Abstract
Kisspeptin, encoded by Kiss1, stimulates reproduction and is synthesized in the hypothalamic anteroventral periventricular and arcuate nuclei. Kiss1 is also expressed at lower levels in the medial amygdala (MeA) and bed nucleus of the stria terminalis (BNST), but the regulation and function of Kiss1 there is poorly understood. γ-Aminobutyric acid (GABA) also regulates reproduction, and female GABAB1 receptor knockout (KO) mice have compromised fertility. However, the interaction between GABAB receptors and Kiss1 neurons is unknown. Here, using double-label in situ hybridization, we first demonstrated that a majority of hypothalamic Kiss1 neurons coexpress GABAB1 subunit, a finding also confirmed for most MeA Kiss1 neurons. Yet, despite known reproductive impairments in GABAB1KO mice, Kiss1 expression in the anteroventral periventricular and arcuate nuclei, assessed by both in situ hybridization and real-time PCR, was identical between adult wild-type and GABAB1KO mice. Surprisingly, however, Kiss1 levels in the BNST and MeA, as well as the lateral septum (a region normally lacking Kiss1 expression), were dramatically increased in both GABAB1KO males and females. The increased Kiss1 levels in extrahypothalamic regions were not caused by elevated sex steroids (which can increase Kiss1 expression), because circulating estradiol and testosterone were equivalent between genotypes. Interestingly, increased Kiss1 expression was not detected in the MeA or BNST in prepubertal KO mice of either sex, indicating that the enhancements in extrahypothalamic Kiss1 levels initiate during/after puberty. These findings suggest that GABAB signaling may normally directly or indirectly inhibit Kiss1 expression, particularly in the BNST and MeA, and highlight the importance of studying kisspeptin populations outside the hypothalamus.
Reproduction is governed by an intricate interaction of many hormones, neuropeptides, and neurotransmitters. Many of these regulatory factors modulate, directly or indirectly, the secretion of GnRH, the final output of the neural network regulating fertility. Kisspeptin, a neuropeptide encoded by the Kiss1 gene, is a key upstream stimulator of GnRH secretion (1–8). In mammals, both kisspeptin and its receptor, Kiss1r (formerly Gpr54), have been demonstrated to be necessary for puberty and reproduction (4, 9, 10). In the rodent hypothalamus, Kiss1 is highly expressed in 2 discrete nuclei: the continuum of the anteroventral periventricular nucleus and rostral periventricular nucleus (AVPV/PeN) and the arcuate nucleus (ARC) (11, 12). Adult females have more Kiss1 mRNA and kisspeptin immunoreactivity in the AVPV/PeN than adult males, regardless of the sex steroid milieu (11, 13, 14), whereas there is no such Kiss1 sex difference in the adult ARC, especially when sex steroids are controlled (13, 15–17). In addition to the ARC and AVPV/PeN, recent evidence indicates that Kiss1 is also expressed, to a lesser extent, in several regions outside the hypothalamus, including the medial amygdala (MeA) (18, 19) and bed nucleus of the stria terminalis (BNST) (14, 20, 21).
Neural Kiss1 expression is strongly regulated by sex steroids, testosterone (T) and estradiol (E2), in a brain region-specific manner: in adult rodents, T and E2 down-regulate Kiss1 levels in the ARC, via either androgen receptor (AR) or estrogen receptor (ER) pathways (18, 22, 23), and up-regulate Kiss1 levels in the AVPV/PeN, through ER pathways (2, 13, 22, 23). As in the AVPV/PeN, Kiss1 levels in the MeA are strongly up-regulated by sex steroids (both T and E2) in rodents of both sexes, likely via ER pathways (18). Likewise, Xu et al (14) similarly reported that E2 stimulates the expression of Kiss1 gene and kisspeptin protein in the rat MeA and BNST. Given their hormonal regulation and neuroanatomic location in known reproductive nuclei, the ARC and AVPV/PeN kisspeptin populations are thought to play important roles in mediating negative and positive feedback effects, respectively, of sex steroids (24, 25). In contrast, the function and regulation of Kiss1 neurons in other brain regions is far less understood. We previously demonstrated that, in addition to their sex steroid responsiveness, Kiss1 neurons in the MeA of adult, gondal-intact rats and mice were more prevalent in males than females (18). Another study reported no Kiss1 signal in the MeA of postnatal rats before 3 weeks of age, suggesting that its expression in this region may arise around puberty (19). However, other than this limited information, little is known about the function, development, or possible regulation of extrahypothalamic Kiss1 neurons by factors other than sex steroids.
GABA, the main inhibitory neurotransmitter in the brain of adult mammals, can regulate the reproductive axis acting through ionotropic GABAA/C and metabotropic GABAB receptors. The presence of both GABAA receptor (GABAAR) and GABAB receptor (GABABR) has been described in GnRH neurons (26–28), and GABA signaling can directly regulate GnRH secretion (29). However, GABA may also modulate GnRH secretion indirectly by inhibiting upstream neuronal afferents of GnRH neurons, such as opiate or noradrenergic neurons (30, 31). Yet, at present, the possible interaction of GABA with kisspeptin neurons is not fully characterized. Whereas several groups have suggested that kisspeptin may interact with, or even directly modulate, GABA signaling to GnRH neurons (32–36), the reciprocal ability of GABA to regulate kisspeptin neurons remains far less studied. Recently, Kurian et al (37) used pharmacologic techniques to demonstrate that before puberty in monkeys, but not after, GnRH release is inhibited by tonic GABA input through kisspeptin neurons; this inhibitory action of GABA on pubertal kisspeptin signaling is exerted through GABAAR, although the presence of GABABR in kisspeptin neurons was not assessed, nor was a possible role of GABABR signaling. Indeed, although GABABR has been shown to modulate GnRH secretion and influence characteristics of fertility in rodents, to our knowledge, no direct link between GABABR and kisspeptin neurons has yet been reported.
We previously demonstrated that adult GABAB1KO female mice, which lack functional GABABR, have had decreased hypothalamic GnRH protein content and compromised cyclicity and fertility that is not due to behavioral alterations in mating (38–40). The kisspeptin system was not previously examined in these mice lacking functional GABABR. Therefore, this study aimed to evaluate whether the reproductive impairments in GABAB1KO mice were partly due to alterations in the brain Kiss1 system that might be brought about by nonfunctional GABABR signaling. We evaluated: 1) GABAB1R colocalization in Kiss1 neurons by double-label in situ hybridization (ISH), 2) neural Kiss1 mRNA expression, in both hypothalamic and extrahypothalamic regions, in adult male and female GABAB1KO mice, determined by ISH and real-time PCR (qPCR), and 3) Kiss1 mRNA expression in prepubertal GABAB1KO mice, to evaluate possible developmental changes in the Kiss1 system in the absence of GABABR signaling.
Materials and Methods
Animals
GABAB1 heterozygous mice in the BALB/C inbred strain were bred to produce wild-type (WT) and GABAB1KO littermates, the latter of which lack the GABAB1 subunit and have nonfunctional GABABR (39). All mice were genotyped by PCR analysis, as described previously (38), and housed in groups on a 12-hour light, 12-hour dark cycle (lights on at 7:00 am), with free access to laboratory chow and water. All animal studies were performed according to protocols for animal use, approved by the Institutional Animal Care and Use Committee (IBYME-CONICET) that follows NIH guidelines.
Adult and postnatal day 14 (PND14; day of birth = PND1) WT and GABAB1KO mice of both sexes were killed by rapid decapitation in the morning (9:00 am to 11:00 am). All adult females were in estrus. Brains were collected and immediately frozen on dry ice and stored at −80°C until cutting for ISH assays or micropunching for qPCR. Blood samples and gonads of adults were collected at time of death and stored at −20°C until being assayed for serum and gonadal E2 and T content.
Single-label and double-label ISH
For ISH assays, 5 coronal series of 20-μm brain sections were cut on a cryostat, thaw mounted onto Superfrost-plus slides, and stored at −80°C. Single-label ISH for Kiss1 was performed as previously described (16, 41), using a validated mouse Kiss1 riboprobe. Briefly, one set of slide-mounted brain sections was fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× SSC (sodium citrate, sodium chloride), delipidated in chloroform, dehydrated in ethanols, and air dried. Radiolabeled (33P) Kiss1 antisense riboprobe (0.04 pmol/mL) was combined with tRNA, heat denatured, added to hybridization buffer, and applied to each slide (100 μL/slide). Slides were coverslipped and placed in a 55°C humidity chamber overnight. The slides were then washed in 4× SSC and placed into RNase A treatment for 30 minutes at 37°C, then in RNase buffer without RNase at 37°C for 30 minutes. After washing in 2× SSC at room temperature, slides were washed in 0.1× SSC at 62°C for 1 hour, dehydrated in ethanols, and air dried. Slides were then dipped in Kodak NTB emulsion, air dried, and stored at 4°C for 4–5 days (depending on the assay) before being developed and coverslipped.
For double-label ISH of GABAB1 subunit and Kiss1, slide-mounted brain sections were treated similarly to single-label ISH with the following modifications (42). Digoxigenin (DIG)-labeled antisense mouse Kiss1 riboprobe was synthesized with DIG labeling mix (Roche). Radiolabeled (33P) antisense GABAB1 subunit (GenBank NM_001177511; 0.05 pmol/mL), designed to bind bp 385–856 of the mouse GABAB1 subunit mRNA, and DIG-labeled Kiss1 (1:500) riboprobes were combined with tRNA, heat denatured, and dissolved together in hybridization buffer. The probe mix was applied to slides (100 μL/slide) and hybridized at 55°C overnight. After the 62°C washes on day 2, slides were incubated in 2× SSC with 0.05% Triton X-100 containing 3% normal sheep serum for 1 hour at room temperature. Slides were then incubated overnight at 21°C with anti-DIG antibody conjugated to alkaline phosphatase (Roche; diluted 1:500 in Buffer 1 containing 1% normal sheep serum and 0.3% Triton X-100). Slides were then washed with Buffer 1 and incubated with Vector Red alkaline phosphatase substrate (Vector Laboratories) for 1 hour. Slides were then air dried, dipped in emulsion, stored at 4°C, and developed and coverslipped 9 days later. No staining was detected with sense probes.
Quantification and analysis of ISH data
Single-label ISH slides were analyzed with an automated image-processing system (Dr. Don Clifton, University of Washington) by a person blind to the treatment group. The software counts the number of silver grain clusters representing Kiss1 cells, as well as the number of silver grains over each cell (a semiquantitative index of Kiss1 mRNA content per cell) (15, 16, 18). Cells were considered Kiss1 positive when the number of silver grains in a cluster exceeded that of background by 3-fold. For double-label assays, DIG-containing Kiss1 cells were identified under red fluorescence microscopy and the grain-counting software was used to quantify silver grains (representing GABAB1 subunit mRNA) overlying each cell. Signal-to-background ratios for individual cells were calculated, and a Kiss1 cell was considered double labeled with GABAB1 subunit if its ratio was greater than 3.
RNA extraction and qPCR
As in a previous report (18), total RNA from 500-μm-thick frozen micropunches of select brain regions (AVPV/PeN [∼Plates 28–32 of Paxinos and Franklin Mouse Atlas], ARC [Plates 44–48], MeA [Plates 44–48], BNST [Plates 30–34], and lateral septum [Plates 25–29]) was extracted using the RNeasy Lipid Tissue Mini kit (QIAGEN). RNA was reverse transcribed using the Omniscript RT kit (QIAGEN), and cDNA was stored at −20°C. qPCR was performed on each cDNA sample using the Bio-Rad iCycler Detection System and Quantitect SYBR Green PCR kit (QIAGEN). To detect Kiss1, specific primers were used (Kiss1 forward: 5′-CAA AAG TGA AGC CTG GAT CC-3′; and Kiss1 reverse: 5′-GTT GTA GGT GGA CAG GTC C-3′) (41). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or β-actin (Actb) was used as housekeeping gene, depending on the brain region (41). Standard curves were generated for each product using cloned cDNAs for Kiss1 and GAPDH to quantify the abundance of cDNA in each sample. For standard curves, a dilution series of cloned Kiss1 and GAPDH templates ranging from 10 to 108 copies were used. The qPCR cycling parameters were 1 cycle of 95°C for 15 minutes, followed by 50 cycles of 94°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Data collection was taken at the 72°C extension phase. To ensure the presence of a single product, a dissociation curve was performed after each run. Data were collected from threshold values using the automatic function of the Bio-Rad MyIQ software. All samples were run in duplicate, and Kiss1 was normalized to GAPDH or Actb, the expression of which is constant and not different between groups. The size of the products was confirmed by 1% agarose gels.
Gonadal and serum hormone levels
To assess gonadal sex steroid content, gonads were obtained from adults at time of death and stored at −20°C until subsequent homogenization, ethyl-ether extraction, and determination of sex hormone levels by RIA, as previously described (38). E2- and T-specific antisera were kindly provided by Dr. G. Niswender (Colorado State University, Fort Collins, CO). Tritiated hormones were purchased from New England Nuclear. Assay sensitivities were: E2, 7 pg; and T, 10 pg. Intra- and interassay coefficients of variation were: E2, 6.8% and 11.7%; and T, 7.8% and 12.3%, respectively.
For serum hormone levels, blood was collected from adult WT and GABAB1KO mice. In females, serum was analyzed for E2 levels by a mouse ELISA (sensitivity: 3.0 pg/mL; coefficient of variation, 4.1%) (43) and in males, serum T was assessed by RIA. Both procedures were performed by the University of Virginia Ligand Assay Core.
Statistics
Data are presented as the mean ± SEM for each group. The differences between means of 2 groups or more were analyzed by Student's t test or two-way ANOVA followed by the Tukey honestly significant difference test, respectively. P < .05 was considered statistically significant.
Results
Assessment of GABAB1 subunit colocalization in hypothalamic Kiss1 neurons
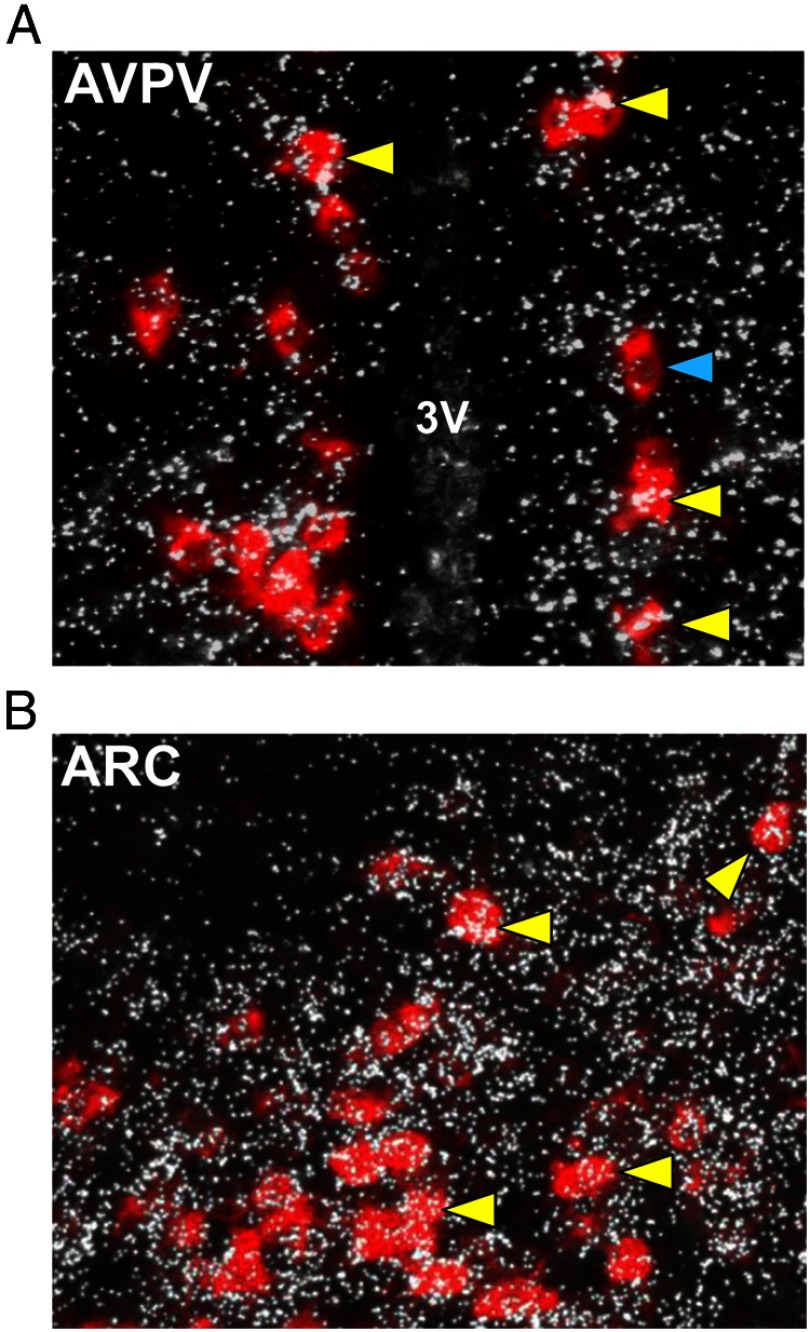
To determine whether GABAB signaling could potentially directly influence the kisspeptin system, we first used double-label ISH to assess whether GABAB1 subunit colocalizes in Kiss1 neurons. Using hypothalamic tissue from adult female mice (estrus; n = 3), we found that virtually all Kiss1 neurons in the AVPV/PeN (97.7 ± 0.89%) coexpress GABAB1 subunit (Figure 1A). Similarly, a majority of Kiss1 neurons in the ARC (71.3 ± 4.0%) also express GABAB1 subunit (Figure 1B). This is the first demonstration of GABAB1 subunit in rodent Kiss1 neurons.
Figure 1.
Representative images of GABAB1 subunit mRNA expression (silver grains) in Kiss1 neurons (red fluorescence) in the AVPV/PeN (panel A) and ARC (panel B) of adult mice. The majority (∼75%-97%) of Kiss1 cells coexpressed GABAB1 subunit in each region. 3V, third ventricle. Yellow arrowheads denote examples of Kiss1-GABAB1 coexpression. Blue arrowheads denote Kiss1 cell without GABAB1.
Kiss1 expression in the AVPV/PeN and ARC of mice lacking GABABR signaling
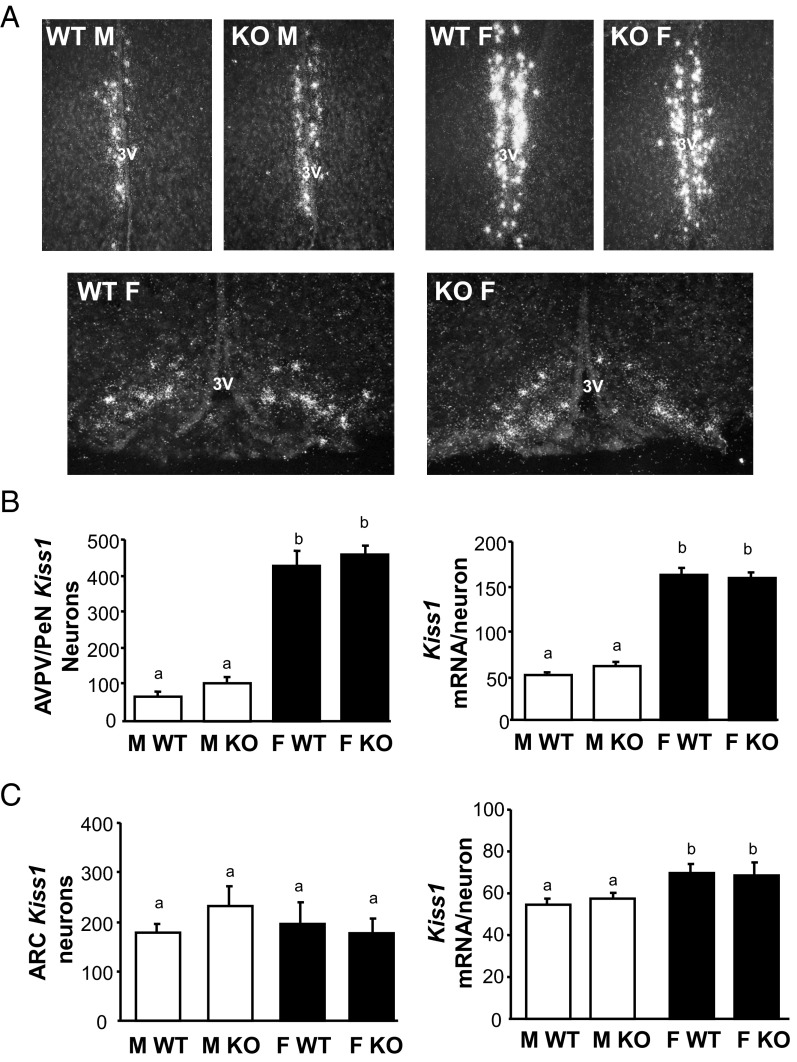
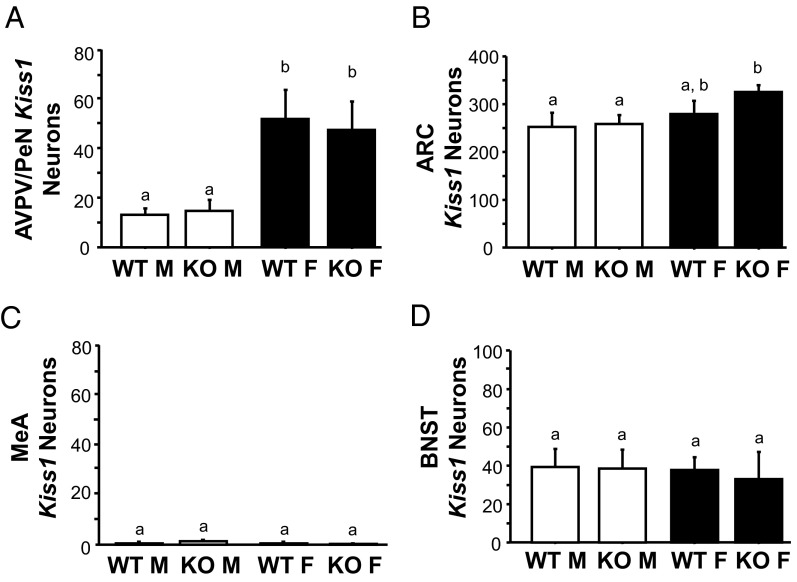
Having demonstrated the coexpression of GABAB1 subunit in most Kiss1 neurons, we next studied whether the absence of functional GABABRs alters Kiss1 mRNA levels in the ARC and AVPV/PeN, the two primary Kiss1 populations linked to reproduction. Single-label ISH for Kiss1 was performed in brains from adult GABAB1KO and WT mice of both sexes (females in estrus; n = 5–7/group). In the AVPV/PeN, Kiss1 expression (both detectable number of Kiss1 cells as well as Kiss1 mRNA per cell) was higher in WT females than WT males, as expected (P < .05; Figure 2, A and B). However, despite known reproductive impairments in GABAB1KO mice, AVPV/PeN Kiss1 expression was similar between genotypes: GABAB1KO females exhibited higher Kiss1 levels than males of both genotypes, similar to that of WT females (Figure 2, A and B). Likewise, Kiss1 levels in the AVPV/PeN of GABAB1KO males were not different from that of WT males (Figure 2B). A similar outcome was detected in the ARC: the number of Kiss1 cells in the ARC did not differ significantly between any group (Figure 2, A and C), and relative Kiss1 mRNA per cell was slightly higher in females than males for both genotypes (Figure 2, A and C).
Figure 2.
Kiss1 expression in the AVPV/PeN and ARC of adult GABAB1KO and WT mice. A, Representative images of Kiss1 expression in the AVPV/PeN (top row) and ARC (bottom row). 3V, third ventricle. B, Mean numbers of Kiss1 neurons in the AVPV/PeN and mean relative Kiss1 mRNA content per neuron in the AVPV/PeN. C, Mean numbers of Kiss1 neurons in the ARC and mean Kiss1 levels per neuron in the ARC. Different letters denote significantly different from each other. F, female; M, male.
Increased Kiss1 expression in the MeA of GABAB1KO mice
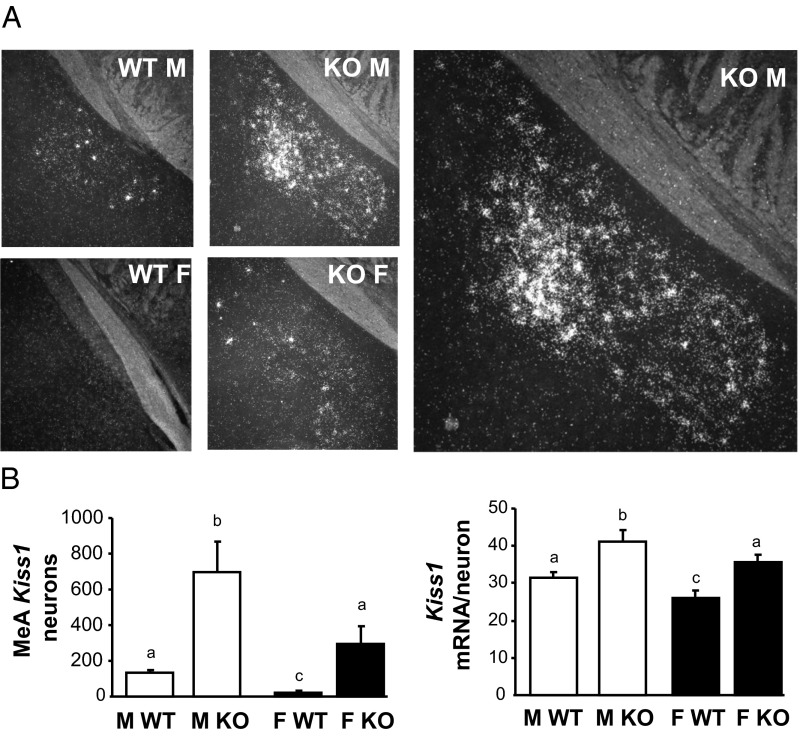
The previous experiment determined that Kiss1 levels were unaltered in the ARC and AVPV/PeN of adult GABAB1KO mice. We therefore next determined, using ISH, whether Kiss1 gene expression in GABAB1KO mice was altered in the MeA, an extrahypothalamic region shown to have moderate Kiss1 expression and perhaps play a role in reproduction (18). As shown previously, adult WT male mice had a moderate number of detectable MeA Kiss1 neurons that was significantly greater than in WT females (P < .05; Figure 3). Interestingly, unlike in the ARC and AVPV/PeN, there was a dramatic alteration in Kiss1 levels in the MeA of GABAB1KO mice. MeA Kiss1 cell number was robustly elevated in GABAB1KO males, being more than 6-fold higher than in WT males (P < .05; Figure 3). Likewise, the number of Kiss1 cells was markedly higher in GABAB1KO females than WT females (>5-fold higher; all females in estrus, P < .05; Figure 3). As with cell number, the relative Kiss1 mRNA level per cell in the MeA was also significantly higher in GABAB1KO mice than in WT mice (P < .05; Figure 3). We found the same result if we analyzed only the posterior ventral or posterior dorsal portion of the MeA (data not shown).
Figure 3.
Kiss1 expression in the MeA of adult GABAB1KO and WT mice A, Representative images of Kiss1 expression in the adult MeA. The large image on the right is a higher magnification of the KO male. B, Mean numbers of Kiss1 neurons in the MeA and mean relative Kiss1 mRNA content per neuron in the MeA. Different letters denote significantly different from each other. F, female; M, male.
Enhanced Kiss1 expression in the BNST and septum of GABAB1KO mice
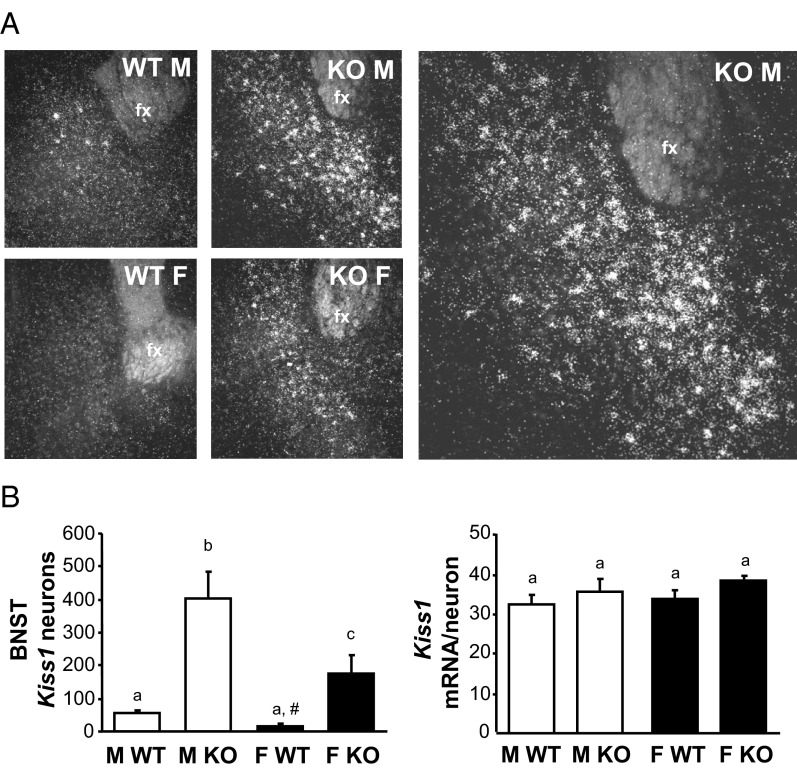
Our surprising finding above of enhanced Kiss1 levels in the MeA of GABAB1KO mice prompted us to examine another extrahypothalamic region, the BNST, that reportedly normally expresses low Kiss1 levels. The BNST lies dorsal-lateral to the AVPV/PeN, communicates with the medial amygdala, and has been implicated in reproductive and social behavior (44, 45). Confirming previous reports, we found low but detectable levels of Kiss1 expression in the BNST of adult WT mice, with no significant sex difference in either Kiss1 cell number or Kiss1 mRNA per cell (Figure 4). Interestingly, as in the MeA, GABAB1KO mice of both sexes had significantly more BNST Kiss1 neurons than WT mice (P < .05 for each sex; Figure 4). This marked increase in BNST Kiss1 cells in GABAB1KO mice was 4- to 8-fold higher than in WT mice (Figure 5). BNST Kiss1 levels were significantly higher in GABAB1KO males than GABAB1KO females (P < .05; Figure 4). Although cell number was robustly higher, the relative expression of Kiss1 mRNA per cell was similar between genotypes (Figure 4).
Figure 4.
Kiss1 expression in the BNST of adult GABAB1KO and WT mice A, Representative images of Kiss1 expression in the adult BNST. The large image on the right is a higher magnification of the KO male. B, Mean numbers of Kiss1 neurons in the BNST and mean relative Kiss1 mRNA content per neuron in the BNST. Different letters denote significantly different from each other. Fx, fornix; F, female; M, male. #, nonsignificant trend, P = .10 vs WT males.
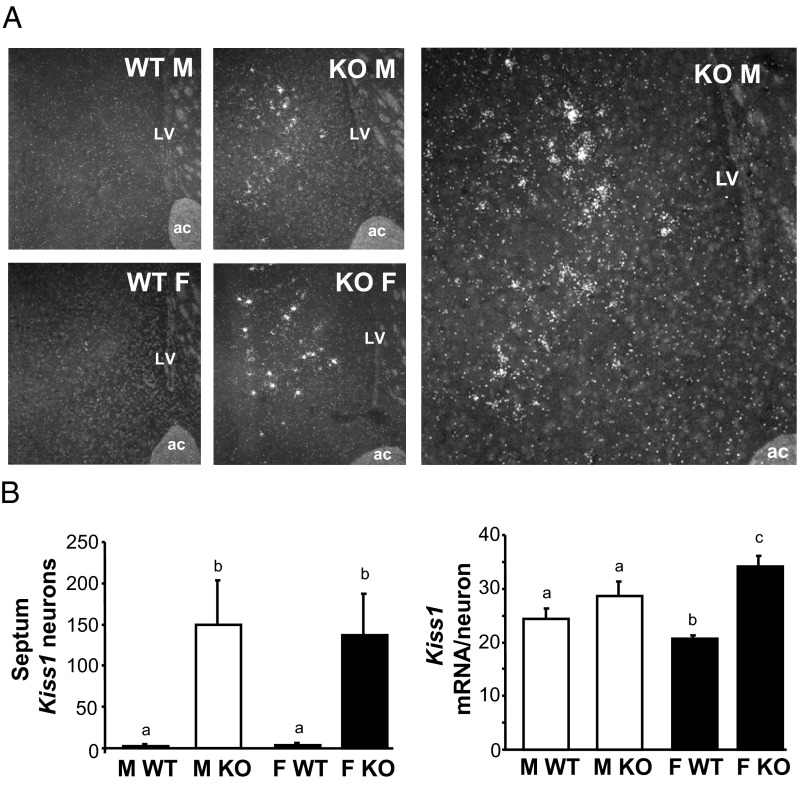
Figure 5.
Kiss1 expression in the lateral septum of adult GABAB1KO and WT mice. A, Representative images of Kiss1 expression in the lateral septum. The large image on the right is a higher magnification of the KO male. B, Mean numbers of Kiss1 neurons in the lateral septum and mean relative Kiss1 mRNA content per neuron in the septum. Different letters denote significantly different from each other. ac, anterior commissure; LV, lateral ventricle; F, female; M, male.
In counting our BNST assay, we noted circumstantially notable Kiss1 expression in some mice in the lateral septal area, just anterior and dorsal to the BNST region. We therefore analyzed Kiss1 expression levels in all mice in this lateral septal region. In WT mice, there was virtually no detection of Kiss1 in either sex in the septum with only a small handful of very weak cells very rarely identified (Figure 5). However, as with the MeA and BNST, adult GABAB1KO mice of both sexes had a dramatically increased number of Kiss1 neurons in this septal area, compared with WT mice (P < .05; Figure 5). In females, Kiss1 mRNA levels per cell in the septum were also significantly higher in GABAB1KO than WT mice (P < .05, Figure 5).
qPCR analysis of Kiss1 expression in hypothalamic and extrahypothalamic regions in GABAB1KOs
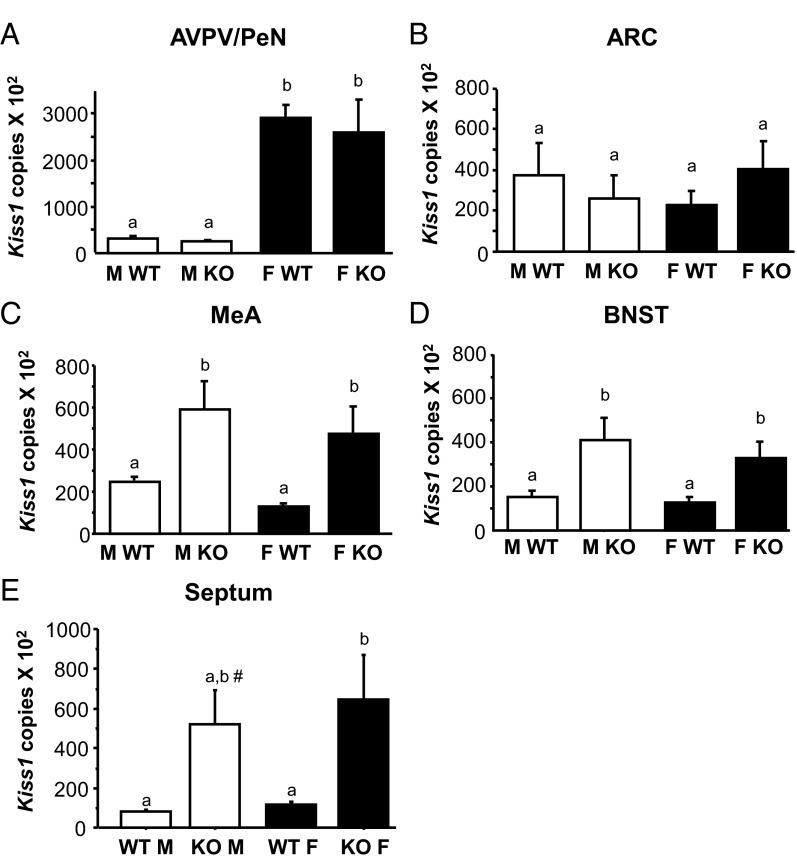
Our ISH data indicated a dramatic enhancement of Kiss1 expression in MeA, BNST, and septum, but not in the 2 main hypothalamic populations (AVPV/PeN and ARC). Because ISH is only semiquantitative, we next used sensitive qPCR to measure absolute Kiss1 mRNA levels in the various Kiss1 populations in adult GABAB1KO mice. Micropunches of tissue from the AVPV/PeN, ARC, MeA, BNST, and septum were collected for male and female (estrus) GABAB1KO and WT mice (n = 5–10/group per region). qPCR analysis of micropunch RNA mirrored the ISH outcomes. In the ARC and AVPV/PeN, Kiss1 levels were identical between genotypes for both sexes (Figure 6). There were no sex differences in Kiss1 levels in the ARC, but in the AVPV/PeN, Kiss1 levels were sexually differentiated as expected (higher in females than males; P < .05). Confirming our ISH findings, absolute Kiss1 levels, as determined by qPCR, were significantly higher in the extrahypothalamic regions of GABAB1KO mice relative to WT mice (Figure 6). These elevated Kiss1 levels were present in all 3 regions (MeA, BNST, and septum) and true for both sexes (P < .05 vs WT for each region). The increase in Kiss1 levels was similar between GABAB1KO males and females for all 3 extrahypothalamic regions.
Figure 6.
Mean numbers of Kiss1 mRNA copies, determined via qPCR, in micropunches from the AVPV/PeN (panel A), the ARC (panel B), the MeA (panel C), the BNST (panel D), and the lateral septum (panel E) taken from adult GABAB1KO and WT mice of both sexes Different letters denote significantly different from each other. #, nonsignificant trend; P = .07 vs WT males and WT females. F, female; M, male.
Assessment of GABAB1 subunit colocalization in extrahypothalamic Kiss1 neurons
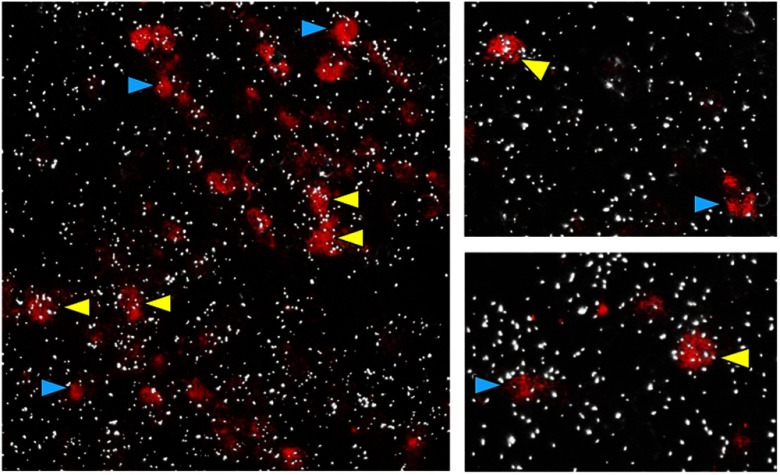
To determine whether GABAB signaling might potentially occur directly in extrahypothalamic Kiss1 cells, we used double-label ISH to assess Kiss1/GABAB1 subunit coexpression in the MeA. This could not be determined in WT mice because the level of Kiss1 expression per cell in extrahypothalamic regions, unlike in the hypothalamus, is not high enough to detect with DIG-labeled ISH probes. However, because Kiss1 levels in the MeA are significantly elevated in GABAB1KOs (Figures 3 and 6), their tissue can be used to better detect Kiss1 cells in this region (our GABAB1 probe targets early exons of the GABAB1 gene that have not been disrupted in the knockout [KO] mice). Using tissue from adult GABAB1KO mice (n = 4 males and 2 females), we found that a majority (∼66%) of detectable Kiss1 neurons in the MeA coexpress GABAB1 subunit (Figure 7), similar in both sexes. Analogous analyses were not possible for Kiss1 neurons in the BNST or septum, because the level of Kiss1 mRNA expression per cell is not high enough in these regions, even in KO mice, for detection of DIG-labeled Kiss1 cells.
Figure 7.
Representative images of GABAB1 subunit mRNA expression (silver grains) in Kiss1 neurons (red fluorescence) in the MeA of adult male mice. Yellow arrowheads denote examples of Kiss1-GABAB1 coexpression. Blue arrowheads denote Kiss1 cell without GABAB1. The majority (∼66%) of MeA Kiss1 cells coexpressed GABAB1 subunit.
Sex steroid levels in adult GABAB1KO mice are normal
Because circulating sex steroids can strongly alter Kiss1 expression, it is possible that the observed alterations in Kiss1 levels in adult GABAB1KO mice might reflect higher circulating sex steroid levels. We therefore measured gonadal content and blood serum levels of E2 (estrous females) and T (males), as well as gonad and uterus weights, in adult GABAB1KO and WT mice. Both gonadal and circulating sex steroid levels were similar between genotypes, even after adjusting for gonadal weight (Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endo.journals.org). In addition, gonad and uterine weights did not differ significantly between genotypes (data not shown). Thus, the high Kiss1 expression in extrahypothalamic regions is not due to higher circulating sex steroids in the GABAB1KO mice.
Kiss1 expression in prepubertal mice lacking GABABR signaling
Because we found large alterations in Kiss1 levels in extrahypothalamic areas (eg, MeA, BNST) in adult GABAB1KO mice, we next tested whether similar increases in expression were present earlier in development, before puberty. We analyzed hypothalamic and extrahypothalamic Kiss1 expression by ISH in PND14 male and female mice (n = 6–7/group). Kiss1 expression at this developmental age was readily detected in the AVPV/PeN and ARC, with no genotype differences in either region, as in adulthood (Figure 8). Surprisingly, unlike in adulthood, no genotype differences in Kiss1 levels were present between GABAB1KO and WT mice in any of the extrahypothalamic brain areas examined in either sex (Figure 8). In the MeA, Kiss1 was virtually undetectable in all prepubertal groups, regardless of sex or genotype. In the BNST, Kiss1 levels in prepubertal mice were low to moderate, with all groups displaying equal Kiss1 expression similar to adult WT levels. Thus, the elevated Kiss1 expression observed in extrahypothalamic regions of adult GABAB1KO mice is not yet present in prepubertal development.
Figure 8.
Mean Kiss1 mRNA expression, determined via ISH, in brains from prepubertal (PND 14) GABAB1KO and WT mice of both sexes A, AVPV/PeN; B, ARC; C, MeA; D, BNST. Different letters denote significantly different from each other. There were no genotype differences detected in any brain region of prepubertal mice. F, female; M, male.
Discussion
The possible interaction between GABABR signaling and Kiss1 neurons has not been previously addressed. Here, we evaluated whether the known reproductive alterations previously observed in GABAB1KO mice were due to impairments in the neural Kiss1 system. Contrary to our hypothesis, and despite the high coexpression of GABAB1R in most Kiss1 neurons, we found that Kiss1 levels in the AVPV/PeN and ARC, assessed by both ISH and qPCR, were identical between genotypes for both sexes. Thus, the reproductive impairments previously observed in adult GABAB1KO females are not due to deficits in Kiss1 expression in the main hypothalamic nuclei that control fertility. However, surprisingly, subsequent ISH and qPCR analyses demonstrated that Kiss1 levels outside the hypothalamus, in the MeA and BNST, as well as the lateral septum, were dramatically increased in GABAB1KO mice of both sexes, an effect that was not due to altered sex steroid levels. Intriguingly, this enhanced Kiss1 expression was not detected in the MeA or BNST of prepubertal mice, indicating that the extrahypothalamic Kiss1 alterations occur during or after puberty. Collectively, these novel findings indicate that impaired GABAB signaling can lead to dramatically altered Kiss1 neurons in a region-specific manner, particularly in the MeA, BNST, and septum. Although it is unknown if these effects on Kiss1 expression are due to direct or indirect (or secondary) effects of GABA signaling on Kiss1 neurons, the presence of GABAB1R in extrahypothalamic Kiss1 neurons suggests the possibility for direct regulation. If so, it is possible that GABAB signaling might normally act to inhibit Kiss1 expression in extra hypothalamic regions. These novel findings underscore the importance of studying the regulation and potential function of other kisspeptin populations outside the hypothalamus.
The presence of GABA receptors in Kiss1 neurons has not been previously determined. Here we demonstrate, for the first time, that the GABAB1 subunit is expressed in a very high percentage of Kiss1 neurons of adult rodents. Despite this high coexpression, the lack of functional GABABRs in GABAB1KO mice surprisingly did not affect Kiss1 expression in the AVPV/PeN or ARC, two important reproductive nuclei. This result was unexpected, given previous findings of subfertility and alterations in GnRH levels and pulsatility in GABAB1KO mice (40). Based on this outcome, we propose that the reproductive impairments previously observed in adult GABAB1KO mice are not due to underlying alterations in Kiss1 expression in the main hypothalamic nuclei, ie, AVPV/PeN and ARC, that control fertility. Rather, other reproductive circuits or factors, like neurokinin B, dynorphin, or glutamate, might be involved, or the observed deficits in GnRH/fertility may be mediated directly by GABABR signaling in GnRH cells. We also cannot rule out that despite normal Kiss1 levels in the ARC or AVPV/PeN, kisspeptin protein levels or secretion patterns in these regions might be altered in GABAB1KO mice.
In addition to the ARC or AVPV/PeN, smaller Kiss1 neuron populations resided in several extrahypothalamic brain areas, such as the MeA and BNST (14, 18–21), although kisspeptin's physiological roles in these regions are still under investigation. Interestingly, in stark contrast to the AVPV/PeN and ARC Kiss1 systems, we found a dramatic increase of Kiss1 expression in these extrahypothalamic regions in GABAB1KO mice. In both the BNST and MeA of GABAB1KO mice, Kiss1 expression was higher in males than in females, although Kiss1 was robustly increased in GABAB1KO mice of both sexes compared with WT mice. The expression of Kiss1 in the MeA and BNST is known to be up-regulated by sex steroids, primarily via ER-signaling pathways (14, 18). However, we found that E2 and T serum levels were similar between genotypes, as was gonadal sex steroid content. Thus, the large increases in Kiss1 expression in MeA and BNST are not due to a secondary effect of higher sex steroid secretion in GABAB1KOs (although we cannot rule out higher levels of local neural steroidogenesis that could, in theory, occur in the MeA or BNST).
The functional implications of the dramatic increases in the Kiss1 cell population in the MeA and BNST in GABAB1KO mice remain to be elucidated, but our results lead us to speculate that under normal adulthood conditions, Kiss1 expression in these areas is negatively regulated, directly or indirectly, by GABABR signaling. Whether this effect of GABABR signaling is direct or indirect on MeA and BNST Kiss1 neurons is currently unknown, although our present finding of GABAB1 coexpression in a majority of MeA Kiss1 cells allows for the potential of a direct route of regulation by GABA, at least for this particular Kiss1 population. This possibility of direct regulation will be important to directly test in future studies for each of the extrahypothalamic regions. In support of this possibility, prior evidence indicated that GABA can signal directly, via GABABR, to neurons in the MeA and BNST (46–49), although whether any of those neurons were Kiss1 neurons was not determined.
The MeA and BNST, both of which express GABABR, participate in wide variety of processes and functions, including the modulation of various social and sexual behaviors through the integration of environmental and hormonal signals. Lesions of the MeA impair male sexual behavior in several rodent species (50, 51), whereas lesions of the posterior amygdala decrease sexual receptivity in female rats (52). The BNST has been shown to participate in proceptive and solicitational behaviors, sexual satiety, and maternal behavior (53–55), the latter of which was also influenced by GABABR signaling (56). Additionally, both the MeA and BNST have been implicated in anxiety behaviors (57–59). Adding complexity to the matter, each subregion of the amygdala sends and receives projections to and from other amygdala subnuclei, as well as other brain nuclei (60–63), including the BNST and hypothalamus (61, 64, 65). Moreover, both the AVPV and medial preoptic nucleus, which play important roles in ovulation and male copulatory behavior, respectively, receive regulatory input from the BNST and MeA (66). Although GABAB1KO females do not demonstrate any deficit in their sexual receptivity, neither maternal and proceptive social behaviors nor male copulatory behaviors have yet been quantitatively studied in these mice. Kisspeptin originating from BNST or MeA could, in theory, participate in the modulation of any number of these social or behavioral processes; however, at present, only 2 studies have addressed the behavioral roles, if any, of kisspeptin. Whereas one study in mice found no direct role for kisspeptin signaling in male or female sexual behaviors (2), another study in rats determined that exogenous kisspeptin treatment increased both anxiety and locomotor behaviors (67). Interestingly, both anxiety and locomotion are similarly increased in GABABKO mice (68–70), but whether elevated endogenous kisspeptin originating from the MeA or BNST is driving these observed behavioral effects is unknown.
Concomitantly with the dramatic increase in Kiss1 observed in the MeA and BNST, we also identified a remarkable increase in Kiss1 expression in the lateral septum of adult GABAB1KO mice, a region where Kiss1 expression is essentially undetectable in WT mice. In this area, no sex difference in Kiss1 expression was observed in the GABAB1KO mice, with both KO males and females showing equally large increases in Kiss1 expression. To our knowledge, this is the first report showing Kiss1 neurons in the lateral septum of non-Kiss1 transgenic mice. Previous studies of transgenic Kiss1 mice have reported low levels of fluorescence (serving as a proxy for Kiss1 neurons) in the septum (71). Although the authors interpreted that expression as “ectopic,” because similar expression had never been reported in normal mice, our present findings argue that such Kiss1 expression in the lateral septum may be real and may only normally be revealed when GABAB signaling is decreased, as in our GABAB1KO mice. Indeed, GABABRs are present in this region (72) and activation of GABABRs can influence lateral septum dopamine release (73), indicating that GABA can act in this region via GABABR. Although the role of Kiss1 neurons in the lateral septum remains to be determined, Kiss1 expression in this region, like that in the MeA and BNST, seems to be under negative (direct or indirect) GABABR regulation in adult animals.
Interestingly, the high Kiss1 expression observed in adult GABAB1KO mice was not present in prepubertal mice of either sex (age PND 14). In the MeA, virtually no Kiss1 neurons were found in any group, including WT mice, suggesting that MeA Kiss1 expression is developmentally regulated to turn on during or after puberty. This matches a recent report in rats demonstrating no Kiss1 expression in the MeA of PND 19 females (19). In the BNST, low to moderate Kiss1 expression was detected in both genotypes, indicating that Kiss1 is already expressed in this nucleus before puberty (even in WT mice), at low levels fairly similar to adult levels. Regardless, the absence of enhanced BNST Kiss1 expression in prepubertal GABAB1KO mice indicates that GABABR's effect on BNST Kiss1, like that in the MeA, is not present at all ages, but developmentally begins sometime after PND 14.
Collectively, our findings indicate that, despite high coexpression of Kiss1 and GABABR, AVPV/PeN and ARC Kiss1 gene expression is not altered in GABAB1KO mice and therefore unlikely to contribute to the subfertility previously observed in these mice. Nevertheless, GABAB1KO mice have dramatic changes in extrahypothalamic neural Kiss1 expression, particularly in the MeA, BNST, and septum. Whether kisspeptin protein or secretion patterns are also altered in these regions remains to be determined. Preliminary immunohistochemical analysis did not successfully identify kisspeptin-immunoreactive cells in the MeA of GABAB1KO mice (V. Lux-Lantos, unpublished observation), although only one pilot assay has been attempted thus far. Although it is unknown whether the observed increases in extrahypothalamic Kiss1 gene expression are due to the loss of direct or indirect (or secondary) effects of GABAB signaling on Kiss1 neurons, the observed presence of GABAB1R in many MeA Kiss1 neurons suggests the potential for direct regulation by GABA (at least in this region). If so, our data suggest that GABAB signaling may normally serve to inhibit Kiss1 expression in extrahypothalamic regions. Because the MeA, BNST, and septal areas are involved in the regulation of reproductive and social behaviors, among other physiological processes like anxiety, the abnormally high Kiss1 expression detected in GABAB1KOs may cause alterations that contribute directly or indirectly to some of the phenotypes observed in these mice. Overall, our findings further emphasize the importance of both studying other kisspeptin populations outside of the hypothalamus and considering developmental vs adulthood differences in kisspeptin neurons and their regulation.
Acknowledgments
This work was supported by grants from The National Science Foundation (IOS-1025893 to A.S.K.); National Institutes of Health (U54 HD012303 to A.S.K.), the National Research Council, Argentina (CONICET; PIP 00363); National Agency for the Promotion of Science and Technology, Argentina (ANPCyT: PICT 2007 no. 01050 to C.L. and PICT 2006 no. 00200 to V.L.-L. and PICT 2008 no. 1383 to G.M.S.); University of Buenos Aires, Argentina (ME 043 and 038); and the Swiss Science Foundation, Switzerland (3100A0–117816 to B.B.). N.P.D.G. is supported by a CONICET PhD fellowship.
N.P.D.G. is grateful to International Society for Neurochemistry Committee for Aid and Education in Neurochemistry for a scholarship to work in Dr A. Kauffman's laboratory.
Disclosure Summary: Authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- BNST
- bed nucleus of the stria terminalis
- DIG
- digoxigenin
- E2
- estradiol
- ER
- estrogen receptor
- GABA
- γ-aminobutyric acid
- GABAAR
- GABAA receptor
- GABABR
- GABAB receptor
- ISH
- in situ hybridization
- KO
- knockout
- MeA
- medial amygdala
- PeN
- periventricular nucleus
- PND
- postnatal day
- qPCR
- real-time PCR
- SSC
- sodium citrate, sodium chloride
- WT
- wild type.
References
- 1. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kauffman AS, Park JH, McPhie-Lalmansingh AA, et al. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27:8826–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013 [DOI] [PubMed] [Google Scholar]
- 6. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lents CA, Heidorn NL, Barb CR, Ford JJ. Central and peripheral administration of kisspeptin activates gonadotropin but not somatotropin secretion in prepubertal gilts. Reproduction. 2008;135:879–887 [DOI] [PubMed] [Google Scholar]
- 8. Hashizume T, Saito H, Sawada T, et al. Characteristics of stimulation of gonadotropin secretion by kisspeptin-10 in female goats. Anim Reprod Sci. 2010;118:37–41 [DOI] [PubMed] [Google Scholar]
- 9. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 11. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 13. Kauffman AS, Gottsch ML, Roa J, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 14. Xu Z, Kaga S, Mochiduki A, et al. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J. 2012;59:161–171 [DOI] [PubMed] [Google Scholar]
- 15. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297:E1212–E1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151:5807–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–1316 [DOI] [PubMed] [Google Scholar]
- 18. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152:2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao J, Patisaul HB. Sex-specific expression of estrogen receptors α and β and Kiss1 in the postnatal rat amygdala. J Comp Neurol. 2013;521:465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21:673–682 [DOI] [PubMed] [Google Scholar]
- 21. Brailoiu GC, Dun SL, Ohsawa M, et al. KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol. 2005;481:314–329 [DOI] [PubMed] [Google Scholar]
- 22. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 23. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 24. Poling MC, Kauffman AS. Organizational and activational effects of sex steroids on kisspeptin neuron development. Front Neuroendocrinol. 2013;34:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith JT. Sex steroid regulation of kisspeptin circuits. Adv Exp Med Biol. 2013;784:275–295 [DOI] [PubMed] [Google Scholar]
- 26. Garyfallou VT, Lemos D, Urbanski HF. Expression profiling of genes encoding glutamate and GABA receptor subunits in three immortalized GnRH cell lines. Brain Res. 2006;1086:50–54 [DOI] [PubMed] [Google Scholar]
- 27. Lagrange AH, Wagner EJ, Rønnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology. 1996;64:114–123 [DOI] [PubMed] [Google Scholar]
- 28. Sliwowska JH, Billings HJ, Goodman RL, Lehman MN. Immunocytochemical colocalization of GABA-B receptor subunits in gonadotropin-releasing hormone neurons of the sheep. Neuroscience. 2006;141:311–319 [DOI] [PubMed] [Google Scholar]
- 29. Maffucci JA, Gore AC. Hypothalamic neural systems controlling the female reproductive life cycle gonadotropin-releasing hormone, glutamate, and GABA. In: Kwang WJ, ed. International Review of Cell and Molecular Biology. Vol 274 Burlington, VT: Academic Press; 2009:69–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donoso AO, Seltzer AM, Navarro CE, Cabrera RJ, López FJ, Negro-Vilar A. Regulation of luteinizing hormone-releasing hormone and luteinizing hormone secretion by hypothalamic amino acids. Braz J Med Biol Res. 1994;27:921–932 [PubMed] [Google Scholar]
- 31. Kalra SP, Kalra PS. Neural regulation of luteinizing hormone secretion in the rat. Endocr Rev. 1983;4:311–351 [DOI] [PubMed] [Google Scholar]
- 32. Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009;150:3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pielecka-Fortuna J, Moenter SM. Kisspeptin increases gamma-aminobutyric acidergic and glutamatergic transmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Endocrinology. 2010;151:291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. García-Galiano D, Pineda R, Roa J, et al. Differential modulation of gonadotropin responses to kisspeptin by aminoacidergic, peptidergic, and nitric oxide neurotransmission. Am J Physiol Endocrinol Metab. 2012;303:E1252–E1263 [DOI] [PubMed] [Google Scholar]
- 35. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. γ-Aminobutyric acid B receptor mediated inhibition of gonadotropin-releasing hormone neurons is suppressed by kisspeptin-G protein-coupled receptor 54 signaling. Endocrinology. 2009;150:2388–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurian JR, Keen KL, Guerriero KA, Terasawa E. Tonic control of kisspeptin release in prepubertal monkeys: implications to the mechanism of puberty onset. Endocrinology. 2012;153:3331–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Catalano PN, Bonaventura MM, Silveyra P, Bettler B, Libertun C, Lux-Lantos VA. GABA(B1) knockout mice reveal alterations in prolactin levels, gonadotropic axis, and reproductive function. Neuroendocrinology. 2005;82:294–305 [DOI] [PubMed] [Google Scholar]
- 39. Schuler V, Lüscher C, Blanchet C, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABAB1. Neuron. 2001;31:47–58 [DOI] [PubMed] [Google Scholar]
- 40. Catalano PN, Di Giorgio N, Bonaventura MM, Bettler B, Libertun C, Lux-Lantos VA. Lack of functional GABAB receptors alters GnRH physiology and sexual dimorphic expression of GnRH and GAD-67 in the brain. Am J Physiol Endocrinol Metab. 2010;298:E683–E696 [DOI] [PubMed] [Google Scholar]
- 41. Semaan SJ, Dhamija S, Kim J, Ku EC, Kauffman AS. Assessment of epigenetic contributions to sexually-dimorphic kiss1 expression in the anteroventral periventricular nucleus of mice. Endocrinology. 2012;153:1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poling MC, Kim J, Dhamija S, Kauffman AS. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology. 2012;153:1827–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152:4443–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92:195–203 [DOI] [PubMed] [Google Scholar]
- 45. Iwasaki H, Jodo E, Kawauchi A, Miki T, Kayama Y, Koyama Y. Role of the lateral preoptic area and the bed nucleus of stria terminalis in the regulation of penile erection. Brain Res. 2010;1357:70–78 [DOI] [PubMed] [Google Scholar]
- 46. Grimes JM, Ricci LA, Melloni RH., Jr Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Horm Behav. 2003;44:271–280 [DOI] [PubMed] [Google Scholar]
- 47. Jaferi A, Zhou P, Pickel VM. Enhanced dendritic availability of μ-opioid receptors in inhibitory neurons of the extended amygdala in mice deficient in the corticotropin-releasing factor-1 receptor. Synapse. 2011;65:8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pleil KE, Lopez A, McCall N, Jijon AM, Bravo JP, Kash TL. Chronic stress alters neuropeptide Y signaling in the bed nucleus of the stria terminalis in DBA/2J but not C57BL/6J mice. Neuropharmacology. 2012;62:1777–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tasan RO, Bukovac A, Peterschmitt YN, et al. Altered GABA transmission in a mouse model of increased trait anxiety. Neuroscience. 2011;183:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210:557–560 [DOI] [PubMed] [Google Scholar]
- 51. Kondo Y. Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiol Behav. 1992;51:939–943 [DOI] [PubMed] [Google Scholar]
- 52. Mascó DH, Carrer HF. Sexual receptivity in female rats after lesion or stimulation in different amygdaloid nuclei. Physiol Behav. 1980;24:1073–1080 [DOI] [PubMed] [Google Scholar]
- 53. Martinez LA, Petrulis A. The bed nucleus of the stria terminalis is critical for sexual solicitation, but not for opposite-sex odor preference, in female Syrian hamsters. Horm Behav. 2011;60:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Perrin G, Meurisse M, Lévy F. Inactivation of the medial preoptic area or the bed nucleus of the stria terminalis differentially disrupts maternal behavior in sheep. Horm Behav. 2007;52:461–473 [DOI] [PubMed] [Google Scholar]
- 55. Parfitt DB, Newman SW. Fos-immunoreactivity within the extended amygdala is correlated with the onset of sexual satiety. Horm Behav. 1998;34:17–29 [DOI] [PubMed] [Google Scholar]
- 56. Arrati PG, Carmona C, Dominguez G, Beyer C, Rosenblatt JS. GABA receptor agonists in the medial preoptic area and maternal behavior in lactating rats. Physiol Behav. 2006;87:51–65 [DOI] [PubMed] [Google Scholar]
- 57. Liu J, Garza JC, Li W, Lu XY. Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int J Neuropsychopharmacol. 2013;16:105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Agmo A. The role of the estrogen receptor α in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav Brain Res. 2010;210:211–220 [DOI] [PubMed] [Google Scholar]
- 59. Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;324:143–179 [DOI] [PubMed] [Google Scholar]
- 61. Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536 [DOI] [PubMed] [Google Scholar]
- 62. Cooke BM, Simerly RB. Ontogeny of bidirectional connections between the medial nucleus of the amygdala and the principal bed nucleus of the stria terminalis in the rat. J Comp Neurol. 2005;489:42–58 [DOI] [PubMed] [Google Scholar]
- 63. Maras PM, Petrulis A. Anatomical connections between the anterior and posterodorsal sub-regions of the medial amygdala: integration of odor and hormone signals. Neuroscience. 2010;170:610–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Coolen LM, Wood RI. Testosterone stimulation of the medial preoptic area and medial amygdala in the control of male hamster sexual behavior: redundancy without amplification. Behav Brain Res. 1999;98:143–153 [DOI] [PubMed] [Google Scholar]
- 65. Manzo J, Cruz MR, Hernández ME, Pacheco P, Sachs BD. Regulation of noncontact erection in rats by gonadal steroids. Horm Behav. 1999;35:264–270 [DOI] [PubMed] [Google Scholar]
- 66. Simerly RB. Hormonal control of neuropeptide gene expression in sexually dimorphic olfactory pathways. Trends Neurosci. 1990;13:104–110 [DOI] [PubMed] [Google Scholar]
- 67. Csabafi K, Jászberényi M, Bagosi Z, Lipták N, Telegdy G. Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behav Brain Res. 2013;241:56–61 [DOI] [PubMed] [Google Scholar]
- 68. Mombereau C, Kaupmann K, Van der Putten H, Cryan JF. Altered response to benzodiazepine anxiolytics in mice lacking GABAB1 receptors. Eur J Pharmacol. 2004;497:119–120 [DOI] [PubMed] [Google Scholar]
- 69. Mombereau C, Kaupmann K, Gassmann M, Bettler B, van der Putten H, Cryan JF. Altered anxiety and depression-related behaviour in mice lacking GABAB2 receptor subunits. Neuroreport. 2005;16:307–310 [DOI] [PubMed] [Google Scholar]
- 70. Sweeney FF, O'Leary OF, Cryan JF. 2013 Activation but not blockade of GABA receptors during early-life alters anxiety in adulthood in BALB/c mice [published online September 17, 2013]. Neuropharmacology. doi:10.1016/j.neuropharm.2013.08.039 [DOI] [PubMed] [Google Scholar]
- 71. Dungan Lemko HM, Elias CF. Kiss of the mutant mouse: how genetically altered mice advanced our understanding of kisspeptin's role in reproductive physiology. Endocrinology. 2012;153:5119–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liang F, Hatanaka Y, Saito H, Yamamori T, Hashikawa T. Differential expression of γ-aminobutyric acid type B receptor-1a and -1b mRNA variants in GABA and non-GABAergic neurons of the rat brain. J Comp Neurol. 2000;416:475–495 [PubMed] [Google Scholar]
- 73. Sotomayor-Zárate R, Araya KA, Pereira P, et al. Activation of GABA-B receptors induced by systemic amphetamine abolishes dopamine release in the rat lateral septum. J Neurochem. 2010;114:1678–1686 [DOI] [PubMed] [Google Scholar]