Abstract
Kisspeptin plays a pivotal role in pubertal onset and reproductive function. In rodents, kisspeptin perikarya are located in 2 major populations: the anteroventral periventricular nucleus and the hypothalamic arcuate nucleus (ARC). These nuclei are believed to play functionally distinct roles in the control of reproduction. The anteroventral periventricular nucleus population is thought to be critical in the generation of the LH surge. However, the physiological role played by the ARC kisspeptin neurons remains to be fully elucidated. We used bilateral stereotactic injection of recombinant adeno-associated virus encoding kisspeptin antisense into the ARC of adult female rats to investigate the physiological role of kisspeptin neurons in this nucleus. Female rats with kisspeptin knockdown in the ARC displayed a significantly reduced number of both regular and complete oestrous cycles and significantly longer cycles over the 100-day period of the study. Further, kisspeptin knockdown in the ARC resulted in a decrease in LH pulse frequency. These data suggest that maintenance of ARC-kisspeptin levels is essential for normal pulsatile LH release and oestrous cyclicity.
Kisspeptin is a neuropeptide encoded by the Kiss1 gene, which is essential for the onset of puberty and regulating reproductive function. Both mice and humans lacking functional kisspeptin receptors (Kiss1r) or Kiss1 genes exhibit low gonadotrophins levels and do not undergo pubertal development (1–3). Administration of exogenous kisspeptin potently stimulates gonadotropin secretion in a number of different species (4–6). Furthermore, the stimulatory effects of kisspeptin appear to be mediated by a direct activation of Kiss1r-expressing GnRH neurons (7, 8). Indeed, a recent study has demonstrated that Kiss1r signaling in GnRH neurons is essential and sufficient for reproductive function (9). Therefore, it has been suggested that kisspeptin is the master regulator of the reproductive axis acting upstream of GnRH neurons.
In rodents, kisspeptin perikarya are located in 2 major populations, the anteroventral periventricular nucleus (AVPV) and the hypothalamic arcuate nucleus (ARC) (10). The AVPV population is thought to play a key role in mediating the positive feedback of estradiol (E2) that results in the generation of the preovulatory GnRH/LH surge (11, 12). The role played by the ARC kisspeptin neurons is less well characterized. Expression studies suggest that ARC kisspeptin neurons may relay negative feedback effects of gonadal steroids and therefore may play a role in the tonic control of gonadotropin secretion (11, 12). Indeed, the ARC has long been considered the principal site of GnRH pulse generation in rodents and, therefore, essential for the maintenance of fertility (13, 14). Evidence suggests that kisspeptin release within the ARC is required for GnRH pulsatility, because intra-ARC administration of a kisspeptin antagonist reduces LH pulse frequency (15). Furthermore, administration of kisspeptin into the ARC stimulates LH release (16). Recent studies suggest the kisspeptin neurons in the ARC may be the source of the neuronal pacemaker, which results in GnRH pulse generation (17, 18).
Kisspeptin knockout (KO) strategies have revealed the absolute requirement of kisspeptin in pubertal development (3). However, the absence of sexual maturation prevents these models from being used to examine the physiological role of kisspeptin in the adult animal. In addition, kisspeptin expression is absent from all tissues in these KO models, making it difficult to determine the role of specific populations of kisspeptin-expressing cells, or the effect of more modest changes in kisspeptin expression. Therefore, in the current study, we aimed to decrease kisspeptin expression specifically within the ARC of adult female rats to a similar degree as the decreases observed after inhibitory physiological stimuli, such as stress (19), inflammation (20), and lactation (21). We used stereotactic injection of adeno-associated virus (AAV) type-2, a small, nonpathogenic, single-stranded DNA virus with a tropism for neurons to drive the expression of antisense kisspeptin in the ARC of adult female rats, and investigated the effects on oestrous cyclicity and release of gonadotropins.
Materials and Methods
Adult female Wistar rats (220–250 g) obtained from Charles River were individually housed under standard conditions described previously (16). All animal procedures were approved by the British Home Office Animals (Scientific Procedures) Act 1986.
Antisense kisspeptin construct and in vitro testing
Full-length kiss1 cDNA was isolated by RT-PCR and cloned into pTR-CGW vector (gift from Dr Verhaggen, Amsterdam, The Netherlands) carrying the ampicillin resistance gene, inverted terminal repeats, human immediate early cytomegalovirus promoter, and wood chuck posttranscriptional regulatory element. The cytomegalovirus immediate early promoter was in the reverse orientation such that antisense cRNA would be produced. The cDNA construct was sequenced over its entire length 3 times to ensure the sequence was correct. The ability of this construct to inhibit kisspeptin production was tested in RIN 1056a cells that had previously been transfected with sense kisspeptin. RIN 1056a-kisspeptin cells were transfected with either antisense kisspeptin or enhanced green fluorescent protein (EGFP) control construct. After a 2-hour serum-free starvation period, kisspeptin release was measured using a specific RIA (6).
Recombinant AAV (rAAV) production and titer
The rAAV kisspeptin antisense (rAAV-kisspeptin-AS) and rAAV-encoding EGFP (rAAV-EGFP) were prepared using the adenovirus-free method as previously described (22). rAAV was produced using the 2-plasmid system (plasmid pDG was obtained from Dr Kleinschmidt, Heidelberg, Germany). The physical particle titer was determined by a dot blot assay. The infectious particle titer was determined using the infectious center assay.
Intranuclear injection
Adult Female Wistar rats anesthetized with 2-L/min oxygen and 4% inhaled isoflurane (Abbott Laboratories Ltd) received stereotactic bilateral injections of 1-μL rAAV-kisspeptin-AS or rAAV-EGFP containing 5 × 1012 genome particles, over 5 minutes, under aseptic conditions, via a burr hole using a 33-gauge stainless steel injector (Plastics One) and an infusion pump (Harvard Apparatus). The ARC injection coordinates were calculated as 3.3 mm posterior to bregma, 0.5 mm lateral to bregma, and 10.5 mm below the skull surface (23). Animals recovered for 4 days after surgery before vaginal cytology, food, and body weight measurements commenced. At 3 weeks after injection, a subset of rats (n = 4) were killed by deep pentobarbitone anesthesia followed by cardiac perfusion with PBS then 4% phosphate-buffered formaldehyde. Brains were removed, equilibrated in 20% sucrose before snap freezing in liquid nitrogen and stored at −70°C until use. Forty-micrometer coronal sections were cut on a freezing sled microtome (Shandon) and alternate sections mounted on poly-lysine slides (BDH). The brain sections slides were rehydrated by immersion in 0.1M PBS and then coverslipped before viewing. The sections were viewed by direct fluorescence using the fluoroscein isothiocyanate filter viewing under a fluorescence microscope (DM4000B; Leica Microsystems).
Oestrous cycle stage determination.
Before injection of rAAV-kisspeptin-AS or rAAV-EGFP, oestrous cyclicity was monitored for 10 days. Only rats exhibiting regular oestrous cycles were injected. Vaginal cytology was monitored daily by vaginal lavage between 9 and 11 am. Cycles were classified as either regular, extended, or abnormal. Briefly, regular cycles lasted between 3 and 5 days, with either 1 or 2 days of oestrus, or 2 or 3 days of diestrus. Extended cycles contained either 3 or 4 days of oestrus, or 4 or 5 days of diestrus. Abnormal cycles contained more than 4 consecutive days of oestrus, or more than 6 days of diestrus. A “complete” cycle was classified as one in which 3 stages of the oestrous cycle were observed in the correct order. Finally, cycle length was determined by the number of days between each occurrence of oestrus (24). Oestrous cycles were monitored between day 4 and 100 after injection. Food and body weights were measured weekly for the duration of this study.
Tissue and plasma collection
At 100 days after injection, animals were decapitated during the diestrous phase of the oestrous cycle to provide stable basal hormone levels. Blood was collected and plasma separated and stored as previously described (16) until measurement of LH, FSH, and E2. Ovaries were fixed and wax embedded and sectioned longitudinally at 4 μm; a section was taken at random and stained with hematoxylin-eosin (25). The sections were examined under a light microscope (Zeiss Axioskop 2 plus), and ovarian morphology was assessed by counting the number of antral follicles and corpus lutea. Brains were rapidly removed and snap frozen in isopentane and stored at −80°C.
LH pulse and surge experiment
An additional cohort of female Wistar rats were injected with rAAV-Kisspeptin-AS or rAAV-EGFP as described above. At the same time, these animals were bilaterally ovariectomized (OVX) and implanted with a E2-filled silicone capsule, which has been shown in a previous study to result in concentrations of E2 within the range observed during the diestrous phase of the oestrous cycle (26). This circumvented the inherent difficulties in examining LH pulses in intact animals, by controlling the gonadal steroid milieu, removing the potential confounding influence of individual variation in E2 feedback on LH levels. Further, the implantation of E2 capsules enabled the measurement of kisspeptin-knockdown at an equivalent level of E2 to that of the intact rats (26). The animals were housed singly and left for 3 weeks to allow maximum expression of the Kisspeptin-AS to be achieved (22). Animals were then fitted with 2 indwelling cardiac catheters via the jugular veins, to facilitate serial blood sampling. The catheters were exteriorized at the back of the head and secured to a cranial attachment; the rats were fitted with a 30-cm-long metal spring tether (Instec Laboratories). The distal end of the tether was attached to a fluid swivel (Instec Laboratories), which allowed the rats freedom to move around the enclosure. Experimentation commenced 3 days after cardiac catheter implantation.
For measurement of LH pulses, rats were attached via one of the 2 cardiac catheters to a computer-controlled automated blood sampling system, which allows for the intermittent withdrawal of small blood samples (25 μL) without disturbing the animals. Once connected, animals were left undisturbed for 1 hour before sampling commenced. Blood sampling commenced between 10 and 11 am, when samples were collected every 5 minutes for 6 hours for LH measurement. After removal of each 25-μL blood sample, an equal volume of heparinized saline (50-U heparin sodium/mL normal saline; Wockhardt) was automatically infused into the animal to maintain patency of the catheter and blood volume. Blood sampling continued throughout the experiment. Blood samples were frozen at −20°C for later assay to determine LH concentrations.
In the same cohort of OVX+E2 mice, we investigated the effects of rAAV-Kisspeptin-AS on LH surges. Steroid priming was initiated 3 days after the LH pulse experiment. To ensure that rAAV-Kisspeptin-AS and rAAV-EGFP animals were exposed to equivalent hormone levels, gonadal steroid priming was achieved using an injection protocol described to result in LH surges in OVX female rats (27). To induce LH surges, E2 and progesterone (Steraloids, Inc) were dissolved in peanut oil and administered sc in a volume of 0.1 mL. On day 1 and 2 at 9 am, rats received injections of 2 μg of E2. On day 3 at 9 am, the rats were injected sc with 500-μg progesterone. Blood sampling commenced on day 3 at 12 pm, and samples were collected every 5 minutes for 7 hours for LH measurement.
Quantification of ARC kisspeptin
Sections (300 μm) were cut on a cryostat, and punches (1 mm diameter) of the ARC were taken from bregma −0.7 to +2.1 mm according to the rat brain atlas (23), after the micropunch method of Palkovits and Brownstein (28). Punches from the ARC were homogenized in an acid ethanol extraction buffer (0.15% hydrochloric acid in 25% ethanol), and kisspeptin content was measured by sensitive and specific RIA. Briefly, Millipore kisspeptin antibody (29) was used at a final dilution of 1.5 × 10−5 (Millipore). 125I-labeled kisspeptin-10 (Advanced Biotechnology Centre, Imperial College London) was prepared by the chloramine T method and purified by reversed-phase high-performance liquid chromatography. All assays were performed in a total volume of 350 μL of phosphate buffer (pH 7.4) containing 0.3% bovine serum albumin and 0.02% Tween 20 and incubated for 3 days. Intra- and interassay variations were established to be 8.2 ± 0.7% and 6.8 ± 1.7%, respectively. The minimum detection limit of the assay was 1 fmol/punch.
Immunoassays
LH was measured using methods and reagents provided by the National Hormone and Pituitary program (Dr A. Parlow, University of California) as previously described (16). FSH was determined using a specific RIA (Izotop) (30). E2 was measured using a mouse/rat E2 ELISA kit (Calbiotech). Neurokinin B (NKB) was quantified in punched tissues using an ELISA kit (Peninsula Laboratories LLC).
Statistical analysis
All data are presented as mean ± SEM. Oestrous cyclicity data and plasma hormone data were analyzed by unpaired t test (GraphPad Prism version 5 for Windows; GraphPad Software). Percentage time spent in each stage of the oestrous cycle was analyzed using the Mann-Whitney U test (Systat). Body weight and food intake data were analyzed using the generalized estimating equation (Stata 9, StataCorp LP). Verification of LH pulses was established by means of the algorithm ULTRA (31). Two intraassay coefficients of variation of the LH RIA were used as the reference threshold for pulse detection. The effect of rAAV-Kisspeptin-AS on pulsatile LH secretion was analyzed by comparing the mean LH pulse frequency over the 6-hour sampling period. The effect of rAAV-Kisspeptin-AS on LH surges was calculated by analyzing the area under the LH profile 4.5 hours after injection of progesterone (90 min from the start of blood sampling). Associations between ARC kisspeptin levels and cycle length, and between ARC kisspeptin levels and LH-pulse interval, were analyzed by Pearson correlation analysis. For all studies, P < .05 was considered significant.
Results
In vitro antisense Kisspeptin activity
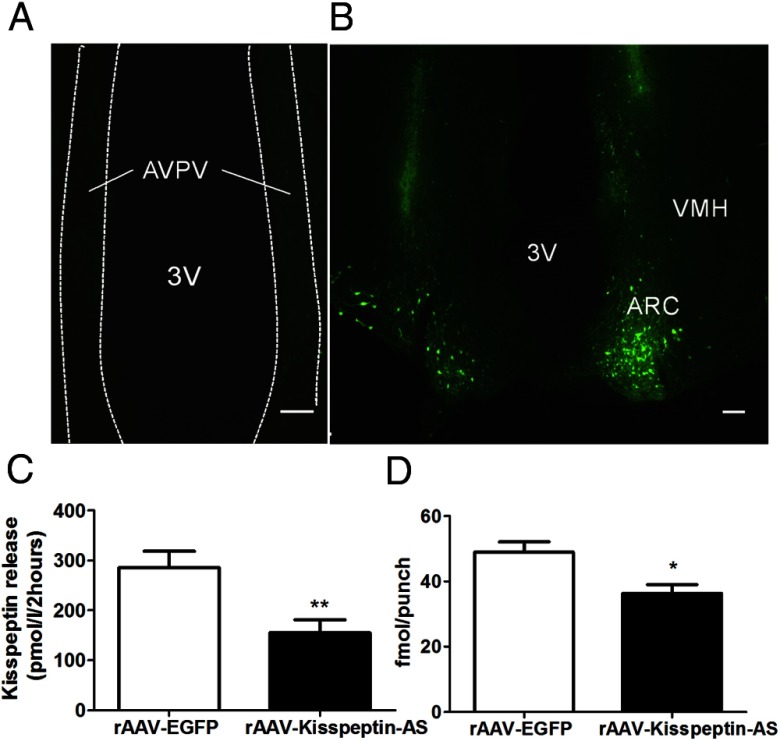
Transient transfection of RIN 1056a cells with full-length antisense kisspeptin significantly reduced kisspeptin secretion compared with control (control, 289.3 ± 34.7 pmol/L vs AS-Kisspeptin, 152.7 ± 24.2 pmol/L; P < .01 Student's t test) (Figure 1C).
Figure 1.
Validation of transgene expression and vitro kisspeptin knockdown. Representative images showing rAAV-EGFP spread in the (A) AVPV and (B) ARC (B). VMH, ventromedial hypothalamus; 3V, third ventricle. Scale bars represent 100 μm. C, Kisspeptin release from the RIN 1056a cell line stably transfected with Kiss1 after transient transfection with EGFP (control) or Kisspeptin-AS after 2 hours of incubation. Data are presented as mean ± SEM (n = 8); **, P < .01 vs kisspeptin-AS. D, The effect of intra-Arc rAAV-EGFP and rAAV-Kisspeptin-AS administration on kisspeptin-IR in the ARC. Quantification of kisspeptin-IR in the ARC of female rats by RIA (n = 12–14). Data are shown as mean ± SEM; *, P < .05.
Anatomical localization of EGFP after ARC injection
After intra-ARC rAAV-EGFP injection, EGFP was visualized in cells with neuronal morphologies in the injected brains. After intra-ARC injection, EGFP-containing cell bodies and axons were observed at a high density within the ARC, without spread to surrounding nuclei such as the ventromedial hypothalamus (Figure 1B). Some slight expression of EGFP was also observed in the injection tract. The injection tracts were easily identifiable, forming a distinctive pattern of autofluorescence. No EGFP-containing neurons were observed in the AVPV, the location of the other major population of kisspeptin-containing neurons in the hypothalamus (Figure 1A) (10).
Effect of AAV-kisspeptin-AS on ARC kisspeptin and NKB expression
Administration of rAAV-Kisspeptin-AS in intact rats resulted in a 27.3% knockdown in ARC kisspeptin content compared with rAAV-EGFP control (48.9 ± 3.2 fmol/punch [rAAV-EGFP] vs 36.3 ± 2.6 fmol/punch [rAAV-Kisspeptin-AS], n = 12–14; P < .05 Student's t test) (Figure 1D). Administration of rAAV-Kisspeptin-AS to OVX+E2 rats resulted in a knockdown of 35.4% in ARC kisspeptin content compared with rAAV-EGFP control (38.4 ± 3.2 fmol/punch [rAAV-EGFP] vs 24.8 ± 2.6 fmol/punch [rAAV-Kisspeptin-AS], n = 5–7; P = .8 Student's t test). Administration of rAAV-Kisspeptin-AS in intact rats had no significant effect on ARC-NKB content compared with rAAV-EGFP control (304.5 ± 53.1 fmol/punch [rAAV-EGFP] vs 402.0 ± 78.8 fmol/punch [rAAV-Kisspeptin-AS]; n = 10–11, P > .05, Student's t test).
Effect of AAV-kisspeptin-AS on ovarian cyclicity
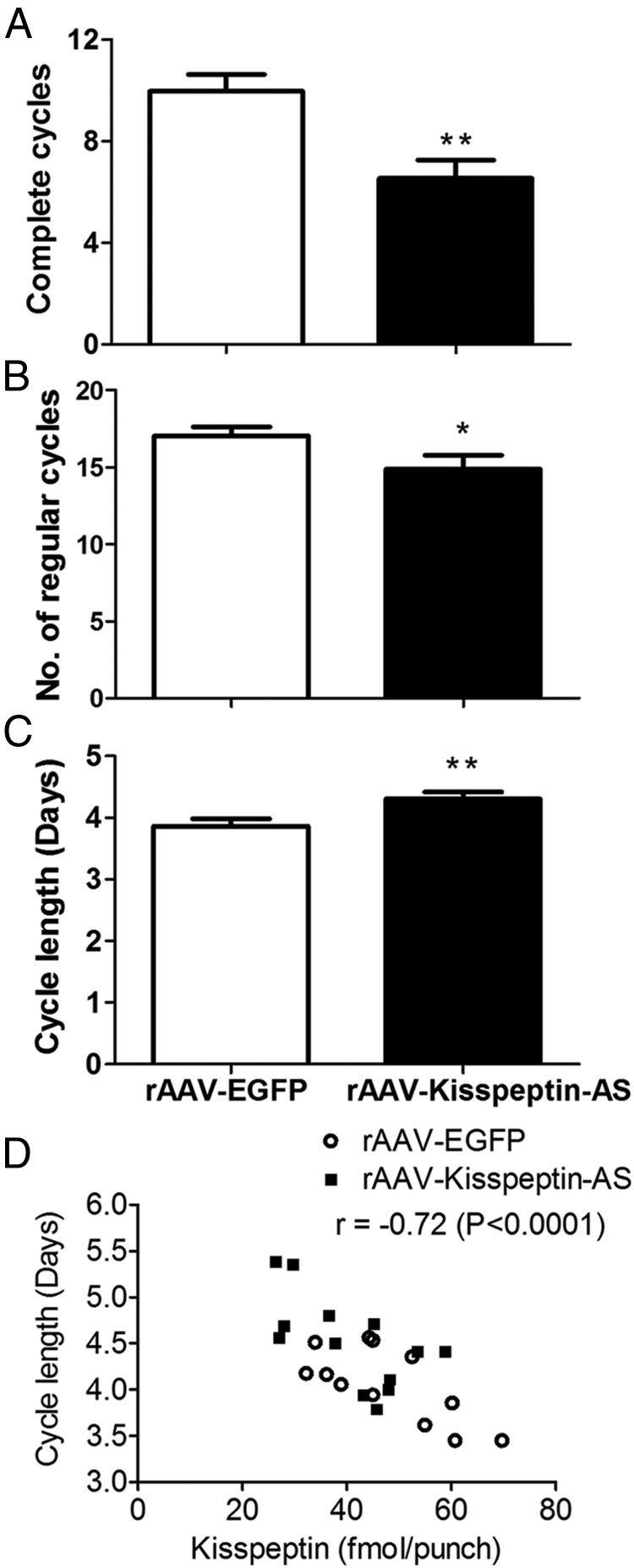
Animals administered intra-ARC rAAV-Kisspeptin-AS had significantly fewer “regular” and complete oestrous cycles between day 4 and 100 after injection compared with EGFP controls (Figure 2, A and B). Animals injected with rAAV-Kisspeptin-AS had significantly longer cycles than controls, as demonstrated by more days between each occurrence of oestrus (Figure 2C). Animals administered rAAV-Kisspeptin-AS displayed a trend for more “extended” and “abnormal” (Table 1) cycles than controls, although these effects did not reach significance. No significant differences between groups were observed in the percentage time spent in proestrus, oestrus, metestrus, or diestrus (Mann-Whitney U test, P > .05). There was no difference in body weight or food intake between groups throughout the study (generalized estimating equation, P > .05). A significant correlation was observed between oestrous cycle length and kisspeptin expression (r = −0.72, P = .0001). This correlation was also significant when analyzed for the individual rAAV-EGFP (r = −0.7, P = .0046) and rAAV-Kisspeptin-AS (r = −0.71, P = .0092) groups (Figure 2D).
Figure 2.
The effect of intra-Arc rAAV-Kisspeptin-AS administration on oestrous cyclicity in adult female Wistar rats. Vaginal cytology was analyzed daily from day 4 to 100 after injection to determine oestrous cycle stage. Number of complete cycles (A), regular cycles (B), and average number of days per cycle (C) were calculated. D, Correlation between oestrous cycle length and ARC-kisspeptin expression in both the EGFP (○) and Kisspeptin-AS groups (■). Data are presented as mean ± SEM; *, P < .05; **, P < .01 vs rAAV-EGFP group, n = 12–14 per group.
Table 1.
Effect of Intra-Arc rAAV-EGFP or rAAV-Kisspeptin-AS Administration on Oestrous Cyclicity in Adult Female Wistar Rats
| rAAV-EGFP | rAAV-Kisspeptin-AS | |
|---|---|---|
| Regular cycles | 17.1 ± 0.5 | 15.0 ± 0.9a |
| Extended cycles | 0.6 ± 0.3 | 1.4 ± 0.3 |
| Abnormal cycles | 0.0 ± 0.0 | 0.2 ± 0.2 |
| Complete cycles | 10.0 ± 0.6 | 6.5 ± 0.8b |
| Average number of days per cycle | 3.8 ± 0.1 | 4.3 ± 0.1b |
The number of regular, extended, abnormal, and complete cycles were calculated. Cycle length was calculated as the average number of days between each occurrence of oestrus. Data are presented as mean ± SEM; n = 12–14 per group.
P < .05 vs rAAV-EGFP group.
P < .01 vs rAAV-EGFP group.
The effect of ARC rAAV-Kisspeptin-AS administration on intact plasma LH, FSH, and E2 levels
No statistically significant differences in plasma LH (0.67 ± 0.12 ng/mL [rAAV-EGFP] vs 0.64 ± 0.07 ng/mL [rAAV-Kisspeptin-AS]; Student's t test, n = 12–14 per group), FSH (13.69 ± 1.39 ng/mL [rAAV-EGFP] vs 12.31 ± 0.87 ng/mL [rAAV-Kisspeptin-AS]; Student's t test, n = 12–14 per group), or E2 (6.86 ± 1.62 pg/mL [rAAV-EGFP] vs 5.44 ± 0.56 pg/mL [rAAV-Kisspeptin-AS]; Student's t test, n = 12–14 per group) were observed between animals administered rAAV-EGFP and rAAV-Kisspeptin-AS at the single time point analyzed, 100 days after injection.
Effect of ARC AAV-kisspeptin-AS on ovarian morphology
There was no significant difference in the total number of antral follicles or corpora lutea in rAAV-Kisspeptin-AS in rats, compared with rAAV-EGFP control animals (Student's t test, P > .05).
Effect of ARC AAV-kisspeptin-AS on pulsatile LH release in OVX+E2 rats
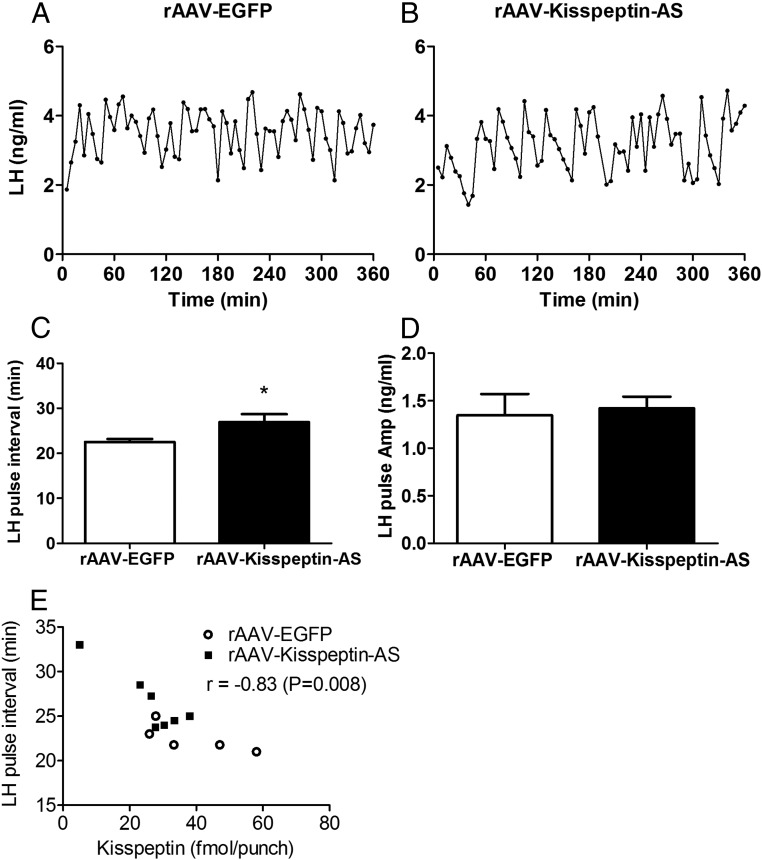
After administration of ARC AAV-kisspeptin-AS in OVX+E2 animals, we observed a significant decrease in LH pulse frequency compared with rAAV-EGFP control animals (22.54 ± 1.3 pulse interval (min) [rAAV-EGFP] vs 26.54 ± 0.7 pulse interval (min) [rAAV-Kisspeptin-AS]; Student's t test, n = 5–7 per group) (Figure 3C). However, no difference was observed in LH pulse amplitude between Kisspeptin-AS and rAAV-EGFP rats (1.35 ± 0.22 ng [rAAV-EGFP] vs 1.42 ± 0.12 ng [rAAV-Kisspeptin-AS]; Student's t test, P > .05, n = 5–7 per group) (Figure 3D). A significant correlation was observed between LH pulse frequency and kisspeptin expression when data from both rAAV-EGFP and rAAV-Kisspeptin-AS groups was analyzed together (Figure 3E).
Figure 3.
The effect of intra-Arc rAAV-Kisspeptin-AS administration on pulsatile LH release in OXV+E2 female Wistar rats. Representative LH profiles illustrating the effect of rAAV-EGFP (A) or rAAV-EGFP and rAAV-Kisspeptin-AS (B) administration into the ARC on LH secretion in OVX+E2 rats. ARC administration of rAAV-KP-AS resulted in a decrease in LH pulses frequency compared with rAAV-EGFP (C), with no effect on LH pulse amplitude (D). E, Correlation between LH pulse frequency and ARC-kisspeptin expression in both the EGFP (○) and Kisspeptin-AS groups (■); *, P < .05 vs rAAV-EGFP (n = 5–7).
Effect of ARC AAV-kisspeptin-AS on E2-induced LH surge
We were able to induce a LH surge in 10 of the 12 of the rats in the study; a ratio which has been observed previously (27). Area under the curve analysis indicated no effect of rAAV-Kisspeptin-AS compared with rAAV-EGFP on the LH surge (1909 ± 286 ng/mL·min [rAAV-EGFP] vs 1786 ± 164 ng/mL·min [rAAV-Kisspeptin-AS]; Student's t test, P > .05).
Discussion
To examine the physiological role of kisspeptin neurons within the ARC, we used rAAV to specifically knockdown expression in this nucleus. Both in vitro and in vivo administration of rAAV-Kisspeptin-AS significantly decreased kisspeptin protein levels. Female rats with ARC-kisspeptin knockdown displayed a significantly reduced number of both regular and complete oestrous cycles over a 100-day period and significantly longer cycles. This suggests that kisspeptin-knockdown lengthened each cycle sufficiently to result in a reduction in the total number of cycles. These data provide support to the suggestion that ARC kisspeptin neurons provide a tonic stimulatory drive to GnRH neurons and therefore oestrous cyclicity. The knockdown in kisspeptin expression observed in the current study may represent a physiologically relevant paradigm, because the magnitude of decrease did not exceed that observed in response to inhibitory stimuli such as inflammation (20) and lactation (21). Our data therefore demonstrate that even modest changes in ARC-kisspeptin levels can have significant effects on reproductive function. However, knockdown of ARC kisspeptin in intact animals did not alter serum LH, FSH, and E2 levels, as measured at a single time point. The decrease in kisspeptin expression observed in the present study may be insufficient to significantly alter gonadotropin and E2 levels. However, the observations were made in gonadal-intact rats, where gonadotropin levels are under the inhibitory influence of gonadal steroid feedback. Therefore, LH and FSH levels may already be close to being maximally suppressed, and a decrease in kisspeptin expression within the ARC may have little effect. Interestingly, both gonadal intact wild types and Kiss1-KO mice display similar E2 levels (3).
Although knockdown of kisspeptin in the ARC did not significantly influence serum LH levels measured at a single time point, we postulated that they might influence LH pulsatility. Appropriate gonadotropin pulse frequency and amplitude is essential for normal follicular development and thus oestrous cyclicity (32). We therefore investigated the role of ARC kisspeptin neurons in the control of pulsatile LH release by knocking down ARC expression through the administration of rAAV-Kisspeptin-AS. Kisspeptin knockdown in the ARC resulted in a modest but significant decrease in LH pulse frequency in OVX+E2 rats, which may underlie the subtle disruptions in oestrous cyclicity observed in the intact animals. These data provide the first direct evidence that kisspeptin neurons within the ARC play a critical role in maintaining LH pulse frequency in the adult female rat. Although the precise mechanisms that underlie the pulsatile release of GnRH/LH have yet to be fully elucidated, evidence suggests the ARC plays a major role. Surgically isolating this nucleus in female rats induces persistent oestrus but maintains pulsatile LH release (13), and multiunit electrical activity volleys recorded from the ARC are associated with the initiation of LH pulses (26, 33, 34). The multiunit electrical activity recording electrodes were near kisspeptin neuronal cell bodies (34), suggesting that kisspeptin neurons in the ARC may act to drive pulsatile GnRH/LH release. This is supported by studies demonstrating pulsatile release of kisspeptin from the basal hypothalamus/stalk-median eminence (35) and that administration of a kisspeptin antagonist into the ARC decreases pulsatile LH release (15). Indeed, the current study suggests that kisspeptin synthesis by neurons within the ARC plays a pivotal role in determining LH pulse frequency. Further, expression of kisspeptin mRNA in the ARC mirrors the pulsatile release of LH under a variety of physiological conditions (11, 12, 19, 21). Finally, using N-methyl-aspartic acid to lesion the ARC in mice has been shown to lengthen the oestrous cycle (36). Reducing the number of cells in the ARC by 30% resulted a cycle prolongation of 1 day (36). These findings mirror the knockdown and cycle disturbances observed in the current study and thus may reflect a decrease in ARC kisspeptin associated with the loss of ARC cells. Kisspeptin neurons in the ARC coexpress dynorphin and NKB, and it has been suggested these neuropeptides act in concert to regulate gonadotropin secretion (37). Thus, the subtle phenotype observed in the current study may possibly reflect a compensatory action of dynorphin and NKB on gonadotropin release and subsequent oestrous cyclicity. Although we did not observe a significant change in ARC-NKB content, further studies are required for a more detailed investigation of any potential compensatory action. Recent studies have examined the role of kisspeptin neurons in adult rodents using cell ablation techniques. Targeted ablation of neurons that express neurokinin 3 receptor by administering a toxin-bound neurokinin 3 receptor agonist into the ARC resulted in the loss of 95% of ARC kisspeptin neurons (38). Such a profound loss of kisspeptin neurons within the ARC resulted in the partial loss of E2-mediated feedback and decreased LH levels in both OVX and OVX+E2 rats. This study provides a valuable insight into the role of ARC kisspeptin neurons in gonadal steroid feedback in OVX mice. However, it does not specifically address the physiological role of kisspeptin within the ARC of gonadal-intact rats, or in the role played by ARC kisspeptin neurons in pulsatile LH release. An alternative kisspeptin cell ablation strategy used transgenic mice, in which kisspeptin cells were selectively susceptible to diphtheria toxin. Administration of diphtheria toxin ablated kisspeptin cells throughout the body, including those in the ovary, pituitary, ARC, amygdala, and the AVPV. Consistent with our findings, this ablation resulted in disrupted oestrous cycles and infertility (39), although the effects observed cannot be attributed to a specific neuronal population. Because cell ablation techniques result in the loss of the entire cell, the phenotype produced in both of these studies will reflect the loss of all neuropeptides/transmitters produced by the cell. The approach used in the present study enabled investigation of the role of kisspeptin specifically, rather than of kisspeptin expressing neurons. Interestingly, a recent study demonstrated that mice with a 95% reduction in kisspeptin expression retain some level of reproductive function (40). This relative insensitivity of the reproductive axis to changes in kisspeptin expression does not accord with the proposed role of kisspeptin as a conduit for physiological inputs, and although it may reflect a potential redundancy in the system, the role of developmental compensation in these mice is likely to be important. Certainly, our data suggest that quite modest changes in kisspeptin synthesis in a single hypothalamic nucleus in adult animals can influence reproductive function.
Numerous studies have indicated that the AVPV population of kisspeptin neurons drives the stimulation of the LH surge in rodents. Increased kisspeptin expression is observed within the AVPV preceding the spontaneous (preovulatory) as well as estrogen-induced LH surges, with increased cFos expression present in AVPV kisspeptin neurons during this period (41, 42). Further, administration of kisspeptin antiserum in the medial preoptic area is able to block the LH surge in rodents (43). As expected, a decrease in ARC kisspeptin expression does not influence E2-induced LH surge. However, given the modest knockdown in kisspeptin expression, we cannot exclude the possibility that the LH surge might be affected with a greater knockdown. These findings suggest the effects of ARC kisspeptin knockdown on oestrous cyclicity are not mediated by disturbances to the LH surge.
In summary, we have demonstrated that decreasing the expression of kisspeptin within the ARC in adult female rats disrupts oestrous cyclicity. These data suggest that maintaining kisspeptin expression in the ARC within relatively conservative levels is essential for preservation of normal reproductive function.
Acknowledgments
This work was funded by grants from the Medical Research Council, Biotechnology and Biological Sciences Research Council, National Institute for Health Research, Wellcome Trust an Integrative Mammalian Biology Capacity Building Award, an FP7- HEALTH- 2009- 241592 European Obesity Consortium studying the Hypothalamus and its Interaction with the Periphery Grant and the Society for Endocrinology. W.S.D. is supported by the National Institute for Health Research Career Development fellowship and A.H.S. by a Wellcome Trust Research Training fellowship.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAV
- adeno-associated virus
- ARC
- hypothalamic arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- E2
- estradiol
- EGFP
- enhanced green fluorescent protein
- KO
- knockout
- NKB
- neurokinin B
- OVX
- ovariectomized
- rAAV
- recombinant AAV
- rAAV-EGFP
- rAAV-encoding EGFP
- rAAV-kisspeptin-AS
- rAAV kisspeptin antisense.
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627 [DOI] [PubMed] [Google Scholar]
- 3. d'Anglemont de Tassigny X, Fagg LA, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104(25):10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077 [DOI] [PubMed] [Google Scholar]
- 5. Thompson EL, Patterson M, Murphy KG, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16(10):850–858 [DOI] [PubMed] [Google Scholar]
- 6. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609–6615 [DOI] [PubMed] [Google Scholar]
- 7. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272 [DOI] [PubMed] [Google Scholar]
- 8. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirilov M, Clarkson J, Liu X, et al. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4:2492. [DOI] [PubMed] [Google Scholar]
- 10. Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30(1):26–33 [DOI] [PubMed] [Google Scholar]
- 11. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692 [DOI] [PubMed] [Google Scholar]
- 12. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984 [DOI] [PubMed] [Google Scholar]
- 13. Blake CA, Sawyer CH. Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology. 1974;94(3):730–736 [DOI] [PubMed] [Google Scholar]
- 14. Ohkura S, Tsukamura H, Maeda K. Effects of various types of hypothalamic deafferentation on luteinizing hormone pulses in ovariectomized rats. J Neuroendocrinol. 1991;3(5):503–508 [DOI] [PubMed] [Google Scholar]
- 15. Li XF, Kinsey-Jones JS, Cheng Y, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4(12):e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patterson M, Murphy KG, Thompson EL, Patel S, Ghatei MA, Bloom SR. Administration of kisspeptin-54 into discrete regions of the hypothalamus potently increases plasma luteinising hormone and testosterone in male adult rats. J Neuroendocrinol. 2006;18(5):349–354 [DOI] [PubMed] [Google Scholar]
- 17. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gottsch ML, Popa SM, Lawhorn JK, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152(11):4298–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kinsey-Jones JS, Li XF, Knox AM, et al. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21(1):20–29 [DOI] [PubMed] [Google Scholar]
- 20. Castellano JM, Bentsen AH, Romero M, et al. Acute inflammation reduces kisspeptin immunoreactivity at the arcuate nucleus and decreases responsiveness to kisspeptin independently of its anorectic effects. Am J Physiol Endocrinol Metab. 2010;299(1):E54–E61 [DOI] [PubMed] [Google Scholar]
- 21. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23(1):52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gardiner JV, Kong WM, Ward H, Murphy KG, Dhillo WS, Bloom SR. AAV mediated expression of anti-sense neuropeptide Y cRNA in the arcuate nucleus of rats results in decreased weight gain and food intake. Biochem Biophys Res Commun. 2005;327(4):1088–1093 [DOI] [PubMed] [Google Scholar]
- 23. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed Sydney; Orlando: Academic Press; 1986 [Google Scholar]
- 24. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97 [DOI] [PubMed] [Google Scholar]
- 25. Bucci TJ, Bolon B, Warbritton AR, Chen JJ, Heindel JJ. Influence of sampling on the reproducibility of ovarian follicle counts in mouse toxicity studies. Reprod Toxicol. 1997;11(5):689–696 [DOI] [PubMed] [Google Scholar]
- 26. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–315 [DOI] [PubMed] [Google Scholar]
- 27. Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology. 2005;146(10):4331–4339 [DOI] [PubMed] [Google Scholar]
- 28. Palkovits M, Brownstein MJ. Maps and Guide to Microdissection of the Rat Brain. New York, NY: Elsevier; 1988 [Google Scholar]
- 29. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett. 2006;401(3):225–230 [DOI] [PubMed] [Google Scholar]
- 30. Biegel LB, Hurtt ME, Frame SR, O'Connor JC, Cook JC. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci. 2001;60(1):44–55 [DOI] [PubMed] [Google Scholar]
- 31. Van Cauter E. Estimating false-positive and false-negative errors in analyses of hormonal pulsatility. Am J Physiol. 1988;254(6 pt 1):E786–E794 [DOI] [PubMed] [Google Scholar]
- 32. Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88 [DOI] [PubMed] [Google Scholar]
- 33. Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39(3):256–260 [DOI] [PubMed] [Google Scholar]
- 34. Ohkura S, Takase K, Matsuyama S, et al. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813–821 [DOI] [PubMed] [Google Scholar]
- 35. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. May PC, Kohama SG, Finch CE. N-methyl-aspartic acid lesions of the arcuate nucleus in adult C57BL/6J mice: a new model for age-related lengthening of the estrous cycle. Neuroendocrinology. 1989;50(5):605–612 [DOI] [PubMed] [Google Scholar]
- 37. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol. 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14(6):704–710 [DOI] [PubMed] [Google Scholar]
- 40. Popa SM, Moriyama RM, Caligioni CS, et al. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology. 2013;154(8):2784–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–378 [DOI] [PubMed] [Google Scholar]
- 43. Kinoshita M, Tsukamura H, Adachi S, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–4436 [DOI] [PubMed] [Google Scholar]