Abstract
Obesity-induced endoplasmic reticulum (ER) stress causes chronic inflammation in adipose tissue and steatosis in the liver, and eventually leads to insulin resistance and type 2 diabetes (T2D). The goal of this study was to understand the mechanisms by which administration of bilirubin, a powerful antioxidant, reduces hyperglycemia and ameliorates obesity in leptin-receptor-deficient (db/db) and diet-induced obese (DIO) mouse models. db/db or DIO mice were injected with bilirubin or vehicle ip. Blood glucose and body weight were measured. Activation of insulin-signaling pathways, expression of inflammatory cytokines, and ER stress markers were measured in skeletal muscle, adipose tissue, and liver of mice. Bilirubin administration significantly reduced hyperglycemia and increased insulin sensitivity in db/db mice. Bilirubin treatment increased protein kinase B (PKB/Akt) phosphorylation in skeletal muscle and suppressed expression of ER stress markers, including the 78-kDa glucose-regulated protein (GRP78), CCAAT/enhancer-binding protein (C/EBP) homologous protein, X box binding protein (XBP-1), and activating transcription factor 4 in db/db mice. In DIO mice, bilirubin treatment significantly reduced body weight and increased insulin sensitivity. Moreover, bilirubin suppressed macrophage infiltration and proinflammatory cytokine expression, including TNF-α, IL-1β, and monocyte chemoattractant protein-1, in adipose tissue. In liver and adipose tissue of DIO mice, bilirubin ameliorated hepatic steatosis and reduced expression of GRP78 and C/EBP homologous protein. These results demonstrate that bilirubin administration improves hyperglycemia and obesity by increasing insulin sensitivity in both genetically engineered and DIO mice models. Bilirubin or bilirubin-increasing drugs might be useful as an insulin sensitizer for the treatment of obesity-induced insulin resistance and type 2 diabetes based on its profound anti-ER stress and antiinflammatory properties.
Obesity, which has become a global health problem, is a strong risk factor for the development of type 2 diabetes (T2D). Overweight compromises the antioxidant defense system, and low antioxidants can be an early sign of disease-prone conditions (1). For example, low levels of serum bilirubin, a powerful antioxidant in the body, are inversely associated with abdominal obesity, metabolic syndromes, systemic inflammation, diabetes (2, 3), diabetes-related nephropathy (4), amputation (5), cardiovascular disease (6), endothelial dysfunction (7), and low-density lipoprotein oxidation (8, 9), in adults, children, and adolescents.
Obesity also induces an insulin-resistant state in adipose tissue, liver, and muscle tissue and causes resistance to insulin-stimulated glucose transport in these tissues (10). Recent studies demonstrate that endoplasmic reticulum (ER) stress contributes to obesity-induced insulin resistance (11, 12). The ER is a central organelle, where transmembrane and secretory proteins are synthesized, folded, and matured (13). Accumulation of unfolded proteins in the ER lumen leads to the unfolded protein response (UPR), which is necessary to reduce ER stress and retain cellular homeostasis. The UPR is mediated via 3 ER transmembrane proteins that transmit signals from the ER to the cytoplasm or nucleus: inositol-requiring enzyme 1, protein kinase RNA-like ER kinase, and activating transcription factor (ATF)6. Activation of these mechanisms suppresses generation of unfolded proteins, facilitates proper protein folding, and activates ER-associated degradation to reduce UPR accumulation in the ER (14, 15). This process is crucial for cellular adaption to ER stress and, if it fails, leads to cell death.
Unfolded proteins are present in liver and adipose tissue of insulin-resistant rodents, and activation of the ER stress pathways in these tissues leads to accumulation of unfolded protein inside the ER eventually causing cell death in obese mice (16). For example, ER stress manifested by elevated levels of immunoglobulin heavy chain-binding protein (BiP [immunoglobulin-binding protein], or the 78-kDa glucose-regulated protein [GRP78]), phosphorylation of protein kinase RNA-like ER kinase, and phosphorylation of a subunit of eukaryotic translation initiation factor 2 leads to increased chronic inflammation, macrophage infiltration, and insulin resistance in adipose tissue in leptin-deficient (ob/ob) mice (16). ER stress is reduced by chemical chaperones, which alleviate chronic inflammation in adipose tissue, reduce body weight, and improve insulin signaling in T2D (17–19). In the liver, ER stress induces hepatic insulin resistance by increasing de novo lipogenesis and fat accumulation and by directly interfering with insulin signaling (20, 21), which are important mechanisms contributing to insulin resistance in obesity.
Induction of heme oxygenase-1 (HO-1) exerts cytoprotective effects in many stress conditions and disease models. Overexpression of HO-1 sensitizes the insulin-signaling pathway in Zucker diabetic obese rats (22) and ob/ob mice (23–30). Bilirubin is generated during heme degradation by the enzyme HO-1 in vivo (31). High bilirubin serum concentrations are associated with increased total antioxidant capacity and protection against oxidative stress-induced diseases (32, 33). However, whether bilirubin improves insulin resistance and obesity and mitigates ER stress observed in murine models of obesity is still unknown. Therefore, we investigated protective effects and mechanisms of bilirubin administration in alleviating hyperglycemia and obesity in leptin-receptor-deficient (db/db) mice and in diet-induced obese (DIO) mice.
Materials and Methods
Animals
Eight-week-old db/db mice and their wild-type littermates were purchased from The Jackson Laboratory; db/db mice were normal chow, and treatment was started at 10 weeks of age. For induction of obesity, C57BL/6 DIO mice and DIO controls were obtained at 12 weeks of age (The Jackson Laboratory), and mice were continuously fed with high-fat diet from OpenSource Diet (no. 12331), which contains 16.4% protein, 25.5% carbohydrate, and 58% fat and sucrose at 700 kcal. The low-calorie diet was the equivalent of a chow diet control (10% of calories derived from fat, no. D12450B; Research Diets). DIO mice were fed with high-fat diet for another 16 weeks before treatment. DIO mice were single housed for the study. All studies were performed in male mice only. The Animal Care Committee at the Medical University of South Carolina approved all animal experiments.
HO-1 induction and bilirubin administration
HO-1 expression was induced by the pharmacological agent, cobalt protoporphyrin (CoPP) (10 or 20 mg/kg, ip every 48 h for 14 d). The selective inhibitor of HO-1 enzymatic activity, zinc protoporphyrin (ZnPP) (20 mg/kg) was used to block the action of HO-1. Bilirubin (Frontier Scientific) was administered to recipients at 20 μmol/kg (11.7 mg/kg, ip) twice per day. CoPP, ZnPP, and bilirubin were prepared as described previously (34, 35).
Mouse monitoring after treatment
Nonfasting serum blood glucose levels and body weights of mice were measured daily at 9 am. A tiny drop of whole-blood samples (∼5–10 μL) were collected from a minimal tail incision from the lateral tail vein at the very end of the mouse's tail and analyzed using a ACCU-CHEK glucometer (Roche). Physical activities of mice were observed on a daily basis once treatment started. At least 2 investigators observed the mice for at least 20 minutes each time. During the observation, mouse behavior, food intake, drinking, and licking were closely observed. Signs of illness-ruffled fur, hunched posture, lethargy, and dehydration were also noted. Food intake (including possible spillage) was measured during a 24-hour period at 7 days after treatment.
Insulin tolerance test (ITT)
Mice were fasted for 5 hours, weighed, and blood was collected from the tail vein to evaluate fasting blood glucose levels. Mice were then given an ip injection of regular human insulin at 0.75 U/kg body weight (Humulin, Eli Lily). Serum blood glucose levels were measured at 15, 30, 60, 90, and 120 minutes after insulin injection using the ACCU-CHEK glucometer (LifeScan).
Tissue harvesting
At the end of each experiment, mice were weighed and injected with saline or insulin (10 U/kg, ip). The gastrocnemius, liver, and epididymal fat tissue were collected 6 minutes after insulin injection, snap frozen in liquid nitrogen, and processed for Western blot or immunohistochemical analyses as described (36).
Real-time RT-PCR analysis
Expression of TNF-α, IL-1β, monocyte chemoattractant protein-1 (MCP-1), BiP, C/EBP homologous protein (CHOP), and AFT4 mRNA were analyzed by real-time RT-PCR as described previously (34). β-Actin expression was quantified in each sample and used as an endogenous control. 6-Carboxy-fluorescein-labeled real-time RT-PCR primers were purchased from Life Technologies (Life Technologies).
Western blot analysis
The plasma membrane was separated from whole-cell lysates using Plasma Membrane Protein Extraction kit (Biovision Technologies). Whole-cell lysate or membrane fractions of cells were quantified using the bicinchoninic acid methods, and samples of equal amount were separated in a 4%–12% BT NuPAGE gel (Life Technologies). Proteins were transferred to a Hybond-P membrane (GE Healthcare) and blocked with 5% nonfat milk, followed by incubation with the following primary antibodies: phospho-Thr308 Akt (PKB/Akt, protein kinase), phospho-Ser473 Akt (Cell Signaling Technology), Akt (Santa Cruz Biotechnology, Inc), and Glut4 (glucose transporter type 4) (Abcam) overnight at 4°C. Blots were then probed with horseradish peroxidase-labeled secondary antibodies (Cell Signal) and visualized by an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech). The intensity of each signal was determined using ImageJ software (National Institutes of Health). For analysis performed in db/db and DIO mice, please see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Immunohistochemistry staining
Mouse livers or epididymal fat were snap frozen in liquid nitrogen. Liver tissue sections were immersed in filtered hematoxylin, rinsed with water, and stained with eosin for another 1–2 minutes. Sections were rinsed with water and dehydrated in ascending alcohol solutions, cleared with xylene, and mounted with cover a slip onto a labeled glass slide with Permount. Macrophage infiltration in epididymal fat tissues was detected with the rat antimouse monoclonal anti-F4/80 antibody (Abcam).
Statistical analysis
Data are expressed as mean ± SEM. Differences between groups were compared for statistical significance by ANOVA or Student's t test; P < .05 denoted significance.
Results
Systemic induction of HO-1 leads to a rapid reduction of hyperglycemia and increased insulin sensitivity in db/db mice
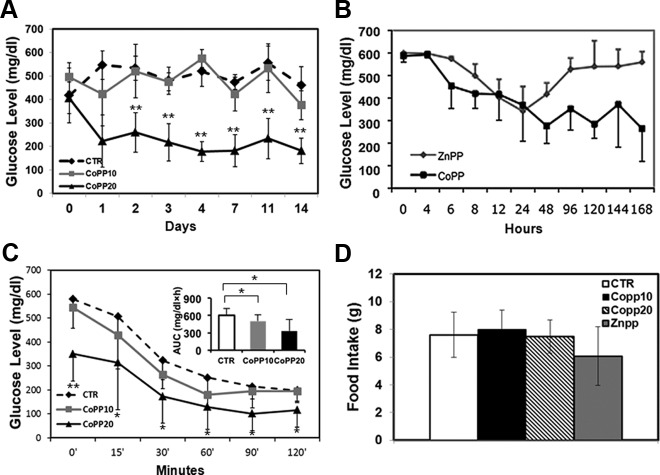
We first evaluated whether systemic induction of HO-1 by CoPP injection would influence hyperglycemia in db/db mice. Treatment with CoPP at 10 mg/kg slightly reduced blood glucose levels in db/db mice from an initial average blood glucose level of more than 500 to 375 ± 61 mg/dL at the end of the experiment (d 14). At a higher dose of CoPP (20 mg/kg), blood glucose levels were reduced to 181 ± 54 mg/dL at 14 days, indicating that systemic induction of HO-1, in part, promotes glucose metabolism in mice with hyperglycemia (Figure 1A). HO-1 induction by CoPP in lean control mice did not alter their blood glucose levels (average, 120 ± 23 mg/dL; data not shown), suggesting that HO-1 only lowers blood glucose levels under hyperglycemic conditions, and its overexpression does not result in a state of hypoglycemia.
Figure 1.
Systemic induction of HO-1 by CoPP injection reduces hyperglycemia and increases insulin sensitivity in db/db mice. A, HO-1 induction by CoPP at 20 mg/kg (defined as CoPP20) reduced blood glucose levels in db/db mice compared with mice that received the vehicle (defined as CTR). B, Blocking HO-1 activity by ZnPP (20 mg/kg) diminished the effect of CoPP (20 mg/kg) in lowering hyperglycemia. C, Treatment with CoPP increased insulin sensitivity as demonstrated by blood glucose levels measured by ITT and the areas under the curve (insets, mg/dL · h). D, No differences in the amount of food consumed within a 24-hour period of time were observed in db/db mice that received CoPP, ZnPP, or vehicle (defined as CTR). At least 8 mice were included in each treatment group. Experiments have been repeated independently at least 3 times; **, P < .01 and *, P < .05 as analyzed by ANOVA and Student's t test.
To confirm that the reduction in blood glucose levels after CoPP injection was indeed due to up-regulation of HO-1 in the db/db mice, HO-1 activity was blocked by ZnPP in a group of mice shortly after HO-1 induction. As evident in Figure 1B, HO-1 induction led to a rapid reduction in blood glucose levels in CoPP-treated mice, whereas blood glucose levels in mice in which HO-1 was blocked by ZnPP remained elevated, indicating that HO-1 activity contributes to the reduction of blood glucose levels in db/db mice.
We performed an ITT test in db/db mice 12 days after CoPP treatment. Mice that received CoPP had reduced serum glucose levels (Figure 1C) as well as reduced areas under the curve (Figure 1C, insets) at most time points after ip insulin injection, indicating efficient insulin-mediated glucose disposal, which suggests that HO-1 reduces hyperglycemia in db/db mice, in part, via promoting insulin sensitivity. To exclude the possibility that the reduced glucose levels in CoPP-treated mice were due to decreased appetite, we measured food intake during a 24-hour period 7 days after treatment. There was no significant difference in food intake between mice that received CoPP and those that received vehicle (Figure 1D). Notably, the body weights of mice that received CoPP were not significantly different from those that received vehicle over the 14-day experimental period (data not shown).
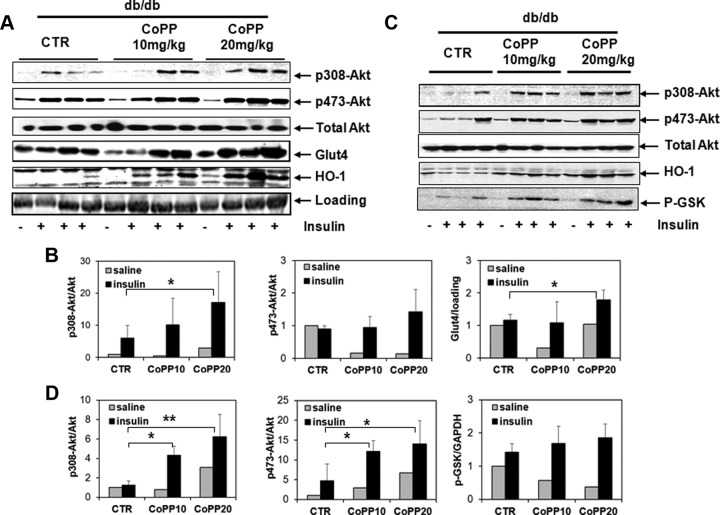
HO-1 induction activates insulin-signaling pathways in skeletal muscle and the liver
Activation of molecules involved in the insulin-signaling pathway was assessed in skeletal muscle and liver of db/db mice treated with or without CoPP. Compared with db/db mice that received vehicle, at 20-mg/kg, CoPP led to a significant increase in phosphorylation of Akt 308 in skeletal muscle, indicating that systemic induction of HO-1 activates the insulin-signaling pathway (Figure 2, A and B). Akt 473 phosphorylation in muscle was not different between control and CoPP-treated mice, although levels were increased only in response to insulin in all groups (Figure 2, A and B).
Figure 2.
Detection of molecules involved in insulin signaling in the skeletal muscles and livers of db/db mice. A, Expression of p308–p473- and total Akt and HO-1, and membrane presence of Glut4 were detected in skeletal muscle from mice treated with saline or insulin by Western blotting. CTR refers to db/db mice that received vehicle only. B, Ratios of phosphorylated Akt in total Akt, expression of Glut4 in each sample relative to the loading control were quantified in muscles. C, Expression of p308-, p473-, total Akt, HO-1, and p-GSK in liver tissues. D, Ratios of phosphorylated Akt in total Akt, phosphorylated GSK (p-GSK)/GAPDH were quantified in livers from CTR, CoPP10, and CoPP20 groups; *, P < .05, saline vs insulin.
We further evaluated whether HO-1 induction enhanced membrane transport of Glut4 in skeletal muscle. db/db control mice showed impaired membrane transport of Glut4 in skeletal muscle, in that the membrane Glut4 level remained largely unchanged after insulin stimulation. In contrast, we observed a significant increase in membrane expression of Glut4 in CoPP-treated mice in response to insulin challenge, especially in mice that received 20-mg/kg CoPP (Figure 2, A and C), indicating that induction of HO-1 promotes membrane transport of Glut4, which at least partly accounts for the enhanced glucose uptake in skeletal muscle.
To ascertain whether overexpression of HO-1 promotes insulin action in liver, phosphorylation of Akt at Thr308 and Ser473 sites was measured in liver homogenate from db/db mice that received vehicle or CoPP. CoPP increased phosphorylation of Akt at both phosphorylation sites to an extent comparable with that observed in wild-type C57BL/6 mice (Figure 2, C and D). In addition, phosphorylation of glycogen synthase kinase (GSK)3, which blocks the ability of GSK3 to inhibit glycogen synthesis, was also increased in liver from db/db mice treated with CoPP after insulin stimulation (Figure 2, C and D). Thus, HO-1 induction in the liver promotes glycogen synthesis by increasing phosphorylation of GSK3, which may eventually contribute to reduced hyperglycemia in db/db mice.
Bilirubin mimics the protective effects of HO-1 in db/db mice
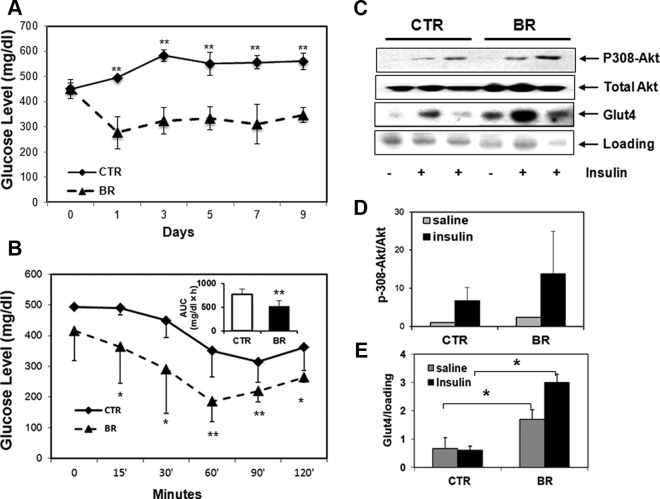
We determined whether the protective effects of HO-1 in the db/db mouse model could be mimicked by administering exogenous bilirubin. Based on previous studies (35), bilirubin at 20 μmol/kg was administered twice per day for 7 days. This treatment significantly reduced hyperglycemia in db/db mice (Figure 3A). db/db mice that received bilirubin treatment showed enhanced insulin sensitivity compared with mice that received vehicle (Figure 3B). Sensitivity was comparable with wild-type controls (data not shown). Therefore, bilirubin appears to mimic the protective effect of HO-1 in reversing hyperglycemia and increasing insulin sensitivity in db/db mice. To assess activation of insulin signaling, we measured phosphorylation of Thr308 Akt in bilirubin-treated mice, and bilirubin-treated mice has more phosphorylated-Akt (p-Akt)/total Akt compared with control (Figure 3, C and D), indicating enhanced activation of insulin signaling in those mice. We also observed significantly greater Glut4 expression on muscle cell membrane from bilirubin-treated mice compared with controls (Figure 3, C and E). These data provide evidence that bilirubin reduces hyperglycemia in part by increasing insulin sensitivity in db/db mice. Similar to HO-1 induction, bilirubin treatment did not lead to a significant change of body weights (data not shown).
Figure 3.
Administration of bilirubin reduces the blood glucose level and increases insulin sensitivity in db/db mice. A, Treatment of db/db mice with bilirubin (BR) significantly reduced blood glucose levels in db/db mice compared with those that received vehicle (defined as CTR). B, Mice that received BR showed significantly lower blood glucose levels at most time points and smaller area under the curve (AUC) compared with control after ITT test. C, Expression of p308 and total Akt, and membrane expression of Glut4 were observed in muscles of mice that received BR compared with controls after insulin stimulation. D, Quantification of p-308Akt in total Akt; and E, relative expression of membrane Glut4 in muscles from db/db mice treated with or without BR. At least 8 mice were included in each treatment group; *, P < .05 and **, P < .01, ANOVA or Student's t test.
ER stress manifested by activation of the UPR-signaling pathway in adipocytes has been reported to be one of the major contributors to insulin resistance in obesity. To test the hypothesis that bilirubin enhances insulin sensitivity via modulating ER stress in db/db mice, we measured expression of ER stress markers, including BiP, CHOP, XBP-1 (X box binding protein), and ATF4, at mRNA level in epididymal fat and liver from db/db mice treated with or without bilirubin. Expression of BiP, CHOP, XBP-1, and AFT4 was significantly reduced in the adipose of db/db mice receiving bilirubin therapy compared mice receiving vehicle (Figure 4A). No significant difference of gene expressions was observed in liver samples from both groups (Figure 4B). These results indicate that bilirubin functions, in part, by regulating ER stress proteins in adipose tissue.
Figure 4.
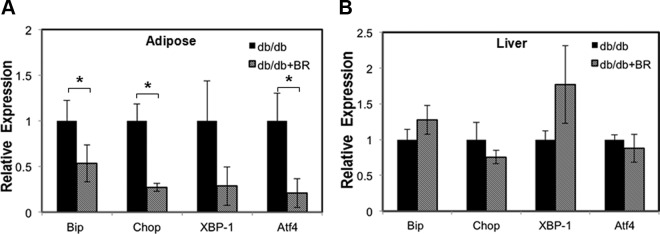
Bilirubin treatment suppresses expression of ER stress markers in db/db mice. A, Relative expression of BiP, Chop, XBP-1, and ATF4 mRNA in muscles of db/db mice treated with vehicle (db/db) or bilirubin (db/db+BR). Relative expression of BiP, Chop, XBP-1, and ATF4 mRNA in livers of db/db mice treated with vehicle (db/db) or bilirubin (db/db+BR). *, P < .05 vehicle vs bilirubin.
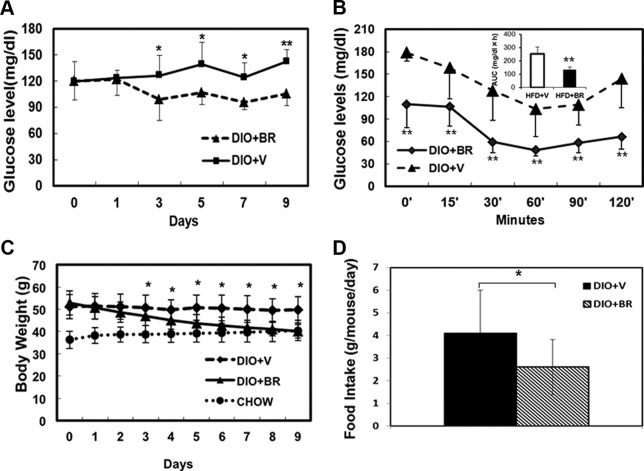
Bilirubin reduces body weight and improves insulin sensitivity in DIO mice
To further assess the effect of bilirubin in improving DIO and insulin resistance, we injected DIO mice with bilirubin. The average fasting blood glucose levels of DIO mice at 28 weeks was 126 ± 27 mg/dL. DIO mice that received bilirubin for 12 days showed significantly reduced blood glucose levels of 107 ± 11 mg/dL compared with 131 ± 14 mg/dL in vehicle-treated controls (P = .001, n = 8 in each group) (Figure 5A). DIO mice treated with bilirubin were significantly more sensitive to insulin stimulation (Figure 5B). The body weights of DIO mice reached an average of 51.26 ± 5.47 g at 28 weeks, which was significantly higher than mice fed normal chow (average, 36.24 ± 3.88 g) (P < .01). The body weights of DIO mice treated with bilirubin was significantly decreased compared with vehicle-treated controls and reduced to a similar level as mice fed normal chow after only 5 days of bilirubin administration. Bilirubin administration continued for the duration of the experiment, with body weights plateauing at around 40 g (Figure 5C). At day 5 after treatment, DIO mice receiving vehicle consumed an average of 4.1 ± 1.8 g of food per day, whereas mice receiving bilirubin ate significantly less than 2.8 ± 2.0 g of food per day (Figure 5D). Unlike mice that received vehicle, bilirubin-treated DIO mice behaved normally, with dry bedding and reduced aggressiveness.
Figure 5.
Bilirubin (BR) treatment reduces blood glucose levels and obesity and increases insulin sensitivity in DIO mice. A, Changes in blood glucose levels in DIO mice treated with BR (DIO+BR) compared with control mice treated with vehicle (DIO+V); *, P < .05 and **, P < .01, BR vs vehicle. B, BR treatment increases insulin sensitivity in DIO mice. Data shown are blood glucose levels at different time points as measured by ITT; inset, areas under the curves. C, Changes in body weights of DIO mice. The body weights of mice were measured daily after BR treatment; *, P < .05, BR vs vehicle. D, Average food intake per mouse per 24-hour period in DIO mice treated with BR or vehicle. At least 8 mice were included in each treatment group.
Bilirubin improves insulin signaling in DIO mice
Phosphorylation of Thr308 Akt in response to insulin stimulation was measured in skeletal muscle and liver of DIO mice treated with or without bilirubin and normal chow controls. In DIO mice that received vehicle, phosphorylation of Akt 308 was reduced after insulin stimulation comparable with chow controls (Figure 6, A and B). In contrast, there was increase in phosphorylation of Akt Thr308 in both skeletal muscle (Figure 6, A and B) and liver in bilirubin-treated DIO mice after insulin stimulation (Figure 6, C and D), indicating that DIO mice treated with bilirubin remained sensitive to insulin despite the high-fat diet.
Figure 6.
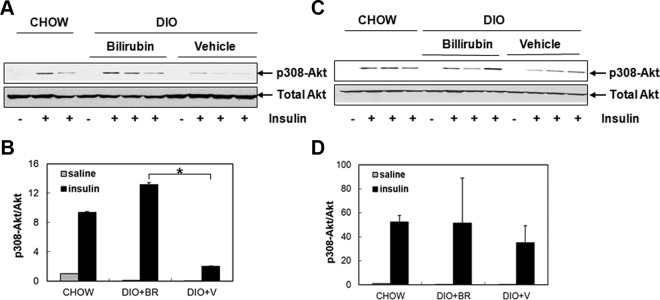
Bilirubin treatment promotes Akt phosphorylation in DIO mice. A, Phosphorylation of Akt at Thr308 was determined in muscle tissues from DIO mice treated with bilirubin, vehicle, or in chow controls 6 minutes after stimulation with either saline or insulin. B, Quantification of p-308Akt in total Akt. C, Expression of p308 Akt was determined in livers from DIO mice treated with bilirubin, vehicle, or in chow controls after stimulation with either saline or insulin. D, Quantification of p-308Akt in total Akt in liver. BR, bilirubin; V, vehicle.
Bilirubin treatment reduces inflammation and ER stress in adipose tissue of DIO mice
Expression of ER stress markers was compared in DIO mice fed with high-fat diet in the presence or absence of bilirubin to those fed normal chow. Expression of BiP, CHOP, XBP-1, and AFT4 were significantly reduced in DIO mice receiving bilirubin therapy compared with DIO mice receiving vehicle (Figure 7A). These results indicate that bilirubin functions, in part, to reduce body weight by regulating ER stress proteins induced in obese mice.
Figure 7.
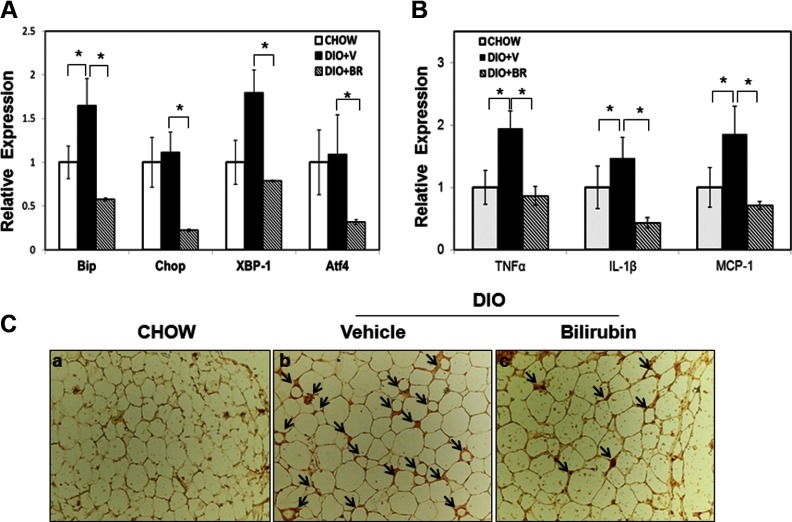
Bilirubin (BR) treatment suppresses expression of ER stress markers and proinflammatory cytokines, and inhibits macrophage infiltration in adipose tissue of DIO mice. A, Relative expression of BiP, Chop, XBP-1, and ATF4 mRNA in DIO mice treated with vehicle (DIO+V) or BR (DIO+BR) and chow control (chow). B, Relative expression of TNF-α, IL-1β, and MCP-1 in mice fed with normal chow (chow), DIO treated with vehicle (DIO+V), and DIO treated with BR (DIO+BR). C, Immunohistochemical staining of epididymal tissues from BR-treated DIO mice showed significantly less macrophage infiltration compared with mice that received vehicle. Arrows point to macrophages (F4/80+ cells). *, P < .05, Student's t test.
We next assessed the relationship between obesity-induced ER stress and chronic inflammation by measuring expression levels of proinflammatory cytokines in epididymal fat. We found that expression of TNF-α, IL-1β, and MCP-1 at mRNA level was significantly decreased in tissues harvested from bilirubin-treated mice compared with tissues from vehicle-treated DIO mice or mice fed normal chow (Figure 7B). Moreover, immunohistochemical staining confirmed that there were significantly fewer F4/80+ macrophages infiltrating adipose tissue of mice that received bilirubin compared with controls (Figure 7C). Collectively, these data suggest that bilirubin treatment functions to decrease body weight and increase insulin sensitivity, in part, by suppressing inflammation and macrophage infiltration and reducing ER stress in adipose tissue.
Bilirubin treatment mitigates steatosis and ER stress in the liver
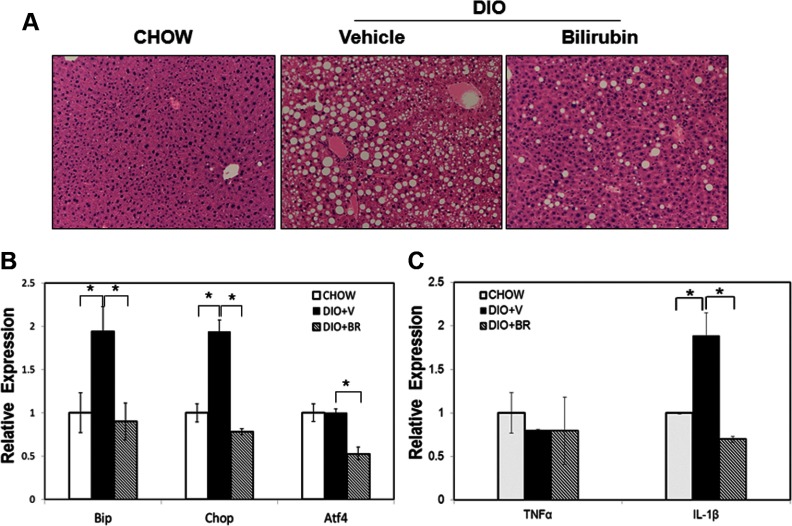
To determine whether bilirubin reduces hepatic steatosis and ER stress in the liver in our model, we examined hematoxylin and eosin-stained liver sections from DIO mice treated with or without bilirubin and from chow controls. Vehicle-treated DIO mice showed massive macrovesicular hepatic steatosis compared with chow controls, which had very few visible fat-containing cells. In striking contrast, bilirubin therapy significantly reduced the number of fat cells within the liver (Figure 8A). We also measured expression of BiP and Chop mRNA and found that they were significantly up-regulated in vehicle-treated DIO mice but were greatly reduced in bilirubin-treated DIO mice. In addition, expression levels of ATF4 were reduced in bilirubin-treated DIO mice as compared with vehicle-treated DIO mice and chow controls (Figure 8B). Expression of IL-1β was induced in adipose tissue from vehicle-treated DIO mice but was inhibited in bilirubin-treated DIO mice. In contrast to adipose tissue, there was little change in expression levels of TNF-α among DIO mice and chow controls (Figure 8C). Taken together, these data indicate that bilirubin treatment reduces liver steatosis, in part, by suppressing ER stress and proinflammatory cytokine expression.
Figure 8.
Bilirubin (BR) ameliorates hepatic steatosis by suppressing ER stress in the liver. A, H&E staining of liver sections from mice fed normal chow (chow), DIO mice treated with vehicle (V), or DIO mice treated with BR (BR). B, Relative expression levels of BiP, Chop, and ATF4 mRNA in liver from mice fed normal chow (chow), DIO mice treated with V (DIO+V), or DIO mice treated with BR (DIO+BR). C, Expression of TNF-α and IL-1β in mice fed normal chow (chow), DIO mice treated with V (DIO+V), or DIO mice treated with BR (DIO+BR). *, P < .05, Student's t test.
Discussion
The present study identifies a novel function for bilirubin as an insulin sensitizer based on its role in reducing hyperglycemia, obesity, and insulin resistance. The results demonstrate that bilirubin mimics the protective effects of HO-1 induction by reducing hyperglycemia and improving insulin sensitivity in the db/db mouse model and reveal that bilirubin can influence obesity and insulin sensitivity in high-fat diet-induced obese mice. Bilirubin improves insulin sensitivity at least in part by suppressing ER stress and chronic inflammation in adipose tissue and liver of obese mice.
The protective effects of HO-1 have been extensively studied in experimental models of T2D, including ob/ob mice and diabetic rats. In these studies, chronic HO-1 induction improved insulin sensitivity and glucose tolerance based on its profound antiinflammatory effects and regulation of adipogenesis (29, 37–40). In our study, HO-1 activation directly regulates insulin signaling in insulin-targeting tissues in db/db mice as evidenced by changes in Akt, a marker used for insulin resistance (41). The kinase responsible for phosphorylation of Thr308 is the phosphoinositide 3 kinase (PI3K)-dependent kinase-1, whereas phosphorylation of Ser473 has been suggested to be regulated by PKB/Akt autophosphorylation in a PI3K-dependent kinase-1-dependent manner (42). We anticipated that both PI3K and PKB/Akt pathways were potentially activated by HO-1 induction. This activation resulted in an increase in Glut4 in skeletal muscle as well as enhanced glucose uptake.
Although induction of HO-1 has beneficial effects in reversing the progress of diabetes, individuals vary in their ability to respond to stress by up-regulating HO-1. A microsatellite polymorphism in the HO-1 promoter is associated with variation (43) and hinders the potential application of HO-1 as a therapeutic modality in this model. Bilirubin, one of the products of HO-1 enzymatic activity and a strong antioxidant and major component in Chinese traditional medicine, has been shown to substitute for the protective effects of HO-1 in many disease models (35, 44–47). Subjects who have life-long hyperbilirubinemia, known as Gilbert's syndrome, exhibit one-third the cardiovascular mortality of those with normal bilirubin levels (48, 49). Experimental hyperbilirubinemia induced by Atazanavir, an HIV-related medication, ameliorates oxidative stress and vascular inflammation and improves endothelial function in T2D patients (7). Our data provide mechanistic insight into how bilirubin increases sensitization resulting in direct effect on obesity.
ER stress-induced lipogenesis leads to insulin resistance in the liver (14, 50–52). Inflammation contributes to ER stress in adipose tissue, including release of proinflammatory cytokines and mediators, such as TNF-α, IL-1β, and generation of reactive oxygen species. These in turn contribute to ER stress and insulin resistance that can be inhibited in the presence of bilirubin (53, 54). The suppression of inflammation by bilirubin corresponds with reduced macrophage infiltration into adipose tissue and suppression of ER stress. In the liver, ER stress contributes to hepatic steatosis caused in part by a reduction in lipogenesis and insulin resistance (20, 21, 55). We observed that large fat-containing cells in the liver of DIO mice were significantly reduced in response to bilirubin. Our observations in adipose tissue thus translate to the liver, where bilirubin improves insulin sensitivity by decreasing ER stress and lipogenesis. Unlike adipose tissue, however, we did not observe increased expression of TNF or MCP-1 in the livers of DIO mice, even though expression levels in bilirubin-treated mice were significantly lower than in mice that received vehicle. Therefore, our study suggests that a reduction in hepatic ER stress mediated by bilirubin was secondary to a decrease in lipogenesis in the liver, as reported elsewhere, and speaks to the remarkable specificity of bilirubin as a cytoprotective molecule (20). The precise role of bilirubin in suppressing ER stress is undergoing further investigation.
In both db/db and DIO mouse models, bilirubin improved glycemic control and increased insulin sensitivity. One notable difference between the DIO and the db/db models is that bilirubin treatment reduced body weights in the DIO mice but not in db/db mice. One interpretation is that bilirubin influences the regulation of the leptin/leptin receptor pathway to control appetite and food intake (Liu, J., X. Dong, H. Dong, and H. Wang, unpublished data), which in the db/db mice was not observed due to lack of the leptin receptor. The lower blood glucose levels that we observed were intriguing and may be explained by the fact that mice fed a high-fat diet in our facility showed increased aggressiveness and had to be kept in individual cages and, therefore, required greater energy expenditure to maintain normal homeostasis, eg, body temperature compared with those housed together.
Collectively, we have identified a critical mechanism by which bilirubin exerts salutary effects in mouse models of obesity and insulin resistance. Given the nontoxic nature of bilirubin, other than during the immediate postnatal period, bilirubin and drugs that elevate HO-1 may serve as potential therapeutic approaches to increase insulin sensitivity and glucose uptake. Bilirubin possesses many advantages compared with other agents currently used to treat T2D due to its strong and unique antioxidant, antiinflammatory, and antiapoptotic effects. The anti-ER stress properties reported here suggest that bilirubin may also be developed as an agent to increase the sensitivity of the cell to insulin resulting in reduced body weight, T2D, and other metabolic diseases (7, 56).
Acknowledgments
We thank Xinyu Zhang for technical assistance.
This work was supported in part by the South Carolina Clinical and Translational Research Institute, the NIH Grant UL1RR029882, and the Juvenile Diabetes Research Foundation Grant 5–2012-149 (to H.Wa. and D.B.A.), the NIH grants EB015744 and DK097544 (to H.Wa.) and by the American Diabetes Association Grant 7–12-BS–94) and the National Institutes of Health (1R01DK083567) (to Y.-B.K.)
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATF
- activating transcription factor
- Bip
- immunoglobulin-binding protein
- CHOP
- C/EBP homologous protein
- CoPP
- cobalt protoporphyrin
- DIO
- diet-induced obese
- ER
- endoplasmic reticulum
- Glut4
- glucose transporter type 4
- GSK
- glycogen synthase kinase
- HO-1
- heme oxygenase-1
- ITT
- insulin tolerance test
- MCP-1
- monocyte chemoattractant protein-1
- PKB/Akt
- protein kinase
- PI3K
- phosphoinositide 3 kinase
- T2D
- type 2 diabetes
- UPR
- unfolded protein response
- XBP-1
- X box binding protein
- ZnPP
- zinc protoporphyrin.
References
- 1. Jenko-Pražnikar Z, Petelin A, Jurdana M, Žiberna L. Serum bilirubin levels are lower in overweight asymptomatic middle-aged adults: an early indicator of metabolic syndrome? Metabolism. 2013;62(7):976–985 [DOI] [PubMed] [Google Scholar]
- 2. Ohnaka K, Kono S, Inoguchi T, et al. Inverse associations of serum bilirubin with high sensitivity C-reactive protein, glycated hemoglobin, and prevalence of type 2 diabetes in middle-aged and elderly Japanese men and women. Diabetes Res Clin Pract. 2010;88(1):103–110 [DOI] [PubMed] [Google Scholar]
- 3. Fukui M, Tanaka M, Shiraishi E, et al. Relationship between serum bilirubin and albuminuria in patients with type 2 diabetes. Kidney Int. 2008;74(9):1197–1201 [DOI] [PubMed] [Google Scholar]
- 4. Ikeda N, Inoguchi T, Sonoda N, et al. Biliverdin protects against the deterioration of glucose tolerance in db/db mice. Diabetologia. 2011;54(8):2183–2191 [DOI] [PubMed] [Google Scholar]
- 5. Chan KH, O'Connell RL, Sullivan DR, et al. Plasma total bilirubin levels predict amputation events in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2013;56(4):724–736 [DOI] [PubMed] [Google Scholar]
- 6. Fukui M, Tanaka M, Yamazaki M, et al. Low serum bilirubin concentration in haemodialysis patients with type 2 diabetes. Diabet Med. 2011;28(1):96–99 [DOI] [PubMed] [Google Scholar]
- 7. Dekker D, Dorresteijn MJ, Pijnenburg M, et al. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2011;31(2):458–463 [DOI] [PubMed] [Google Scholar]
- 8. Boon AC, Hawkins CL, Bisht K, et al. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic Biol Med. 2012;52(10):2120–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshino S, Hamasaki S, Ishida S, et al. Characterization of the effect of serum bilirubin concentrations on coronary endothelial function via measurement of high-sensitivity C-reactive protein and high-density lipoprotein cholesterol. Heart Vessels. 2013;28(2):157–165 [DOI] [PubMed] [Google Scholar]
- 10. Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest. 2006;116(7):1767–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCurdy CE, Schenk S, Holliday MJ, et al. Attenuated Pik3r1 expression prevents insulin resistance and adipose tissue macrophage accumulation in diet-induced obese mice. Diabetes. 2012;61(10):2495–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Roos B, Rungapamestry V, Ross K, et al. Attenuation of inflammation and cellular stress-related pathways maintains insulin sensitivity in obese type I interleukin-1 receptor knockout mice on a high-fat diet. Proteomics. 2009;9(12):3244–3256 [DOI] [PubMed] [Google Scholar]
- 13. Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54(4):795–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao SS, Kaufman RJ. Targeting endoplasmic reticulum stress in metabolic disease. Expert Opin Ther Targets. 2013;17(4):437–448 [DOI] [PubMed] [Google Scholar]
- 16. Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461 [DOI] [PubMed] [Google Scholar]
- 17. Ozawa K, Miyazaki M, Matsuhisa M, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54(3):657–663 [DOI] [PubMed] [Google Scholar]
- 18. Nakatani Y, Kaneto H, Kawamori D, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280(1):847–851 [DOI] [PubMed] [Google Scholar]
- 19. Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan SM, Sun RQ, Zeng XY, et al. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased ER stress. Diabetes. 2013;62(6):2095–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kammoun HL, Chabanon H, Hainault I, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119(5):1201–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ndisang JF, Lane N, Jadhav A. The heme oxygenase system abates hyperglycaemia in Zucker diabetic fatty rats by potentiating insulin-sensitizing pathways. Endocrinology. 2009;150(5):2098–2108 [DOI] [PubMed] [Google Scholar]
- 23. Chen YH, Lin SJ, Lin MW, et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet. 2002;111(1):1–8 [DOI] [PubMed] [Google Scholar]
- 24. Brucmandale CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52(9):2338–2345 [DOI] [PubMed] [Google Scholar]
- 25. Arredondo M, Jorquera D, Carrasco E, Albala C, Hertrampf E. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with iron status in persons with type 2 diabetes mellitus. Am J Clin Nutr. 2007;86(5):1347–1353 [DOI] [PubMed] [Google Scholar]
- 26. Shakeri-Manesch S, Zeyda M, Huber J, Ludvik B, Prager G, Stulnig TM. Diminished upregulation of visceral adipose heme oxygenase-1 correlates with waist-to-hip ratio and insulin resistance. Int J Obes (Lond). 2009;33(11):1257–1264 [DOI] [PubMed] [Google Scholar]
- 27. Kim DH, Burgess AP, Li M, et al. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines tumor necrosis factor-α and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther. 2008;325(3):833–840 [DOI] [PubMed] [Google Scholar]
- 28. Li M, Peterson S, Husney D, et al. Interdiction of the diabetic state in NOD mice by sustained induction of heme oxygenase: possible role of carbon monoxide and bilirubin. Antioxid Redox Signal. 2007;9(7):855–863 [DOI] [PubMed] [Google Scholar]
- 29. Nicolai A, Li M, Kim DH, et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53(3):508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterson SJ, Frishman WH, Abraham NG. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev. 2009;17(3):99–111 [DOI] [PubMed] [Google Scholar]
- 31. Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24(8):449–455 [DOI] [PubMed] [Google Scholar]
- 32. Vitek L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol. 2012;3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci USA. 1988;85(24):9748–9752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H, Lee SS, Gao W, et al. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes. 2005;54(5):1400–1406 [DOI] [PubMed] [Google Scholar]
- 35. Wang H, Lee SS, Dell'Agnello C, et al. Bilirubin can induce tolerance to islet allografts. Endocrinology. 2006;147(2):762–768 [DOI] [PubMed] [Google Scholar]
- 36. Lee DH, Shi J, Jeoung NH, et al. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem. 2009;284(18):11776–11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li M, Kim DH, Tsenovoy PL, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57(6):1526–1535 [DOI] [PubMed] [Google Scholar]
- 38. Peterson SJ, Drummond G, Kim DH, et al. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49(8):1658–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ndisang JF, Jadhav A. Up-regulating the hemeoxygenase system enhances insulin sensitivity and improves glucose metabolism in insulin-resistant diabetes in Goto-Kakizaki rats. Endocrinology. 2009;150(6):2627–2636 [DOI] [PubMed] [Google Scholar]
- 40. Ndisang JF, Lane N, Syed N, Jadhav A. Up-regulating the heme oxygenase system with hemin improves insulin sensitivity and glucose metabolism in adult spontaneously hypertensive rats. Endocrinology. 2010;151(2):549–560 [DOI] [PubMed] [Google Scholar]
- 41. Dongiovanni P, Ruscica M, Rametta R, et al. Dietary iron overload induces visceral adipose tissue insulin resistance. Am J Pathol. 2013;182(6):2254–2263 [DOI] [PubMed] [Google Scholar]
- 42. Persad S, Attwell S, Gray V, et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276(29):27462–27469 [DOI] [PubMed] [Google Scholar]
- 43. Exner M, Schillinger M, Minar E, et al. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther. 2001;8(5):433–440 [DOI] [PubMed] [Google Scholar]
- 44. Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290(3):F563–F571 [DOI] [PubMed] [Google Scholar]
- 45. Sarady-Andrews JK, Liu F, Gallo D, et al. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L1131–L1137 [DOI] [PubMed] [Google Scholar]
- 46. Slebos DJ, Ryter SW, Choi AM. Heme oxygenase-1 and carbon monoxide in pulmonary medicine. Respir Res. 2003;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ollinger R, Wang H, Yamashita K, et al. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid Redox Signal. 2007;9(12):2175–2185 [DOI] [PubMed] [Google Scholar]
- 48. Lin JP, O'Donnell CJ, Schwaiger JP, et al. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation. 2006;114(14):1476–1481 [DOI] [PubMed] [Google Scholar]
- 49. Vítek L, Jirsa M, Brodanová M, et al. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis. 2002;160(2):449–456 [DOI] [PubMed] [Google Scholar]
- 50. Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012;81:767–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118(10):3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev. 2008;29(3):317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501 [DOI] [PubMed] [Google Scholar]
- 54. Kasap B, Soylu A, Ertoy Baydar D, Kiray M, Tugyan K, Kavukcu S. Protective effects of bilirubin in an experimental rat model of pyelonephritis. Urology. 2012;80(6):1389.e17–e22 [DOI] [PubMed] [Google Scholar]
- 55. Ren LP, Chan SM, Zeng XY, et al. Differing endoplasmic reticulum stress response to excess lipogenesis versus lipid oversupply in relation to hepatic steatosis and insulin resistance. PLoS One. 2012;7(2):e30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Geraldes P, Yagi K, Ohshiro Y, et al. Selective regulation of heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. J Biol Chem. 2008;283(49):34327–34336 [DOI] [PMC free article] [PubMed] [Google Scholar]