Abstract
A variety of functions have been proposed for progesterone receptor membrane component 1 (PGRMC1), including acting as a component of a membrane progestin receptor and as an adaptor protein. Here we show that stable overexpression of human PGRMC1 in nuclear progesterone receptor (PR)-negative breast cancer cell lines causes increased expression of PGRMC1 and membrane progesterone receptor α (mPRα) on cell membranes that is associated with increased specific [3H]progesterone binding. The membrane progestin binding affinity and specificity were characteristic of mPRα, with a Kd of 4.7 nM and high affinity for the mPR-specific agonist, Org OD 02–0, and low affinity for corticosteroids. Progestin treatment caused activation of G proteins, further evidence for increased expression of functional mPRs on PGRMC1-transfected cell membranes. Immunocytochemical and coimmunoprecipitation studies showed a close association of PGRMC1 with mPRα in cell membranes. Transfection of PGRMC1 into spontaneously immortalized rat granulosa cells was associated with membrane expression of PGRMC1 and mPRα as well as antiapoptotic effects of progestins that were abolished after cotransfection with small interfering RNA for mPRα. These data demonstrate that PGRMC1 can act as an adaptor protein, transporting mPRα to the cell surface, and that the progestin binding and apoptotic functions previously ascribed to PGRMC1 are dependent on cell surface expression of mPRα. Collectively, the results suggest PGRMC1 and mPRα are components of a membrane progesterone receptor protein complex. Increased expression of estrogen receptor β was also observed in the membranes of PGRMC1-transfected cells, suggesting that PGRMC1 can act as an adaptor protein for multiple classes of steroid receptors.
Many actions of progesterone are too rapid to be readily explained by the classic genomic mechanism of steroid action involving activation of the intracellular transcription factors, progesterone receptors PR-A and PR-B, which typically occurs over a time scale of hours. Extensive evidence has accumulated that progesterone, like other steroid hormones, can also initiate rapid, cell surface-mediated actions within minutes by activating membrane receptors and their intracellular signal transduction pathways (1–3). For instance, cell surface-initiated (nonclassical) progesterone actions have been demonstrated on sperm motility and the acrosome reaction (4), oocyte meiotic maturation (5), GnRH secretion (6), reproductive behaviors (7), and apoptosis of granulosa, breast cancer and neuronal cells (8–11). Some of these alternative progesterone actions are nongenomic, whereas others may ultimately lead to altered gene transcription through activation of second messengers such as MAPKs resulting in cAMP response element-binding protein phosphorylation and by alteration of PR transactivation through regulation of coactivators such as steroid receptor coactivator 2 (12). Biochemical binding characteristics of putative membrane receptors mediating some of these rapid progesterone actions have been described, but in many cases their identities remain unclear (2, 3, 13). Although nonclassical progesterone signaling can be mediated by PRs in the cytoplasm through an interaction with Src kinase (14), strong progesterone responses have been reported in PR-null mice (6, 7) and in cells and tissues lacking PRs (15). Therefore, cell surface-initiated progesterone actions demonstrated in many cells must involve other receptor mechanisms.
There is substantial evidence that progesterone initiates rapid, cell-surface actions in PR-negative cells through 2 distinct membrane protein families, 7–8 transmembrane membrane progesterone receptors (mPRs) (5, 13, 16), and progesterone receptor membrane components (PGRMC) 1 and 2, which have a single transmembrane domain (8, 13). mPRs are members of the progesterone and adipoQ receptor (PAQR) family (17, 18) and comprise 5 subtypes (mPRα, -β, -γ, -δ, and -ϵ), all of which display high-affinity (Kd ∼5 nM), specific progestin binding on plasma membranes of vertebrate cells (11, 17). Recent studies in several laboratories have clearly established that specific progestin binding is an intrinsic property of mPRs (3, 13). Recombinant mPRs produced in mammalian, yeast, and prokaryotic expression systems all display the binding characteristics of progestin receptors (5, 16, 17, 19). Moreover, recombinant human, zebrafish, and seatrout mPRαs produced in the same mammalian expression system display different progestin binding specificities, showing greatest affinities for their endogenous progestin hormones, which differ among the 3 species (13, 17). The mPRs are ubiquitously expressed in vertebrate tissues (18), and all 5 mPR subtypes are expressed on plasma membranes of vertebrate cells, are coupled to G proteins, and initiate a variety of intracellular signaling pathways associated with G protein activation (5, 11, 12, 17, 20). Therefore, mPRs are plausible candidates for the membrane progesterone receptors mediating rapid, cell surface-mediated actions of progestins in every cell type in which nonclassical progestin actions have been described.
Although PGRMC1 is clearly a component of nonclassical progesterone signaling, its exact role is uncertain, and clear evidence that it functions as a specific progesterone receptor is lacking (13, 21–23). Recombinant PGRMC1 only has moderate steroid specificity for progesterone and displays relative high binding affinity for testosterone and cortisol when it is produced in Chinese hamster ovary cells (24) and binds dexamethasone when expressed in COS-7 cells (25). Moreover, the mechanisms by which PGRMC1 mediates its antiapoptotic actions and intracellular signaling are unclear (8, 23), and limited data on signaling pathways through PGRMC1 suggest that its action is indirect. A variety of other ligands have been shown or proposed to bind to PGRMC1 including heme, cholesterol metabolites, several compounds that are pharmacologically active such as haloperidol, and the σ 2 ligand WC-21 (23, 26, 27). No specific progesterone binding to recombinant PGRMC1 produced in a prokaryotic expression system (Escherichia coli) was detected, whereas the recombinant protein bound heme, indicating that a functional protein was produced (28). One possible explanation of these findings is that a protein present in eukaryotic cells that forms a complex with PGRMC1 required for progesterone binding is lacking in E. coli (13). Recent studies show that PGRMC1 forms complexes with many proteins such as multiple P-450 proteins, including those involved on sterol synthesis, proteins in cholesterol synthetic pathways such as Scap (steroid regulatory element-binding protein cleavage activating protein) and insulin-induced gene (Insig), epidermal growth factor receptor (EGFR) (29), and plasminogen activator RNA-binding protein (PAIRBP1), previously known as RDA288 (21, 23, 30). In addition, evidence has been obtained that PGRMC1 is involved in wide diversity of functions including axonal guidance, steroid synthesis and metabolism, cholesterol regulation and endocystosis, and EGFR functions (21, 23). Collectively these findings are inconsistent with the specific role for PGRMC1 as a progesterone membrane receptor proposed in some recent papers (8, 31) and suggest instead that the protein has more general functions, including acting as an adaptor protein for a wide variety of proteins.
There are no published studies that attempt to reconcile these 2 competing models of nonclassical progesterone actions through mPRs and PGRMC1. We hypothesized that mPRs and PGRMC1 may be components of the same membrane progesterone receptor complex, the progesterone-binding pocket and G protein coupling residing in mPRs, and PGRMC1 acting as an adaptor protein, regulating transport of mPR to the plasma membrane and facilitating its signaling through the formation of a PGRMC1-mPR complex. In support of this idea recent studies demonstrate that both mPRs and PGRMC1 mediate similar antiapoptotic functions of progestins in vertebrate granulosa and cancer cells (8–10, 32, 33) and both show increased expression in tumors (9, 33). Therefore, in the present study the effects of overexpression of PGRMC1 on plasma membrane expression and functions of mPRs were investigated in granulosa and breast cancer cells. Triple-negative MDA-MB-231 human breast cancer cells were selected for these studies because, unlike other cells expressing mPRs, membrane progesterone receptor functions cannot be demonstrated in these cells and no cell surface expression of mPRs can be detected (11, 17). The potential involvement of mPRα in mediating antiapoptotic actions of progesterone in rat spontaneously immortalized granulosa cells (SIGCs) was also investigated because the role of PGRMC1 as an intermediary in these progesterone actions has been extensively investigated in this cell model (8, 30). In addition possible up-regulation of other steroid receptors on MDA-MB-231 cell membranes by PGRMC1 was investigated.
Materials and Methods
Chemicals
Steroids were purchased from Sigma-Aldrich or Steraloids, Inc., and Org OD 02–0 was obtained from Organon. R5020, [2,4,6,7-3H]progesterone ([3H]P4, 102.1 Ci/mmol, and [2,4,6,7-3H]estradiol-17β ([3H]E2, 84 Ci/mmol) were purchased from Amersham Pharmacia Biotech. All other chemicals were purchased from Sigma-Aldrich unless otherwise stated.
Expression of recombinant PGRMC1 in mammalian cell lines
Full-length cDNA clones encoding human PGRMC1 were purchased from OriGene and subcloned into a PCMV6-NEO mammalian expression vector (OriGene). Correct insertion was confirmed by sequencing. Human MDA-MB-231, SKBR3, and MCF-7 breast cancer cells (American Type Culture Collection, Manassas, VA) and rat SIGCs (kindly provided by Dr. Robert Burghardt, Texas A&M University, College Station, TX) were transfected with the PGRMC1 construct (PG) or empty vector (V) using Lipofectamine 2000 (Invitrogen,) and selected with geneticin (G-148; 500 μg/mL; Invitrogen) to generate stable cell lines with high PGRMC1 expression by limiting dilution (13). Breast cancer cells and SIGCs were cultured in DMEM/Ham's F-12 medium containing 10% charcoal-stripped FBS (Invitrogen). SKBR3 cells, not transfected with PGRMC1 or empty vector, were transiently transfected with PGRMC1 small interfering RNA (siRNA) (100 nM), or nontarget siRNA using Lipofectamine 2000 twice at 0 hours and 16 hours, following the manufacturer's procedures (Dharmacon), and experiments were conducted 2 days later. Estrogen receptor (ER)β expression was decreased in PG-transfected MDA-MB-231 cells by transfection with ERβ siRNA and mPRα expression in SIGCs was decreased by transfection with mPRα siRNA (100 nM, Dharmacon) twice as described above, and experiments were conducted on the third day.
Preparation of plasma membranes and other subcellular fractions
Cells were homogenized by sonication in buffer containing a 0.1% protease inhibitor cocktail, and subcellular fractions were isolated by differential centrifugation as described previously (5, 17, 34).
Progesterone and estrogen membrane receptor binding assays
Saturation analysis of [3H]P4 and [3H]E2 binding (range: 0.125 nM to 8 nM) alone (total binding [TB]), or in the presence of 450–750 nM nonradiolabeled steroid (nonspecific binding [NSB]), to plasma membranes of V and PG cells after 30 minutes incubation at 4°C was determined by filtration assay as described previously (17, 35). For competitive binding assays, tubes contained [3H]P4 (4 nM) and steroid competitors (range 10−9 to 10−5 M), and displacement of [3H]P4 binding was expressed as a percentage of maximum specific binding.
Activation of G proteins
G protein activation in response to progesterone treatment (100 nM for 15 minutes at 25°C) was determined by measuring increases in specific binding of [35S]GTPγS to plasma membranes (∼50 μg protein) of V, PG, and mPRα-transfected cells as described previously (17, 35).
Western blot analysis and immunocytochemistry
Proteins (10 μg) were resolved on 12% SDS-PAGE gels and transferred to nitrocellulose membranes (17). Blotted membranes were blocked with 5% nonfat milk in buffer for 1 hour prior to incubation with the antibodies. The human mPRα antibody has been described and validated previously (17). The ERβ antisera, raised in rabbits to N-terminal amino acid sequence 1–150 of human ERβ (H-150, Santa Cruz Biotechnology) does not cross-react with ERα (36, 37). PGRMC1 antibody was generated in rabbits to a 15-mer sequence of human PGRMC1 (CDEEEPKDESARKND) conjugated to keyhole limpet hemocyanin and rat mPRα antisera to the peptide sequence CEPVFTVDRAEVPRLF. Antibodies (dilution: 1:2500; ERβ: 1:300) were incubated overnight at 4°C. The specificity of the immunoreactions was confirmed by blocking with peptide antigens (2–3 μg peptide/μL antibody diluted 1:20 in PBS) preincubated at 26°C with antibodies for 1.5–2 hours and by demonstrating weaker immunoreactive bands after siRNA treatments. Blots were incubated for 1 hour at 26°C with goat antirabbit secondary antibody (Cell Signaling Technology), and visualized by treatment with enhanced chemiluminescence substrate (SuperSignal, Pierce).
Immunocytochemistry was conducted as described previously, with few modifications (11, 17). Cells were incubated with primary antibodies (dilution 1:500) in 2% BSA overnight at 4°C. The specificity of the immunoreactions was confirmed by preabsorbing antisera with peptide antigens (1–5 μg peptide/1 μL antibody). Cells were subsequently incubated with AlexaFluor 488 goat antirabbit or AlexFluor 647 donkey antigoat secondary antibody (Molecular Probes; dilution 1:1000), and fluorescent-labeled receptor proteins were visualized using a Nikon fluorescent microscope.
Biotinylation of surface proteins and pull-down assay
Surface proteins were biotinylated for 30 minutes at 4°C with 1 mg/mL sulfo-NHS-LC biotin (Pierce Chemical Co), solubilized in lysis buffer, absorbed overnight on immobilized strepavidin-agarose slurry, eluted in sodium dodecyl sulfate, and electrophoresced followed by Western blot analysis as described previously (38).
Coimmunoprecipitation of PGRMC1 with mPRα
Coimmunoprecipitation was performed as described previously for coimmunoprecipitation of receptors with G proteins (17). Plasma membranes were solubilized with radioimmune precipitation assay buffer and incubated overnight at 4°C with goat anti-mPRα or PGRMC1 antibodies and control goat IgG (1:100, Santa Cruz Biotechnology) and then incubated with protein A/G plus-agarose beads for 2 hours. Immunoprecipitates were eluted by boiling, and samples were separated and blotted onto nitrocellulose membranes following standard Western blot procedures. Membranes were blocked with 5% nonfat milk and incubated at 4°C overnight with rabbit human PGRMC1 or mPRα (1:2500) antibodies. Proteins were visualized as described above for Western blot analysis.
RT-PCR of steroid receptors
Total RNA was extracted with Trireagent. Reverse transcription was performed on 1–3 μg total RNA using Superscript II reverse transcriptase (Invitrogen), and oligo (dT)s. PCR was conducted using Platinum PCR SuperMix (Invitrogen). Gene-specific primers for PGRMC1, (sense, 5′-GCTCAACCTGCTGCTGCTT; antisense, 5′-GCACTCTCATCTTTTGGTTC), mPRα (17), and ERβ, (sense, 5′-GAGGCTGCGAGAAATAACTGC; antisense, 5′-GCTGGATATTCATGGTGGCTG) PCR was performed as described previously (11).
Statistics
Saturation curves of steroid binding in radioreceptor assays were analyzed by nonlinear regression, and the dissociation constants (Kd) and binding capacities (Bmax) were calculated using GraphPad Prism Software (version 3.02). Results are expressed as means ± SEM of 4–6 observations, and all experiments were repeated at least 3 times with separate batches of cells on different days. Student's paired t test was used for paired comparisons (GraphPad Prism Software).
Results
Plasma membrane expression of mPRα on MDA-MB-231 cells stably transfected with PGRMC1
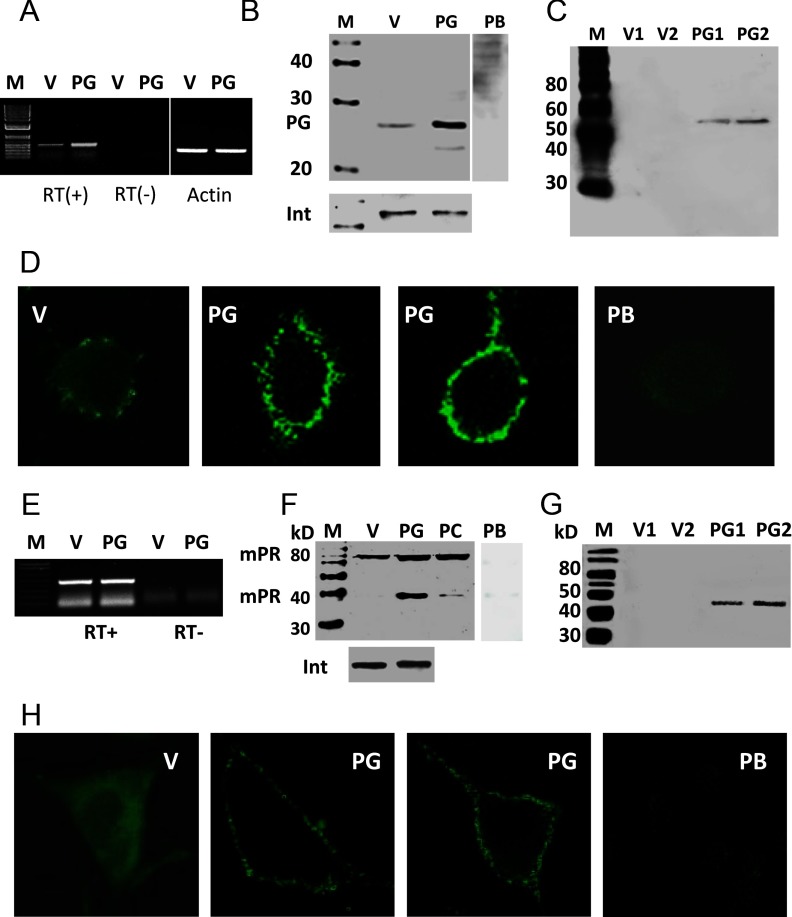
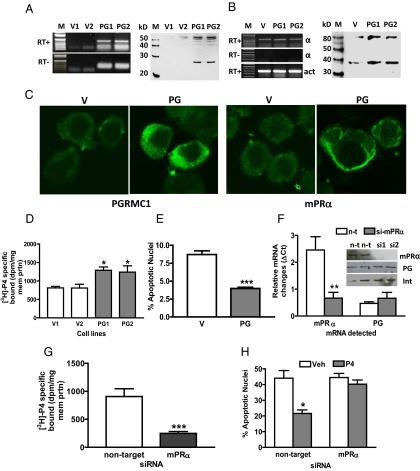
RT-PCR produced a strong band of the expected size for PGRMC1 in PR-negative MDA-MB-231 cells stably transfected with PGRMC1 (PG) which was weaker in V-transfected cells and absent in RT-controls (Figure 1A). Quantitative PCR showed that PGRMC1 mRNA expression was 2-fold higher in PG cells compared with that in V cells (Supplemental Figure 1A published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Western blot analysis showed a single approximately 28-kDa band corresponding to the predicted molecular weight of PGRMC1, which was stronger in cell membranes of PG cells and absent after pretreatment of the PGRMC1 antibody with peptide antigen (Figure 1B). An immunoblot of biotinylated cell membrane proteins with the PGRMC1 antibody showed bands at approximately 55 kDa, likely representing a PGRMC1 dimer (22), indicating that the protein is expressed on the surface of PG cells (Figure 1C). Immunocytochemical analysis of V cells showed that PGRMC1 is distributed throughout the cytoplasm with some plasma membrane staining, whereas PGRMC1 was concentrated near the cell membrane in PG cells (Figure 1D). Fluorescence staining of PG cells was blocked after preincubation of the PGRMC1 antibody with peptide antigen, confirming the specificity of the immunoreaction (Figure 1D).
Figure 1.
Expression of PGRMC1 and mPRα in MDA-MB-231 cells stably transfected with PGRMC1. A and E, Detection of PGRMC1 (A) and mPRα (E) mRNA in PGRMC1 (PG)- and vector (V)-transfected cells by RT-PCR. B and F, Western blot of plasma membranes from PG and V cells with PGRMC1 (B) and mPRα (F) antibodies. C and G, Western blot analysis of biotinylated cell-surface proteins with the PGRMC1 (C) mPRα (G) antibodies. D and H, Immunocytochemistry of PGRMC1 (D) and mPRα (H) proteins in PG and V cells. Int, integrin; PB, peptide block; PC, mPRα-transfected control cells; PG1,PG2,V1,V2, different stably transfected cell lines; M, DNA size marker and protein molecular weight marker. In total 5 stable cell lines expressing PGRMC1 were established, and overexpression of mPRα was observed in every case.
No increase in mPRα mRNA expression was detected in PG cells compared with that in V cells by RT-PCR (Figure 1E) or by quantitative PCR (Supplemental Figure 1B), whereas Western blot analysis showed stronger mPRα protein bands in plasma membranes of PG cells compared with V controls (Figure 1F). Both 40-kDa and 80-kDa protein bands were detected in PG cell membranes and in MDA-MB-231 cells stably transfected with mPRα (positive controls [PC]), whereas only the 80-kDa band, which is presumably the mPRα dimmer, was present in V membranes (Figure 1F). The specificity of the immunoreaction was confirmed by preincubating the mPRα antiserum with peptide antigen (peptide block). Biotinylation of surface proteins followed by Western blot analysis with mPRα antibody showed a single band corresponding to the mPRα monomer, indicating cell-surface expression of mPRα on PG cells, but not on V cells (Figure 1G). Immunocytochemistry with mPRα antiserum showed diffuse cytoplasmic and perinuclear localization of mPRα in V cells (Figure 1H), whereas mPRα was concentrated in plasma membranes of PG cells (Figure 1H), which was also shown by confocal microscopy (Supplemental Figure 1C).
[3H]P4 binding characteristics of plasma membranes of MDA-MB-231 cells transfected with PGRMC1
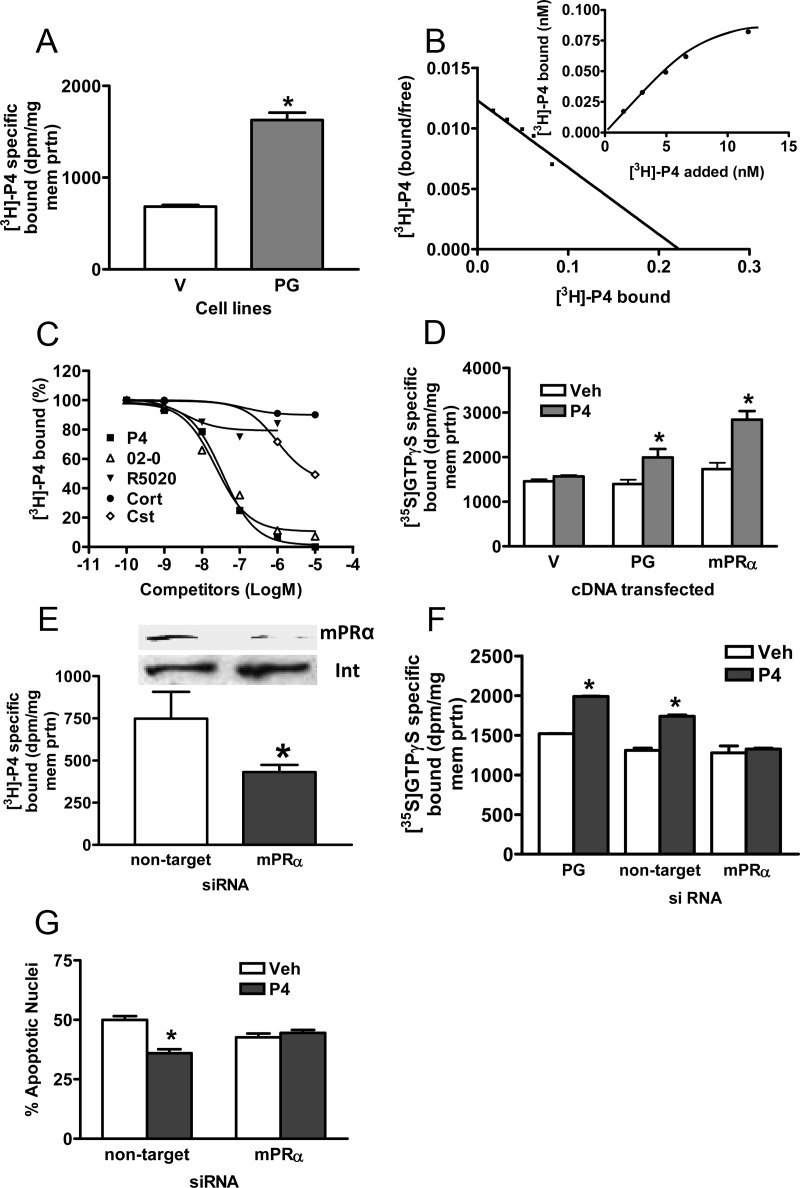
Cell-surface expression of PGRMC1 and mPRα proteins on PG cells was associated with a significant approximately 2.5-fold increase in specific [3H]P4 binding compared with binding to V cell membranes in single-point binding assays (Figure 2A). Saturation and Scatchard plot analyses showed the presence of a high-affinity, saturable, single binding site with a dissociation constant (Kd = 4.69 nM) similar to that of mPRα and a Bmax of 0.31 nM (Figure 2B). Competition studies showed the steroid specificity of binding to PG cells is characteristic of mPRα and differs from that of PR (35). Progesterone and the selective mPRα agonist, Org OD 02–0 (35), were equally effective competitors for [3H]P4 binding, with IC50s of approximately 5 × 10−8 M, whereas the nuclear PR agonist, R5020, did not compete for P4 binding at concentrations up to 10−6 M (17, 35; Figure 2C). Cortisol was ineffective as a competitor for P4 binding and corticosterone displayed very weak binding affinity with an IC50 of about 10−5 M, 500-fold higher than that of progesterone (Figure 2C). Membrane localization of mPRα and PGRMC1 was also accompanied by the acquisition of progesterone-dependent G protein activation, shown by an increase in membrane-bound specific [35S]GTPγS binding (Figure 2D), which was similar to that in MDA-MB-231 cells transfected with mPRα (Figure 2D). Transfection of PG cells with mPRα siRNA decreased membrane mPRα expression and specific [3H]P4 binding (Figure 2E), and blocked the specific [35S]GTPγS binding (Figure 2F) and antiapoptotic (Figure 2G) responses to progesterone treatments.
Figure 2.
Progestin binding to plasma membranes and G protein activation in MDA-MB-231 cells stably transfected with PGRMC1 (PG). A, Specific [3H]P4 binding to PG- and vector (V)-transfected cell membranes in a single point assay. B, Representative saturation curve and Scatchard plot of specific [3H]P4 binding to plasma membranes of PG cells. C, Representative competition curves for progestin binding expressed as a percentage of maximum progesterone binding. D, Specific membrane binding of [35S]GTPγS in response to 100 nM progesterone. E, Effects of transfection of PG cells with mPRα siRNA on membrane mPRα expression (upper) and specific [3H]P4 membrane binding (lower). F. Effects of transfection of PG cells with mPRα siRNA on specific membrane binding of [35S]GTPγS in response to progesterone. G, Effects of transfection of PG cells with mPRα siRNA on progesterone attenuation of serum starvation-induced apoptosis. P4, progesterone; 02–0, Org OD 02–0; R5020, promegestone; Cort, cortisol; Cst, corticosterone; veh, vehicle control. D, mPRα, MDA-MB-231 cells stably transfected with mPRα. E, mPRα, PG cells transfected with mPRα siRNA; nontarget, PG cells transfected with control pool of siRNA. P < .05 compared with V (A), corresponding vehicle-treated (D, F, and G), compared with nontarget (E), n = 6 (Students t test). All the experiments were repeated 3 or more times and similar results were obtained in each experiment.
Coimmunoprecipitation of PGRMC1 and mPRα from plasma membranes of MDA-MB-231 cells transfected with PGRMC1
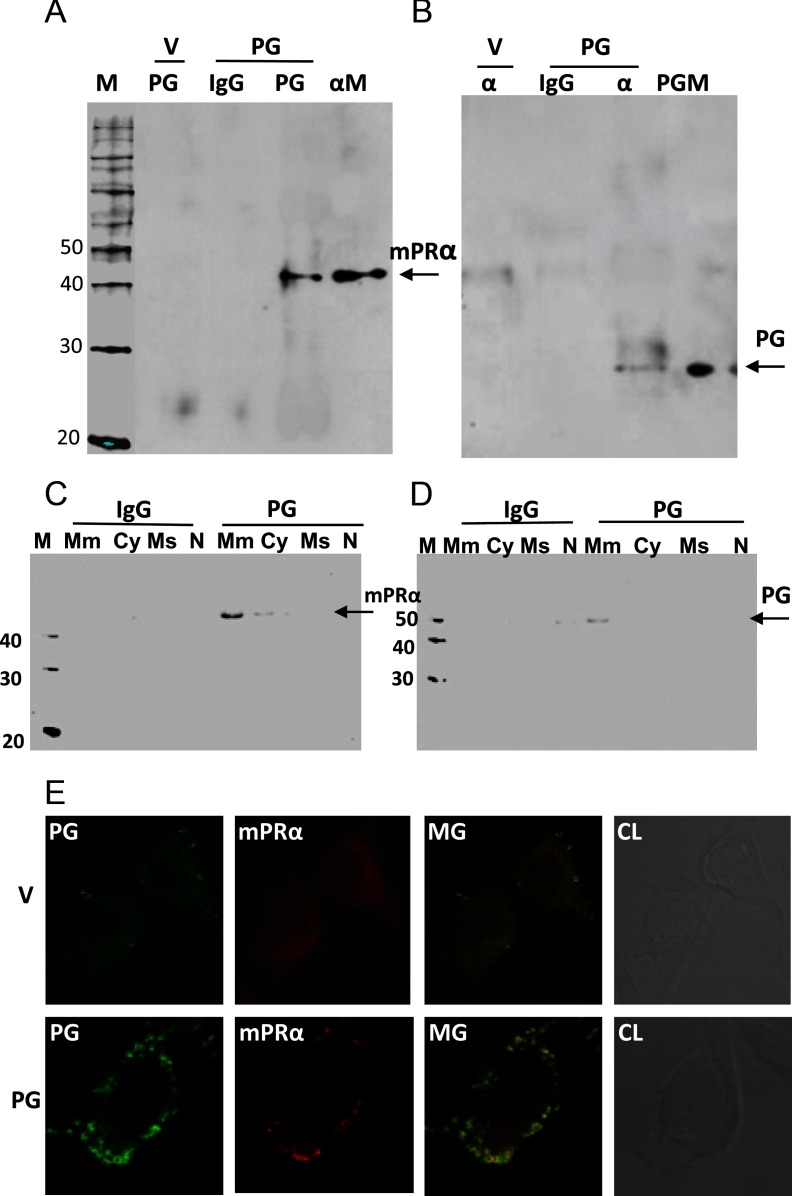
Coimmunoprecipitation experiments indicated that PGRMC1 interacts with, and likely is coupled to, mPRα in PG cells. Immunoprecipitation of plasma membrane proteins of PG cells with goat PGRMC1 antibody (Santa Cruz Biotechnology), followed by Western blotting with rabbit mPRα antisera, showed a single immunoreactive band at 40 kDa, the predicted molecular weight of the mPRα monomer, and the absence of a band in the goat IgG controls (Figure 3A). mPRα was associated with PGRMC1 in the plasma membrane fraction, with a weaker band in the cytosolic fraction of PG cells (Figure 3C). Similarly, immunoprecipitation with goat mPRα antibody (Santa Cruz Biotechnology), followed by immunodetection with rabbit PGRMC1 antibody on a Western blot, showed a band of the correct size for PGRMC1 (∼29 kDa), whereas no immunoreactive band was detected in the control goat IgG-immunoprecipitated protein fraction (Figure 3B). PGRMC1 was associated with mPRα in the plasma membrane fraction of PG cells (Figure 3D). Immunocytochemistry of PG cells with PGRMC1 and mPRα antibodies showed their colocalization on the plasma membrane, with both close and overlapping areas of intense staining apparent in the merged image, further suggesting a close association between PGRMC1 and mPRα (Figure 3E, MG). Cell surface expression of mPRβ and mPRγ also appeared to be increased in PG cells although the increase was minor compared with that of mPRα (Supplemental Figure 2), and neither mPRβ nor mPRγ coimmunoprecipitated with PGRMC1 (results not shown).
Figure 3.
Coimmunoprecipitation and colocalization of PGRMC1 and mPRα on plasma membranes of MDA-MB-231 cells stably transfected with PG and V. A and C, Representative result of immunoprecipitation with specific PGRMC1 antibody (PG) or IgG followed by immunodetection of mPRα by Western blot analysis. B and D, Representative results of immunoprecipitation with specific mPRα antibody (α) or IgG followed by immunodetection of PGRMC1 by Western blot analysis. E, Colocalization by immunocytochemistry of PGRMC1 and mPRα in cells transfected with PG and V. M, molecular weight marker; αM, Western blot analysis of mPRα-transfected plasma membranes with mPRα antibody; PGM, Western blot analysis of PG-transfected plasma membranes with PG antibody; Mm, plasma membrane fraction; Cy, cytosolic fraction; Ms, microsomal fraction; N, nuclear fraction; MG, merge; CL, control light image.
Functional characteristics of mPRα in SKBR3 and MCF-7 cells stably transfected with PGRMC1
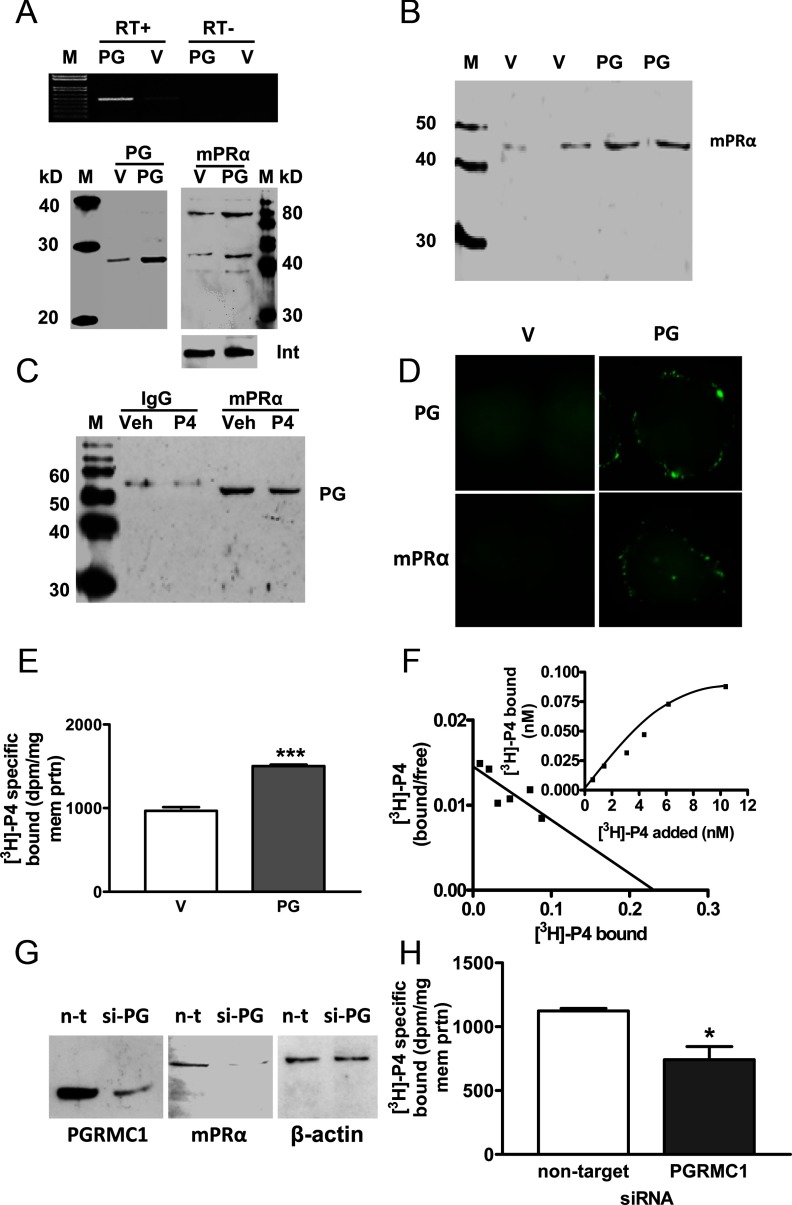
Similar up-regulation of cell-surface mPRα protein expression and [3H]P4 binding was observed when PGRMC1 was stably expressed in PR-negative SKBR3 cells. Increased expression of PGRMC1 in plasma membranes of transfected (PG) cells compared with V controls was accompanied by increased expression of the 40-kDa and 80-kDa mPRα protein bands on Western blots (Figure 4A), and immunoblot analysis of biotinylated proteins showed greater cell-surface expression of mPRα (Figure 4B). PGRMC1 protein was immunoprecipitated with the mPRα antibody, indicating a close association between the 2 proteins (Figure 4C). There was an apparent decrease in staining intensity of the band after progesterone treatment, consistent with decreased membrane-bound mPRα due to its internalization after progesterone binding, and decreased immunoprecipitation of plasma membrane proteins associated with mPRα (10, 17) (Figure 4C). Immunocytochemical analysis showed localization of both PGRMC1 and mPRα near the plasma membrane of PG cells, whereas diffuse, weak intracellular staining for both proteins was observed in V cells (Figure 4D). This pattern of protein expression was associated with a significant increase in specific [3H]P4 binding compared with the V controls (Figure 4E). Saturation analysis showed the presence of a high affinity (Kd = 4.7 nM), saturable single binding site characteristic of recombinant mPRα (Figure 4F). SKBR3 cells (not PG or V) transiently transfected with PGRMC1 siRNA showed decreased expression of both PGRMC1 and mPRα (Figure 4G) and decreased [3H]P4 binding compared with the nontarget controls (Figure 4H).
Figure 4.
Expression, colocalization of PGRMC1 and mPRα and progesterone binding in SKBR3 cells stably expressed with PGRMC1 (PG). A, Detection of PGRMC1 (PG) mRNA (top) and membrane-bound PGRMC1 protein (bottom left) and mPRα protein (bottom right) in PG- and in vector (V)-transfected cells by RT-PCR and Western blot analysis. B, Western blot analysis of biotinylated cell-surface proteins with the mPRα antibody. C, Coimmunoprecipitation of PGRMC1 and mPRα. Pull-down with specific mPRα antibodies followed by immunodetection of PGRMC1 by Western blot analysis. P4, pretreated with 100 nM progesterone for 30 minutes prior to immunoprecipitation. D, Immunocytochemistry of PGRMC1 (upper) and mPRα (lower) in PG and V cells. E and F, Progesterone binding to PG and V cells. E, Specific [3H]P4 binding to transfected cell membranes in a single point assay. F, Representative saturation curve and Scatchard plot of specific [3H]P4 binding to plasma membranes of transfected cells. G and H, Effects of transfection of SKBR3 cells (not PG or V) with PGRMC1 siRNA (si-PG) or nontarget (n-t) siRNA on protein expression of PGRMC1 and mPRα (G) and specific [3H]P4 binding (H). Veh, vehicle; M, DNA size marker and molecular weight marker; int, integrin; ***, P < .01 compared to V control, n = 6; *, P < .05 compared with nontarget control, n = 6. All the experiments were repeated 3 or more times and similar results were obtained in each experiment.
Similar effects of overexpression of PGRMC1 were observed in a PR-positive breast cancer cell line, MCF-7 cells (Supplemental Figure 3). Expression of mPRα on cell membranes was increased, and this was associated with the appearance of a saturable, high-affinity P4 binding site (Kd = 5.05 nM) with the characteristics of mPR.
Functional characteristics of mPRα in SIGCs stably transfected with PGRMC1
SIGCs showed low expression of both PGRMC1 and mPRα transcripts and protein levels (Figure 5, A and B), whereas stable expression of PGRMC1 in SIGCs resulted in increased expression of both PGRMC1 and mPRα proteins on the cell surface (Figure 5, A and B). Increased mPRα levels were also detected on cell membranes by immunocytochemical staining (Figure 5C). As observed with breast cancer cells, increased cell-surface expression of these 2 proteins was accompanied by the appearance of high levels of specific membrane-bound [3H]P4 binding (Figure 5D). In addition, stably transfected cells showed an antiapoptotic response to progesterone treatment, confirming the findings of Peluso et al (8) (Figure 5E). Transient transfection of siRNA to mPRα decreased mPRα expression (Figure 5F) and specific [3H]P4 binding to background levels (Figure 5G) and attenuated the antiapoptotic response to progesterone treatment (Figure 5H), suggesting that both progesterone binding and its antiapoptotic actions are dependent upon the presence of mPRα
Figure 5.
Expression of PGRMC1 and mPRα in SIGCs stably transfected with PGRMC1 (PG). A, detection of PGRMC1 mRNA in PG- and V-transfected cells by RT-PCR and PGRMC1 protein in transfected cell membranes by Western blot analysis. B, Detection of mPRα mRNA and mPRα protein in PG and V cell membranes (for rat PGRMC1 and mPRα RT-PCR primers see Supplemental Figure 4). C, Immunocytochemistry of PGRMC1 (left) and mPRα (right) proteins in PG and V cells. D, Specific [3H]P4 binding to plasma membranes of PG and V cells in a single point assay. E, Effects of progesterone treatment (1 nM, 16 hours) on serum starvation-induced apoptosis of PG and V cells. F, Effects of transfection of PG SIGC cells with mPRα siRNA (si-mPRα) or nontarget siRNA (n-t) on membrane mPRα and PGRMC1 mRNA (bottom) and protein expression (top). G, Comparison of specific [3H]P4 binding to SIGCs transfected with mPRα or nontarget siRNA in a single-point binding assay. H, Effects of progesterone (P4) treatment (1 nM, 16 hours) on apoptosis of serum-starved SIGCs after transfection with mPRα or nontarget siRNA. V1,V2, PG1, PG2: separate clones of transfected SIGCs; M, DNA size marker and molecular weight marker; Veh, vehicle; Int, integrin. *, P < .05 compared with V controls (D) or corresponding Veh controls (H), n = 6; **, P < .01 compared with corresponding controls (F), n = 6; ***, P < .001 compared with corresponding V controls (E) or nontarget controls (G), n = 6. All the experiments were repeated 3 or more times and similar results were obtained in each experiment.
Plasma membrane expression of ERβ on MDA-MB-231 cells stably transfected with PGRMC1
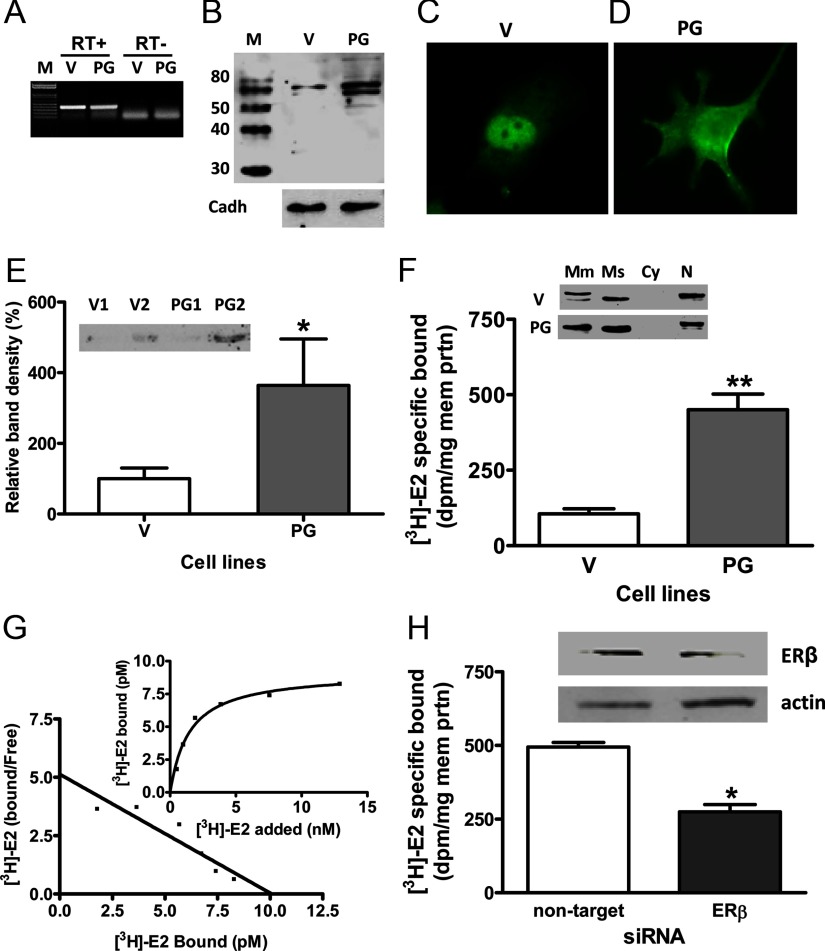
There was no apparent increase in ERβ mRNA expression in MDA-MB-231 cells transfected with PGRMC1 (PG) compared with vector (V) controls (Figure 6A). On the other hand cell-surface expression of ERβ was increased in PG cells, as shown by Western blot analysis of membrane preparations (Figure 6, B and F), immunocytochemical analysis (Figure 6D), and immunoblotting of biotinylated surface proteins (Figure 6E). Increased cell surface expression of ERβ protein was accompanied by significantly increased amounts of specific [3H]E2 binding in plasma membrane fractions of PG cells compared with V controls (Figure 6F) which was shown by saturation analysis to be saturable and high affinity, with a Kd of 1.46 nM (Figure 6G). Treatment with ERβ siRNA decreased the staining intensity of the immunoreactive band and specific [3H]E2 binding in PG cells (Figure 6H), thereby confirming the specificity of the ERβ antibody and the PGRMC1 effect.
Figure 6.
Expression of ERβ in MDA-MB-231 cells stably transfected with PGRMC1 (PG). A and B, Detection of ERβ mRNA (A) in PG- and vector (V)-transfected cells by RT-PCR and ERβ protein in transfected cell membranes (B) by Western blot analysis. C and D. Immunocytochemistry of ERβ protein in V (C) and PG cells (D). E, Western blot analysis of biotinylated cell-surface proteins with the ERβ antibody. F, Detection of ERβ in different subcellular fractions of PG and V cells (upper) and specific [3H]E2 binding to PG cell membranes in a single point assay (lower). G, Representative saturation curve and Scatchard plot of specific [3H]E2 binding to plasma membranes of PG cells. H, Effects of transfection of PG cells with ERβ siRNA (si-ERβ) or nontarget siRNA on membrane ERβ mRNA and protein expression (top) and specific [3H]E2 binding in a single point binding assay (bottom). M, molecular weight marker; Mm, plasma membrane fraction; Ms, microsomal; fraction; Cy, cytoplasmic fraction; N, nuclear fraction; V1,V2, PG1, PG2, separate clones of transfected MDA-MB-231 cells. **, P < .01; *, P < .05 compared with V or nontarget controls; n = 6. All the experiments were repeated 3 or more times and similar results were obtained in each experiment
Discussion
PGRMC1, as its name indicates, is a component of the membrane progesterone receptor, but its binding partner is currently unknown (21–23). Several lines of evidence in the present study strongly suggest that the other protein component in this progesterone membrane receptor complex is mPRα. mPRα and other mPRs are good candidates for membrane progestin receptors because they are ubiquitously expressed in vertebrate cells and have been shown by several research groups to display all the characteristics of specific progestin membrane receptors (5, 11, 17, 19, 38–40). The coimmunoprecipitation and ICC results clearly show that PGRMC1 is closely associated with mPRα in plasma membranes of several breast cancer cell lines and SIGCs. The nature of the likely coupling of PGRMC1 to mPRα is unknown but probably involves the heme binding domain of PGRMC1 as reported for its binding to other proteins (21, 23, 28) and as previously suggested for the progesterone receptor complex (8). Overexpression of PGRMC1 results in increased plasma localization of mPRα and increased amounts of mPRα associated with PGRMC1 in these cells. Importantly, increased plasma membrane expression of these 2 proposed progesterone receptor components is associated with increased specific [3H]P4 binding to plasma membrane fractions. Further, the steroid competition studies demonstrate that the progesterone receptor binding is characteristic of mPRα and not of PGRMC1 (17, 24). Both PGRMC1 and mPRα have previously been reported to mediate antiapoptotic actions of progestins in breast cancer and granulosa cells (8–10, 30, 31). The mPRα siRNA results in both types of cells clearly demonstrate that these antiapoptotic progestin actions and significant specific progestin membrane binding are dependent upon sufficient mPRα expression on their plasma membranes. Collectively, the results suggest that mPRα is the long sought after partner with PGRMC1 that together constitute the progestin receptor complex on plasma membranes required for nonclassical progesterone actions.
Most published data suggest that the progesterone-binding pocket resides in the mPRα protein, although definitive evidence is currently lacking. High-affinity, specific progestin binding has been demonstrated by several research groups to recombinant mPRα produced in mammalian and yeast cell expression systems, as well as in a prokaryotic system (E. coli) (5, 16, 17, 19, 38–40). In contrast, steroid binding to recombinant PGRMC1 produced in mammalian cells does not display high specificity for progesterone (24, 25), and progesterone binding could not be detected to recombinant PGRMC1 produced in an E. coli expression system in which heme binding was demonstrated (28), suggesting a protein required for progesterone binding is lacking in E. coli (13). It is noteworthy that in SIGCs where previous results suggested that PGRMC1 binds progesterone (8), progesterone binding to membranes of SIGCs overexpressing PGRMC1 was decreased to near background levels in the present study when mPRα expression was down-regulated by treatment with mPRα siRNA. Recent results showing that the putative σ-2 binding site is part of a protein complex containing PGRMC1 (27) further support the proposal that ligand specificity does not reside within the PGRMC1 component of the receptor complex.
It has been clearly established that progesterone signaling initiated at the plasma membrane through activation of G proteins in numerous target cells is mediated through mPRα (3, 10, 12, 13, 17). A G protein-binding domain has been partially characterized on the third intracellular loop of mPRα at a similar position to that shown to be important for G protein signaling in G protein-coupled receptors (17). G protein activation by progestins through mPRs has been demonstrated in a wide variety of vertebrate cell types, and the type of G protein activated shows both mPR subtype- and cell-dependent differences (3, 6, 9–13, 17, 20). In contrast, there are no reports of G protein activation through PGRMC1. mPRα and other mPRs have been coimmunoprecipitated with G proteins from plasma membranes prepared from breast cancer, myometrial, neuronal, and granulosa cells, oocytes, and sperm (3, 9–13, 17), whereas no evidence has been reported previously or obtained in the present study (results not shown) that PGRMC1 is coupled to G proteins. Finally, there is extensive data on G protein α- and β/γ-dependent signaling through the mPRs, which is abrogated with inhibitors of G protein activation such as pertussis toxin (3, 5, 6, 12, 13). On the other hand, no clear evidence has been obtained for activation of G protein-dependent signaling through PGRMC1, and the signaling pathways remain to be identified (13, 22, 41).
The modulation cell-surface expression and functions of mPRα by PGRMC1 in the present study are similar to those reported for EGFR (29) and further support the proposed role of PGRMC1 as an adaptor protein (21). The finding that a fluorescent σ-2 ligand colocalizes with PGRMC1 in the endoplasmic reticulum and mitochondria indicates that PGRMC1 receptor protein complexes are present at multiple intracellular locations (27). A close association between PGRMC1 and mPRα was anticipated because PGRMC1 interacts and forms complexes with a wide variety of proteins, including multiple P-450 proteins, PAIRBP1, Insig, and Scap (21, 23, 28, 30, 42). Although the results of previous studies indicate that the protein partners with PGRMC1 are cell specific, in the current study PGRMC1 was found to enhance mPRα expression and functions in all 4 cell lines investigated, representing both cancer and noncancer cell lines, which suggests they are common binding partners in a broad range of target cells. The mPRs and PGRMC1 are widely expressed in mammalian tissues with overlapping distributions (13, 18, 21). In view of the present findings it is important to determine whether the nonclassical actions of progesterone formerly attributed to PGRMC1 and mPRs alone are instead mediated by a PGRMC1-mPRα receptor complex.
The observation that the antiapoptotic actions of progesterone in SIGCs are blocked by pretreatment with siRNAs for either PGRMC1 (8) or mPRα is consistent with a mechanism of progesterone action through such a PGRMC1-mPRα receptor complex. Although PAIRBP1 also interacts with PGRMC1 and clearly participates in the antiapoptotic actions of progesterone in these cells (8), a recent study demonstrated that progesterone binding to SIGCs and cellular localization of PGRMC1 are not dependent on its interaction with PAIRBP1(41). These results suggest that PAIRBP1 is not a component of the membrane progesterone receptor but instead is a downstream component of the signaling pathway leading to antiapoptosis. Experiments using mPR siRNAs and with the specific mPR agonist, Org OD 02–0, have clearly shown that mPRα and other mPRs also mediate antiapoptotic actions of progesterone and activation of MAPK and Akt in fish granulosa, breast cancer, and neuronal cells (9–11, 43). Activation of these signaling pathways is a plausible mechanism by which progesterone exerts it antiapoptotic actions through mPRs because MAPK and Akt are known to inhibit apoptosis through up-regulation of antiapoptotic members of the Bacl-2 family and inhibition of the proapoptotic gene BAD (43). Interestingly, progesterone-induced changes in the expression of these genes in SIGCs have been shown to involve PGRMC1 (44). However, caspase gene expression was not affected through PGRMC1 in SIGCs (44), and caspase 3 activity was unaltered through mPRα in breast cancer cells (9). It is concluded from these results that antiapoptotic progestin actions mediated through these 2 membrane proteins are very similar (43) and may be mediated through a single receptor complex. A requirement for PGRMC1 to form a functional membrane progestin receptor with mPRα also provides a plausible explanation for the lack of mPR-dependent progestin binding and signaling in MDA-MB-231 breast cancer cells, despite the identification of multiple mPRs in these cells. Plasma membrane expression and receptor functions of mPRα in these cells can be induced by overexpressing PGRMC1 and also by overexpressing mPRα, indicating that increased availability of either protein promotes the formation of a functional membrane progesterone receptor complex on the cell membrane.
An unexpected finding of the present study is that overexpression of PGRMC1 in MDA-MB-231 cells is also associated with increased specific [3H]E2 binding and expression of ERβ on the plasma membrane. The saturation and Scatchard analyses show that this specific [3H]E2 binding is high affinity, limited capacity and occupies a single binding site that is characteristic of membrane ERs. This ER binding is not associated with a membrane-bound form of ERα because MDA-MB-231 cells do not express ERα (45). The increase in membrane [3H]E2 binding also does not appear to be associated with G protein-coupled ER because we did not detect an increase in G protein-coupled estrogen receptor protein expression on the membranes of PGRMC1-transfected cells over that in vector-transfected cells (results not shown). Moreover, the estrogen binding is unlikely to be due to the presence any N-terminal truncated forms of ERβ because the antibody recognizes the N-terminal region of the ERβ (45) and detects protein bands on Western blots of approximately 60 kDa, which corresponds to the molecular weight of full-length ERβ1 (59 kDa) (46). Direct coupling of PGRMC1 to ERβ remains to be demonstrated. However, the results of the biotinylation experiments in PGRMC1-transfected cells confirming membrane localization of ERβ were anticipated because ERβ has been detected on the plasma membranes of a variety of cell types and tissues (36, 47, 48) and has been shown or proposed to be the principal nuclear ER mediating nongenomic estrogen actions in neural tissues (36, 49). The present findings potentially have wide-ranging implications for our understanding of the regulation of membrane ERβ expression and membrane-initiated nongenomic estrogen actions. Investigations on the role of PGRMC1 in enhancing the cell surface expression of ERβ are warranted in other cells such as in other breast cancer cell lines with greater ERβ expression and in cardiovascular cells in which ERβ has been shown to act through this extranuclear mechanism (48, 50)
In conclusion, this study demonstrates that PGRMC1 functions as an adaptor protein for both a 7-transmembrane receptor, mPRα, and a nuclear steroid receptor, ERβ, increasing their expression and receptor functions on the cell surface. Additional studies on the role of PGRMC1 in the plasma membrane expression of other 7-transmembrane steroid receptors and other members of the nuclear receptor superfamily will be required to determine the broad significance of these findings. However, the present results are consistent with an essential and central role for PGRMC1 in enhancing the cell surface-mediated, nongenomic actions of steroids through its influence on the cellular expression of their receptors and as a partner in the membrane receptor complex required for steroid signaling.
Acknowledgments
This work was supported by National Institutes of Health Grant ESO 12961 (to P.T.).
Disclosure Summary: The authors have nothing to disclose
Footnotes
- E2
- 17β-estradiol
- EGFR
- epidermal growth factor receptor
- ER
- estrogen receptor
- mPR
- membrane progestin receptor
- P4
- progesterone
- PAIRBP
- plasminogen activator RNA-binding protein
- PG
- PGRMC1 construct
- PGRMC1
- progesterone receptor membrane component 1
- SIGC
- spontaneously immortalized granulosa cell
- siRNA
- small interfering RNA
- V
- vector.
References
- 1. Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev. 1998;19:3–17 [DOI] [PubMed] [Google Scholar]
- 2. Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41 [DOI] [PubMed] [Google Scholar]
- 3. Thomas P. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen Comp Endocrinol. 2012;175:367–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uhler ML, Leung A, Chan SY, Wang C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction. Fertil Steril. 1992;58:1191–1198 [PubMed] [Google Scholar]
- 5. Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sleiter N, Pang Y, Park C, et al. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology. 2009;150:3833–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frye CA, Sumida K, Lydon JP, O'Malley BW, Pfaff DW. Mid-aged and aged wild-type and progestin receptor knockout (PRKO) mice demonstrate rapid progesterone and 3α,5α-THP-facilitiated lordosis. Psychopharmacology. 2006;185:423–432 [DOI] [PubMed] [Google Scholar]
- 8. Peluso JJ, Romak J, Liu X. Progesterone receptor membrane component-1(PGRMC1) is the mediator of progesterone's antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology. 2008;149:534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dressing GE, Alyea R, Pang Y, Thomas P. Membrane progesterone receptors (mPRs) mediate progestin induced antimorbidity in breast cancer cells and are expressed in human breast tumors. Horm Cancer. 2012;3:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dressing GE, Pang Y, Dong J, Thomas P. Progestin signaling through mPRα in Atlantic croaker granulosa/theca cell cocultures and its involvement in progestin inhibition of apoptosis. Endocrinology. 2010;151:5916–5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pang Y, Dong J, Thomas P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and ϵ (mPRδ and mPRϵ) and mPRδ involvement in neurosteroid inhibition of apoptosis. Endocrinology. 2013;154:283–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karteris E, Zervou S, Pang Y, et al. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–1534 [DOI] [PubMed] [Google Scholar]
- 13. Thomas P. Characteristics of membrane progestin receptor α (mPRα) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boonyaratanakornkit V, Scott MP, Ribon V, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280 [DOI] [PubMed] [Google Scholar]
- 15. Dosiou C, Hamilton AE, Pang Y, et al. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196:67–77 [DOI] [PubMed] [Google Scholar]
- 16. Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas P, Pang Y, Dong J, et al. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor α subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718 [DOI] [PubMed] [Google Scholar]
- 18. Tang YT, Hu T, Arterburn M, et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380 [DOI] [PubMed] [Google Scholar]
- 19. Smith JL, Kupchak BR, Garitaonandia I, et al. Heterologous expression of human mPRα, mPRβ and mPRγ in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73:1160–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pang Y, Thomas P. Progesterone signals through membrane progesterone receptors (mPRs) in MDA-MB-468 and mPR-transfected MDA-MB-231 breast cancer cells which lack full-length and N-terminally truncated isoforms of the nuclear progesterone receptor. Steroids. 2011;76:921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36 [DOI] [PubMed] [Google Scholar]
- 22. Lösel RM, Besong D, Peluso JJ, Wehling M. Progesterone membrane component 1–many tasks for a versatile protein. Steroids. 2008;73:929–934 [DOI] [PubMed] [Google Scholar]
- 23. Rohe HJ, Ahmed IS, Twist KE, Craven RJ. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P-450 activation and drug binding. Pharmacol Ther. 2009;121:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyer C, Schmid R, Scriba PC, Wehling M. Purification and partial sequencing of high-affinity progesterone-binding sites(s) from porcine liver membranes. Eur J Biochem. 1996;239:726–731 [DOI] [PubMed] [Google Scholar]
- 25. Marek CJ, Wallace K, Durwald E, et al. Low affinity glucocorticoid binding site ligands as potential anti-fibrogenics. Comp Heptatol. 2009;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyer C, Schmieding K, Falkenstein E, Wehling M. Are high-affinity progesterone binding site(s) from porcine liver microsomes members of the sigma receptor family? Eur J Pharmacol. 1998;347:293–299 [DOI] [PubMed] [Google Scholar]
- 27. Xu J, Zeng C, Chu W, et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat Commun. 2011;2:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Min L, Strushkevich NV, Harnastai IN, et al. Molecular identification of adrenal inner zone antigen as a heme-binding protein. FEBS J. 2005;272:5832–5843 [DOI] [PubMed] [Google Scholar]
- 29. Ahmed IS, Rohe HJ, Twist KE, Craven RJ. Pgrmc1 (progesterone membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J Biol Chem. 2010;285:24775–24782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peluso JJ, Pappalardo A, Fernandez G, Wu CA. Involvement of an unnamed protein, RDA288, in the mechanism through which progesterone mediates its antiapoptotic action in spontaneously immortalized granulosa cells. Endocrinology. 2004;145:3014–3022 [DOI] [PubMed] [Google Scholar]
- 31. Neubauer H, Adam G, Seeger H, et al. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric. 2009;12:230–239 [DOI] [PubMed] [Google Scholar]
- 32. Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone's antiapoptotic action. Endocrinology. 2006;147:3133–3140 [DOI] [PubMed] [Google Scholar]
- 33. Peluso JJ. Progesterone signaling mediated through progesterone receptor membrane component-1 in ovarian cells with special emphasis on ovarian cancer. Steroids. 2011;76:903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filardo E, Quinn J, Pang Y, et al. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245 [DOI] [PubMed] [Google Scholar]
- 35. Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P. Comparison between steroid binding to membrane progesterone receptor α (mPRα) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRα-specific agonists. Steroids. 2010;75:314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Milner TA, Ayoola K, Drake CT, et al. Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95 [DOI] [PubMed] [Google Scholar]
- 37. García Pedrero JM, Del Rio B, Martínez-Campa C, Muramatsu M, Lazo PS, Ramos S. Calmodulin is a selective modulator of estrogen receptors. Mol Endocrinol. 2002;16:947–960 [DOI] [PubMed] [Google Scholar]
- 38. Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors α and β in transfected cells. J Endocrinol. 2006;190:247–260 [DOI] [PubMed] [Google Scholar]
- 39. Ashley RL, Arreguin-Arevalo JA, Nett TM. Binding characteristics of the ovine membrane progesterone receptor α and expression of the receptor during the estrous cycle. Reprod Biol Endocrinol. 2009;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tokumoto T, Tokumoto M, Oshima T, et al. Characterization of multiple membrane progestin receptor (mPR) subtypes from the goldfish ovary and their roles in the induction of oocyte maturation. Gen Comp Endocrinol. 2012;177:168–176 [DOI] [PubMed] [Google Scholar]
- 41. Peluso JJ, Yuan A, Liu X, Lode V. Plasminogen activator inhibitor 1 RNA-binding protein interacts with progesterone receptor membrane component 1 to regulate progesterone's ability to maintain viability of spontaneously immortalized granulosa cells and rat granulosa cells. Biol Reprod. 2013;88:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suchanek M, Radzikowska A, Thiele C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat Methods. 2005;2:261–267 [DOI] [PubMed] [Google Scholar]
- 43. Thomas P, Pang Y. Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology. 2012;96:162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peluso JJ, Liu X, Gawkowska A, Lodde V, Wu CA. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol Cell Endocrinol. 2010;320:153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neubauer H, Ruoff A, Paessler N, Solomayer E, Wallwiener D, Fehm T. A laminin-rich basement membrane matrix influences estrogen receptor β expression and morphology of MDA-MB-231 breast cancer cells. Oncol Rep. 2009;21:475–481 [PubMed] [Google Scholar]
- 46. Saunders PT, Millar MR, Williams K, et al. Expression of oestrogen receptor β (ERβ) protein in human breast cancer biopsies. Br J Cancer. 2002;86:250–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nishio M, Kuroki Y, Watanabe Y. Subcellular localization of estrogen receptor β in mouse hippocampus. Neurosci Lett. 2004;355:109–112 [DOI] [PubMed] [Google Scholar]
- 48. Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERβ has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946 [DOI] [PubMed] [Google Scholar]
- 49. Wang G, Drake CT, Rozenblit M, et al. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res. 2006;1094:163–178 [DOI] [PubMed] [Google Scholar]
- 50. Marino M, Ascenzi P. Membrane association of estrogen receptor α and β influences 17β-estradiol-mediated cancer cell proliferation. Steroids. 2008;73:853–858 [DOI] [PubMed] [Google Scholar]