Abstract
Intrauterine mammalian development depends on the preservation of placental function. The expression of the protein N-myc downstream–regulated gene 1 (NDRG1) is increased in placentas of human pregnancies affected by fetal growth restriction and in hypoxic primary human trophoblasts, where NDRG1 attenuates cell injury. We sought to assess the function of placental NDRG1 in vivo and tested the hypothesis that NDRG1 deficiency in the mouse embryo impairs placental function and consequently intrauterine growth. We found that Ndrg1 knock-out embryos were growth restricted in comparison to wild-type or heterozygous counterparts. Furthermore, hypoxia reduced the survival of female, but not male, knock-out embryos. Ndrg1 deletion caused significant alterations in placental gene expression, with a marked reduction in transcription of several lipoproteins in the placental labyrinth. These transcriptional changes were associated with reduced fetal:maternal serum cholesterol ratio exclusively in hypoxic female embryos. Collectively, our findings indicate that NDRG1 promotes fetal growth and regulates the metabolic response to intrauterine hypoxic injury in a sexually dichotomous manner.
Eutherian fetal development and growth depend on the placenta, which regulates maternal-fetal exchange and executes essential transport, metabolic, immune, and endocrine functions. Placental injury stemming from hypoxia, oxidative stress, or inflammatory insults can impede normal intrauterine development, leading to fetal death or fetal growth restriction (FGR) and the resulting perinatal and neonatal morbidity and mortality, medically induced prematurity, and adult metabolic disease (1–4). Despite the significant burden of disease associated with placental dysfunction, mechanisms underlying placental function and the adaptive response to injury remain poorly understood.
N-Myc Downstream Regulated Gene 1 (NDRG1) is a 43-kDa, evolutionarily conserved stress-response protein (5, 6). A homozygous nonsense mutation of NDRG1 results in the demyelinating peripheral neuropathy Charcot-Marie-Tooth 4D (CMT4D), with a similar phenotype in Ndrg1-null mice (7, 8). NDRG1-deficient mice also display impaired mast cell differentiation and degranulation (9). Despite numerous studies, including a particular emphasis on mechanisms of metastasis suppression (reviewed in [10]), the molecular function of NDRG1 remains elusive. Although conserved peptide sequences place NDRG1 in the α/β hydrolase superfamily, there is no evidence of such enzymatic activity by this protein. Expression of NDRG1 is stimulated by multiple diverse signals, including tunicamycin (11), metals (cobalt, nickel, calcium, and iron chelators) (12, 13), nitric oxide (14), vitamin D (15), vitamin C (16), retinoids (17), androgen, and estrogen (18–21), and DNA damaging compounds (actinomycin D, doxorubicin, geldanamycin) (22–24). Several prior findings implicate NDRG1 as relevant to cholesterol metabolism: 1) NDRG1 interacts with apolipoproteins A1 and A2, key proteins in high-density lipoprotein (HDL) synthesis and reverse cholesterol transport (6); 2) humans with mutations are affected with hypercholesterolemia and a sex-specific defect in reverse cholesterol transport (6, 25); and 3) the demyelinating phenotype of CMT4D mutation implicates NDRG1 in cholesterol metabolism, as high concentrations of cholesterol are required for myelin synthesis (26, 27).
Previous work in our laboratory demonstrated that NDRG1 is up-regulated in primary term human trophoblasts (PHT) cultured in hypoxia (28). In addition, we and others have found increased expression of NDRG1 in placentas from pregnancies affected by FGR (29, 30). We also showed that overexpression of NDRG1 in PHT cells enhances cell differentiation, while silencing of NDRG1 potentiates hypoxia-induced apoptosis (31), suggesting that placental expression of NDRG1 constitutes an adaptive response to injury. Having found that NDRG1 bolstered differentiation and protected trophoblasts from hypoxic injury in vitro, we sought to test the hypothesis that NDRG1 is critical for placental function and fetal growth in vivo. We found that Ndrg1 deletion in the mouse caused FGR and led to an increased rate of hypoxia-induced death of female, but not male, embryos. We also found that Ndrg1 deletion caused significant alterations in placental gene expression, with a marked reduction in transcription of several lipoproteins in the placental labyrinth. These changes were associated with a female-specific reduction in the ratio of fetal:maternal serum cholesterol. Collectively, these findings indicate that 1) NDRG1 regulates the placental adaptive response to injury in vivo, 2) NDRG1 supports the maintenance of intrauterine cholesterol homeostasis, and 3) these effects are influenced by fetal sex.
Materials and Methods
Mouse breeding, genotyping, and exposure to hypoxia
The Institutional Animal Care and Use Committee of the University of Pittsburgh approved our protocols. The generation of the Ndrg1-null mouse line was described by Okuda et al (8). Timed breeding of C57BL/6 mice heterozygous for the Ndrg1-null allele was performed by pairing heterozygous males and females overnight, with separation the next morning, designated as embryonic day 0.5 (E0.5). Pregnancy was assumed based on a weight gain of 10% or greater at E10.5. Mice were given standard chow and water ad libitum and kept on a 12-hour light, 12-hour dark cycle in room air. For hypoxia exposure, pregnant dams were placed in a Polymer Hypoxic Glove Box with a Purge Airlock system and CO2 and O2 control indicators (Coy Laboratory Products), designed for experiments in live rodents, with controlled and monitored humidity and gas composition. Exposure to hypoxia was initiated on E12.5, when fetal survival and growth definitively depend on placental function (32). The O2 level was set at 12% for hypoxic animals (33), whereas normoxic controls remained in ambient air. Dams were killed on E18.5 by cervical dislocation after isoflurane anesthesia. Embryos and placentas were immediately weighed, and tissues were subsequently processed for further analysis. Genomic DNA was extracted from embryo tails by the alkaline lysis and boiling method (34) and analyzed for Ndrg1 genotype (8). Fetal sex was determined by PCR amplification of SRY (35).
In situ hybridization
To detect NDRG1 mRNA in tissues, we performed in situ hybridization, as previously published (36). Briefly, 10-μm frozen sections of optimal cutting temperature (OCT) compound (Tissue-Tek/Sakura)-embedded murine placentas were rehydrated, digested with proteinase K (Sigma) at 10 μg/mL for 15 minutes at 37°C, treated with 0.2 N HCl for 10 minutes at room temperature, acetylated (0.25% acetic anhydride in triethanolamine for 10 min at room temperature), and then hybridized with cRNA probes overnight at 60°C. Slides were washed with saline-sodium citrate, digested with 20 μg/mL ribonuclease A (Sigma) for 30 minutes at 37°C, washed, and blocked using 1% blocking reagent (Roche) in maleic acid buffer, followed by incubation with anti-DIG-AP antibody (Roche) at 0.5 U/mL for 2 hours at room temperature, washed, and then reacted with BM purple (Roche) with 1 mM levamisole overnight.
RNA isolation and RT-quantitative PCR (qPCR) analysis
To extract RNA, we homogenized placental tissue using a T10 Basic S1 homogenizer (IKA Works). After homogenization, RNA for RT-qPCR was extracted from placental homogenates using Tri-Reagent (Molecular Research Center) according to the manufacturer's instructions. After removal of contaminating DNA using DNA-free (Invitrogen), extracted RNA was quantified using a NanoDrop-1000 spectrophotometer (Fisher-Thermo). One microgram of RNA served as the template for RT using the SuperScript VILO kit (Invitrogen) according to the manufacturer's instructions. The RT product was diluted to a total volume of 100 μL, and 3 μL was used for PCR using 250 nM concentrations for forward and reverse gene-specific primers (Table 1) and SYBR Green PCR Master Mix (Applied Biosystems) in a total reaction volume of 10 μL. Reactions were run in duplicate and analyzed using an Applied Biosystems GeneAmp 7900 Sequence Detection System. Dissociation curves were run on all reactions, and samples were normalized to L32 expression (37). Relative expression changes were calculated using the ΔΔCt method (38).
Table 1.
Primers Used for PCR
| Transcript | Direction | Sequence |
|---|---|---|
| SRY | F | ATTTATGGTGTGGTCCCGTG |
| R | AAGCTTTGCTGGTTTTTGGA | |
| L32 | F | CCTCTGGTGAAGCCCAAGATC |
| R | TCTGGGTTTCCGCCAGTTT | |
| Apoa1 | F | GCTCAAGAGCAACCCTACCTT |
| R | GCTTTCTCGCCAAGTGTCTTC | |
| Apoa2 | F | GCAGACGGACCGGATATGC |
| R | GCTGCTCGTGTGTCTTCTCA | |
| Apoa4 | F | TCAGAAGACGGATGTCACTCA |
| R | ATGCGGTCACGTAGGTCCT | |
| Apoa5 | F | TCCTCGCAGTGTTCGCAAG |
| R | GAAGCTGCCTTTCAGGTTCTC | |
| Apoc2 | F | ACCTGTACCAGAAGACATACCC |
| R | CCTGCGTAAGTGCTCATGG | |
| Apoc4 | F | TGTTCTTGGTCAGCTTTGTAGC |
| R | AGGCTGTGGGTCTTGTTTAGG |
Western blot analysis
Western immunoblotting was performed as previously described (36). Proteins were extracted from placental tissue by homogenization in lysis buffer. Protein lysates were electrophoresed using 10% SDS-polyacrylamide gel at 130 V and then transferred to polyvinyldifluoride membranes (Bio-Rad) at 23 V overnight. After blocking with 5% nonfat dried milk in Tris-buffered saline with 0.05% Tween-20, membranes were incubated overnight with rabbit anti-NDRG1 antibody (0.25 μg/mL; Invitrogen) at 4°C. The membranes were washed and incubated with peroxidase-conjugated goat antirabbit IgG (40 ng/mL; Santa Cruz Biotech) for 2 hours at room temperature. Detection was performed with SuperSignal West Dura kit (Thermo Scientific).
Laser capture microdissection
Frozen sections (10 μm) of OCT-embedded murine placentas were fixed on polyethylene naphthalate membrane slides (Life Technologies) and stained with cresyl violet. Using the Leica CTR6500 laser capture microdissection system (Leica), the placental labyrinth was visualized, circumscribed, and collected by gravity-assisted microdissection. After the collection of tissue, samples were immediately placed on ice. RNA was then extracted and qPCR performed as outlined above.
Cholesterol assay
After killing, maternal whole blood was collected by intracardiac aspiration. Fetal blood samples were obtained by collecting drained whole blood after decapitation. Whole blood from 152 embryos and 25 pregnant dams was heated to 37°C for 30 minutes to promote coagulation and then stored at 4°C overnight. Samples were centrifuged at 1000 rpm for 10 minutes, and the supernatant serum was collected for analysis.
For quantification of placental cholesterol concentrations, we removed nonadherent decidual tissue from one-fourth of each placenta's basal plate. The remaining tissue was weighed, and homogenized in a glass tube using a Teflon pestle (Thomas Scientific). Cholesterol was extracted from tissue for measurement with the Cholesterol Quantitation Kit (BioVision) according to the manufacturer's instructions, using a VersaMax microplate reader (Molecular Devices). This assay relies on reference standards for establishment of optical density–concentration curves. All assays were performed in duplicate, with the coefficient of variation between duplicates consistently below 10%.
Statistics
Statistical analyses were performed using Stata (StataCorp). ANOVA with post-hoc Bonferroni correction for multiple comparisons was used to compare placenta and embryo weights across genotypes. The correlation between fetal serum cholesterol concentration and weight was quantified with the pairwise correlation coefficient. Genotype frequency distributions were compared using Fisher's exact test. Gene expression was compared in wild-type (WT) and knock-out (KO) mice after RT-qPCR using Student's t test. Multivariate linear regression was used to compare fetal serum cholesterol levels across Ndrg1 genotypes while controlling for the effect of fetal sex, hypoxia, and litter size.
Results
NDRG1 is expressed in the mouse placenta
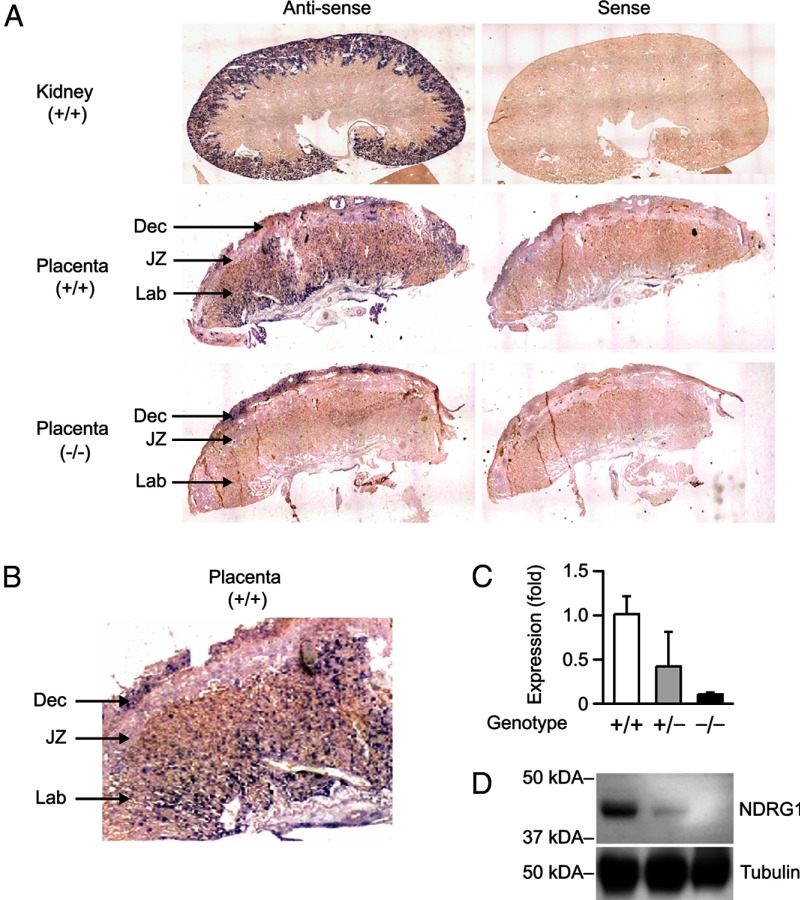
Although the expression of NDRG1 in the murine kidney and peripheral nerves has been previously validated (8), NDRG1's expression in the murine placenta has not been hitherto explored. Using in situ hybridization with adult mouse kidney as a positive control (Figure 1A), we found that Ndrg1 was expressed in the mouse placenta, localized predominantly to the labyrinth and adjacent, maternally derived uterine decidua, but not to the junctional zone (Figure 1B). These findings were confirmed using immunohistochemistry (data not shown). In Ndrg1-null placentas derived from heterozygous dams, we detected Ndrg1 expression in the decidua, as expected, but not in the fetal-derived placental tissue (Figure 1A, lower panel). We confirmed the expected level of RNA and protein expression in WT, heterozygote (Het), and KO placental homogenates using RT-qPCR and immunoblotting (Figure 1, C–D).
Figure 1.
NDRG1 is expressed in WT and absent in KO mouse placentas. (A) In situ hybridization showing Ndrg1 expression in WT adult kidney, WT placenta, and KO placenta. (B) Ndrg1 expression in WT placenta. (C) RT-qPCR showing expression of Ndrg1 in WT, Het, and KO placenta (n = 3). (D) Western immunoblot showing expression of NDRG1 in WT, Het, and KO placenta (representative of three experiments). Tubulin was used as a loading control. Dec, decidua; JZ, junctional zone; Lab, labyrinth.
NDRG1 deletion causes FGR
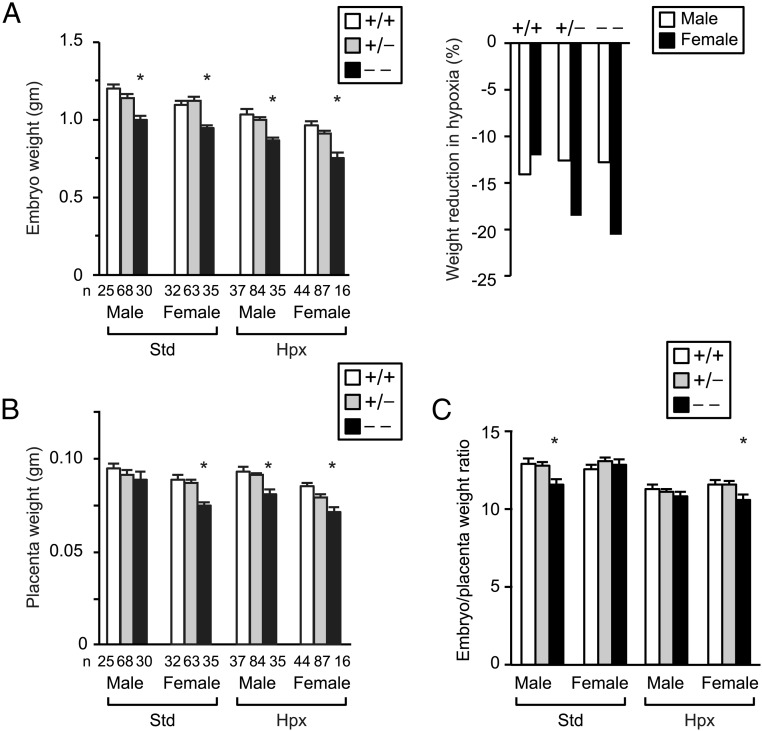
To investigate the effect of Ndrg1 deletion on fetoplacental development in vivo, we performed timed breeding of heterozygous males and females and analyzed the offspring after killing of pregnant dams on E18.5. KO embryos were growth restricted in comparison to WT and Het embryos (Figure 2A). We previously showed that silencing of endogenous Ndrg1 in hypoxic PHT cells enhanced cell injury, and NDRG1 overexpression protected trophoblasts from hypoxic injury (31). To determine the effect of hypoxia on KO embryos, we placed pregnant dams in an atmosphere of 12% O2 between E12.5 and E18.5 and compared fetal and placental weights across the different genotypes. Hypoxia caused a universal reduction in embryo weight (P < .001 by multivariable linear regression). Notably, when stratified by fetal sex, KO females demonstrated a trend toward greatest weight reduction on hypoxic exposure (Figure 2A, right panel). With the exception of male embryos bred in standard conditions, NDRG1 deficiency also caused a significant reduction in placental weight (Figure 2B). In addition, in standard conditions the ratio of embryo:placenta weight was reduced in male KO embryos. Hypoxia caused a significant reduction of the embryo:placenta weight ratio in all strata, which was further exacerbated by Ndrg1 deletion in female embryos (Figure 2C). Although KO placentas were smaller, we found no significant effect of Ndrg1 deletion on placental histologic morphology (not shown).
Figure 2.
Ndrg1 deletion causes FGR and reduced placental weight. (A) Mean embryo weight (g) at E18.5, stratified by sex, genotype, and O2 level (left). Reduction in fetal weight resulting from exposure to hypoxia, stratified by fetal sex and genotype (right). (B) Mean placenta weight (g) at E18.5, stratified by fetal sex, genotype, and O2 level. (C) Mean ratio of embryo:placenta weight at E18.5. *, P < .05 compared with WT of same sex and oxygen level by ANOVA with Bonferroni correction. Hypoxia (Hpx) denotes placement of pregnant dams in 12% O2 between E12.5 and E18.5 and compared with standard (Std) conditions. The numbers (n) below the bars denote the number of embryos analyzed for each paradigm. Note that hypoxia caused a universal reduction in embryo weight and embryo:placenta weight ratio (P < .001 by multivariable linear regression).
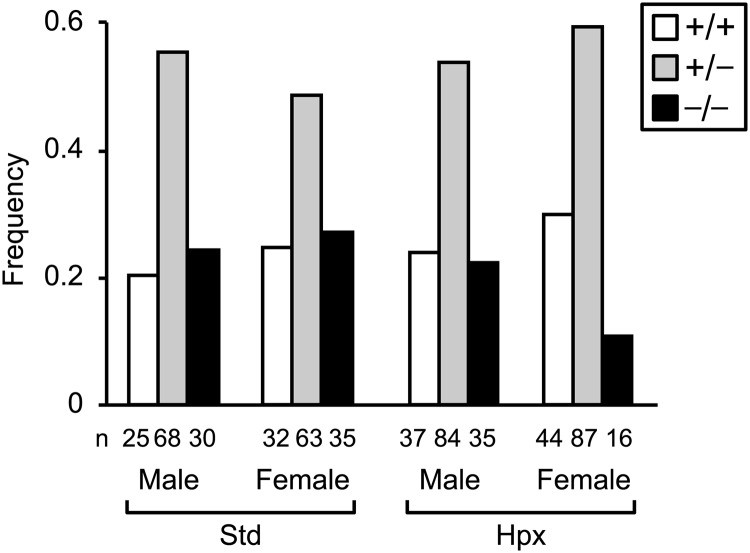
To determine if deletion of Ndrg1 impacted intrauterine survival, we examined the genotype of all intact embryos on E18.5. Isolated placentas and partially resorbed embryos were not counted. In control litters exposed to room air, the genotypes of male and female progeny from heterozygous matings were distributed at the expected Mendelian frequency (Figure 3), indicating that loss of NDRG1 did not impact survival. However, with exposure to hypoxia, we observed a significant reduction in the number of female KO embryos that survived to E18.5 (P < .01, Fisher's exact test), with no effect in males, indicating that NDRG1 deficiency confers susceptibility to hypoxia-induced fetal death in a sex-dependent manner.
Figure 3.
Ndrg1 deletion causes hypoxia-induced intrauterine death in female embryos; genotype frequencies after stratification by O2 level and fetal sex. Genotype frequencies deviated from expected Mendelian frequency exclusively in hypoxic females (P < .01, Fisher's exact test). The numbers (n) below the bars denote the number of embryos analyzed for each paradigm. Standard (Std) and Hypoxia (Hpx) conditions are as described in Figure 2.
Ndrg1 deletion affects expression of placental apolipoproteins
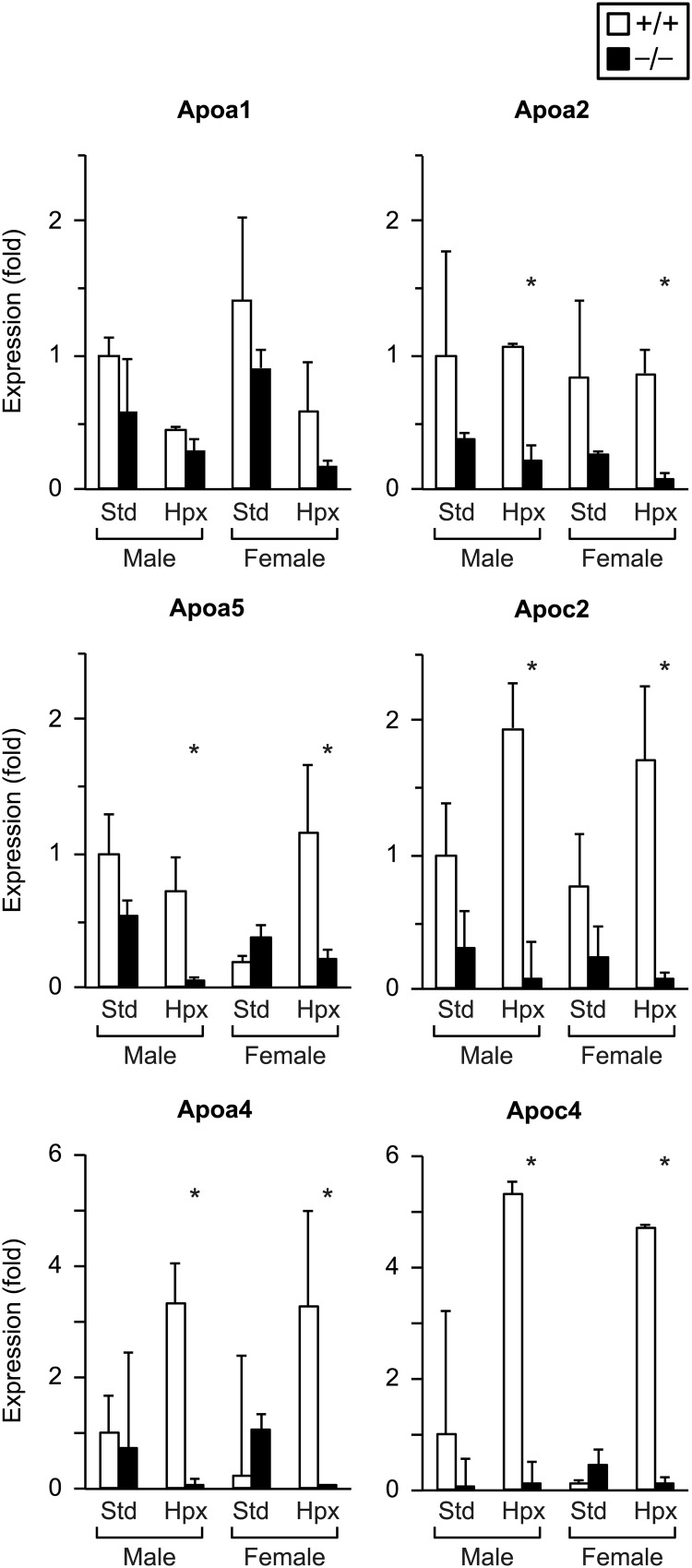
Having shown that NDRG1 deficiency influences intrauterine growth and the fetal response to hypoxia, we sought to examine the expression changes of candidate genes that might contribute to the phenotype of NDRG1-deficient embryos. Notably, NDRG1 is known to interact with HDL-associated apolipoproteins and has been implicated in regulation of cholesterol metabolism and cellular stress response (5, 6, 25). We therefore focused on expression of key apolipoproteins involved in cholesterol transport and the response to oxidative stress (39–42). To identify transcriptional changes specifically associated with Ndrg1 deletion, we performed laser capture microdissection to isolate tissue from the placental labyrinth, where Ndrg1 expression was localized (Figure 1A). Figure 4 depicts changes in expression of major apolipoproteins in WT vs KO placentas. In standard conditions, Ndrg1 deletion had no significant effect on expression of these transcripts in the placental labyrinth. However, with exposure to hypoxia, NDRG1 deficiency led to reduced expression of Apoa2, Apoa4, Apoa5, Apoc2, and Apoc4. Fetal sex had no effect on apolipoprotein expression in the labyrinth.
Figure 4.
The impact of Ndrg1 deletion on apolipoprotein expression in the placental labyrinth; RT-qPCR results (n = 2–4, each group). *, P < .05 by Student's two-tailed t test in WT vs KO of same sex at same oxygen level.
Fetal serum cholesterol is reduced in hypoxic Ndrg1-null pups
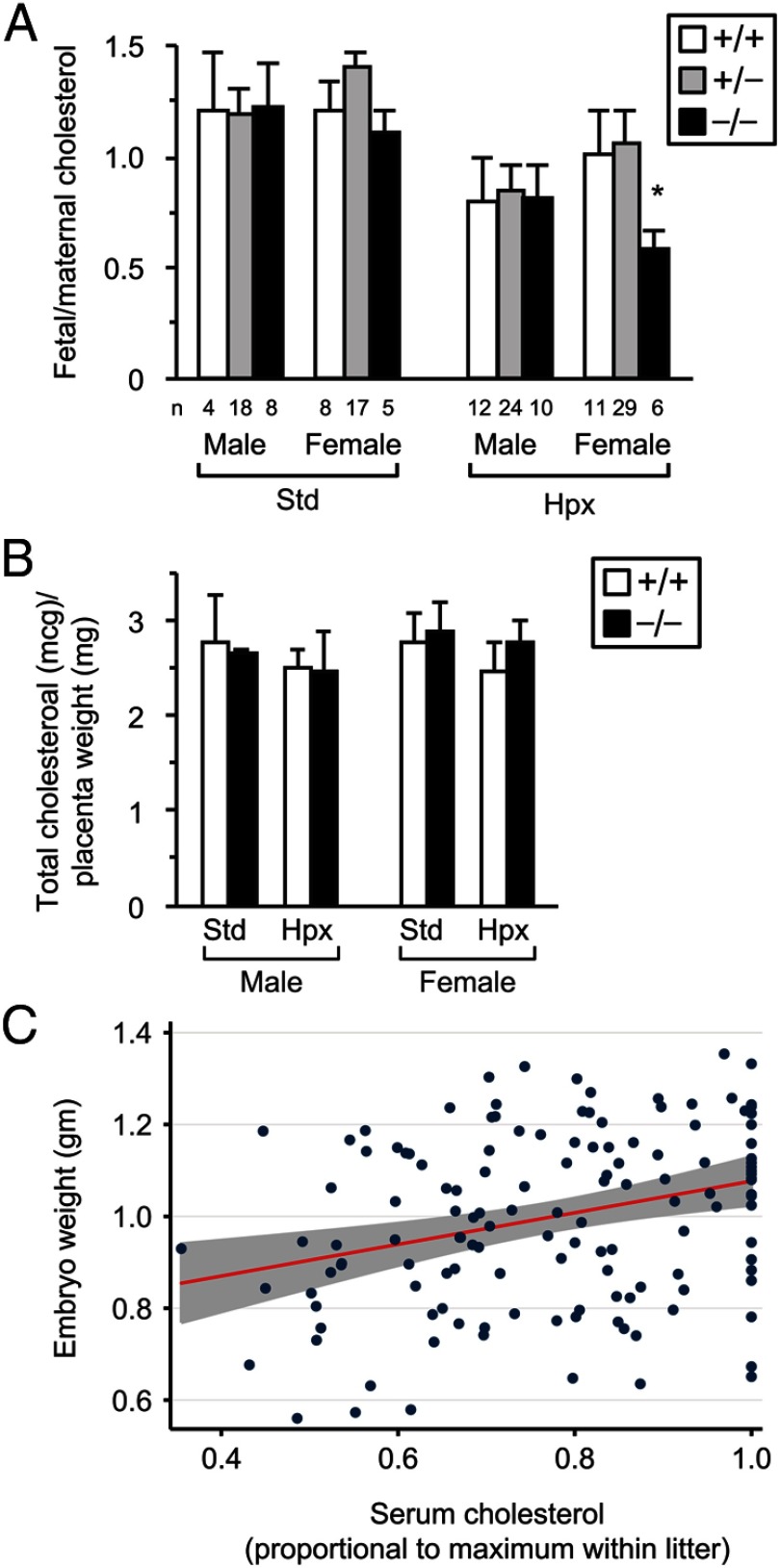
To determine if these changes in apolipoprotein expression were associated with altered fetoplacental cholesterol levels, we measured total cholesterol in fetal and maternal serum. When normalized to maternal serum cholesterol, hypoxia caused a universal reduction in fetal serum cholesterol levels (P < .001 by multivariable linear regression, Figure 5A). Although the effect of hypoxia on fetal serum cholesterol was not influenced by NDRG1 in male embryos, female embryos displayed reduced relative cholesterol levels in KO embryos compared with WT or Het animals (P = .001). Notably, despite differences in serum cholesterol levels, we found no difference in the amount of cholesterol accumulated in placental tissue stemming from differences in sex, oxygen level, or Ndrg1 expression (Figure 5B). Finally, fetal serum cholesterol levels were positively correlated with fetal weight (Figure 5C; pairwise correlation coefficient 0.2542, P = .0016).
Figure 5.
NDRG1 deficiency modulates fetal serum cholesterol. (A) Mean fetal:maternal ratio of serum cholesterol concentration. *, P = .001 by Student's t test compared with WT and Het. Note also that hypoxia caused a universal reduction in fetal:maternal serum cholesterol ratio (P < .001 by multivariable linear regression). The numbers (n) below the bars denote the number of embryos analyzed for each paradigm. Standard (Std) and Hypoxia (Hpx) conditions are as described in Figure 2. (B) Total cholesterol content in placental tissue (n = 3). Standard (Std) and Hypoxia (Hpx) conditions are as described in Figure 2. (C) Fetal serum cholesterol (proportional to maximum value within litter) correlates with embryo weight; pairwise correlation coefficient 0.2542, P = .0016.
Discussion
Our findings establish the pregnancy-related phenotype of NDRG1 deficiency, demonstrating FGR stemming from Ndrg1 deletion, and support our prior in vitro findings of a protective role for NDRG1 in the maintenance of placental function (31). Ndrg1 is expressed in the murine placental labyrinth, the region that mediates maternal-fetal exchange and is largely analogous to human syncytiotrophoblasts. These data, coupled with the increased expression of NDRG1 in hypoxic trophoblasts and placentas from pregnancies complicated by FGR (28–31), further support the conclusion that increased placental expression of NDRG1 constitutes an adaptive response to hypoxic injury. Future experiments using Cre recombinase to specifically delete a floxed Ndrg1 allele in the embryo or placenta will distinguish the relative contribution of fetal vs placental gene deletion to the observed phenotype (43).
Placental metabolism, steroid processing, and perinatal outcomes are largely influenced by fetal sex (44–48). In the human placenta, gene expression diverges according to sex (49) and, in mice, the response to environmental exposures, such as fat content in maternal diet, impacts placental gene expression and intrauterine survival in a sexually dichotomous manner (44, 50). The Liver X Receptor (LXR) is a key transcriptional regulator of cholesterol metabolism and regulates the transcription of the ApoE/C-I/C-IV/C-II gene cluster (42, 51, 52). Functional crosstalk between the LXR and sex steroid receptors has been demonstrated to influence cholesterol metabolism (53). Of relevance, the LXRβ-deficient mouse displays motor neuron degeneration exclusively in males and NDRG1-null humans affected with CMT 4D demonstrate a male-specific reduction in serum HDL (6, 54). Differences in circulating sex hormone concentrations and/or receptor expression may contribute to the sex differences in hypoxia-induced mortality and serum cholesterol that we observed in hypoxic KO animals. Alternatively, sexually discrepant regulation of genes on the X chromosome may underlie the observed phenotypic differences. The escape of specific genes from X chromosome inactivation in females can be affected by environmental stressors, including hypoxia, and is differentially regulated in specific extraembryonic cell lineages (55, 56). Hypoxia and/or NDRG1 deficiency may lead to female-specific alterations in expression of X chromosome genes that are maladaptive with exposure to a second insult, causing exacerbation of injury specifically in female, Ndrg1-null, hypoxic embryos. Our data indicate that the phenotypic differences in the effect of NDRG1 deficiency in male and female embryos do not stem from differences in apolipoprotein expression, as transcription of apolipoproteins was comparably affected by Ndrg1 deletion and hypoxia in the labyrinth of male and female placentas.
We demonstrated that hypoxic injury reduces fetal cholesterol serum levels, and that this reduction is exacerbated by NDRG1 deficiency in females. The change we observed in fetal:maternal serum cholesterol ratio was not mediated by the accumulation of cholesterol in the placenta, as placental cholesterol levels were unchanged with hypoxia or Ndrg1 deletion. This finding suggests that fetal cholesterol decreased as a result of a reduction in either net cholesterol uptake from the maternal circulation or reduced de novo fetal cholesterol synthesis and highlights the need for further insight into maternal-fetal cholesterol trafficking.
There is a well-established association between FGR, lipid peroxidation, and hypocholesterolemia (57–59). Tissue hypoxia precipitates accumulation of reactive oxygen species, which promotes the unregulated oxidation of cholesterol and LXR activation (60–62). Thus, it is likely that placental hypoperfusion and hypoxia, critical mediators of FGR, contribute to the associated derangements in cholesterol metabolism. The placental metabolic response to uncontrolled cholesterol oxidation and/or hypoxic injury may promote trophoblast survival at the expense of fetal nutrient delivery and growth. Our findings implicate NDRG1 as a regulator of this response and highlight the negative effect of disrupted cholesterol metabolism on intrauterine growth and development.
Acknowledgments
We thank Tianjiao Chu for analysis and advice regarding statistics; Patrick Reidy, Elena Sadovsky, and Huijie Sun for technical assistance; and Lori Rideout for assistance with manuscript preparation.
Reprints will not be available.
This project is supported by a grant from the American Association of OBGYN Foundation/Society for Maternal-Fetal Medicine (to J.L.), Pennsylvania Department of Health Research Formula Funds (to J.L.), National Institutes of Health Grants K12HD063087 (to J.L.), P01 HD069316 (to Y.S. and Y.B.), R01 HD045675 (to Y.S.), and R01 ES011597 (to Y.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CMT4D
- Charcot-Marie-Tooth 4D
- E
- embryonic day
- FGR
- fetal growth restriction
- HDL
- high-density lipoprotein
- Het
- heterozygote
- KO
- knock-out
- LXR
- Liver X Receptor
- PHT
- primary term human trophoblasts
- qPCR
- quantitative PCR
- WT
- wild-type.
References
- 1. Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–496 [DOI] [PubMed] [Google Scholar]
- 2. Barker DJ. Fetal growth and adult disease. Br J Obstet Gynaecol. 1992;99:275–276 [DOI] [PubMed] [Google Scholar]
- 3. McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–1238 [DOI] [PubMed] [Google Scholar]
- 4. Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000;182:198–206 [DOI] [PubMed] [Google Scholar]
- 5. Kachhap SK, Faith D, Qian DZ, et al. The N-Myc down regulated Gene1 (NDRG1) Is a Rab4a effector involved in vesicular recycling of E-cadherin. PLoS ONE. 2007;2:e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunter M, Angelicheva D, Tournev I, et al. NDRG1 interacts with APO A-I and A-II and is a functional candidate for the HDL-C QTL on 8q24. Biochem Biophys Res Commun. 2005;332:982–992 [DOI] [PubMed] [Google Scholar]
- 7. Kalaydjieva L, Gresham D, Gooding R, et al. N-myc downstream-regulated gene 1 is mutated in hereditary motor and sensory neuropathy-Lom. Am J Hum Genet. 2000;67:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okuda T, Higashi Y, Kokame K, Tanaka C, Kondoh H, Miyata T. Ndrg1-deficient mice exhibit a progressive demyelinating disorder of peripheral nerves. Mol Cell Biol. 2004;24:3949–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taketomi Y, Sunaga K, Tanaka S, et al. Impaired mast cell maturation and degranulation and attenuated allergic responses in Ndrg1-deficient mice. J Immunol. 2007;178:7042–7053 [DOI] [PubMed] [Google Scholar]
- 10. Ellen TP, Ke Q, Zhang P, Costa M. NDRG1, a growth and cancer related gene: regulation of gene expression and function in normal and disease states. Carcinogenesis. 2008;29:2–8 [DOI] [PubMed] [Google Scholar]
- 11. Kokame K, Kato H, Miyata T. Homocysteine-respondent genes in vascular endothelial cells identified by differential display analysis. GRP78/BiP and novel genes. J Biol Chem. 1996;271:29659–29665 [DOI] [PubMed] [Google Scholar]
- 12. Le NT, Richardson DR. Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: a link between iron metabolism and proliferation. Blood. 2004;104:2967–2975 [DOI] [PubMed] [Google Scholar]
- 13. Chen Z, Zhang D, Yue F, Zheng M, Kovacevic Z, Richardson DR. The iron chelators Dp44mT and DFO inhibit TGF-β-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1). J Biol Chem. 2012;287:17016–17028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hickok JR, Sahni S, Mikhed Y, Bonini MG, Thomas DD. Nitric oxide suppresses tumor cell migration through N-Myc downstream-regulated gene-1 (NDRG1) expression: role of chelatable iron. J Biol Chem. 2011;286:41413–41424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsugaki T, Zenmyo M, Hiraoka K, et al. N-myc downstream-regulated gene 1/Cap43 expression promotes cell differentiation of human osteosarcoma cells. Oncol Rep. 2010;24:721–725 [DOI] [PubMed] [Google Scholar]
- 16. Karaczyn A, Ivanov S, Reynolds M, Zhitkovich A, Kasprzak KS, Salnikow K. Ascorbate depletion mediates up-regulation of hypoxia-associated proteins by cell density and nickel. J Cell Biochem. 2006;97:1025–1035 [DOI] [PubMed] [Google Scholar]
- 17. Chen S, Han YH, Zheng Y, et al. NDRG1 contributes to retinoic acid-induced differentiation of leukemic cells. Leuk Res. 2009;33:1108–1113 [DOI] [PubMed] [Google Scholar]
- 18. Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041 [DOI] [PubMed] [Google Scholar]
- 19. Pflueger D, Rickman DS, Sboner A, et al. N-myc downstream regulated gene 1 (NDRG1) is fused to ERG in prostate cancer. Neoplasia. 2009;11:804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fotovati A, Fujii T, Yamaguchi M, et al. 17β-estradiol induces down-regulation of Cap43/NDRG1/Drg-1, a putative differentiation-related and metastasis suppressor gene, in human breast cancer cells. Clin Cancer Res. 2006;12:3010–3018 [DOI] [PubMed] [Google Scholar]
- 21. Fujii T, Yokoyama G, Takahashi H, et al. Preclinical and clinical studies of novel breast cancer drugs targeting molecules involved in protein kinase C signaling, the putative metastasis-suppressor gene Cap43 and the Y-box binding protein-1. Curr Med Chem. 2008;15:528–537 [DOI] [PubMed] [Google Scholar]
- 22. Xu X, Sutak R, Richardson DR. Iron chelation by clinically relevant anthracyclines: alteration in expression of iron-regulated genes and atypical changes in intracellular iron distribution and trafficking. Mol Pharmacol. 2008;73:833–844 [DOI] [PubMed] [Google Scholar]
- 23. Banz VM, Medová M, Keogh A, et al. Hsp90 transcriptionally and post-translationally regulates the expression of NDRG1 and maintains the stability of its modifying kinase GSK3β. Biochim Biophys Acta. 2009;1793:1597–1603 [DOI] [PubMed] [Google Scholar]
- 24. Jung EU, Yoon JH, Lee YJ, et al. Hypoxia and retinoic acid-inducible NDRG1 expression is responsible for doxorubicin and retinoic acid resistance in hepatocellular carcinoma cells. Cancer Lett. 2010;298:9–15 [DOI] [PubMed] [Google Scholar]
- 25. Dackovic J, Keckarevic-Markovic M, Komazec Z, et al. Hereditary motor and sensory neuropathy Lom type in a Serbian family. Acta Myo. 2008;27:59–62 [PMC free article] [PubMed] [Google Scholar]
- 26. Verheijen MH, Camargo N, Verdier V, et al. SCAP is required for timely and proper myelin membrane synthesis. Proc Natl Acad Sci USA. 2009;106:21383–21388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saher G, Brügger B, Lappe-Siefke C, et al. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8:468–475 [DOI] [PubMed] [Google Scholar]
- 28. Roh CR, Budhraja V, Kim HS, Nelson DM, Sadovsky Y. Microarray-based identification of differentially expressed genes in hypoxic term human trophoblasts and in placental villi of pregnancies with growth restricted fetuses. Placenta. 2005;26:319–328 [DOI] [PubMed] [Google Scholar]
- 29. Choi SJ, Oh SY, Kim JH, Sadovsky Y, Roh CR. Increased expression of N-myc downstream-regulated gene 1 (NDRG1) in placentas from pregnancies complicated by intrauterine growth restriction or preeclampsia. Am J Obstet Gynecol. 2007;196:45.e1–e7 [DOI] [PubMed] [Google Scholar]
- 30. Gratton RJ, Gluszynski M, Nygard K, Mazzuca DM, Graham CH, Han VK. Reducing agent and tunicamycin-responsive protein (RTP) mRNA expression in the placentae of normal and pre-eclamptic women. Placenta. 2004;25:62–69 [DOI] [PubMed] [Google Scholar]
- 31. Chen B, Nelson DM, Sadovsky Y. N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem. 2006;281:2764–2772 [DOI] [PubMed] [Google Scholar]
- 32. Barak Y, Nelson MC, Ong ES, et al. PPAR γ is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595 [DOI] [PubMed] [Google Scholar]
- 33. Tomlinson TM, Garbow JR, Anderson JR, Engelbach JA, Nelson DM, Sadovsky Y. Magnetic resonance imaging of hypoxic injury to the murine placenta. Am J Physiol Regul Integr Comp Physiol. 2010;298:R312–R319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanley T, Merlie JP. Transgene detection in unpurified mouse tail DNA by polymerase chain reaction. BioTechniques. 1991;10:56. [PubMed] [Google Scholar]
- 35. Pomp D, Good BA, Geisert RD, Corbin CJ, Conley AJ. Sex identification in mammals with polymerase chain reaction and its use to examine sex effects on diameter of day-10 or -11 pig embryos. J Anim Sci. 1995;73:1408–1415 [DOI] [PubMed] [Google Scholar]
- 36. Mishima T, Miner JH, Morizane M, Stahl A, Sadovsky Y. The expression and function of fatty acid transport protein-2 and -4 in the murine placenta. PLoS One. 2011;6:e25865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maity A, Solomon D. Both increased stability and transcription contribute to the induction of the urokinase plasminogen activator receptor (uPAR) message by hypoxia. Exp Cell Res. 2000;255:250–257 [DOI] [PubMed] [Google Scholar]
- 38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-δ δ C(T)) method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 39. Qin X, Swertfeger DK, Zheng S, Hui DY, Tso P. Apolipoprotein AIV: a potent endogenous inhibitor of lipid oxidation. Am J Physiol. 1998;274:H1836–H1840 [DOI] [PubMed] [Google Scholar]
- 40. Duka A, Fotakis P, Georgiadou D, et al. ApoA-IV promotes the biogenesis of apoA-IV-containing HDL particles with the participation of ABCA1 and LCAT. J Lipid Res. 2013;54:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soran H, Hama S, Yadav R, Durrington PN. HDL functionality. Curr Opin lipidol. 2012;23:353–366 [DOI] [PubMed] [Google Scholar]
- 42. Mak PA, Laffitte BA, Desrumaux C, et al. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors α and β. J Biol Chem. 2002;277:31900–31908 [DOI] [PubMed] [Google Scholar]
- 43. Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(suppl 1):S97–SS101 [DOI] [PubMed] [Google Scholar]
- 44. Rosenfeld CS, Grimm KM, Livingston KA, Brokman AM, Lamberson WE, Roberts RM. Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc Natl Acad Sci USA. 2003;100:4628–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Andoh T, Uda H, Yoshimitsu N, et al. The sex differences in cord-blood cholesterol and fatty-acid levels among Japanese fetuses. J Epidemiol. 1997;7:226–231 [DOI] [PubMed] [Google Scholar]
- 46. Hagemenas FC, Kittinger GW. The effect of fetal sex on placental biosynthesis of progesterone. Endocrinology. 1974;94:922–924 [DOI] [PubMed] [Google Scholar]
- 47. Sathishkumar K, Balakrishnan M, Chinnathambi V, Chauhan M, Hankins GD, Yallampalli C. Fetal sex-related dysregulation in testosterone production and their receptor expression in the human placenta with preeclampsia. J Perinatol. 2012;32:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(suppl):S33–S39 [DOI] [PubMed] [Google Scholar]
- 49. Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci USA. 2006;103:5478–5483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci USA. 2010;107:5557–5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y, Breevoort SR, Angdisen J, et al. Liver LXRα expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J Clin Invest. 2012;122:1688–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993 [DOI] [PubMed] [Google Scholar]
- 53. Krycer JR, Brown AJ. Cross-talk between the androgen receptor and the liver X receptor: implications for cholesterol homeostasis. J Biol Chem. 2011;286:20637–20647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Andersson S, Gustafsson N, Warner M, Gustafsson JA. Inactivation of liver X receptor β leads to adult-onset motor neuron degeneration in male mice. Proc Natl Acad Sci USA. 2005;102:3857–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang SY, Dobkin C, He XY, Brown WT. Transcription start sites and epigenetic analysis of the HSD17B10 proximal promoter. BMC Biochem. 2013;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Corbel C, Diabangouaya P, Gendrel AV, Chow JC, Heard E. Unusual chromatin status and organization of the inactive X chromosome in murine trophoblast giant cells. Development. 2013;140:861–872 [DOI] [PubMed] [Google Scholar]
- 57. Leduc L, Delvin E, Ouellet A, et al. Oxidized low-density lipoproteins in cord blood from neonates with intra-uterine growth restriction. Eur J Obstet Gynecol Reprod Biol. 2011;156:46–49 [DOI] [PubMed] [Google Scholar]
- 58. Spencer JA, Chang TC, Crook D, et al. Third trimester fetal growth and measures of carbohydrate and lipid metabolism in umbilical venous blood at term. Arch Dis Child Fetal Neonatal Ed. 1997;76:F21–F25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pecks U, Brieger M, Schiessl B, et al. Maternal and fetal cord blood lipids in intrauterine growth restriction. J Perinat Med. 2012;40:287–296 [DOI] [PubMed] [Google Scholar]
- 60. Miyata T, Takizawa S, van Ypersele de Strihou C. Hypoxia. 1. Intracellular sensors for oxygen and oxidative stress: novel therapeutic targets. Am j Physiol Cell Physiol. 2011;300:C226–C231 [DOI] [PubMed] [Google Scholar]
- 61. Brown AJ, Jessup W. Oxysterols: sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol Aspects Med. 2009;30:111–122 [DOI] [PubMed] [Google Scholar]
- 62. Lehmann JM, Kliewer SA, Moore LB, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140 [DOI] [PubMed] [Google Scholar]