Abstract
Developmental models of GH deficiency (GHD) and excess indicate that GH is positively associated with β-cell mass. Therefore, the reduction in GH levels observed with age and weight gain may contribute to the age-related decline in β-cell function. To test this hypothesis, β-cell mass and function were assessed in a mouse model of adult-onset, isolated GHD (AOiGHD). β-Cell mass did not differ between low-fat (LF)-fed AOiGHD and controls. However, high fat-fed AOiGHD mice displayed impaired expansion of β-cell mass and a reduction of bromodeoxyuridine-labeled islet cells, whereas in vitro β-cell function (basal and glucose-stimulated insulin secretion [GSIS]) did not differ from controls. In contrast, duration of AOiGHD differentially altered in vitro β-cell function in LF-fed mice. Specifically, islets from young LF-fed AOiGHD mice showed significant reductions in insulin content and basal insulin secretion, but GSIS was similar to that of controls. A similar islet phenotype was observed in a developmental model of isolated GHD (GH-releasing hormone knockout). Given that LF- and high fat-fed AOiGHD mice, as well as GH-releasing hormone knockout mice, display improved insulin sensitivity, islet changes may be due to reduced insulin demand, rather than primary β-cell dysfunction. However, islets from older LF-fed AOiGHD mice exhibited impaired GSIS, associated with reduced expression of genes important to maintain glucose sensing, suggesting that factors secondary to AOiGHD can alter β-cell function with age. AOiGHD mice exhibited postprandial hypertriglyceridemia and increased pancreatic expression of lipid/inflammatory stress response genes (activating transcription factor 3 and peroxisome proliferator activator receptor β/δ). Therefore, we speculate that these changes may initially protect the AOiGHD β-cell, but with age, lipotoxicity may impair β-cell function.
GH levels are positively associated with longitudinal growth during the adolescent years and decline progressively after puberty, at a rate of approximately 14% per decade of life (1), a decline that is exacerbated by weight gain (2). In addition to being critical for somatic growth, GH is also thought to be required for normal expansion of the pancreatic β-cell population during development, based on studies showing reduced β-cell mass in mouse models with developmental defects in GH production and signaling (3, 4). Direct effects of GH on β-cell proliferation and function are further supported by in vitro studies using rodent islet cultures (5–7). Therefore, reduced GH secretion in the elderly and obese adults may contribute to β-cell dysfunction. Indirect evidence supporting this hypothesis is the fact that humans with long-term adult-onset GH deficiency (AOGHD) (8) or with developmental isolated GHD show impaired glucose tolerance (IGT) (9, 10) and may have an increase prevalence of diabetes mellitus (11). However, in these individuals factors other than GHD, such as stress of surgery and/or radiation, head trauma, and additional pituitary hormone deficiencies and their imperfect hormonal replacement, could also contribute to their metabolic decline (12, 13).
In order to test the specific role GH plays in maintaining adult metabolism, our laboratory has developed and validated a mouse model of adult-onset, isolated GHD (AOiGHD), in which GH levels are reduced after sexual maturation to approximately 30% of controls (14). In this model, GHD is associated with low/normal IGF-I levels (14, 15), resembling a subpopulation of patients with AOGHD (16). We have previously reported that AOiGHD mice, unlike developmental models of GHD/resistance (3, 17), are not dwarf or overtly obese. However, similar to the developmental GH-deficient (18) and GH-resistant models (GH receptor knock-out [GHRKO]) (3), AOiGHD mice show increased insulin sensitivity and develop diet-dependent glucose intolerance with inappropriately low insulin levels (14), suggesting impaired β-cell mass and/or function. To determine whether the in vivo changes in metabolic function observed in AOiGHD mice are associated with alterations in β-cells, the current study examined: 1) β-cell mass in 1-year-old AOiGHD mice and their GH-sufficient controls maintained on either a low-fat (LF) or a high-fat (HF) diet; 2) islet mass and β-cell proliferation of AOiGHD and control mice after short-term (4 and 16 wk) HF feeding; and 3) time-dependent changes in in vivo metabolic function and in vitro islet function of LF- and HF-fed AOiGHD and control mice. To compare the impact of adult vs developmental GHD on in vitro islet function, we also examined β-cell function in GHRH-KO mice (17) and their littermate controls.
Materials and Methods
Animals
Experimental mouse studies were approved by the Institutional Animal Care and Use Committee of University of Illinois at Chicago, Jesse Brown Veterans Affairs Medical Center, and/or Johns Hopkins University. Male AOiGHD mice were generated as previously described (14). In all experiments outlined below, AOiGHD was induced by diphtheria toxin (DT)-selective somatotrope death in 12-week-old mice that were heterozygotes for both the rat GH promoter, Cre recombinase (rGHpCre+/−) transgene and the inducible DT receptor (iDTR+/−) transgene. DT-treated rGHpCre−/− iDTR+/− mice serve as controls.
Experiment 1
Islets per pancreas and β-cell mass were measured in the pancreases collected from 1-year-old LF-fed (10% kcal from fat, catalog number 12450B; Research Diets) or HF-fed (45% kcal from fat, catalog number 12451; Research Diets) male AOiGHD mice (39 wk of GHD) and their littermate controls, generated in a previous study (14). The pancreases were weighed and fixed overnight in 10% neutral-buffered formalin (Fisher Scientific), then stored in 70% ethanol until further processing for immunohistochemistry.
Experiment 2
To investigate the impact of AOiGHD on islet cell proliferation, mice were maintained on a LF diet after weaning (starting at 4 wk of age) and AOiGHD induced at 12 weeks of age, at which time mice were switched to a HF diet (60% kcal from fat, catalog number 12492; Research Diets). After 4 or 16 weeks of HF feeding, mice were implanted with osmotic minipumps (Alzet, Durect Corp) to infuse bromodeoxyuridine (20 mg/mL, 1 μL/h; Sigma-Aldrich, Inc) over a 72-hour period in order to maximize the labeling of slowly proliferating islet cells. After BrdU infusion, the pancreases were excised and fixed overnight in methacarn (60% methanol, 30% chloroform, and 10% acetic acid) (19) and stored in 70% ethanol until further processing and immunostaining for BrdU.
Experiment 3
In order to investigate the time-dependent impact of AOiGHD and diet on in vivo metabolic function and in vitro islet function, mice were maintained on a LF diet after weaning (starting at 4 wk of age) and AOiGHD induced at 12 weeks of age. At 22 weeks of age, a subset of mice was switched to a HF diet (60% kcal from fat). All mice were weighed every 4 weeks, and body composition was assessed by nuclear magnetic resonance spectroscopy (MiniSpec LF50 Bruker Optics) starting at 12 weeks of age. Islets were collected from 22-week-old LF-fed mice (10 wk of GHD) or 46-week-old, LF- or HF-fed mice (34 wk of GHD) after a 4-hour fast (food withdrawn at 8 am), for determination of islet gene expression, insulin and proinsulin content, as well as basal and secretagogue-induced insulin release; 22- and 46-week-old mice were chosen based on previous studies done in AOiGHD mice (14). Blood was collected at the time of killing, for determination of glucose, insulin, and lipids. Livers were weighed and aliquots assessed for lipid content.
Experiment 4
In order to compare the impact of AOiGHD vs developmental GHD, glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed on 7- and 46-week-old male GHRH-KO mice and their littermate controls. In addition, islets were isolated from chow-fed 16- to 18-week-old male GHRH-KO mice, for assessment of islet gene expression, as well as basal and glucose-stimulated insulin release.
Experiment 5
To assess postprandial lipid homeostasis, 40-week-old LF-fed AOiGHD (28 wk of GHD) were fasted for 24 hours and refed ad libitum for 6 hours. A subset of mice was killed after fasting and the rest after refeeding. Plasma and pancreas were stored frozen for further analysis.
Immunohistochemistry and islet morphometry analysis
Formalin- and methacarn-fixed pancreases were paraffin embedded. Five-micrometer sections were taken every 200 μm for analysis. For insulin immunostaining, 2 formalin-fixed sections per pancreas were deparaffinized, hydrated in graded-ethanol/water solutions, and then treated with 10mM citrate buffer at 125°C for 5 minutes followed by 3% H2O2, for 5 minutes at room temperature. Samples were blocked in Tris-buffered saline (TBS) containing 1.5% goat serum and 0.01% Tween 20 for 20 minutes, and sections were incubated (4°C overnight in TBS 0.01% Tween 20) with a 1:500 dilution of a rabbit antiinsulin polyclonal antibody (catalog number 4590; Cell Signaling Technology, Inc). Specific signal was revealed with the Vectastain ABC kit (PK-4001; Vector Laboratories), and sections were counterstained with hematoxylin. For BrdU immunostaining, 2 methacarn-fixed sections per pancreas were deparaffinized, hydrated in graded-ethanol/water solutions, treated with 2N HCl for 90 minutes, neutralized with 0.1M sodium borate, and then blocked with 1X× Power Block (BioGenex) diluted in TBS for 2 minutes. Sections were then incubated for 2 hours with a 1:50 dilution of mouse anti-BrdU monoclonal antibody (catalog number 555627; BD Biosciences), specific signal revealed using the Dako ARK kit (Dako), and counterstained with hematoxylin. Total pancreas area, insulin-stained area, islet area, and BrdU+ nuclei were measured using Olympus BX43 and CKX41 microscopes and CellSens software (Olympus). To obtain an accurate measurement of islet number and β-cell mass, 100–270 islets per pancreas were counted. To determine β-cell size, nuclei within insulin-stained islet area were counted (at least 1000 cells/pancreas), to estimate an average of the β-cell size. To determine the proportion of islet cells that underwent DNA synthesis over a 72-hour period (labeled with BrdU), a total of 2500–3500 cells was identified per section, and the proportion of BrdU+ to (BrdU− + BrdU+) cells was obtained.
Isolation and culture of islets
After cervical dislocation under isofluorane anesthesia, a midline incision was made, and the pancreas was infused with 2–4 mL of 0.5-mg/mL collagenase P (Roche Applied Science) in Hanks' balance salt solution (HBSS) (Invitrogen), delivered into the common bile duct using a 30-gauge needle. Pancreases were pooled (2–3) and digested in 5-mL collagenase solution in 50-mL centrifuge tubes at 37°C for 15 minutes with gentle agitation every 5 minutes. After strong shaking (5 s), the digestion solution was diluted in ice-cold HBSS, centrifuged, and the pellet suspended in ice-cold HBSS and filtered through a 400-μm mesh, then centrifuged (1500 rpm for 1 min at 4°C). A discontinuous density gradient was used to separate islets from exocrine tissue. Specifically, the digestion pellet was resuspended in 5 mL of 1.108-g/mL Ficoll gradient (Mediatech, Inc Corning), and 2 mL each of 1.096-, 1.069-, and 1.037-g/mL Ficoll gradient (Mediatech, Inc Corning) were consecutively added, followed by centrifugation at 2500 rpm for 20 minutes without brake at 4°C. The layer containing the purified islets was removed, and islets were washed over a 40-μm nylon cell strainer (Becton Dickinson) with HBSS. After 2 washes in HBSS, the pellet (∼300–400 islets) was resuspended in 15 mL of serum containing 2.8-G RPMI 1640 media (RPMI 1640 containing 2.8mM D-glucose, 10% fetal bovine serum, 0.5% BSA, 2mM L-Glu [Sigma-Aldrich, Inc], and 1% antibiotic/antimicotic [Invitrogen]). Islets were plated on 100-mm diameter nonadherent petri dishes (Becton Dickinson) and cultured at 37°C, 5% CO2 overnight. Overnight cultured islets were washed in serum-free 2.8-G RPMI 1640 and incubated for an additional 2 hours at 37°C, 5% CO2, and 50 similar size islets distributed per well in nonadherent 12-well plates (Nunc) in 2-mL serum-free 2.8-G RPMI 1640 for assessment of gene expression.
Glucose-stimulated insulin secretion (GSIS) in vitro
For assessment of basal and secretagogue-stimulated insulin secretion, 20 similar size islets were placed in Millicell culture inserts (8-μm pore size; Millipore) in tissue culture-treated 24-well plates (Becton Dickinson) and incubated in 1 mL of 2.8-G Krebs-Ringer HEPES buffer (KRHB) (0.5mM MgCl2, 4.5mM KCl, 0.12mM NaCl, 0,7mM Na2HPO4, 0,15mM NaH2PO4, 10mM HEPES, and 0.5% free-fatty acids-free BSA [Sigma-Aldrich, Inc]) for 1 hour to stabilize insulin secretion. Culture inserts containing islets were changed to fresh, warmed 2.8-G KRHB for 30 minutes (to assess basal insulin release), then to 16.8mM D-glucose (16.8 G) KRHB for 30 minutes (to assess GSIS). In some experiments, after a 16.8-G challenge, islets were immediately treated with 16.8-G, 250μM palmitate (Sigma-Aldrich, Inc) and 20nM glucagon-like peptide (GLP)-1 (7–36)-amide (Phoenix Pharmaceuticals) KRHB for 30 minutes (to assess maximum secretory capacity). After each treatment, media were recovered, centrifuged, and the supernatant was stored at −20°C for determination of basal and stimulated insulin release. After the final treatment, islets were recovered in 1.5% HCl, 70% ethanol and stored at −20°C for determination of islet insulin content.
Metabolic/hormonal endpoints
Plasma glucose from blood collected by lateral tail vein knick or submandibular vein was measured using AlphaTrack meter and glucose strips (Abbot Laboratories). Insulin, proinsulin (Mercodia), and GH (Millipore) were measured using mouse/rat ELISA kits. Mouse/rat insulin ELISA kit shows cross-reactivity with proinsulin I (43%) and proinsulin II (60%). Triglycerides, nonesterified fatty acids, total cholesterol, and ketones were measured using colorimetric assay reagents (Wako Life Sciences, Inc). In GHRH-KO mice, GTTs (2 g/kg oral) were performed after an overnight fast, and ITTs (1 U/kg ip) were performed under ad libitum-fed conditions between 8 and 11 am. Blood samples were taken at t = 0 and 30 minutes in oral GTT to assess GSIS in vivo.
mRNA isolation and quantitative real-time RT-PCR
RNA was extracted from pancreatic islets using Absolutely RNA miniprep kit (Agilent Technologies) or from whole pancreas using TRIzol reagent (Invitrogen) and Deoxyribonuclease (Promega). DNA-free RNA was reverse transcribed using RevertAid First strand cDNA synthesis kit (Thermo Scientific) and cDNA amplified by quantitative real-time RT-PCR using Brilliant III Ultrafast SYBR green (Agilent Technologies). Primer sequences are supplied in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. mRNA copy number of all transcripts from islets and whole pancreas were adjusted by a normalization factor calculated from the mRNA copy number of 3 separate housekeeping genes (β-actin, glyceraldehyde 3-phosphate dehydrogenase, and cyclophilin-A mRNA) using GeNorm 3.3, as previously described (20, 21).
Statistical analysis
Student's t tests were performed to analyze AOiGHD's effect within diet and/or age. Two-way ANOVA followed by Bonferroni post hoc test was performed to compare AOiGHD/diet effect at the end of the study or AOiGHD/duration of diet within LF or HF feeding. P values of less than 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software).
Results
AOiGHD does not impact β-cell mass under LF-fed conditions but impairs β-cell mass expansion in response to HF feeding, which is associated with reduced β-cell size and proliferation.
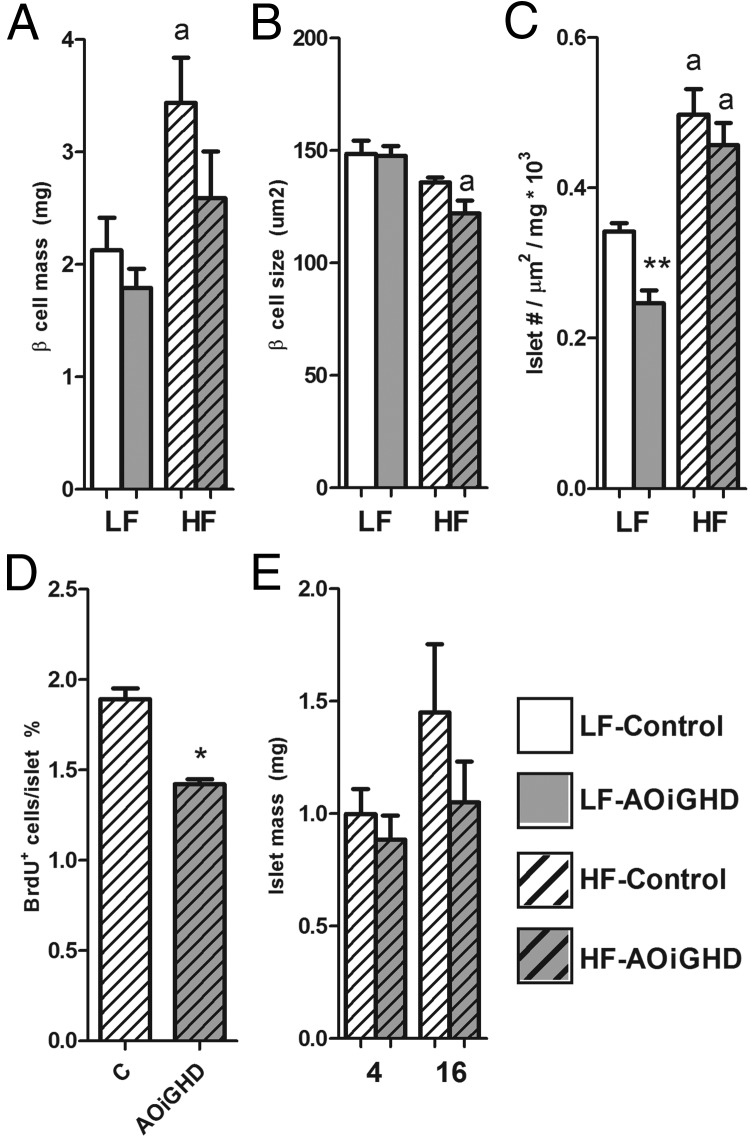
LF-fed, AOiGHD mice (1 y of age) had similar β-cell mass and β-cell size, compared with diet-matched littermate controls (Figure 1, A and B). However, AOiGHD mice showed fewer islets than control mice (P = .0133; two-way ANOVA) (Figure 1C), which could be attributed to fewer numbers of small islets (0–5000 μm2, ∼1–30 cells) (Supplemental Figure 1).
Figure 1.
Immunohistochemical analysis of pancreatic sections of LF- and HF-fed AOiGHD male mice. β-Cell mass (A), β-cell size (B), and islet number/area per pancreas (C) of 1-year-old LF-fed (open bars) and HF-fed (crosshatched bars) AOiGHD mice. BrdU+ islet cells in 16-week HF-fed AOiGHD mice (D) and islet mass of 4- and 16-week HF-fed AOiGHD mice (E). Values are mean ± SEM of 5–6 mice/group per diet. Asterisks indicate differences between AOiGHD and controls. *, P < .05; **, P < .01. a, significant difference between LF- and HF-fed group within genotype.
By two-way ANOVA, long-term HF feeding had an overall stimulatory effect on β-cell mass (P = .0061) (Figure 1A) and islet numbers (P < .0001) (Figure 1C) and an inhibitory effect on β-cell size (P = .0017) (Figure 1B), as previously reported (22–24). However, the stimulatory effect of HF feeding on β-cell mass in AOiGHD mice did not reach significance (P = .1122) (Figure 1A), whereas the inhibitory effect of HF feeding on β-cell size was more pronounced in AOiGHD mice, compared with controls (P = .0071) (Figure 1B).
To test whether the impaired expansion of β-cell mass in HF-fed AOiGHD mice may be due to a reduction in cell proliferation, the proportion of BrdU-labeled islet cells was determined after 16 weeks of HF feeding (Figure 1D), and islet mass was assessed after 4 and 16 weeks of HF feeding (Figure 1E). The proportion of BrdU-positive cells was significantly lower in AOiGHD islets compared with controls (Figure 1D). Islet mass after 4 or 16 weeks of HF feeding did not significantly differ from controls (Figure 1E).
Long-term, but not short-term, AOiGHD impairs in vitro GSIS in a diet-dependent fashion
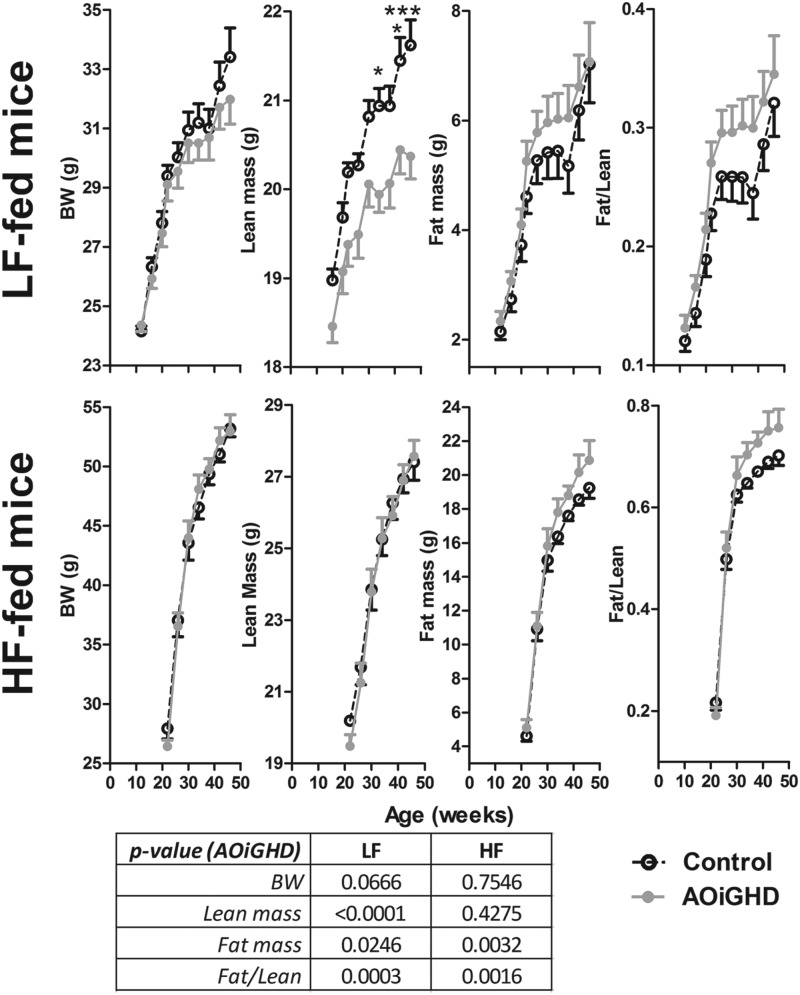
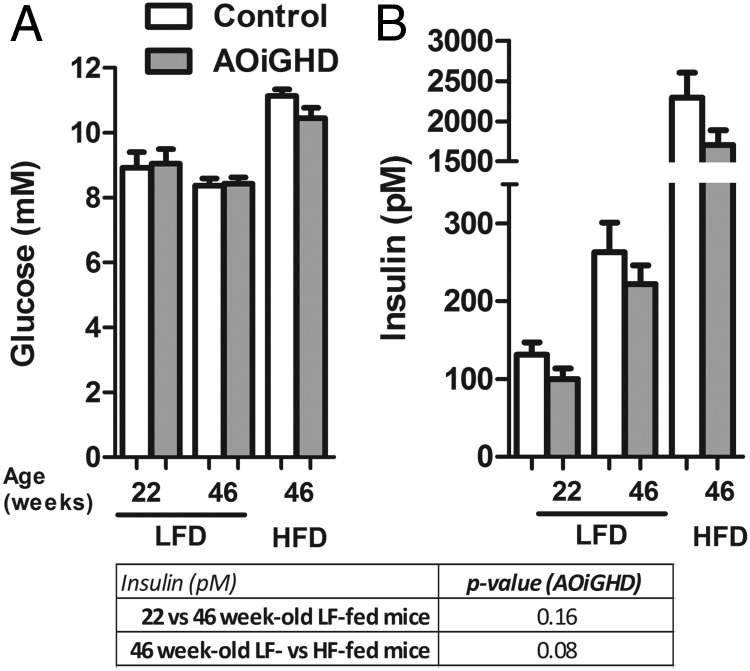
To directly assess the age- and diet-dependent impact of AOiGHD on β-cell function, islets were isolated from LF-fed, AOiGHD and control mice at 22 weeks of age (10 wk of GHD, Supplemental Figure 2) and LF- and HF-fed AOiGHD and control mice at 46 weeks of age (34 wk of GHD, 24 wk of HF feeding). The impact of age and diet on body composition and metabolic endpoints is shown in Supplemental Table 2 and Figures 2 and 3). At 22 and 46 weeks of age, GH levels were reduced in LF-fed AOiGHD mice compared with diet- and age-matched controls (Supplemental Table 2). HF feeding suppressed GH levels in control mice but did not alter the already low GH levels observed in AOiGHD mice. IGF-I levels did not differ between controls and AOiGHD mice. However, HF feeding increased IGF-I levels independent of GH status (Supplemental Table 2). The reduction of GH in LF-fed AOiGHD mice was associated with reduced lean mass (Figure 2). In addition, AOiGHD mice exhibited an increase in fat/lean ratio independent of diet (Figure 2). Glucose levels did not significantly differ between control and AOiGHD mice within age and diet but increased with HF feeding (Figure 3). Insulin rose with age and HF feeding in both groups but tended to be suppressed in AOiGHD across age and diet.
Figure 2.
Body composition (body weight, fat/lean, lean, and fat mass) of LF- or HF-fed AOiGHD from 12 to 46 weeks of age. Values are means ± SEM of 10–14 mice/group per diet and were analyzed by two-way ANOVA within diet, and P values for the overall effect of AOiGHD across diet are provided in the lower panel. Asterisks (*, P < .05; ***, P < .001) indicate values that significantly differ between genotype, within diet, as assessed by Bonferroni's post hoc comparisons. BW, body weight.
Figure 3.
Glucose (A) and insulin (B) levels in 4-hour fasted, 22- and 46-week-old LF-fed and 46-week-old HF-fed control and AOiGHD mice. LFD, LF diet; HFD, HF diet. Values are means ± SEM of 10–14 mice/group per diet and were analyzed by two-way ANOVA within diet or age, and P values for the overall effect of AOiGHD on insulin are provided in the lower panel as assessed by Bonferroni's post hoc comparisons.
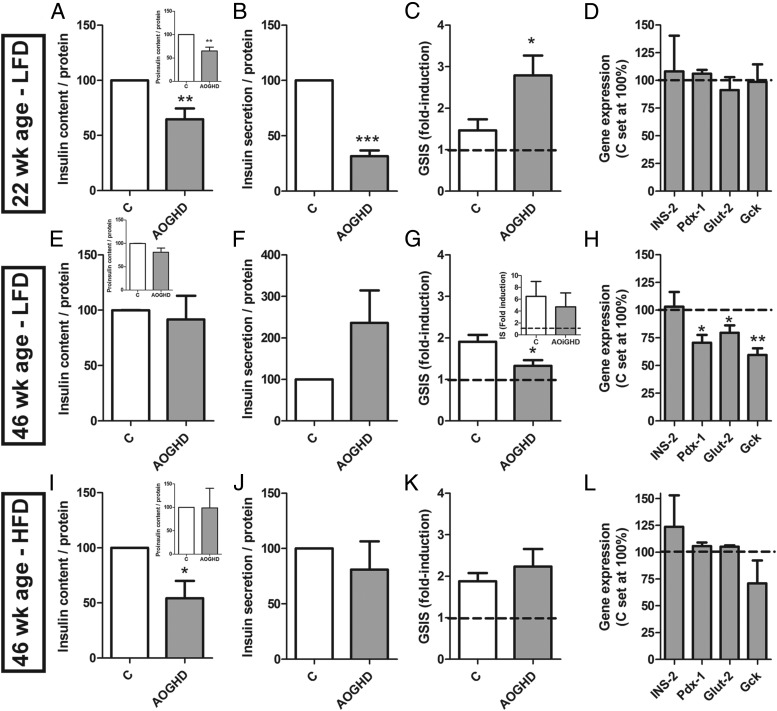
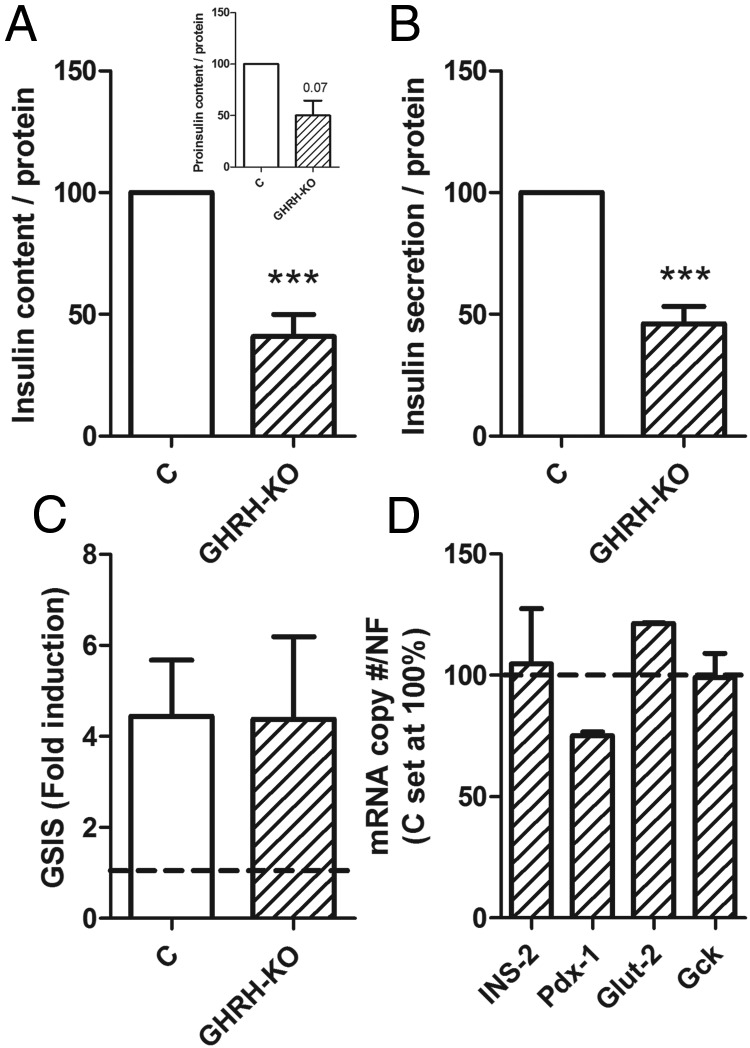
Islets isolated from 22-week-old LF-fed AOiGHD mice showed reduced insulin and proinsulin content and basal insulin secretion. Despite this reduction, AOiGHD islets released relatively more insulin in response to glucose (GSIS), compared with control islets (Figure 4, A–C). Islets from 18-week-old GHRH-KO mice also showed reduced insulin content and basal secretion, whereas GSIS and gene expression did not differ from controls (Figure 5, A–D). Like AOiGHD, in vivo GHRH-KO mice showed improved insulin sensitivity (Supplemental Figure 3).
Figure 4.
In vitro basal and secretagogue-induced insulin secretion and insulin/proinsulin content of male AOiGHD islets. Insulin and proinsulin content, basal (2.8mM glucose)-induced insulin secretion, 16.8mM GSIS and gene expression of 22-week-old LF-fed (A–D), 46-week-old LF-fed (E–H), and 46-week-old HF-fed (I–L) control (C) and AOiGHD mice; 16.8mM glucose, 250μM palmitate, 20nM GLP-1-induced insulin secretion of 46-week-old LF-fed control and AOiGHD mice (G, inset). Insulin, proinsulin content, and insulin secretion were normalized with islet protein content assessed by Bradford and represented as percentage of control values within experiments (set at 100%). GSIS (fold induction) is the ratio of insulin concentration in 16.8mM and 2.8mM glucose-KRHB after 30 minutes of incubation. All values are means ± SEM of 3–4 independent experiments with 3–8 independent wells/genotype per diet, with the exception of gene expression of 46-week-old HF-fed mice (n = 2). Asterisks indicate significant differences between AOiGHD and controls. *, P < .05; **, P < .01; ***, P < .001. INS-2, insulin; Pdx-1, pancreatic and duodenal homeobox; Glut-2, glucose transporter 2; Gck, glucokinase.
Figure 5.
In vitro GSIS and insulin/proinsulin content of male GHRH-KO islets Insulin and proinsulin content (A), basal (2.8mM glucose) insulin secretion (B), 16.8mM GSIS (C), and gene expression (D) of islets from 16- to 18-week-old control (open columns) and GHRH-KO (crosshatched columns) mice. Insulin, proinsulin content, and insulin secretion were normalized with islet protein content assessed by Bradford and are represented as percentage of control values within experiments (set at 100%). GSIS (fold induction) is the ratio of insulin concentration in 16.8mM and 2.8mM glucose-KRHB after 30 minutes of incubation. All values are means ± SEM of 4–5 independent experiments with 4–8 independent wells/genotype with the exception of gene expression analysis (n = 2). Asterisks indicate significant differences between GHRH-KO and controls. ***, P < .001. NF, normalization factor; INS-2, insulin; Pdx-1, pancreatic and duodenal homeobox; Glut-2, glucose transporter 2; Gck, glucokinase.
Islets isolated from 34-week-old LF-fed AOiGHD mice (34 wk of GHD) showed similar insulin and proinsulin content and basal insulin secretion as compared with age-matched control islets (Figure 4, E and F). However, GSIS was impaired in AOiGHD islets (Figure 4G), consistent with a reduction in pancreatic and duodenal homeobox 1, glucose transporter 2, and glucokinase expression (Figure 4H). Similar changes in gene expression were observed in whole pancreatic extracts of 40-week-old, LF-fed AOiGHD mice, after an overnight fast (Supplemental Figure 4). Despite impaired GSIS, AOiGHD islets were fully responsive to combine treatment with glucose, GLP-1, and palmitate (Figure 4G, inset).
In the context of HF feeding, islets isolated from 46-week-old AOiGHD mice did not differ from those isolated from diet- and age-matched controls, with the exception of reduced insulin content (Figure 4, I–L).
AOiGHD mice show impaired postprandial triglyceride clearance and enhanced pancreatic expression of lipid responsive genes.
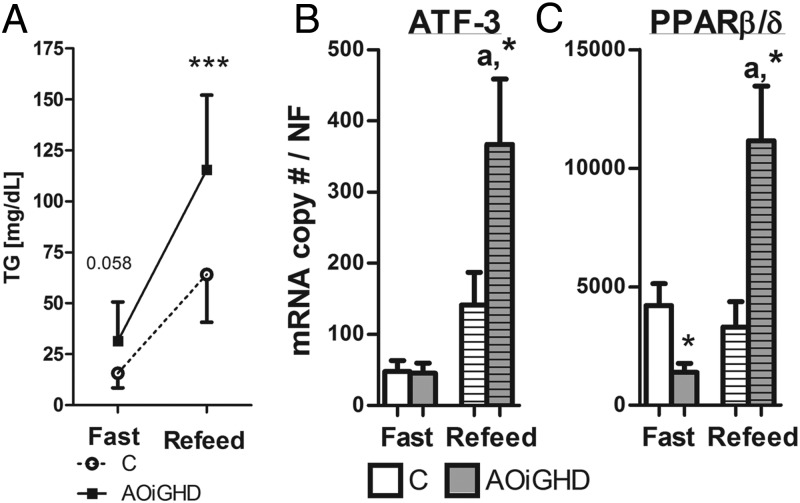
Circulating and hepatic lipids did not differ after a 4-hour fast in AOiGHD mice, compared with diet- and age-matched controls (Supplemental Table 2). However, AOiGHD mice display elevated triglycerides after a fast/refeeding challenge (Figure 6A), whereas food intake, nonesterified fatty acids, cholesterol, glucose, or ketones after refeeding did not differ from controls (Supplemental Table 3). Elevated postprandial triglycerides levels were associated with a dramatic increase in the expression of 2 lipid responsive genes, activating transcription factor 3 (ATF-3) (25) and peroxisome proliferator activator receptor (PPAR)β/δ (26), in pancreatic extracts from refed AOiGHD mice, compared with refed controls (Figure 6, B and C).
Figure 6.
Plasma triglyceride (Panel A), pancreas ATF-3 (Panel B), and PPARβ/δ (Panel C) expression in 24-hour fasted and 6-hour refed, 40-week-old LF-fed AOiGHD mice. Values are means ± SEM of 6–9 mice/genotype per group. Asterisks indicate significant differences between AOiGHD and controls within group. *, P < .05; ***, P < .001. a, significant differences between refed and fasted expression within genotype. C, Control.
Discussion
Early in vitro studies demonstrated human GH stimulated islet (β-cell) proliferation and increased insulin synthesis and secretion, with the impact of GH being more pronounced in fetal islet cultures, compared with adult islet cultures (5–7). Subsequent studies demonstrated that the direct effects of human GH on islet proliferation and function were at least in part due to signals mediated through the prolactin receptor (4, 5, 27–29). However, in rodent models of developmental GHD, β-cell mass is reduced (3, 4), whereas in models of GH elevation, β-cell mass is increased (4, 30). Because rodent GH directly signals through the GH receptor (GHR), but not the prolactin receptor, these observations support a role for GH/GHR in regulating β-cell expansion during development. However, changes in β-cell mass or function in GH-modified models may be more related to changes in insulin demands, because developmental GHD leads to improved insulin sensitivity (3), whereas pathologic elevations in GH cause insulin resistance (31). An indirect role for GH mediation of β-cell development is supported by recent studies showing β-cell-specific knockout of the GHR (βGHRKO mice) did not significantly alter systemic insulin sensitivity, glucose tolerance, or β-cell mass when mice were fed a standard chow diet (24). However, GH/GHR does appear to be important in the well-characterized compensatory expansion of β-cell mass in response to HF feeding (32), because βGHRKO mice show dramatically impaired β-cell expansion in response to HF feeding, which was associated with impaired in vivo GSIS and response to GTT (24). Although the evidence is intriguing, the βGHRKO mice were generated using mice that carry the rat insulin-promoter, Cre recombinase transgene (RIP-Cre), which was previously shown to be expressed in the hypothalamus (mainly in the arcuate nucleus) (33). Although it was shown that GHR protein (by Western blotting and immunocytochemistry) in βGHRKO did not differ from controls (24), it cannot be excluded that a select subpopulation of neurons may have been impacted, which may in turn impact islet function (34, 35). Furthermore, the relative expansion of β-cell mass in response to HF feeding is intact in global GHRKO mice (23), indicating the GHR is not essential for β-cell expansion. Therefore, the real importance of GH/GHR in directly mediating β-cell proliferation/function remains a subject of debate and requires further study.
As reviewed above, evidence is accumulating that GH plays a role (indirect or direct) in mediating β-cell proliferation and function during development. However, the question remains: does GH play a role in maintaining adult β-cells? This question is clinically relevant in that GH levels decline with advancing age and weight gain, and both elderly and obese humans have a higher risk of developing diabetes. In addition, older AOGHD patients display IGT (8) and have an increased prevalence of diabetes (11). However, in many cases, AOGHD is associated with pituitary surgery and/or radiation, head trauma, and additional pituitary hormone deficiencies, all of which may contribute to metabolic dysfunction independent of reduced GH (12, 13). Therefore, it is difficult to determine from the clinical data whether changes in metabolic environment are specifically related to GHD or other associated pathologies. To circumvent these problems, we used the AOiGHD mouse as a model system, because isolated GHD can be induced after sexual maturation by DT-mediated destruction of pituitary somatotropes, without altering the appearance and function of other pituitary cell types and without hypothalamic involvement (14). Similar to that which occurs in many AOGHD, elderly or obese patients, the reduction in GH in the AOiGHD mouse is not complete and IGF-I levels are only modestly reduced or within the normal range (see Refs. 14, 15 and this study). We have previously reported that both LF- and HF-fed AOiGHD mice remain more insulin sensitive, compared with diet-matched GH-intact controls (14), as assessed by ITTs. However, in the context of HF feeding, AOiGHD mice have reduced fed and fasted (overnight) insulin levels, associated with impaired glucose clearance after an ip glucose injection (14). From these initial observations, we originally hypothesized that impaired glucose clearance observed in HF-fed AOiGHD is due to defects in β-cell expansion and/or function. In the current study, we observed that the expansion of AOiGHD β-cell mass was modestly impaired after long-term HF feeding, and the proportion of islet cells undergoing DNA replication was lower in AOiGHD mice, after short-term HF feeding, compared with age-matched controls. However, GSIS was not altered in islets from older HF-fed AOiGHD mice. These results, coupled with the fact that glucose levels of HF-fed AOiGHD did not differ from controls, while insulin remained relatively lower, suggest that the modest reduction in β-cell mass and proliferation observed is more reflective of a reduction in systemic insulin demand, rather than β-cell dysfunction. Therefore, impaired glucose clearance observed in HF-fed AOiGHD mice may be more related to changes in hepatic glucose uptake/production. However, this hypothesis remains to be tested.
Given HF feeding/obesity dramatically alters multiple systems that may serve to override any effect of AOiGHD, including the reduction of GH (see Ref. 36 and this study), we also examined β-cell function after short-term (10 wk) and long-term (34 wk) AOiGHD, in LF-fed mice. In this context, we observed that the duration of AOiGHD differentially alters in vitro β-cell function. Specifically, in islets from young AOiGHD mice, insulin/proinsulin content and basal insulin release was reduced, whereas GSIS was enhanced, without alteration in expression of genes important in glucose sensing. Of note, islets from a developmental model of GHD (GHRH-KO mice) showed a similar in vitro phenotype. Systemic insulin sensitivity is improved in AOiGHD and GHRH-KO mice, compared with their genotype-matched controls, as reflected by improved response to ITTs, as well as normal glucose but reduced insulin levels under fed and fasted conditions. Therefore, the reduced insulin content and basal insulin secretion observed in islets from GHD mice is unlikely due to β-cell dysfunction as much as adaptation to in vivo insulin demands. However, with age, insulin content and basal secretion is normalized in AOiGHD islet cultures, which may be due to the age-related reduction in insulin sensitivity (ie, greater insulin demands), which develops in the C57Bl/6 mouse strain (37). Interestingly, GSIS was impaired in islet cultures of older LF-fed AOiGHD mice, which was associated with reduced expression of genes important in glucose sensing. This may be relevant in the human population, because it has recently been reported that a lifetime of isolated GHD, due to an inactivating mutation in the GHRH receptor gene, improves homeostatic model assessment-insulin resistance scores (9) but does not prevent the development of diabetes and increases the prevalence of IGT (9, 10). In these same individuals, HOMA-β scores are reduced (9). Although the reduction in insulin output in these subjects could be due to impaired compensatory expansion of β-cell mass, as observed in βGHRKO mice (24), our current results indicate that defects in β-cell function may also be involved. However, because these defects were not observed in younger AOiGHD mice, this suggests that age-related changes secondary to GHD may play a role in β-cell dysfunction. It should be noted that metabolic phenotype of developmental GHD may differ from that of developmental GH resistance in that it has been observed that a family with a GHR inactivating mutation show a major reduction in diabetes (38).
Dyslipidemia is prevalent in developmental isolated GHD (39) and AOGHD (40–42) humans. Although AOiGHD mice do not show major changes in circulating lipids in the basal state (14), hyperlipidemic/hyperglycemic challenge in vivo (fast-refeed experiment) revealed postprandial hypertriglyceridemia, which also has been reported in AOGHD humans (40–42). Evidence that this acute elevation in circulating triglycerides is physiologically relevant is the observation that the expression of ATF-3 and PPARβ/δ, previously shown to be up-regulated/activated by lipid stimulation and inflammatory stress in β-cells (25, 26, 43), was dramatically up-regulated in pancreatic extracts of refed AOiGHD mice, compared with refed controls. Although β-cell-specific changes in ATF-3 and PPARβ/δ expression were not examined in the current study, it has been previously reported that both of these transcriptional regulators play a protective role in maintaining/augmenting β-cell function in response to metabolic/inflammatory stress (25, 26, 44–47). In fact, a recent study demonstrates that PPARβ/δ protects β-cells from palmitate-induced apoptosis (48) and improves GSIS (43, 48). Therefore, we might speculate that these positive effects of ATF-3 and/or PPARβ/δ may explain in part the improved GSIS observed in islets of young LF-fed AOiGHD mice. However, repeated episodes of hypertriglyceridemia could lead to lipotoxicity, where accumulation of lipids in β-cells may impair β-cell function (49–51). Although these initial observations are intriguing, further studies are required to determine why AOiGHD exhibit postprandial hypertriglyceridemia and whether this is associated with alterations in β-cell lipid metabolism.
In summary, our results demonstrate that AOiGHD mice display altered β-cell function and proliferation, but indirect evidence suggests that the observed changes are secondary to changes in insulin sensitivity and lipid metabolism. However, additional studies, including examination of adult-onset β-cell-specific inactivation of the GHR, will be required to directly test this hypothesis.
Acknowledgments
This work was supported by Fundación Alfonso Martin Escudero (J.C.-C.); the Sara Borrell Program Grant CD11/00276 (to M.D.G.); Junta de Andalucía, Spain (CTS-5051 and PI-0369–2012), Ministerio de Economía y Competitividad, Spain (BFU2010–19300), and The Spanish Biomedical Research Centre in Physiopathology of Obesity and Nutrition (to R.M.L.); and by Department of Veterans Affairs, Office of Research and Development Merit Award BX001114 and National Institutes of Health Grant R01DK088133 (to R.D.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AOGHD
- adult-onset GHD
- AOiGHD
- adult-onset, isolated GHD
- ATF-3
- activating transcription factor 3
- DT
- diphtheria toxin
- GHR
- GH receptor
- GHRH-KO
- GH-releasing hormone knockout
- GLP
- glucagon-like peptide
- GSIS
- glucose-stimulated insulin secretion
- GTT
- glucose tolerance test
- HBSS
- Hanks' balance salt solution
- HF
- high fat
- IGT
- impaired glucose tolerance
- KRHB
- Krebs-Ringer HEPES buffer
- ITT
- insulin tolerance test
- LF
- low fat
- PPAR
- peroxisome proliferator activator receptor
- TBS
- Tris-buffered saline.
References
- 1. Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73(5):1081–1088 [DOI] [PubMed] [Google Scholar]
- 2. De Marinis L, Bianchi A, Mancini A, et al. Growth hormone secretion and leptin in morbid obesity before and after biliopancreatic diversion: relationships with insulin and body composition. J Clin Endocrinol Metab. 2004;89(1):174–180 [DOI] [PubMed] [Google Scholar]
- 3. Liu JL, Coschigano KT, Robertson K, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287(3):E405–E413 [DOI] [PubMed] [Google Scholar]
- 4. Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136(5):2013–2021 [DOI] [PubMed] [Google Scholar]
- 5. Brelje TC, Scharp DW, Lacy PE, et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132(2):879–887 [DOI] [PubMed] [Google Scholar]
- 6. Nielsen JH. Effects of growth hormone, prolactin, and placental lactogen on insulin content and release, and deoxyribonucleic acid synthesis in cultured pancreatic islets. Endocrinology. 1982;110(2):600–606 [DOI] [PubMed] [Google Scholar]
- 7. Nielsen JH, Linde S, Welinder BS, Billestrup N, Madsen OD. Growth hormone is a growth factor for the differentiated pancreatic β-cell. Mol Endocrinol. 1989;3(1):165–173 [DOI] [PubMed] [Google Scholar]
- 8. Beshyah SA, Gelding SV, Andres C, Johnston DG, Gray IP. β-Cell function in hypopituitary adults before and during growth hormone treatment. Clin Sci (Lond). 1995;89(3):321–328 [DOI] [PubMed] [Google Scholar]
- 9. Oliveira CR, Salvatori R, Barreto-Filho JA, et al. Insulin sensitivity and β-cell function in adults with lifetime, untreated isolated growth hormone deficiency. J Clin Endocrinol Metab. 2012;97(3):1013–1019 [DOI] [PubMed] [Google Scholar]
- 10. Vicente TA, Rocha IE, Salvatori R, et al. Lifetime congenital isolated GH deficiency does not protect from the development of diabetes. Endocr Connect. 2013;2(2):112–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abs R, Mattsson AF, Thunander M, et al. Prevalence of diabetes mellitus in 6050 hypopituitary patients with adult-onset GH deficiency before GH replacement: a KIMS analysis. Eur J Endocrinol. 2013;168(3):297–305 [DOI] [PubMed] [Google Scholar]
- 12. Danilowicz K, Bruno OD, Manavela M, Gomez RM, Barkan A. Correction of cortisol overreplacement ameliorates morbidities in patients with hypopituitarism: a pilot study. Pituitary. 2008;11(3):279–285 [DOI] [PubMed] [Google Scholar]
- 13. Melmed S. Idiopathic adult growth hormone deficiency. J Clin Endocrinol Metab. 2013;98(6):2187–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luque RM, Lin Q, Cordoba-Chacon J, et al. Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes. PLoS One. 2011;6(1):e15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gahete MD, Córdoba-Chacón J, Luque RM, Kineman RD. The rise in growth hormone during starvation does not serve to maintain glucose levels or lean mass but is required for appropriate adipose tissue response in female mice. Endocrinology. 2013;154(1):263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shalet SM. Partial growth hormone deficiency in adults; should we be looking for it? Clin Endocrinol (Oxf). 2010;73(4):432–435 [DOI] [PubMed] [Google Scholar]
- 17. Alba M, Salvatori R. A mouse with targeted ablation of the growth hormone-releasing hormone gene: a new model of isolated growth hormone deficiency. Endocrinology. 2004;145(9):4134–4143 [DOI] [PubMed] [Google Scholar]
- 18. Yakar S, Setser J, Zhao H, et al. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest. 2004;113(1):96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGinley JN, Knott KK, Thompson HJ. Effect of fixation and epitope retrieval on BrdU indices in mammary carcinomas. J Histochem Cytochem. 2000;48(3):355–362 [DOI] [PubMed] [Google Scholar]
- 20. Cordoba-Chacon J, Gahete MD, Pozo-Salas AI, et al. Peripubertal-onset but not adult-onset obesity increases IGF-I and drives development of lean mass, which may lessen the metabolic impairment in adult obesity. Am J Physiol Endocrinol Metab. 2012;303(9):E1151–E1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahrén J, Ahrén B, Wierup N. Increased β-cell volume in mice fed a high-fat diet: a dynamic study over 12 months. Islets. 2010;2(6):353–356 [DOI] [PubMed] [Google Scholar]
- 23. Robertson K, Kopchick JJ, Liu JL. Growth hormone receptor gene deficiency causes delayed insulin responsiveness in skeletal muscles without affecting compensatory islet cell overgrowth in obese mice. Am J Physiol Endocrinol Metab. 2006;291(3):E491–E498 [DOI] [PubMed] [Google Scholar]
- 24. Wu Y, Liu C, Sun H, et al. Growth hormone receptor regulates β cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J Clin Invest. 2011;121(6):2422–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Busch AK, Cordery D, Denyer GS, Biden TJ. Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic β-cell function. Diabetes. 2002;51(4):977–987 [DOI] [PubMed] [Google Scholar]
- 26. Cohen G, Riahi Y, Shamni O, et al. Role of lipid peroxidation and PPAR-δ in amplifying glucose-stimulated insulin secretion. Diabetes. 2011;60(11):2830–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goffin V, Shiverick KT, Kelly PA, Martial JA. Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr Rev. 1996;17(4):385–410 [DOI] [PubMed] [Google Scholar]
- 28. Brelje TC, Allaire P, Hegre O, Sorenson RL. Effect of prolactin versus growth hormone on islet function and the importance of using homologous mammosomatotropic hormones. Endocrinology. 1989;125(5):2392–2399 [DOI] [PubMed] [Google Scholar]
- 29. Brelje TC, Sorenson RL. Role of prolactin versus growth hormone on islet B-cell proliferation in vitro: implications for pregnancy. Endocrinology. 1991;128(1):45–57 [DOI] [PubMed] [Google Scholar]
- 30. Lloyd RV, Jin L, Chang A, et al. Morphologic effects of hGRH gene expression on the pituitary, liver, and pancreas of MT-hGRH transgenic mice. An in situ hybridization analysis. Am J Pathol. 1992;141(4):895–906 [PMC free article] [PubMed] [Google Scholar]
- 31. Bogazzi F, Raggi F, Russo D, et al. Growth hormone is necessary for the p53-mediated obesity-induced insulin resistance in male C57BL/6JxCBA mice. Endocrinology. 2013;154(11):4226–4236 [DOI] [PubMed] [Google Scholar]
- 32. Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. J Clin Invest. 2006;116(7):1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song J, Xu Y, Hu X, Choi B, Tong Q. Brain expression of Cre recombinase driven by pancreas-specific promoters. Genesis. 2010;48(11):628–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Minami S, Kamegai J, Sugihara H, Suzuki N, Wakabayashi I. Growth hormone inhibits its own secretion by acting on the hypothalamus through its receptors on neuropeptide Y neurons in the arcuate nucleus and somatostatin neurons in the periventricular nucleus. Endocr J. 1998;45(suppl):S19–S26 [DOI] [PubMed] [Google Scholar]
- 35. Rosario W, Wautlet AW, Patterson C, et al. Revealing a functionally-relevant map between the brain and pancreatic islets. 73rd Scientific Sessions of the American Diabetes Association; June 21–25, 2013; Chicago, IL 333-OR [Google Scholar]
- 36. Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147(6):2754–2763 [DOI] [PubMed] [Google Scholar]
- 37. Goren HJ, Kulkarni RN, Kahn CR. Glucose homeostasis and tissue transcript content of insulin signaling intermediates in four inbred strains of mice: C57BL/6, C57BLKS/6, DBA/2, and 129X1. Endocrinology. 2004;145(7):3307–3323 [DOI] [PubMed] [Google Scholar]
- 38. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Menezes Oliveira JL, Marques-Santos C, et al. Lack of evidence of premature atherosclerosis in untreated severe isolated growth hormone (GH) deficiency due to a GH-releasing hormone receptor mutation. J Clin Endocrinol Metab. 2006;91(6):2093–2099 [DOI] [PubMed] [Google Scholar]
- 40. Perotti M, Caumo A, Brunani A, et al. Postprandial triglyceride profile after a standardized oral fat load is altered in growth hormone (GH)-deficient adult patients and is not improved after short-term GH replacement therapy. Clin Endocrinol (Oxf). 2012;77(5):721–727 [DOI] [PubMed] [Google Scholar]
- 41. Twickler TB, Cramer MJ, Dallinga-Thie GM, Chapman MJ, Erkelens DW, Koppeschaar HP. Adult-onset growth hormone deficiency: relation of postprandial dyslipidemia to premature atherosclerosis. J Clin Endocrinol Metab. 2003;88(6):2479–2488 [DOI] [PubMed] [Google Scholar]
- 42. Twickler TB, Wilmink HW, Schreuder PC, et al. Growth hormone (GH) treatment decreases postprandial remnant-like particle cholesterol concentration and improves endothelial function in adult-onset GH deficiency. J Clin Endocrinol Metab. 2000;85(12):4683–4689 [DOI] [PubMed] [Google Scholar]
- 43. Tang T, Abbott MJ, Ahmadian M, Lopes AB, Wang Y, Sul HS. Desnutrin/ATGL activates PPARδ to promote mitochondrial function for insulin secretion in islet β cells. Cell Metab. 2013;18(6):883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gurzov EN, Barthson J, Marhfour I, et al. Pancreatic β-cells activate a JunB/ATF3-dependent survival pathway during inflammation. Oncogene. 2012;31(13):1723–1732 [DOI] [PubMed] [Google Scholar]
- 45. Zmuda EJ, Qi L, Zhu MX, Mirmira RG, Montminy MR, Hai T. The roles of ATF3, an adaptive-response gene, in high-fat-diet-induced diabetes and pancreatic β-cell dysfunction. Mol Endocrinol. 2010;24(7):1423–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salvadó L, Serrano-Marco L, Barroso E, Palomer X, Vázquez-Carrera M. Targeting PPARβ/δ for the treatment of type 2 diabetes mellitus. Expert Opin Ther Targets. 2012;16(2):209–223 [DOI] [PubMed] [Google Scholar]
- 47. Winzell MS, Wulff EM, Olsen GS, Sauerberg P, Gotfredsen CF, Ahren B. Improved insulin sensitivity and islet function after PPARδ activation in diabetic db/db mice. Eur J Pharmacol. 2010;626(2–3):297–305 [DOI] [PubMed] [Google Scholar]
- 48. Yang Y, Tong Y, Gong M, et al. 2013 Activation of PPARβ/δ protects pancreatic β cells from palmitate-induced apoptosis by upregulating the expression of GLP-1 receptor. Cell Signal. 26(2):268–278 [DOI] [PubMed] [Google Scholar]
- 49. Fontés G, Zarrouki B, Hagman DK, et al. Glucolipotoxicity age-dependently impairs β cell function in rats despite a marked increase in β cell mass. Diabetologia. 2010;53(11):2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. β-Cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-β-cell relationships. Proc Natl Acad Sci USA. 1994;91(23):10878–10882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44(8):863–870 [DOI] [PubMed] [Google Scholar]