Abstract
Uterine leiomyomata (LMs) are the most common tumor affecting the female reproductive organs. The most notable pathophysiologic feature of this tumor is the excessive accumulation of rigid extracellular matrix (ECM) composed mainly of collagen types I and III. It is believed that the rigidity of the collagen-rich ECM causes symptoms such as abnormal bleeding and pelvic pain/pressure. However, the molecular pathogenesis for this ECM-rich tumor has yet to be elucidated. We have established that miR-29b was consistently down-regulated in LM compared with myometrium (MM). Hence, the function of miR-29b in LM was examined in vivo using adult female ovariectomized NOD-scid IL2Rγnull mice for subrenal xenograft models. In LM xenografts, restoring miR-29b inhibited the accumulation of ECM and the development of solid tumors. Although the miR-29b knockdown in MM cells increased the expression of collagens, it did not transform MM cells into tumorigenic, indicating that the down-regulation of miR-29b is essential but not sufficient for LM tumorigenesis. In addition, 17β-estradiol and progesterone down-regulated miR-29b and up-regulated mRNAs for multiple collagens in LM xenografts. Thus, we conclude that ECM production in LMs is regulated by steroid hormones via down-regulation of miR-29b, which is one of the mechanisms underlying the excessive accumulation of ECM.
Uterine leiomyomata (LMs) or fibroids are the most common tumor of the female reproductive organs with a cumulative incidence by age 50 of nearly 70% in Caucasian women and more than 80% in African-American women (1). LMs can cause severe symptoms such as abnormal uterine bleeding and pelvic pain, which are mainly due to the large volume and the rigidity of the tumor, reflecting the excess accumulation of collagen-rich extracellular matrix (ECM) (2). In LMs, mRNAs for COL1A1, COL1A2, COL3A1, COL5A1, COL5A2, and COL7A1 are up-regulated compared with the myometrium (MM) (3). Recently, our xenograft study demonstrated that cotreatment with 17β-estradiol (E2) and progesterone (P4) stimulates LM growth through cell proliferation and hypertrophy as well as the ECM accumulation (4). However, the molecular mechanism for ECM accumulation in LM remains unknown.
In addition to mRNAs, several microRNAs (miRNAs) are dysregulated in LMs (5). The dysregulation of miRNAs can lead to various human disorders including cardiovascular disease (6) and cancer (7). In this study, we established that miR-29b, a major transcript in the miR-29 family in MM, was consistently down-regulated in LMs. miR-29b has been classified as a tumor suppressor (8–10). Therefore, its down-regulation may play a critical role in LM growth. Also, miR-29b has been shown to repress the expression of multiple collagens via binding to the 3′-untranslated region of mRNAs (11–13). Furthermore, dysregulation of the miR-29 family is associated with fibrosis in the liver (14, 15), lung (16), kidney (17), and cardiovascular system (18), suggesting that the down-regulation of miR-29b may contribute to the overproduction/accumulation of collagens in these diseases.
In our current study, the function of miR-29b in the tumorigenesis of LM was tested in vivo using human LM and MM xenograft models. Our results establish the essential role of miR-29b down-regulation in the pathogenesis of LMs.
Materials and Methods
Tissue collection
The use of patient tissues was approved by the Institutional Review Board for Human Research at Northwestern University. LM and MM tissues were obtained from hysterectomy or myomectomy patients (39–52 years of age) at Prentice Women's Hospital. All samples were confirmed to be free of malignancy by histopathological examination with a diagnosis of usual type LMs.
Cell cultures
Primary cultures of LM and MM were obtained from 23 patients as previously described with minor modifications (4). Briefly, MM and LM tissues were cut into pieces (∼9 mm3), washed twice with Dulbecco's PBS containing 1% antibiotic-antimycotic solution (ABS) (Life Technologies), and digested in Hanks's Balanced Salt solution containing 1.5 mg/mL collagenase (Sigma-Aldrich, 83.3 μg/mL DNase I (Sigma-Aldrich), and 1% ABS at 37°C for 5 hours. After filtration through a 100-μm nylon cell strainer (BD Falcon), cells were plated at 5–10 × 106 /100-mm dishes and cultured in DMEM/F12 with 10% fetal bovine serum, 1% ABS at 37°C, and 5% CO2.
Lentiviral construction and infection
The miR-29b lentiviral vector was constructed by cloning the pre-miR-29b cDNA into the pGIPZ (Thermo Scientific Open Biosystems). The miR-29b knockdown (KD) lentiviral vector (miRZip-29b) and pGreen Puro Scramble Hair pin control vector were purchased from System Biosciences, Inc. Lentiviruses were produced as described previously (19). Primary LM and MM cells (1 × 106 cells/dish) were cultured overnight and then incubated with Opti-MEM Reduced-Serum Medium (Life Technologies) containing 6 μg/mL polybrene for 1 hour followed by the concentrated lentiviral suspension for 48 hours. The transduced cells were selected by culturing with 1 μg/mL puromycin for 3 days and then prepared into cell pellets.
Cell pellets preparation for subrenal grafting
All animal procedures were approved by Northwestern University's Animal Care and Use Committee. LM and MM cells were suspended into rat-tail collagen solution (BD Bioscience) at 5–10 × 105 cells per 20 μL. The 20-μL cell suspension was polymerized by incubating for 30 minutes at 37°C. After overnight floating culture in 10% fetal bovine serum DMEM/F12, the cell pellets were grafted under the renal capsule of adult ovariectomized NOD-scid IL2Rγnull mice (The Jackson Laboratory) that were implanted sc with slow-releasing 80 mg E2+P4 hormone pellets (0.8 mg E2, 75.2 mg P4, and 5 mg cholesterol). The hormone pellets were obtained by drying E2, P4, and cholesterol (Sigma-Aldrich) dissolved in diethyl ether into powder and pressed into pellets using the Para Pellet Press (Para Instrument Co.). Hormone release was assessed by plasma E2 and P4 levels of the host at The Ligand Assay and Analysis Core Facility, University of Virginia. In each experiment, 6–14 xenografts/ group were grafted into 3–6 mice/group. The experiment was repeated more than 3 times using primary cultures from different patients. In the statistical analysis, the average value of 3–8 xenografts/group in each experiment was considered as a single measurement.
Tumor formation analyses
Xenograft images on the kidney surface were taken from the x-, y-, and z-axes using a dissecting microscope connected to a computer with Leica Application Suite version 3.8 software (Leica Microsystems). Tumor volume was assessed only for the portion that extended out on the surface of the kidney as half ellipsoids (π/6 × length × width × height) (4). The circularity of tumor-kidney boundary was used as a parameter to indicate the degree of circumvention. Height/area was used as a reverse indicator of flatness.
Quantitative RT-PCR
Total RNA was isolated using the mirVana miRNA isolation kit (Ambion). For the quantitative analyses of miR-29b, 50–100 ng of total RNA was reverse transcribed using specific primers for miR-29b and U6 (internal control) from the TaqMan MicroRNA reverse transcription kit (Applied Biosystems). Real-time quantitative PCR (qPCR) was performed using the ABI7900 with the TaqMan 2× Universal PCR master mix. For collagen mRNAs, 1 μg of total RNA was used to generate cDNA using the qSCript cDNA synthesis kit (Quanta Biosciences). qPCR for collagens and their internal controls (glyceraldehydes 3-phosphate dehydrogenase [GAPDH], β-actin, and U6) was performed using the Power SYBR Green PCR master mix (Applied Biosystems). The qPCR primers are listed in Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. The qPCR cycle was programmed as 50°C for 2 minutes, 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Relative levels (ΔCt) were calculated by subtracting the cycle threshold (Ct) for internal control from the Ct for miR-29b and collagens. The mRNA and miRNA levels were normalized to GAPDH and U6, respectively. Nonetheless, β-actin, GAPDH, and U6 showed the same trend, and the choice between the 3 controls did not make a difference in the conclusion. Results were obtained from 3–4 independent experiments performed in triplicate.
Immunofluorescence
Immunofluorescence was performed as previously described (20). Anti-αsmooth muscle actin (1:500, Abcam), anti-Ki-67 (1:100, DAKO), anti-collagen I (1:200), and collagen III (1:200, LifeSpan BioSciences) were used as primary antibodies. Alexa-Fluro488 donkey antimouse and Alexa-Fluro594 donkey antirabbit (1:1000, Jackson ImmunoResearch Laboratories) were used for secondary antibodies. Images were obtained with an Olympus Fluoview FV10i Confocal Microscope system (Olympus America, Inc).
Proliferation rate
The Ki-67 labeling index was determined among αsmooth muscle actin-positive cells in 2 sections/xenograft from 4 independent repeats using different patient samples.
Masson's Trichrome staining
The xenograft sections were stained simultaneously using Masson's Trichrome staining kit (Sigma-Aldrich). Images of the entire xenograft section were generated by merging multiple images taken at ×40 magnification using a Keyence Z900.
Statistical analysis
Values reported are means ± SD. The statistical significance was determined by an ANOVA or Student's t test.
Results
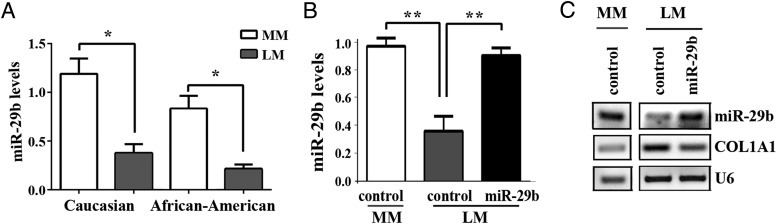
To validate miR-29b down-regulation in LM (5), the miR-29b expression levels were further analyzed from 20 Caucasian and 19 African-American patients by quantitative RT-PCR. The miR-29b levels were significantly lower in LM compared with MM tissues from both groups (Figure 1A). The miR-29b levels in primary LM and MM cell cultures were consistent with those in the original tissues (Figure 1, B and C). Simultaneously with the down-regulation of miR-29b, COL1A1 was up-regulated in LM compared with MM (Figure 1C). mRNA levels for COL1A1, COL1A2, COL3A1, COL5A3, and COL7A1 were also higher in the primary LM than MM cells (Supplemental Figure 1).
Figure 1.
miR-29b is down-regulated in LM A, miR-29b levels in LM and MM. Quantitative RT-PCR analysis for miR-29b levels in LM and MM tissues from 20 Caucasian and 19 African-American patients. Data are shown as the mean ± SD (*, P < .05). B, Mature miR-29b level in LM cells was restored to the level in MM by the lentiviral vector (**, P < .01). Results were obtained from 3–4 independent experiments performed in triplicate. C, Restoration of miR-29b reduces the COL1A1 level in primary LM cells. Images represented were taken from at least 3 experiments.
We examined whether miR-29b was involved in the regulation of collagen expression in LM cells by restoring miR-29b to the level in MM cells using a miR-29b lentivirus (Figure 1B). The restoration of miR-29b in the LM cells reduced the COL1A1 level proportionally (Figure 1C), confirming a function of miR-29b in repressing collagen expression in cultured LM cells.
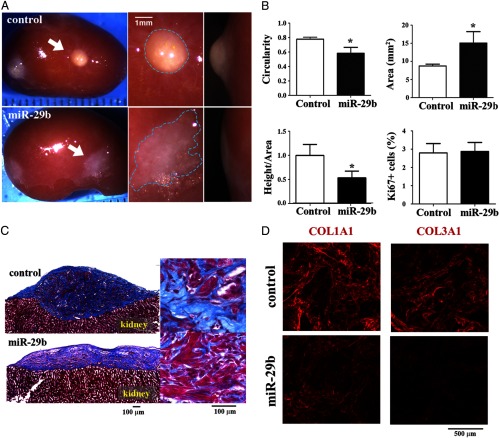
The effect of miR-29b restoration was further assessed in vivo using the LM xenograft model (4). Like LMs in patients, the control LM xenografts appeared as white, well-circumscribed, and solid spherical nodules partially embedded in the kidney (Figure 2A, upper panel). Conversely, LM xenografts that were restored with miR-29b failed to form solid tumors and grew flat as irregular shapes spreading on the kidney's surface (Figure 2A, lower panel). As assessed by the circularity of the kidney-xenograft boundary (1.0 indicates perfect circle), the degree of roundness was significantly higher in the control than in miR-29b xenografts (Figure 2B, circularity). Due to the small size and soft texture of miR-29b-restored LM xenografts, direct measurement of rigidity was unfeasible. Instead, the ratio of external height/area was used to evaluate the solidity of the tumor, which also showed a substantial decrease when miR-29b was restored (Figure 2B, height/area). The flat growth of the miR-29b LMs appeared to reflect the low rigidity of the tissue due to a reduction in the accumulation of the collagen-rich ECM as clearly demonstrated by the reduced blue-stained area with Masson's trichrome staining (Figure 2C). Immunofluorescence analysis for COL1A1 and COL3A1 by confocal microscopy further confirmed a reduction in the levels of these ECM proteins in the miR-29b group (Figure 2D and Supplemental Figure 2). Therefore, down-regulation of miR-29b is essential for the pathogenesis of LMs by increasing the ECM accumulation. As assessed by the growth area on the kidney surface, the miR-29b xenografts were significantly larger than controls (Figure 2B, area). However, the Ki-67 labeling index was similar between the control and miR-29b groups (Figure 2B, Ki67+ cells; Supplemental Figure 3). Therefore, the higher proliferation rate of LM compared with MM cells is not a result of the down-regulation of miR-29b.
Figure 2.
miR-29b down-regulation is essential for LM formation A, Restoration of miR-29b inhibits LM formation in vivo. Upper panels, Control LM xenografts formed solid spherical LM nodules (arrows). Lower panels, miR-29b-restored LM xenografts grew flat on the surface of the host kidney (arrows). The represented images from 5 xenograft experiments using LM tissues from 5 different patients. B, Restoration of miR-29b flattens LM xenografts without affecting the cell proliferation rate. Circularity, area, the ratio between height and area, and Ki-67 labeling index were expressed as the mean ± SD (*, P < .05). Data reflected a flat growth in miR-29b xenografts. C, miR-29b restoration reduces the accumulation of ECM. Smooth muscle cells were stained red with Biebrich Scarlet-Acid Fuschin. Collagens were stained blue with Aniline Blue. The area of ECM (blue) was significantly reduced in the miR-29b xenografts. D, miR-29b restoration reduces COL1A1 and COL1A3 levels. Confocal images for COL1A1 and COL1A3 were obtained from control and miR-29b groups (×120 magnification). Multichannel images are shown in Supplemental Figure 2.
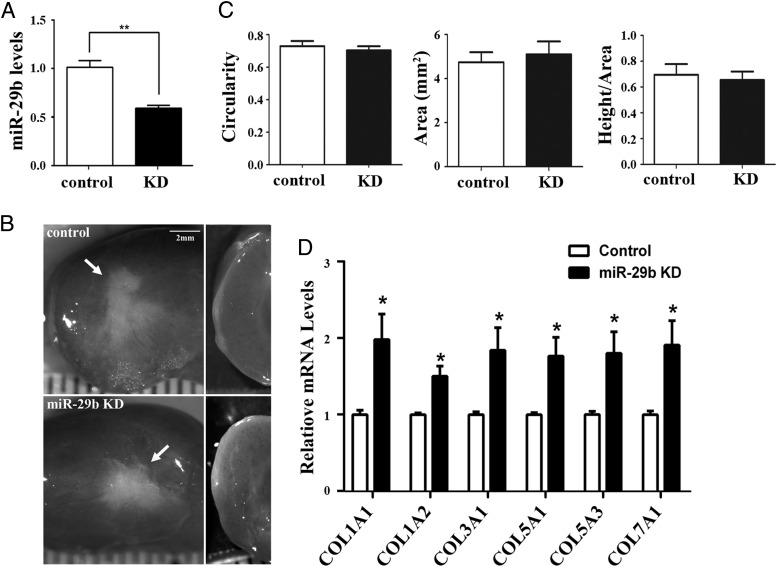
To further confirm the function of miR-29b in the tumorigenesis of LM, xenografts were generated from MM cells transduced with miR-29b KD or empty (control) lentiviral vector and were grown for 8 weeks in the host cotreated with E2+P4. Although the level of miR-29b was significantly reduced in miR-29b KD xenografts (Figure 3A), no observable differences were detected in the appearance and growth of miR-29b KD and control xenografts (n > 12) (Figure 3B). The solidity, roundness, and growth area were also comparable between control and miR-29b KD groups (Figure 3C). However, miR-29b KD increased the mRNAs for COL1A1, COL1A2, COL3A1, COL5A1, COL5A3, and COL7A1 in MM xenografts (Figure 3D), indicating that the level of miR-29b is the dominant determinant of collagen production in both MM and LM in vivo.
Figure 3.
miR-29b KD does not transform MM cells A, miR-29b KD in MM xenografts. Levels of miR-29b were assessed in xenografts of MM cells transduced with empty or KD vector (**, P < .01). B and C, miR-29b KD is not tumorigenic. Both miR-29b KD and control xenografts grew flat on the surface of the host kidney (arrows). The images are from 3 independent xenograft experiments using MM tissues from 3 different patients. B, Circularity, area, and the ratio between height and area (mean ± SD) were comparable between miR-29b KD and control groups (C). D, miR-29b KD significantly up-regulates collagen mRNAs. Quantitative RT-PCR data were normalized by GAPDH and expressed as the value 1.0 (*, P < .05) in the control group.
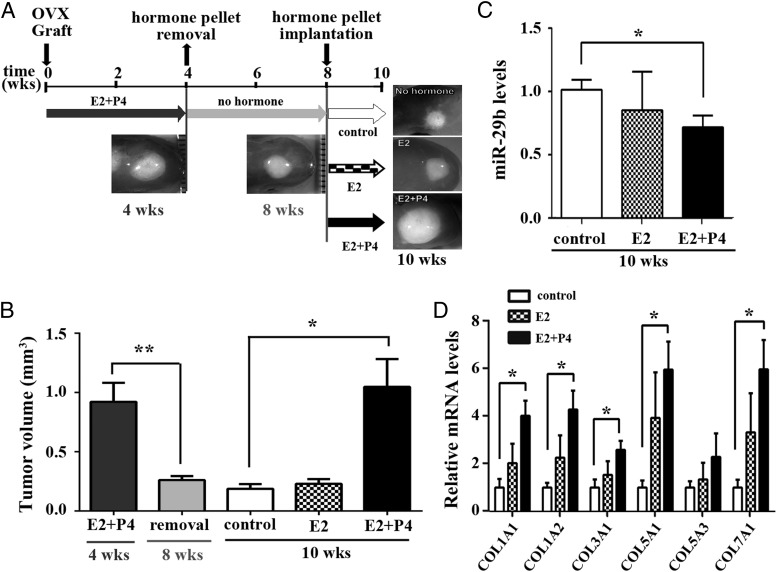
Our previous study showed that E2+P4 stimulate the growth of LMs through cell proliferation, hypertrophy, and the accumulation of ECM (4). Accordingly, we examined whether the levels of miR-29b and collagen mRNAs were regulated by steroid hormones. Initially, LM xenografts were grown in the host mice supplemented with E2+P4 for 4 weeks in order to establish solid tumors. Some tumors were harvested at this time point (Figure 4A, 4 wks), and the remaining mice had their hormone pellets removed, and the xenografts were grown for an additional 4 weeks without hormone supplements (Figure 4A, 8 wks). As previously demonstrated, LM xenografts reduced in volume significantly in response to hormone withdrawal (Figure 4B, 8 wks). After 4 weeks of growth in ovariectomized hosts without hormone supplements, the hosts were treated with no hormone (control), E2, or E2+P4 for an additional 2 weeks (Figure 4A, 10 wks). When E2+P4 treatment was restored, xenografts grew to their original size before the hormone withdrawal within 2 weeks (Figure 4B). In contrast, the tumor volume in the E2 and control groups (10 weeks) remained unchanged compared with that at 8 weeks (Figure 4B). At 10 weeks, miR-29b levels were significantly lower in the E2+P4 than the control group (Figure 4C). Simultaneously, collagen mRNAs were significantly higher in the E2+P4 than the control group (Figure 4D), indicating that E2+P4 treatment induces collagen expression through the down-regulation of miR-29b in LM in vivo.
Figure 4.
Hormonal regulation of miR-29b in LM A, Schematics of hormone treatment protocol. Hosts were ovariectomized (OVX) and supplemented with E2+P4 pellets at the time of grafting. After 4 weeks, pellets were removed. At 8 weeks, mice were treated with no hormone, E2, or E2+P4 for 2 weeks. The experiment was repeated using 4 different LM tissues. B, Volume change in LM xenografts. Tumor volumes are shown as the mean ± SD. C and D, E2+P4 down-regulates miR-29b and up-regulates collagens. The levels of mature miR-29b (C) and collagen mRNAs (D) were reversely correlated in LMs. *, P < .05; **, P < .01.
Discussion
Neoplasms are often associated with gene dysregulation. In this study, we have established that miR-29b is consistently down-regulated in LMs compared with MMs. However, it only indicates a correlation, and it is unclear whether the reduced miR-29b level is involved in LM pathogenesis. Therefore, we examined the function of miR-29b in LM and MM utilizing the xenograft model. The use of the xenograft model was essential, because LMs increase their size by proliferation, hypertrophy, and ECM accumulation (4), all of which cannot be addressed in vitro. In addition, cultured LM and MM cells lose their original characteristics including the expression of steroid receptors (21, 22); thus the response of LM and MM cells to steroid hormones would be compromised in vitro. Our current study has established the molecular links between the down-regulation of miR-29b and ECM formation in LMs, one of the key pathologic features in the tumorigenesis of LMs. However, down-regulation of miR-29b was essential, but not sufficient, to transform MM cells into LMs, indicating involvement of other factors in the pathogenesis of LMs.
Repression of collagens by the miR-29 family through binding to the 3′-untranslated region has been shown in other cell types in vitro (11–13). Our study confirmed that collagen levels are inversely correlated with the level of miR-29b in LM and MM in vivo. However, unlike acute myeloid leukemia cells (9), the growth rate of LM cells was unaffected by the level of miR-29b, indicating that miR-29b is not a growth regulator in LM and MM cells. The mechanism for the down-regulation of miR-29b in LM is unclear. However, our finding of steroid hormone-controlled miR-29b down-regulation provides additional evidence in the complex molecular regulation in this progesterone-driven neoplasm. Further study of the mechanism is warranted. LM growth is partially due to excessive ECM deposition; thus therapeutic interventions should also consider targeting ECM production rather than solely inhibiting cell proliferation. Overall, our findings indicate that miR-29b could be a potential therapeutic target that would provide a prospective modality in treating symptomatic LM.
Acknowledgments
We thank Lennell Reynolds in the Cell Image Facility Core, Dongjun Ren in the Miller's laboratory from Northwestern University, and Jessica Choy for technical assistance. We acknowledge the Ligand Assay and Analysis Core Laboratory, University of Virginia Center for Research in Reproduction, for hormone level analysis.
This work was supported by National Institutes of Health Grants RO1 HD064402 (to T.K.), RO1 CA154358 (to T.K.), and R03 HD057380 (to J.W.).
Current address for Z.L.: Institute of Cell Biology, Shandong University School of Medicine, Shandong, China.
Authors Contributions: J.J.W., and T.K. conceived the study; W.Q., Z.L., V.A.S., J.J.W. and T.K. designed the experiment; W.Q., Z.L., V.A.S., S.A.D. and Y.L. performed experiments, W.Q., Z.L., V.A.S., S.A.D., J.J.W. and T.K. collected and analyzed data; W.Q., S.A.D., M.E.F. and T.K. wrote the manuscript.
Disclosure Summary: The authors declare that they have no conflict of interest.
Footnotes
- ABS
- antibiotic-antimycotic solution
- E2
- 17β-estradiol
- ECM
- extracellular matrix
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- KD
- knockdown
- LM
- leiomyoma
- miRNA
- micro-RNA
- MM
- myometrium
- P4
- progesterone
- qPCR
- quantitative PCR.
References
- 1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107 [DOI] [PubMed] [Google Scholar]
- 2. Norian JM, Owen CM, Taboas J, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151:2433–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang T, Zhang X, Obijuru L, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–347 [DOI] [PubMed] [Google Scholar]
- 6. Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest. 2013;123:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pencheva N, Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol. 2013;15:546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Zhang X, Li H, Yu J, Ren X. The role of miRNA-29 family in cancer. Eur J Cell Biol. 2013;92:123–128 [DOI] [PubMed] [Google Scholar]
- 9. Garzon R, Heaphy CE, Havelange V, et al. MicroRNA 29b functions in acute myeloid leukemia. Blood. 2009;114:5331–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao JJ, Lin J, Lwin T, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Taylor NE, Lu L, et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55:974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Z, Hassan MQ, Jafferji M, et al. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284:15676–15684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwiecinski M, Noetel A, Elfimova N, et al. Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis in hepatic stellate cells by miRNA-29 induction. PLoS ONE. 2011;6:e24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun. 2011;412:74–79 [DOI] [PubMed] [Google Scholar]
- 15. Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218 [DOI] [PubMed] [Google Scholar]
- 16. Cushing L, Kuang PP, Qian J, et al. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin W, Chung AC, Huang XR, et al. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Nandi S, Martel A, Antoun A, Ioshikhes I, Blais A. Discovery, optimization and validation of an optimal DNA-binding sequence for the Six1 homeodomain transcription factor. Nucleic Acids Res. 2012;40:8227–8239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SY, Cordeiro MH, Serna VA, et al. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013;20:987–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Severino MF, Murray MJ, Brandon DD, Clinton GM, Burry KA, Novy MJ. Rapid loss of oestrogen and progesterone receptors in human leiomyoma and myometrial explant cultures. Mol Hum Reprod. 1996;2:823–828 [DOI] [PubMed] [Google Scholar]
- 22. Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod. 2006;12:187–207 [DOI] [PubMed] [Google Scholar]