study. The rats were group housed with standard rat food and tap water ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were carried out under the guidelines of the American Physiological Society and the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
The rats were assigned to five groups: normal, control diabetes (STZ), and three doses of C21-treated diabetes (C21-L, C21-M, and C21-H + STZ). Experiments lasted 2 weeks. Normal rats received only a bolus injection of citrate buffer as the control for STZ. Control diabetic and C21-treated diabetic rats were sc infused with saline or three doses of C21 (C21-L: 0.24 mg/kg·d; C21-M: 0.48 mg/kg·d; C21-H: 0.96 mg/kg·d) for 14 days using osmotic minipumps. On day 7 of infusion, these rats received a single injection of STZ (40 mg/kg in citrate buffer, sublingual vein) to induce diabetes. On the last day of C21 infusion, half of the rats were euthanized to collect blood samples for serum insulin measurement and save pancreata for islet morphology and biochemical measurements. The other half was used for oral glucose tolerance tests and the insulin secretory response to a glucose challenge. During the entire 14 days, the rats were placed in metabolic cages to measure daily water and food intake, urine and fecal excretion, and body weight.
Oral glucose tolerance test (OGTT), glucose concentration, and insulin concentration
An OGTT was performed after an overnight fast (16 h). A glucose solution (50% in water) was administered orally (2 g/kg body weight) and a 300-μL blood sample was obtained from the tail vein at 0, 10, 20, 40, 80, and 160 minutes after glucose loading for the measurement of glucose and insulin. Glucose concentration was evaluated using test strips (ReliOn; ARKRAY USA, Inc) based on the glucose oxidase method. Insulin concentration was measured with an ELISA kit (ALPCO Diagnostics).
Islet isolation
Rat islets were isolated as previously reported (14). Briefly, after removal from the abdominal cavity, the pancreata were washed in sterile ice-cold Hanks' balanced salt solution (HBSS; pH 7.4) containing penicillin (100 U/mL), streptomycin (0.1 mg/mL), and Fungizone (0.25 mg/mL; Life Technologies). The pancreata were then placed in another HBSS containing 1 mg/mL collagenase (type V; Sigma) and shaken (200 cycles/min) in a water bath (37°C) for 8–10 minutes to digest the tissues. The enzymatic action was terminated by adding 15 mL ice-cold HBSS without collagenase. The digested pancreatic tissue was then gently dispersed by pipetting. The big islets were picked up by hand under a microscope and the small islets were collected by centrifugation. The pure islets were stored at −80°C for the following biochemical analysis.
Cellular experiments
To establish a direct effect of C21 on β-cells, we used the INS-1E β-cells (29) (generously provided by Dr Pierre Maechler, University of Geneva, Geneva, Switzerland) to evaluate the influence of C21 on oxidative stress and apoptotic-relevant events under STZ treatment. The INS-1E cells (5 × 105 cells/well) were cultured in six-well plates with RPMI 1640 medium with 5% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 2 mM glutamine, 10 mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were assigned to four groups: control, STZ, C21 + STZ, and PD123319 + C21 + STZ. The final concentration of reagents was 5 mM for STZ, 1 μM for C21, and 0.1 mM for PD123319. The cells were pretreated with C21 for 2 days and then treated with STZ for another 2 days. To block the AT2R, PD12319 was added to the medium 2 hours before C21 administration. These cells were then harvested to evaluate superoxide (O2−·) by dihydroethidium (DHE) staining, gp91 and superoxide dismutase 1 (SOD1) protein expression by Western blotting, and apoptosis by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining.
3-[4,5-Dimethylthiazol-2yl]-2,5-diphenyl tetrazolium bromide (MTT) assay
Another group of INS-1E cells were used to evaluate the effects of C21 on cell survival using a colorimetric MTT assay. INS-1E cells (5 × 105 cells/well) were seeded in 96-well, flat-bottom plates with the medium containing C21 at three concentrations (0.1, 1, and 10 μM) or PD123319 (0.1 mM) + C21 (1 μM). After 2 days of incubation, STZ was added to the medium at final concentrations of 0, 0.1, 0.2, 0.5, 1, 2, 5, or 10 mM. After another 2 days of incubation, cell viability was estimated using the MTT assay. Briefly, the medium was replaced with the MTT solution (100 μL, 5 mg/mL), and the cells were then incubated at 37°C for 45 minutes. After removal of the MTT solution, cells were treated with 100 μL dimethylsulfoxide for 15 minutes at room temperature. The absorbance was measured at 490 nm in a microplate reader.
Molecular and biochemical experiments
Quantitative real-time RT-PCR was used to evaluate mRNA expression. Western blot analysis was used for protein expression and immunofluorescence for protein localization in the samples obtained from the above animal and cellular experiments.
Quantitative real-time RT-PCR
The proinsulin 2 mRNA expression in the pancreas was evaluated by real-time RT-PCR. Total RNA was extracted from the pancreas with TRIZOL reagent (Invitrogen), which was then reverse transcribed into double-stranded cDNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories). Templates (50 ng cDNA) were subjected in triplicate to real-time PCR using a thermocycler (PTC-200 Peltier thermal cycler with CHROMO 4 continuous fluorescence detector; Bio-Rad Laboratories) and Brilliant III Ultra-Fast QPCR master mix (Agilent Technologies). Specific primers of rat proinsulin 2 (Rn.PT.51.18628503.g) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Rn.PT.39a.11180736.g) from the Integrated DNA Technologies were used for relative quantification of proinsulin 2 mRNA expression. Proinsulin 2 mRNA was first normalized against GAPDH mRNA as Δcycle threshold, and then the relative expression was compared to the control group using the ΔΔcycle threshold method for quantification with Opticon Monitor software (Bio-Rad Laboratories).
Western blotting analysis
The target proteins and their primary antibodies were insulin (rabbit polyclonal antibody, sc-9168; Santa Cruz), AT2R (rabbit monoclonal antibody, ab92445; Abcam), AT1R (rabbit polyclonal antibody, sc-1173; Santa Cruz Biotechnology), gp91 (mouse monoclonal antibody, 611414; BD Biosciences), SOD1 (goat polyclonal antibody, sc-8637; Santa Cruz Biotechnology), caspase-3 (rabbit polyclonal antibody, sc-7148; Santa Cruz Biotechnology), and adipocyte protein 2 (aP2; rabbit polyclonal antibody, ab66682; Abcam). The GAPDH (mouse monoclonal antibody, sc-32233; Santa Cruz Biotechnology) served as an internal control. Western blot was carried out as described in our previous publications (8–10, 14). Briefly, the tissue or cell samples were homogenized in RIPA buffer, and total protein was extracted from the homogenates. Protein concentration was measured using a protein assay kit and then adjusted by adding 2 × 4% sodium dodecyl sulfate sample buffer to obtain equal concentrations among these samples. The samples were then loaded on a 10% SDS-PAGE gel (30 μg protein per well) and subjected to electrophoresis. The fractionized protein on the gel was electrically transferred onto a polyvinyl difluoride membrane. The membrane was first probed with the primary antibody to the target protein and then with GAPDH primary antibody. After incubation with primary antibodies, the membranes were probed with secondary antibodies followed by treatment with enhanced chemiluminescence substrate (Pierce). The bands on the membrane were visualized and analyzed using a UVP BioImaging System. The final reported data are the insulin band densities divided by the GAPDH density.
Immunofluorescence staining
The localization of target proteins (AT2R, insulin, and aP2) in the pancreas and epididymal adipose tissue were detected using immunofluorescence staining. The rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with PBS, followed by 4% paraformaldehyde in PBS. The pancreas and epididymal adipose tissue was removed, and 40-μm sections were made in a cryostat. The sections were washed with PBS and permeabilized for 30 minutes at room temperature with a solution containing 0.3% Triton X-100 dissolved in PBS. The samples were blocked with blocking solution containing 10% normal goat serum and 0.3% Triton X-100 in PBS at room temperature for 2 hours. The samples were then incubated with primary antibodies (for pancreas: rabbit anti-AT2R, sc-9040; Santa Cruz Biotechnology; goat antiinsulin A, sc-7839; Santa Cruz Biotechnology; for epididymal adipose tissue: goat anti-AT2R, sc-7420; Santa Cruz Biotechnology; rabbit anti-fatty acid binding protein 4, ab66682; Abcam) in 10% normal goat serum and 0.3% Triton X-100 in PBS at 4°C overnight. After three washes with PBS, the samples were incubated for 2 hours with secondary fluorescent antibodies (for pancreas: donkey antirabbit, A21206; donkey antigoat, A11058; Invitrogen; for epididymal adipose tissue: donkey antirabbit, A21207; donkey antigoat, A11055; Invitrogen). The samples were mounted with antifade reagent with 4′,6′-diamino-2-phenylindole (UltraCruz mounting medium, sc-24941; Santa Cruz Biotechnology). The slides were examined with a laser confocal microscope (objective: ×40; Leica TSC STED).
Evaluation of apoptosis
Quantification of β-cell apoptosis was performed using an in situ cell death detection ELISAplus assay (Roche Diagnostics) according to the recommended protocol. In brief, the pancreatic sections or INS-1E cells (grown on coverslips) were incubated with insulin primary antibody (sc-9040, goat antiinsulin A: 1:500) overnight at 4°C. After three washes using PBS, the samples were incubated with 50 μL TUNEL reaction mixture at 37°C in the dark for 60 minutes and then with secondary antibody (donkey antigoat, A11058, 1:500; Invitrogen) for another 60 minutes followed by three washes with PBS. The samples were mounted with antifade reagent with 4′,6′-diamino-2-phenylindole (UltraCruz mounting medium, sc-24941; Santa Cruz Biotechnology). The slides were examined with a laser confocal microscope (objective: ×40; Leica TSC STED). TUNEL assays were carried out a minimum of three times.
Superoxide imaging and analysis
Dihydtroethidium was used to detect the accumulation of O2−· in the pancreatic islets and cultured INS-1E cells. To evaluate O2−· in islets, the pancreas was removed, quickly frozen, embedded into optimum cutting temperature compound, and cryostat sectioned (50 μm) directly onto chilled microscope slides. Sections were then incubated with DHE (10 μM) in a light-protected humidified chamber at 37°C for 30 minutes. To determine the O2−· of INS-1E cells, the cells (5 × 105 cells/well) were cultured in six-well plates with the medium containing C21 (1 μM) or PD123319 (0.1 mM) + C21 (1 μM) for 2 days, followed by adding STZ (5 mM) into the medium for another 2 days of incubation. During the last 30 minutes of incubation, DHE was added to the medium. After incubation with DHE, the pancreatic sections or cultured INS-1E cells were washed twice using PBS to remove the extracellular dye and then imaged using a laser confocal microscope (Leica TSC STED). The excitation wavelength was 488 nm and emission fluorescence was detected with the use of a 585-nm filter. The images were analyzed using ImageJ software (National Institutes of Health) to quantify the relative fluorescent intensity of individual islet or INS-1E cells.
Statistical analyses
All data are expressed as mean ± SEM. Significant differences between groups were determined by one-way ANOVA followed post hoc by the Bonferroni test. Values of P < .05 were considered as statistically significant.
Results
Body weight, water intake, and urine excretion
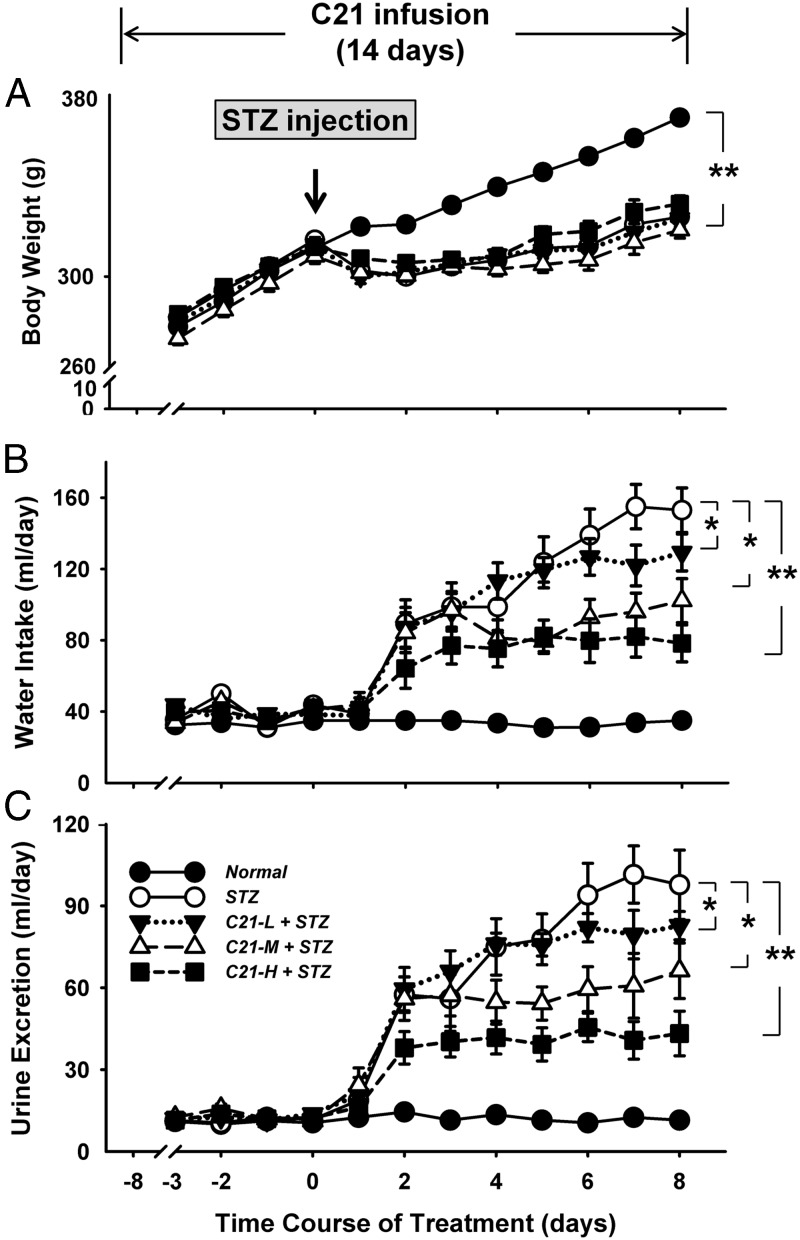
The body weights of rats are shown in panel A of Figure 1. There were no differences in body weights among these five groups before STZ injection, suggesting that C21 treatments, at least during the first 7 days of infusion, had no influence on this parameter. After STZ injection, from day 1 until the end of experiment, the body weights were significantly lower in both control STZ rats and C21-treated STZ rats as compared with normal rats. However, there were no significant difference between control STZ rats and C21-treated STZ rats.
Figure 1.
Body weight (A), water intake (B), and urine excretion (C) over the 2-week experimental period. *, P < .05 and **, P < .01 between the indicated groups (n = 12 each group). C21-L, -M, and -H, Low-, middle-, and high-dose C21.
The daily water intake and urine excretion data are shown in panels B and C of Figure 1. Before STZ injection, there were no significant differences in these two parameters among the five groups. From day 2 after STZ injection, however, water intake and urine excretion were significantly elevated in control STZ rats and C21-treated STZ rats as compared with their own baseline or to normal rats. Interestingly, compared with the control STZ rats, C21-treated STZ rats displayed a significantly lower water intake and urine excretion from day 2 (high dose C21), day 4 (middle dose C21), and day 7 (low dose C21) after STZ injection. In addition, starting from day 5, these two parameters were further elevated in control STZ rats but stayed at the plateau level at the high and middle doses in C21-treated STZ rats, resulting in an increased difference in water intake and urine excretion between control STZ rats and these two doses of C21-treated STZ rats. No significant difference in food intake and fecal excretion was observed among these five groups.
Blood glucose and plasma insulin concentration in the fed state
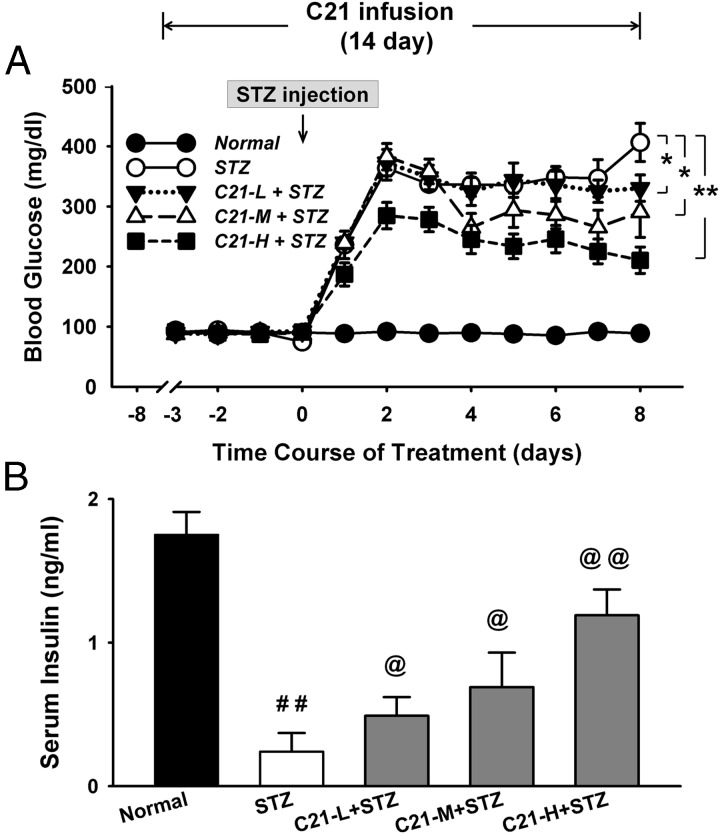
The daily blood glucose concentration is shown in panel A of Figure 2. There were no significant differences in baseline concentration among these five groups. The glucose concentration, however, was dramatically elevated at day 1 and reached a peak at day 2 after STZ injection in both control STZ and C21-treated STZ rats. On the other hand, the C21-treated rats displayed a significantly lower glucose level from day 2 (high dose C21), day 4 (middle dose C21), or day 8 (low dose C21) as compared with the control STZ rats.
Figure 2.
Blood glucose concentration over the 2-week experimental period (A) and serum insulin concentration on day 8 after STZ injection (B). *, P < .05 and **, P < .01 between the indicated groups (n = 12 each group); ##, P < .01 compared with normal group; @, P < .05 and @@, P < .01 compared with the STZ group (n = 6 each group). C21-L, -M, and -H, Low-, middle-, and high-dose C21.
Panel B of Figure 2 shows the serum insulin concentration on the last day of C21 infusion (day 8 after STZ injection). The insulin levels were significantly lower in STZ and C21-treated STZ rats than that in normal rats. The C21-treated STZ rats, however, exhibited a significantly higher insulin concentration as compared with the control STZ rats in a dose-dependent manner.
OGTT and insulin secretory response to glucose challenge
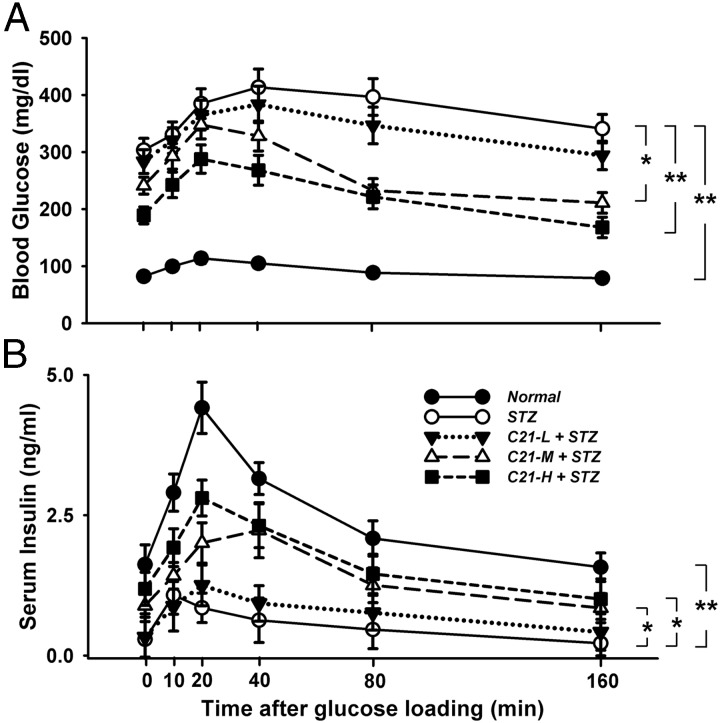
Panel A of Figure 3 shows the time course of blood glucose concentration after a 16-hour fast followed by a single oral glucose load. The fasting blood glucose (0 minute time point) was significantly higher in both STZ and C21-treated STZ rats as compared with normal rats, whereas the high and middle doses in C21-treated STZ rats displayed significantly lower fasting blood glucose than control STZ rats. After glucose loading, the glucose concentrations were elevated in all five groups, with the highest level in control STZ rats and the lowest level in normal rats. In normal rats and high and middle C21-treated STZ rats, the blood glucose reached the peak concentration at 20 minutes, whereas the control STZ rats and low-dose C21-treated STZ rats displayed their peak glucose levels at 40 minutes after glucose loading. At 80 minutes, the blood glucose returned to the fasting level in normal rats and the high and middle C21-treated STZ rats, whereas it remained higher than the fasting level in control STZ rats and low-dose C21-treated STZ rats.
Figure 3.
OGTT and glucose-evoked insulin secretory response on day 8 after STZ injection. A, Blood glucose concentration. B, Serum insulin concentration. *, P < .05 and **, P < .01 between the indicated groups (n = 6 each group). C21-L, -M, and -H, Low-, middle-, and high-dose C21.
Panel B of Figure 3 shows the time course of serum insulin concentration during the OGTT. Normal rats displayed the highest insulin levels in both the fasting state and after glucose loading, whereas the control STZ rats exhibited the lowest levels of insulin. C21-treated rats displayed a dose-dependent increase in the serum insulin levels.
After establishing the dose-dependent beneficial effects of C21 on STZ-induced diabetes in in vivo experiments, we further analyzed the underlying mechanisms. Unless specifically indicated, the pancreas and epididymal adipose samples used in the following experiments were obtained from the rats treated with the middle dose of C21 (0.48 mg/kg·d).
Islet morphology and expression of insulin and Ang II receptors in pancreas
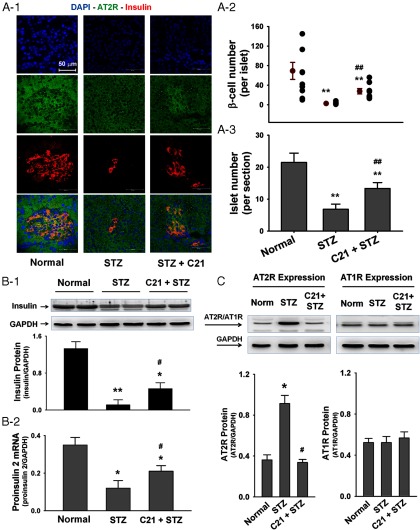
Panel A1 of Figure 4 shows the representative structure of pancreatic islets and the adjacent acinar cells by immunofluorescence staining. Green represents the AT2R and red is the insulin immunoreactive signal indicating the islets. The representative islet size was bigger in normal rats but smaller in control STZ rats as compared with the C21-treated STZ rats. Panels A2 and A3 of Figure 4 show the β-cell count per islet and the islet amount per section. These two parameters were significantly less in control STZ rats as compared with normal rats and C21-treated STZ rats. Interestingly, from panel A2 of Figure 4, it can be seen that the variability of β-cell number in individual islets is significantly higher in normal rats (10–145 β-cells/islet) as compared with control STZ rats (1–8 β-cells/islet) and C21-treated STZ rats (13–56 β-cells/islet).
Figure 4.
Effects of C21 on islet morphology and pancreatic insulin and Ang II receptor expressions. A, Islet morphology (A1), the β-cell count per islet (A2), and the islet number per section (A3) by immunofluorescence staining. B, The insulin protein expression by Western blot (B1) and the proinsulin 2 mRNA expression (B2) by real-time RT-PCR in pancreatic extracts. C, AT2R and AT1R protein expression in pancreatic islets by Western blot. *, P < .05 and **, P < .01 compared with normal group; #, P < .05 and ##, P < .01 compared with STZ group (n = 6 each group).
Panels B1 and B2 of Figure 4 show the expression of insulin protein and proinsulin 2 mRNA in pancreatic extracts. These two indices of insulin biosynthesis were significantly lower in control STZ rats as compared with the normal rats. However, C21-treated STZ rats displayed a significantly higher insulin protein and proinsulin 2 mRNA expression levels than control STZ rats did.
Panel C of Figure 4 shows the AT2R and AT1R receptor protein expressions in pancreatic islets. Control STZ rats displayed a significantly higher AT2R protein as compared with normal rats. This up-regulated AT2R expression but was attenuated in C21-treated STZ rats. On the other hand, we did not detect significant differences in AT1R expression among these three groups.
Oxidative stress and apoptosis in islets
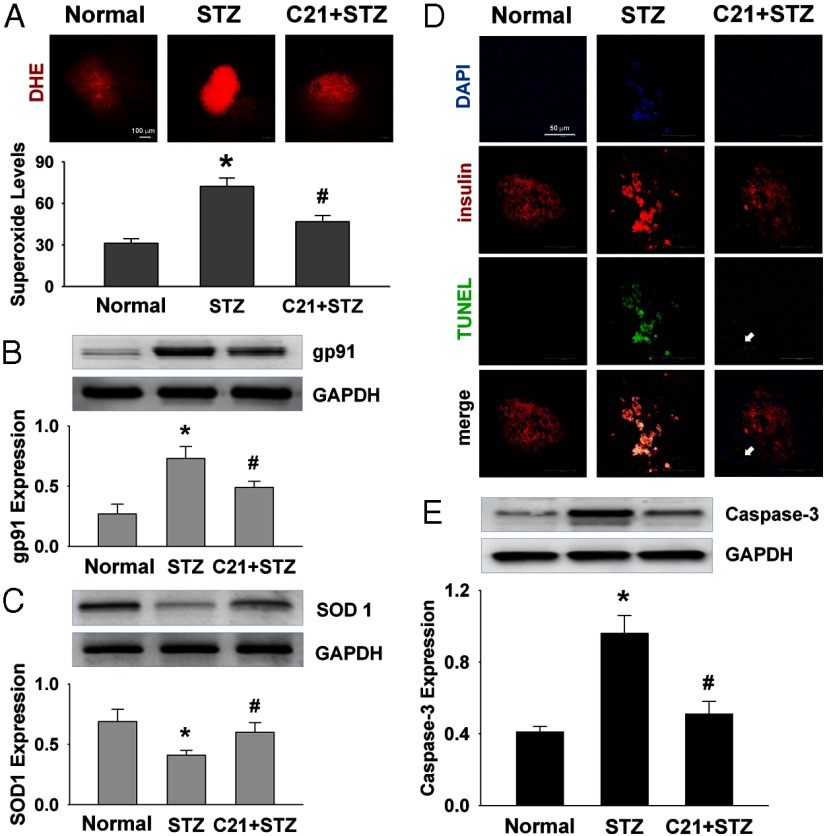
To analyze the mechanisms underlying the preservation of islets by C21, we evaluated oxidative stress and apoptotic events. Panel A of Figure 5 shows the O2−· levels in islets by DHE staining. There was a significantly higher red fluorescence in control STZ rats as compared with the normal rats. This elevated O2−· level was attenuated in C21-treated STZ rats. On the other hand, gp91 expression was significantly up-regulated, whereas SOD1 expression was down-regulated in the islets of control STZ rats as compared with the normal rats. These alterations were significantly attenuated in the C21-treated STZ rats (panels B and C).
Figure 5.
Effects of C21 on superoxide and apoptosis in pancreatic islets. A, Superoxide levels by DHE staining. B and C, Protein expressions of gp91 and SOD1 by Western blot. D, Apoptosis by TUNEL staining. E, Caspase-3 protein expression. *, P < .05 compared with normal group and #, P < .05 compared with the STZ group (n = 6 each group).
Panel D of Figure 5 shows apoptosis in the pancreas by TUNEL staining. In normal rats, no positive apoptotic cells were detected in both islet cells and the adjacent acinar cells. However, in control STZ rats, most β-cells (red color) exhibited a positive apoptotic signal (green color). In C21-treated STZ rats, however, only a few scattered apoptotic cells were found at the left bottom portion of the islet in which the islet's structure appears to be partially disrupted (indicated by the arrow). In addition, caspase-3 expression was significantly up-regulated in the islets of control STZ rats as compared with the normal rats, and this up-regulation was significantly attenuated in the C21-treated rats (panel E).
Oxidative stress and apoptosis in INS-1E cells
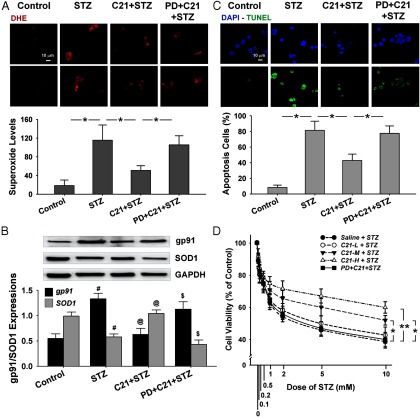
Figure 6 shows the effect of C21 pretreatment on STZ-induced O2−· production and apoptosis in cultured INS-1E cells by DHE and TUNEL staining. The upper subpanel of panel A shows representative images indicating higher density of red fluorescence in both the nucleus and cytosol of STZ-treated cells as compared with the control. This increased fluorescent density in the STZ-treated cells was markedly reduced by C21 pretreatment as seen in the mean data shown in the bottom subpanel. On the other hand, the effect of C21 was completely abolished by PD 123319. These data suggest that STZ increased superoxide production in β-cells, which was attenuated by C21 via AT2R activation. Panel B shows a significant up-regulation of gp91 and a down-regulation of SOD1 protein expression in INS-1E cells by STZ. These alterations were attenuated by pretreatment with C21, and PD 123319 reversed the C21 effect.
Figure 6.
Effects of C21 on oxidative stress, apoptosis, and cell viability in cultured INS-1E cells. A, Superoxide levels by DHE staining. B, Protein expressions of gp91 and SOD1 by Western blot. C, Apoptosis by TUNEL staining. D, Cell viability by MTT assay. *, P < .05 and **, P < .01 between the indicated groups; #, P < .05 vs control; @, P < .05 vs STZ; $, P < .05 vs C21 + STZ. The cells counted in panels A and C are from 14 to 31. The experiments for panels B and D were done in triplicate. C21-L, -M, and –H, Low, middle, and high concentration of C21.
Panel C shows apoptosis in cultured INS-1E cells by TUNEL staining. In the control group only a few apoptotic cells were observed. In the STZ-treated group however, 81.5% of the cells displayed apoptosis whereas the ratio of apoptotic cells dropped to 42.9% when the cells were pretreated with C21. PD 123319 abolished the effect of C21.
Panel D of Figure 6 shows the effects of C21 pretreatment on STZ-induced cell death in INS-1E cells using the MTT assay. STZ evoked a dose-dependent reduction in cell viability in control cells and all three doses of C21-pretreated cells (C21 at 0.1, 1, and 10 μM). Within the dose range of 0.1–1 mM STZ, there was no difference in cell viability between control and C21-pretreated cells. However, when STZ concentration was increased to 2 mM or higher, the high and middle doses, but not low dose, of C21-pretreated cells displayed significantly improved viability as compared with the control, which was abolished by PD 123319. These results suggest that C21 dose dependently protected β-cells by AT2R activation against the toxic effect of STZ.
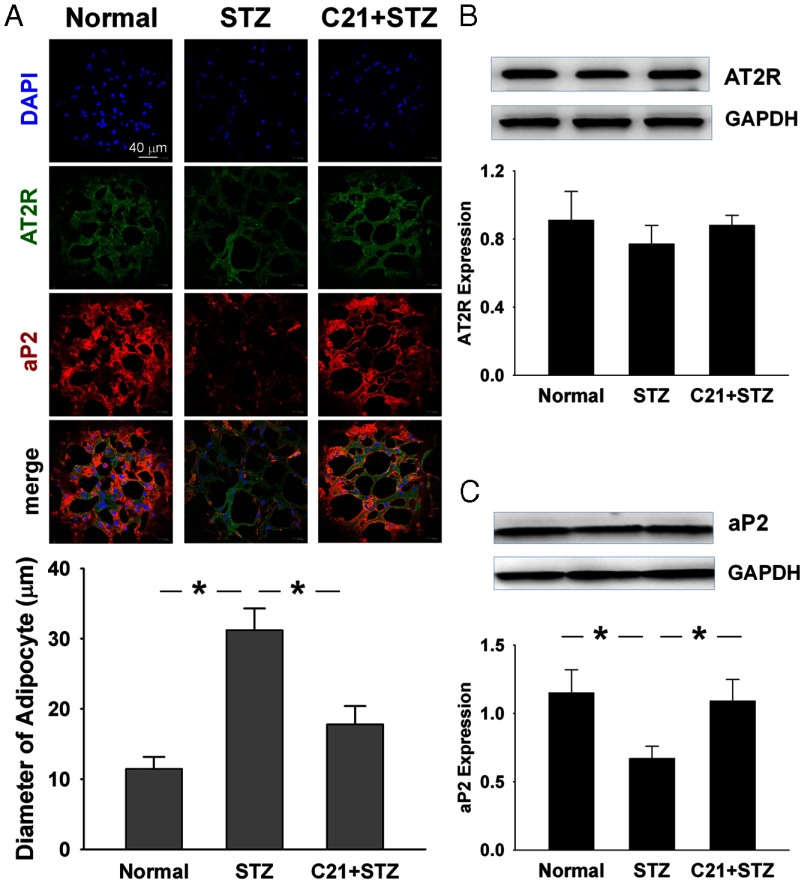
Adipocyte size and differentiation
C21 has been recently demonstrated to profoundly alter adipocyte morphology in the mouse with type 2 diabetes (30) and in the rats with high-fat/high fructose-induced insulin resistance (31). We therefore also evaluated epididymal adipocyte size and aP2 expression, which are shown in Figure 7. The adipocyte diameter was larger in the control STZ rats as compared with the normal rats, whereas C21 significantly suppressed this alteration. In addition, the expression of aP2, an adipocyte differentiation marker, was significantly down-regulated in control STZ rats as compared with normal rats. This decreased aP2 expression was significantly suppressed by C21. Although the AT2R protein was clearly detected in the epididymal adipose tissue by immunofluorescence and Western blot, no significant difference was found among normal, control STZ, and C21-treated STZ rats.
Figure 7.
Effect of C21 on adipocyte size and differentiation of epididymal adipose tissue. A, Adipocyte size by immunofluorescence staining. B and C, Protein expressions of AT2R and aP2. *, P < .05 between the indicated groups.
Discussion
The major finding of the present study is that pretreatment with C21, the first highly selective nonpeptide AT2R agonist (23), in male Sprague Dawley rats significantly ameliorated STZ-induced diabetes. We found that, compared with control diabetic rats, C21-treated diabetic rats exhibited lower blood glucose and higher serum insulin concentration in fed, fasting, and oral glucose loading states in a dose-dependent manner. These rats also displayed lower water intake and urinary volumes, higher islet number, larger islet volume, and higher expression of insulin protein and proinsulin 2 mRNA. In addition, C21-treated diabetic rats had less O2−·and apoptosis in their islets and increased adipocyte differentiation in their epididymal adipose tissue. The beneficial effects of C21 directly on β-cells were confirmed in the INS-1E cell line. These results suggest that activation of AT2R by C21 ameliorated STZ-induced diabetes by protecting pancreatic islets by antioxidation and antiapoptosis.
A local RAS has been reported in rodent and human islets, in which all of the key components were identified (32–34), suggesting a potential involvement of this system in the endocrine pancreas. Indeed, the major effector of RAS, Ang II, and its two primary receptor subtypes, the AT1R and AT2R, has been documented to regulate islet function (35). Interestingly, several large clinical trials repeatedly demonstrated a dramatic reduction in the incidence of diabetes mellitus in hypertension or chronic heart failure patients treated with an AT1R antagonist (36–39). However, the underlying mechanisms are not completely known. This beneficial effect was attributed to the sympathetic inhibition and hemodynamic improvement by AT1R blockade, which promoted insulin secretion by increasing islet blood flow (40) or enhanced insulin action by increasing skeletal muscle blood flow (41). The other potential mechanism is local antioxidation by directly blocking AT1R in pancreatic islets because β-cells are extremely susceptible to oxidative stress (42) due their very low superoxide dismutase expression (43). Based on the current study, we suggest another possibility that when the AT1R is occupied by antagonist, more free Ang II may then activate the AT2R in β-cells to exert a protective effect.
C21 is the first nonpeptide AT2R agonist reported in 2004 (23). This compound is currently being taken through a drug developmental program and expected to enter a clinical phase I study soon (44). Accumulating evidence from animal experiments has documented its beneficial influence on various pathological conditions, especially in cardiovascular disorders. In rats with coronary ligation, C21 has been demonstrated to improve systolic and diastolic ventricular function and reduce myocardial infarct size (45). In hypertensive rats, this chemical significantly reduced vascular injury and myocardial fibrosis (25), evoked mesenteric vasorelaxation (46), and prevented aortic stiffening and collagen accumulation (47). The kidney is the second most studied organ in which C21 exerted potential therapeutic effects. In stroke-prone spontaneously hypertensive rat, the hypertension-induced renal dysfunction development was significantly delayed and kidney damage was ameliorated by C21 treatment (48). In the two-kidney, one-clip Goldblatt hypertensive rats, C21 reversed most of the renal pathological changes, such as increased kidney weight, up-regulated inflammatory markers, and decreased nitric oxide production (49). In addition to the cardiovascular and renal systems, the central nervous system has also been documented as a therapeutic target of C21. It was recently demonstrated that C21 treatment decreased dopamine synthesis in the rat striatum (50), delayed the cognitive decline in an Alzheimer's disease mouse model (51), and improved functional recovery in rat spinal cord injury model (26). Most recently, C21 has been found to ameliorate insulin resistance in type 2 diabetic mice by improving adipocyte dysfunction and protecting pancreatic β-cells (30). The present study provides new evidence demonstrating a protective effect of C21 on pancreatic islet and β-cells in STZ-induced diabetic rats.
STZ is widely used to induce type 1 diabetes mellitus in animals. After administration of STZ, the animal develops diabetes overnight (52). By using this model, we found that C21 dose dependently attenuated hyperglycemia and suppressed the polyuria and polydipsia. The beneficial effect of high-dose C21 appeared on day 2, whereas the effects of middle and low doses of C21 did not emerge until day 4 and day 8 after STZ injection. These results suggest that C21 exerted its preventive and therapeutic effects in this diabetic model in both acute (reducing the acute β-cell death induced by STZ toxicity) and chronic manners (increasing new β-cell number by better glucose control), depending on the dose of C21 applied.
Pancreatic islets are believed to display a relatively low activity of SOD (43) and consequently are especially vulnerable to the actions of superoxide (42). In the STZ animal model, oxidative stress and β-cell apoptosis play a critical role in the pathogenesis of diabetes (27, 28). Indeed, antioxidation and antiapoptosis have been demonstrated to contribute to the preventive effects of cardiotrophin 1 (53) and T (54) on STZ-induced diabetes. In the pancreatic islets of C21-treated STZ rats, we found a decreased O2−· production, down-regulated gp91 expression, and up-regulated SOD1 expression as well as suppressed caspase-3 expression and attenuated β-cell apoptosis. These data suggest that the therapeutic effects of C21 on STZ-induced diabetes are attributed, at least partially, to the antioxidative and antiapoptotic mechanisms in pancreatic islets. Indeed, these beneficial effects of C21 directly on β-cells were confirmed in the cultured INS-1E cells.
In addition to insulin deficiency, STZ-induced diabetes is also characterized by insulin resistance (55). The expression of aP2, a marker of adipocyte progenitors (56), has been reported to be down-regulated in STZ-induced diabetic rats (57). On the other hand, the AT2R seems play a critical role in maintaining normal adipose function because AT2R-deficient mice exhibit significantly lower adipocyte differentiation and less adipocyte number as compared with the WT controls (58). Indeed, activation of the AT2R by C21 ameliorates insulin resistance in type 2 diabetic mice (30) and in rats with insulin resistance (31) by promoting adipocyte differentiation and restoring normal adipocyte size. In the present experiment, we demonstrated that C21 up-regulated aP2 expression and decreased the diameter of adipocytes in epididymal adipose tissue, suggesting that the increase in insulin sensitivity may also contribute to the beneficial effects of C21 on STZ-induced diabetes.
In summary, we demonstrated that C21 dose dependently ameliorated the major manifestations of diabetes, including hyperglycemia, polyuria, and polydipsia, in the STZ-induced diabetic rat. These therapeutic effects are most likely attributed to a direct protection of C21 on pancreatic islets through antioxidative and antiapoptotic mechanisms. Improved adipocyte function might also participate in this protective effect. This study suggests that activation of the AT2R signaling pathway may represent a novel therapeutic option in diabetes. Given its previously documented beneficial effects on cardiovascular disorders (25, 45–47), often concomitant with diabetes, the clinical application of C21 appears to be an attractive therapeutic paradigm.
Acknowledgments
Dr Pierre Maechler (the University of Geneva) generously provided us the INS-1E cell line. Compound 21 used in this study was a gift from Vicore Pharma. The authors also appreciate the critical suggestions for this study and valuable revision of the manuscript by Dr Irving H. Zucker.
This work was supported by the National Institutes of Health Grant R01HL093028.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ang II
- angiotensin II
- aP2
- adipocyte protein 2
- AT1R
- angiotensin type 1 receptor
- AT2R
- angiotensin type 2 receptor
- C21
- Compound 21
- DHE
- dihydroethidium
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- HBSS
- Hanks' balanced salt solution
- MTT
- 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl tetrazolium bromide
- O2−·
- superoxide
- OGTT
- oral glucose tolerance test
- RAS
- renin-angiotensin system
- SOD1
- superoxide dismutase 1
- STZ
- streptozotocin
- TUNEL
- terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling.
References
- 1. de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472 [PubMed] [Google Scholar]
- 2. Wright JW, Harding JW. Brain renin-angiotensin—a new look at an old system. Prog Neurobiol. 2011;95:49–67 [DOI] [PubMed] [Google Scholar]
- 3. Leung PS, Chappell MC. A local pancreatic renin-angiotensin system: endocrine and exocrine roles. Int J Biochem Cell Biol. 2003;35:838–846 [DOI] [PubMed] [Google Scholar]
- 4. Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88 [DOI] [PubMed] [Google Scholar]
- 5. Wang L, Leung PS. The role of renin-angiotensin system in cellular differentiation: implications in pancreatic islet cell development and islet transplantation. Mol Cell Endocrinol. 2013;381:261–271 [DOI] [PubMed] [Google Scholar]
- 6. Chu KY, Lau T, Carlsson PO, Leung PS. Angiotensin II type 1 receptor blockade improves β-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes. 2006;55:367–374 [DOI] [PubMed] [Google Scholar]
- 7. Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes. 2004;53:989–997 [DOI] [PubMed] [Google Scholar]
- 8. Yu L, Zheng M, Wang W, Rozanski GJ, Zucker IH, Gao L. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J Renin Angiotensin Aldosterone Syst. 2010;11:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu L, Shao C, Gao L. Developmental expression patterns for angiotensin receptors in mouse skin and brain. J Renin Angiotensin Aldosterone Syst. 2013;14(3):255–262 [DOI] [PubMed] [Google Scholar]
- 10. Gao J, Chao J, Parbhu KJ, et al. Ontogeny of angiotensin type 2 and type 1 receptor expression in mice. J Renin Angiotensin Aldosterone Syst. 2012;13:341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension. 2000;35:155–163 [DOI] [PubMed] [Google Scholar]
- 12. Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension. 2012;59:409–414 [DOI] [PubMed] [Google Scholar]
- 13. Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shao C, Zucker IH, Gao L. Angiotensin type 2 receptor in pancreatic islets of adult rats: a novel insulinotropic mediator. Am J Physiol Endocrinol Metab. 2013;305(10):E1281–E191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chappell MC, Diz DI, Jacobsen DW. Pharmacological characterization of angiotensin II binding sites in the canine pancreas. Peptides. 1992;13:313–318 [DOI] [PubMed] [Google Scholar]
- 16. Chappell MC, Bosch SM, Hansen BC, Ferrario CM, Diz DI. Differential expression of AT2 angiotensin II receptors in the primate pancreas. J Hypertension. 1994;12:S181 [Google Scholar]
- 17. Ran J, Hirano T, Adachi M. Chronic ANG II infusion increases plasma triglyceride level by stimulating hepatic triglyceride production in rats. Am J Physiol Endocrinol Metab. 2004;287:E955–E961 [DOI] [PubMed] [Google Scholar]
- 18. Chhabra KH, Xia H, Pedersen KB, Speth RC, Lazartigues E. Pancreatic angiotensin-converting enzyme 2 improves glycemia in angiotensin II-infused mice. Am J Physiol Endocrinol Metab. 2013;304:E874–E884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitsuishi M, Miyashita K, Muraki A, Itoh H. Angiotensin II reduces mitochondrial content in skeletal muscle and affects glycemic control. Diabetes. 2009;58:710–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buchanan TA, Thawani H, Kades W, et al. Angiotensin II increases glucose utilization during acute hyperinsulinemia via a hemodynamic mechanism. J Clin Invest. 1993;92:720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramracheya RD, Muller DS, Wu Y, et al. Direct regulation of insulin secretion by angiotensin II in human islets of Langerhans. Diabetologia. 2006;49:321–331 [DOI] [PubMed] [Google Scholar]
- 22. Siebelmann M, Wensing J, Verspohl EJ. The impact of ANG II and IV on INS-1 cells and on blood glucose and plasma insulin. J Recept Signal Transduct Res. 2010;30:234–245 [DOI] [PubMed] [Google Scholar]
- 23. Wan Y, Wallinder C, Plouffe B, et al. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008 [DOI] [PubMed] [Google Scholar]
- 24. Unger T, Dahlof B. Compound 21, the first orally active, selective agonist of the angiotensin type 2 receptor (AT2): implications for AT2 receptor research and therapeutic potential. J Renin Angiotensin Aldosterone Syst. 2010;11:75–77 [DOI] [PubMed] [Google Scholar]
- 25. Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2012;59:291–299 [DOI] [PubMed] [Google Scholar]
- 26. Namsolleck P, Boato F, Schwengel K, et al. AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression. Neurobiol Dis. 2013;51:177–191 [DOI] [PubMed] [Google Scholar]
- 27. Van Dyke K, Jabbour N, Hoeldtke R, Van Dyke C, Van Dyke M. Oxidative/nitrosative stresses trigger type I diabetes: preventable in streptozotocin rats and detectable in human disease. Ann NY Acad Sci. 2010;1203:138–145 [DOI] [PubMed] [Google Scholar]
- 28. Van Dyke K, Ghareeb E, Van Dyke M, Sosa A, Hoeldtke RD, Van Thiel DH. Luminescence experiments involved in the mechanism of streptozotocin diabetes and cataract formation. Luminescence. 2008;23:386–391 [DOI] [PubMed] [Google Scholar]
- 29. Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145:667–678 [DOI] [PubMed] [Google Scholar]
- 30. Ohshima K, Mogi M, Jing F, et al. Direct angiotensin II type 2 receptor stimulation ameliorates insulin resistance in type 2 diabetes mice with PPARγ activation. PLoS One. 2012;7:e48387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shum M, Pinard S, Guimond MO, et al. Angiotensin II type 2 receptor promotes adipocyte differentiation and restores adipocyte size in high-fat/high-fructose diet-induced insulin resistance in rats. Am J Physiol Endocrinol Metab. 2013;304:E197–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tahmasebi M, Puddefoot JR, Inwang ER, Vinson GP. The tissue renin-angiotensin system in human pancreas. J Endocrinol. 1999;161:317–322 [DOI] [PubMed] [Google Scholar]
- 33. Leung PS, Chan WP, Wong TP, Sernia C. Expression and localization of the renin-angiotensin system in the rat pancreas. J Endocrinol. 1999;160:13–19 [DOI] [PubMed] [Google Scholar]
- 34. Chappell MC, Millsted A, Diz DI, Brosnihan KB, Ferrario CM. Evidence for an intrinsic angiotensin system in the canine pancreas. J Hypertens. 1991;9:751–759 [DOI] [PubMed] [Google Scholar]
- 35. Skipworth JR, Szabadkai G, Olde Damink SW, Leung PS, Humphries SE, Montgomery HE. Review article: pancreatic renin-angiotensin systems in health and disease. Aliment Pharmacol Ther. 2011;34:840–852 [DOI] [PubMed] [Google Scholar]
- 36. Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766 [DOI] [PubMed] [Google Scholar]
- 37. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031 [DOI] [PubMed] [Google Scholar]
- 38. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003 [DOI] [PubMed] [Google Scholar]
- 39. Devereux RB, Dahlof B, Kjeldsen SE, et al. Effects of losartan or atenolol in hypertensive patients without clinically evident vascular disease: a substudy of the LIFE randomized trial. Ann Intern Med. 2003;139:169–177 [DOI] [PubMed] [Google Scholar]
- 40. Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41:127–133 [DOI] [PubMed] [Google Scholar]
- 41. Wiernsperger N. Vascular defects in the aetiology of peripheral insulin resistance in diabetes. A critical review of hypotheses and facts. Diabetes Metab Rev. 1994;10:287–307 [DOI] [PubMed] [Google Scholar]
- 42. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622 [DOI] [PubMed] [Google Scholar]
- 43. Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981;199:393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steckelings UM, Larhed M, Hallberg A, et al. Non-peptide AT2-receptor agonists. Curr Opin Pharmacol. 2011;11:187–192 [DOI] [PubMed] [Google Scholar]
- 45. Kaschina E, Grzesiak A, Li J, et al. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532 [DOI] [PubMed] [Google Scholar]
- 46. Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159:709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paulis L, Becker ST, Lucht K, et al. Direct angiotensin II type 2 receptor stimulation in Nω-nitro-L-arginine-methyl ester-induced hypertension: the effect on pulse wave velocity and aortic remodeling. Hypertension. 2012;59:485–492 [DOI] [PubMed] [Google Scholar]
- 48. Gelosa P, Pignieri A, Fandriks L, et al. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens. 2009;27:2444–2451 [DOI] [PubMed] [Google Scholar]
- 49. Matavelli LC, Huang J, Siragy HM. Angiotensin AT(2) receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mertens B, Vanderheyden P, Michotte Y, Sarre S. Direct angiotensin II type 2 receptor stimulation decreases dopamine synthesis in the rat striatum. Neuropharmacology. 2010;58:1038–1044 [DOI] [PubMed] [Google Scholar]
- 51. Jing F, Mogi M, Sakata A, et al. Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J Cereb Blood Flow Metab. 2012;32:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duran-Reyes G, Pascoe-Lira D, Vilar-Rojas C, et al. Diabetogenic effect of STZ diminishes with the loss of nitric oxide: role of ultraviolet light and carboxy-PTIO. Pharmacology. 2004;71:17–24 [DOI] [PubMed] [Google Scholar]
- 53. Jimenez-Gonzalez M, Jaques F, Rodriguez S, et al. Cardiotrophin 1 protects β cells from apoptosis and prevents streptozotocin-induced diabetes in a mouse model. Diabetologia. 2013;56:838–846 [DOI] [PubMed] [Google Scholar]
- 54. Palomar-Morales M, Morimoto S, Mendoza-Rodriguez CA, Cerbon MA. The protective effect of testosterone on streptozotocin-induced apoptosis in β cells is sex specific. Pancreas. 2010;39:193–200 [DOI] [PubMed] [Google Scholar]
- 55. Dall'Aglio E, Chang H, Hollenbeck CB, Mondon CE, Sims C, Reaven GM. In vivo and in vitro resistance to maximal insulin-stimulated glucose disposal in insulin deficiency. Am J Physiol. 1985;249:E312–E316 [DOI] [PubMed] [Google Scholar]
- 56. Shan T, Liu W, Kuang S. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. FASEB J. 2013;27:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Melki SA, Abumrad NA. Expression of the adipocyte fatty acid-binding protein in streptozotocin-diabetes: effects of insulin deficiency and supplementation. J Lipid Res. 1993;34:1527–1534 [PubMed] [Google Scholar]
- 58. Iwai M, Tomono Y, Inaba S, et al. AT2 receptor deficiency attenuates adipocyte differentiation and decreases adipocyte number in atherosclerotic mice. Am J Hypertens. 2009;22:784–791 [DOI] [PubMed] [Google Scholar]