Abstract
The immortal C2C12 cell line originates from dystrophic mouse thigh muscle and has been used to study the endocrine control of muscle cell growth, development, and function, including those actions regulated by myostatin. Previous studies suggest that high concentrations of recombinant myostatin generated in bacteria inhibit C2C12 proliferation and differentiation. Recombinant myostatin generated in eukaryotic systems similarly inhibits the proliferation of primary myosatellite cells, but consequently initiates, rather than inhibits, their differentiation and is bioactive at far lower concentrations. Our studies indicate that 2 different sources of recombinant myostatin made in eukaryotes stimulate, not inhibit, C2C12 proliferation. This effect occurred at different cell densities and serum concentrations and in the presence of IGF-I, a potent myoblast mitogen. This stimulatory effect was comparable to that obtained with TGFβ1, a related factor that also inhibits primary myosatellite cell proliferation. Attenuating the myostatin/activin (ie, Acvr2b) and TGFβ1 receptor signaling pathways with the Alk4/5 and Alk5 inhibitors, SB431542 and SB505142, respectively, similarly attenuated proliferation induced by serum, myostatin or TGFβ1 and in a dose-dependent manner. In serum-free medium, both myostatin and TGFβ1 stimulated Smad2 phosphorylation, but not that of Smad3, and a Smad3 inhibitor (SIS3) only inhibited proliferation in cells cultured in high serum. Thus, myostatin and TGFβ1 stimulate C2C12 proliferation primarily via Smad2. These results together question the physiological relevance of the C2C12 model and previous studies using recombinant myostatin generated in bacteria. They also support the alternative use of primary myosatellite cells and recombinant myostatin generated in eukaryotes.
The atrophic actions of myostatin in muscle have been demonstrated using many different in vitro and in vivo systems and in different vertebrate species (1). The myokine inhibits several aspects of skeletal and cardiac muscle cell growth that include progenitor cell proliferation as well as protein synthesis in mature myotubes and muscle fibers. However, its ability to regulate muscle cell development, specifically differentiation, is controversial because studies have demonstrated myostatin to either inhibit or initiate this process. A better mechanistic understanding of myostatin action is critical because attenuating the myokine is a proposed therapeutic target for many muscle wasting or neuromuscular disease states (2, 3).
Early studies using C2C12 myoblasts, an immortal cell line derived from dystrophic mouse thigh muscle (4), suggest that recombinant myostatin generated in bacteria inhibits both proliferation and differentiation (5–8). However, this is in stark contrast to results obtained with chick, rainbow trout, and mouse myosatellite cells (also known as muscle stem or satellite cells) in which myostatin inhibits proliferation and, as a consequence, initiates terminal differentiation (9–13). It also conflicts with studies using myostatin null (mstn−/−) myosatellites or those with impaired myostatin signaling due to Smad3 deficiency, because differentiation is compromised in these highly proliferative cells (13, 14).
Posttranslational modification of the myostatin propeptide involves proteolytic cleavage of the carboxy-terminal domain away from the latency-associated peptide domain and the dimerization of the former via disulfide linkages (1). Mature myostatin generated in bacteria must therefore be denatured and refolded because bacteria lack the oxidative environment to form disulfide bonds and thus, mature myostatin. We hypothesized that discrepancies in myostatin action may either be due to differences in the bioactivity of recombinants generated in bacteria vs eukaryotes or to innate differences in primary vs immortal cell lines. Indeed, our studies suggest that recombinant myostatin generated in eukaryotes stimulates, not inihibits, C2C12 myoblast proliferation. They also suggest caution when using recombinant myostatin generated in bacteria and support the alternative use of eukaryotic recombinants and primary myosatellites.
Materials and Methods
Cell culture
C2C12 myoblasts were cultured in DMEM (Life Technologies) supplemented with 4 mM l-glutamine and different amounts of fetal bovine serum (FBS) (Sigma-Aldrich), although serum-free medium contained BSA at a final concentration of 0.1%. Cells were plated at different densities and treated with 2 different sources of recombinant human myostatin, both generated in eukaryotic systems. This includes a commercial source (R&D Systems) and recombinants generated as previously described (15). Cells were also treated with TGFβ1, IGF-1 (both from R&D Systems), the Alk4/5 and Alk5 inhibitors SB431542 and SB505142, respectively (Sigma-Aldrich), the Smad3 inhibitor SIS3 (Merck), or with equal volumes of dimethylsulfoxide as a control. For proliferation assays, cells were plated in 96-well plates, treatments lasted for 48 hours, and cell number was determined using Cell Titer 96 (Promega Corp.).
Western blotting
Cells were grown on 6-well plates in DMEM/10% FBS until 50% confluent, washed twice in PBS, and then incubated for 1 hour in serum-free medium with 0.1% BSA and various combinations of hormones and inhibitors. After treatment, cells were washed in PBS and lysed in M-PER Mammalian Protein Extraction Reagent (Thermo Scientific). Equal amounts of protein from each lysate were analyzed by Western blotting using 10% polyacrylamide gels and polyvinylidene difluoride membranes. The latter were blocked with 5% nonfat dry milk and probed with antisera for Smad2, Smad3, phospho-Smad2, phospho-Smad3, or glyceraldehyde 3-phosphate dehydrogenase (all from Cell Signaling Technology). After washing, a horseradish peroxidase-conjugated antirabbit secondary antibody (The Jackson Laboratory) and Amersham ECL (GE Healthcare) were used to visualize bands. Phosphosphorylated Smad2/3 was quantified first, and membranes were then stripped using Restore PLUS (Thermo Scientific) before reprobing with the other antisera.
Statistical analysis
Data were analyzed by one- or two-way ANOVA followed by Tukey's honest significant difference test for multiple comparisons. In each figure, different letters denote significant differences between treatments, and all data were processed using Prism software version 6.0 (GraphPad Software, Inc).
Results
Regulation of C2C12 proliferation
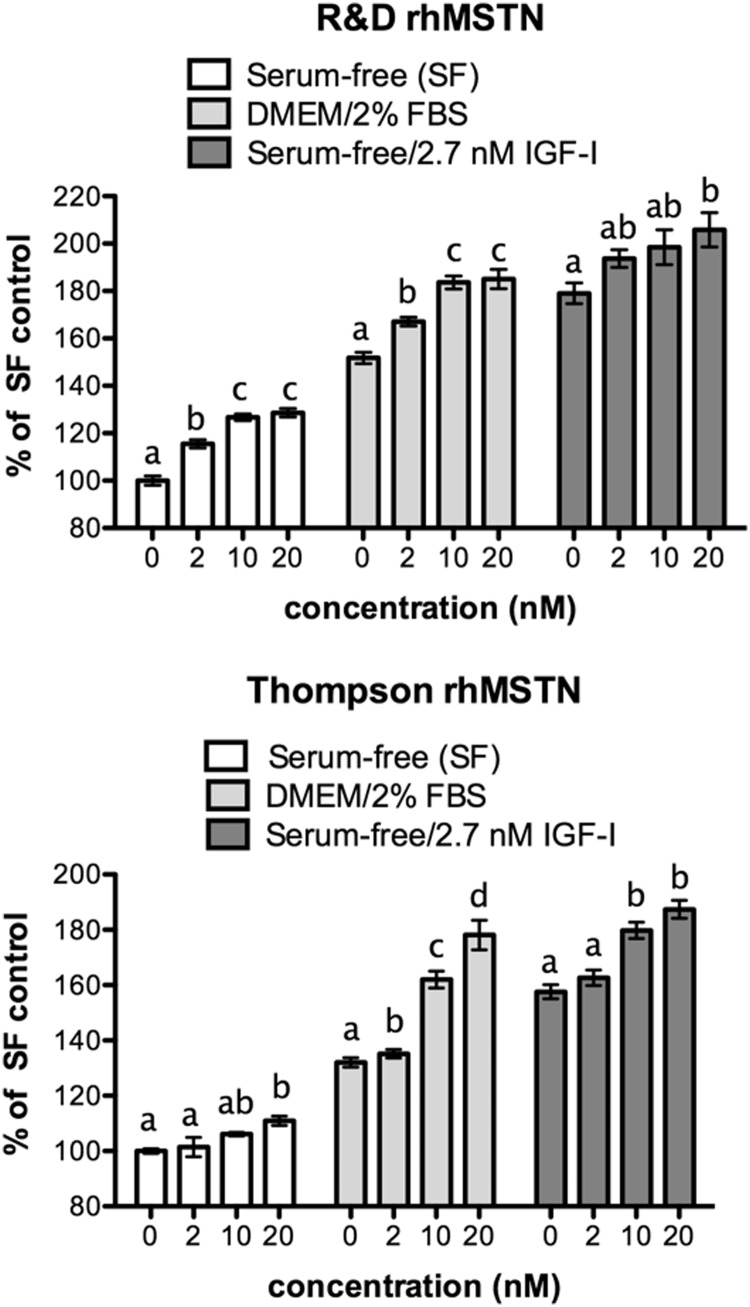
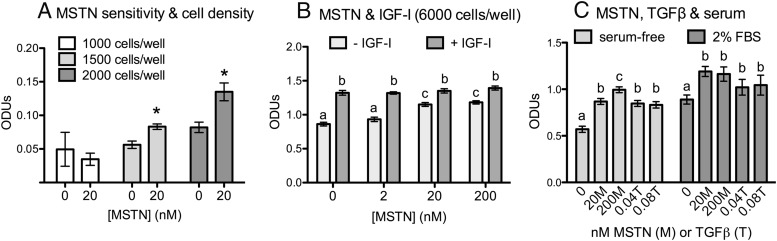
Both of the recombinant myostatin peptides tested stimulated myostatin proliferation under various treatment conditions (Figure 1). This occurred whether cell growth was compromised, as in serum-free conditions, or when already stimulated, as in the presence of serum or when stimulated with IGF-I. In all conditions, the stimulatory effect was dose dependent and the relative efficacy of each recombinant was similar with significant effects often occurring even at the 2 nM dose. Myostatin also stimulated proliferation at low cell densities when growth rate is again suppressed (Figure 2A, 1500 and 2000 cells per well). At high cell densities (6000 cells per well), IGF-I stimulated confluency by 48 hours and the additive effect of myostatin and IGF-I was lost as cells became contact inhibited (Figure 2B). Nevertheless, myostatin alone stimulated proliferation, in a dose-dependent manner, even at this density.
Figure 1.
Myostatin stimulates C2C12 proliferation. Cells were plated in DMEM/10% FBS, at a density of 3000 cells per well in a 96-well plate and cultured for 24 hours Medium was then replaced as indicated and cells were cultured for 48 hours with different concentrations of recombinant human myostatin (rhMSTN) and/or IGF-I. Two different myostatin recombinants were used: a commercially available peptide (R&D Systems) and another (Thompson) generated in our laboratory (15). Cell number was quantified and results are expressed as percent of serum-free (SF) control to account for interassay variance. Statistical comparisons are limited to within-treatment groups, and different letters indicate significant differences whereas the same letters signify no difference (P ≤ .05).
Figure 2.
Cell density, serum, and growth factor regulation of C2C12 proliferation. A and B, The indicated number of cells were plated in DMEM/10% FBS, in 96-well plates and cultured for 24 hours. Cells were then cultured in serum-free (SF) DMEM/0.1% BSA with recombinant human myostatin (rhMSTN) (15) alone or in combination with 2.7 nM IGF-I. C, Cells were also cultured with or without 2% FBS and similarly treated with different concentrations of rhMSTN (M) or TGFβ1 (T). In each experiment, cells were treated for 48 hours before measuring cell number (optical density units [ODUs]). Significant differences are indicated by asterisks (panel A; compared with 0 controls) or by different letters whereas the same letters signify no difference (P ≤ .05).
TGFβ1 has previously been demonstrated to also stimulate C2C12 proliferation (16–18). We repeated this finding (Figure 2C) and further determined that TGFβ1 stimulates proliferation in the presence or absence of serum and that C2C12 cells are at least 500 times more sensitive to TGFβ1 than to myostatin because the effect of 0.04 nM TGFβ1 was similar to that of 20 nM myostatin.
Signal attenuation and proliferation
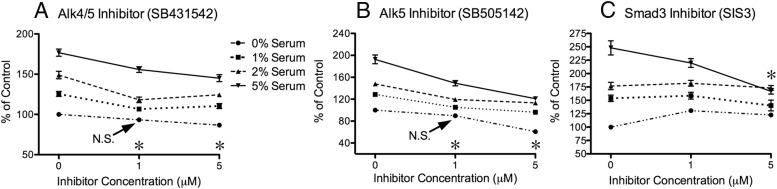
Myostatin and TGFβ1 both activate Alk4 and Alk5 signaling pathways via distinct transmembrane receptors that ultimately phosphorylate Smad2 and Smad3 (1, 19). We therefore determined whether the proliferative effects of both were mediated by canonical signaling pathways using an inhibitor of both Alk4 and Alk5 (SB431542), another of Alk5 specifically (SB505142), and a third that inhibits Smad3 (SIS3). In the absence of myostatin or TGFβ1, the Alk inhibitors attenuated serum-stimulated proliferation in cells cultured with 1%, 2% or 5% FBS (Figure 3, A and B), suggesting that attenuation of basal myostatin/activin and TGFβ1 receptor signaling alone can inhibit proliferation. In fact, the degree of growth inhibition was directly related to the degree of serum stimulation. By contrast, attenuating Smad3 had no effect on cell growth, except in cells stimulated with 5% FBS and cultured with the highest dose of SIS3 (5 μM) that inhibited proliferation (Figure 3C).
Figure 3.
Inhibiting proliferation via Alk4, Alk5, and Smad3 attenuation. Subconfluent cells were cultured for 48 hours with the indicated amounts of serum/FBS (key in panel A also applies to panels B and C) and concentrations of inhibitors. Changes in cell number are expressed as percent of control (0% serum, 0 inhibitor) to account for interassay variance. A and B, Asterisks next to inhibitor concentrations designate significant differences (P ≤ .05) for all means at that concentration and the serum group's respective controls unless otherwise indicated (N.S., not significant). C, Asterisk indicates a significant difference between 0 and 5 μM doses in the 5% serum group.
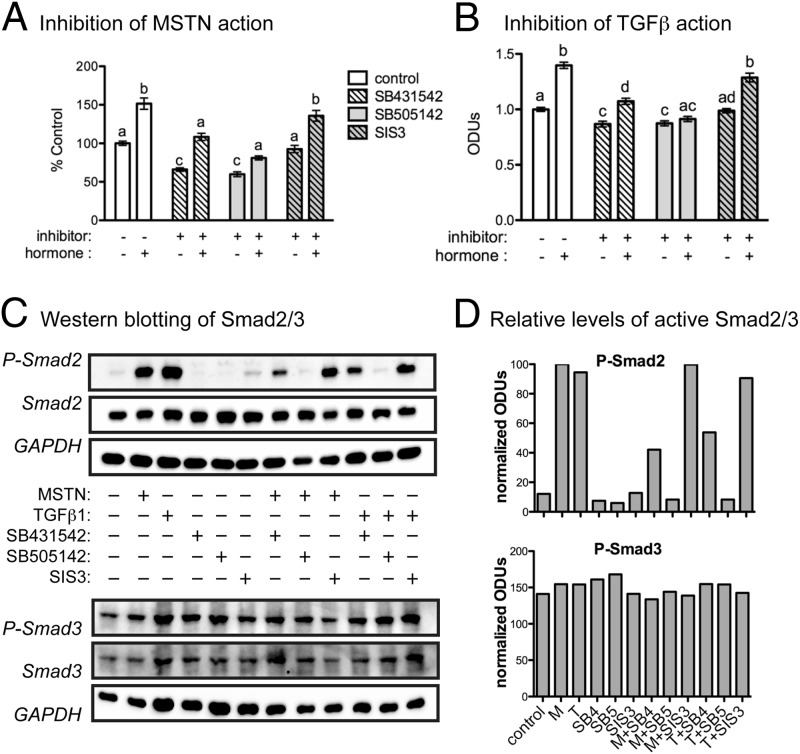
Because the 2 doses of inhibitors tested did not, for the most part, differ in efficacy, the lower dose (1 μM) was used to assess the specificity of action for each cytokine. Both Alk inhibitors attenuated basal as well as myostatin- and TGFβ1-stimulated proliferation and SIS3 was again without effect (Figure 4, A and B). Myostatin and TGFβ1 also stimulated Smad2 phosphorylation, which was prevented by the Alk inhibitors (Figure 4, C and D). Both SB431542 and SB505142 were similarly potent and suggest that attenuating Alk5 alone can suppress myostatin and TGFβ1 action in these cells. Although changes in Smad2 phosphorylation mirrored those in cell proliferation, Smad3 phosphorylation was unresponsive to any treatment. These results together confirm that myostatin and TGFβ1 stimulate C2C12 proliferation via the canonical Alk4/5 and Smad2/3 pathway, although Alk5 and Smad2 appear to be the primarily mediators, at least under the experimental conditions described.
Figure 4.
Myostatin and TGFβ1 activation of Smad2/3. A and B, Cells were cultured in serum-free (SF) DMEM/0.1% BSA for 48 hours in the absence (−) or presence (+) of 20 nM recombinant human myostatin (rhMSTN) (15), 0.04 nM TGFβ1, 1 μM of the indicated inhibitors, or combinations of hormones and inhibitors (Alk4/5, 431532; Alk5, 505142; Smad3, SIS3). Significant differences in cell number, among all groups, are indicated by different letters whereas the same letters signify no difference (P ≤ .05). C, Cells were treated in SF DMEM/0.1% BSA with the indicated combinations of hormones and inhibitors for 1 hour. Levels of total and phospho (P)-Smad2/3 were then estimated by Western blotting of cellular lysates. D, Relative levels of Smad2/3 activation were estimated by normalizing optical density units (ODUs) of P-Smad2/3 to total Smad2/3 and glyceraldehydes-3 phosphate dehydrogenase (GAPDH). Bars are labeled on the x-axis in the same order as samples in panel C (M, myostatin; T, TGFβ1; SB4, 431542; SB5, SB505142).
Discussion
Previous studies using very high doses (2–10 μg/mL, ∼80–400 nM) of recombinant myostatin generated in bacteria suggest that myostatin inhibits C2C12 proliferation (5, 7, 8). This conflicts with our studies using 2 independent sources of recombinant myostatin generated in eukaryotic cells. Indeed, we demonstrate that myostatin stimulates C2C12 proliferation in a dose-dependent manner (Figure 1), that this action is conserved by TGFβ1, that it occurs whether cells are incubated with or without serum and at different cell densities (Figure 2), and finally, that it is mediated by canonical signaling pathways (Figures 3 and 4). Furthermore, our results were generated with far lower concentrations of recombinants (2–20 nM) than used in the previous studies.
The conflicting results could be due to problems associated with using bacterial recombinants or to the immortal nature of C2C12 cells. Bacteria lack the oxidative environment required to generate the disulfide-linked myostatin dimer. Thus, recombinant myostatin generated in Escherichia coli must be denatured and refolded chemically, which produces final preparations composed of both properly and improperly folded recombinants. The previous studies may have used preparations composed primarily of improperly folded peptides because the effective concentrations used were up to 200-fold higher than those reported herein. More recently, the same group used 5 μg/mL and 10 ng/mL of recombinant myostatin generated in E. coli and Chinese hamster ovary cells, respectively, in the same study (20). This is consistent with studies of recombinants generated in our own laboratory (data not shown) using CAGA-luciferase assays (21). Although speculative, such peptides could presumably function as dominant-negatives in a manner similar to that of the Alk inhibitors (Figure 3), and this could explain why the effects are opposite to those reported herein. Nevertheless, bacterial recombinants are potentially still viable and may be generated with an optimized refolding protocol. Their use, however, should be first validated with an appropriate bioassay that could include growth stimulation of C2C12 cells.
Reports of myostatin inhibiting C2C12 differentiation (5, 6, 22) are somewhat inaccurate because these studies demonstrate a delay in the process only after it has already been induced with confluencey and serum withdrawal. In fact, C2C12 cells are immortal, proliferate rapidly, and will not differentiate unless induced. This is an important distinction because true inhibition would either initiate a state of quiescence or prevent cell cycle withdrawal and thereby stimulate proliferation. Moreover, the differentiation process differs in myosatellite and C2C12 cells because the former spontaneously differentiate even when subconfluent and in the presence of serum. Several studies using primary myosatellites from a variety of vertebrate species have consistently demonstrated myostatin to inhibit proliferation and, as a consequence, initiate and stimulate differentiation (9–13). In fact, myostatin promotes the terminal differentiation of muscle progenitors in chicken and mouse embryos (9), and the knockdown of myostatin delays the differentiation of chick myosatellites (12). Furthermore, recombinant myostatin stimulates differentiation in proliferating trout myosatellites (10, 11). Differentiation is also delayed in myosatellites isolated from mstn−/− mice (13) and in cells from Smad3-deficient mice (14). These results directly conflict with those using C2C12 cells and suggest alternatively that myostatin stimulates, not inhibits, muscle cell differentiation.
The TGFβ ligands 1–3 are well known to stimulate C2C12 proliferation (16–18). This is associated with increased nuclear localization of proliferating cell nuclear antigen and, by contrast, decreased nuclear localization of MyoD, which also degrades quicker with TGFβ1 stimulation (16). However, TGFβ ligands are also equally well known to inhibit the proliferation of primary myosatellite cells (23–26), which mirrors the effects of recombinant myostatin generated in eukaryotes. These results question the validity of applying conclusions derived from studies with C2C12 cells to the normal myogenic process because the shared actions of both cytokines are clearly opposite in C2C12 vs primary cells. By contrast, both cytokines regulate mechanisms of fibrosis in vitro and in vivo, and TGFβ1 stimulates C2C12 transdifferentiation into fibrotic cells (27–29). Thus, actions described for TGFβ and myostatin in C2C12 cells may be more relevant to fibrosis than myogenesis. Nevertheless, researchers should be encouraged to use recombinant myostatin generated in eukaryotes or to verify that E. coli recombinants are equipotent, as well as to use primary muscle cell culture systems when investigating endocrine mechanisms of myogenesis.
Acknowledgments
These studies were supported by grants from the National Science Foundation (1147275; to B.D.R.), the National Institutes of Health (R01GM084186; to T.B.T.), and the Muscular Dystrophy Association (216602 to B.D.R.; 240087 to T.B.T.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FBS
- fetal bovine serum
- SIS3
- Smad3 inhibitor.
References
- 1. Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev. 2008;29(5):513–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bradley L, Yaworsky PJ, Walsh FS. Myostatin as a therapeutic target for musculoskeletal disease. Cell Mol Life Sci. 2008;65(14):2119–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han HQ, Mitch WE. Targeting the myostatin signaling pathway to treat muscle wasting diseases. Curr Opin Support Palliat Care. 2011;5(4):334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270(5639):725–727 [DOI] [PubMed] [Google Scholar]
- 5. Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277:49831–49840 [DOI] [PubMed] [Google Scholar]
- 6. Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res. 2003;286(2):263–275 [DOI] [PubMed] [Google Scholar]
- 7. Taylor WE, Bhasin S, Artaza J, et al. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab. 2001;280(2):E221–E228 [DOI] [PubMed] [Google Scholar]
- 8. Thomas M, Langley B, Berry C, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275(51):40235–40243 [DOI] [PubMed] [Google Scholar]
- 9. Manceau M, Gros J, Savage K, et al. Myostatin promotes the terminal differentiation of embryonic muscle progenitors. Genes Dev. 2008;22(5):668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garikipati DK, Rodgers BD. Myostatin stimulates myosatellite cell differentiation in a novel model system: evidence for gene subfunctionalization. Am J Physiol Regul Integr Comp Physiol. 2012;302(9):R1059–R1066 [DOI] [PubMed] [Google Scholar]
- 11. Garikipati DK, Rodgers BD. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation: evidence for endocrine-regulated transcript processing. J Endocrinol. 2012;215(1):177–187 [DOI] [PubMed] [Google Scholar]
- 12. Sato F, Kurokawa M, Yamauchi N, Hattori MA. Gene silencing of myostatin in differentiation of chicken embryonic myoblasts by small interfering RNA. Am J Physiol Cell Physiol. 2006;291(3):C538–C545 [DOI] [PubMed] [Google Scholar]
- 13. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162(6):1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ge X, McFarlane C, Vajjala A, et al. Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res. 2011;21(11):1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cash JN, Rejon CA, McPherron AC, Bernard DJ, Thompson TB. The structure of myostatin:follistatin 288: insights into receptor utilization and heparin binding. EMBO J. 2009;28(17):2662–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schabort EJ, van der Merwe M, Loos B, Moore FP, Niesler CU. TGF-β's delay skeletal muscle progenitor cell differentiation in an isoform-independent manner. Exp Cell Res. 2009;315(3):373–384 [DOI] [PubMed] [Google Scholar]
- 17. Haugk KL, Roeder RA, Garber MJ, Schelling GT. Regulation of muscle cell proliferation by extracts from crushed muscle. J Anim Sci. 1995;73(7):1972–1981 [DOI] [PubMed] [Google Scholar]
- 18. Quinn LS, Steinmetz B, Maas A, Ong L, Kaleko M. Type-1 insulin-like growth factor receptor overexpression produces dual effects on myoblast proliferation and differentiation. J Cell Physiol. 1994;159(3):387–398 [DOI] [PubMed] [Google Scholar]
- 19. Attisano L, Wrana JL. Signal transduction by the TGF-β superfamily. Science. 2002;296(5573):1646–1647 [DOI] [PubMed] [Google Scholar]
- 20. Lokireddy S, McFarlane C, Ge X, et al. Myostatin induces degradation of sarcomeric proteins through a Smad3 signaling mechanism during skeletal muscle wasting. Mol Endocrinol. 2011;25(11):1936–1949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Cash JN, Angerman EB, Kirby RJ, et al. Development of a small-molecule screening method for inhibitors of cellular response to myostatin and activin a. J Biomol Screen. 2013;18(7):837–844 [DOI] [PubMed] [Google Scholar]
- 22. Ríos R, Carneiro I, Arce VM, Devesa J. Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol. 2002;282(5):C993–C999 [DOI] [PubMed] [Google Scholar]
- 23. Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-β. J Cell Physiol. 1987;133(3):567–572 [DOI] [PubMed] [Google Scholar]
- 24. Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-β, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138(2):311–315 [DOI] [PubMed] [Google Scholar]
- 25. Greene EA, Allen RE. Growth factor regulation of bovine satellite cell growth in vitro. J Anim Sci. 1991;69(1):146–152 [DOI] [PubMed] [Google Scholar]
- 26. Hathaway MR, Hembree JR, Pampusch MS, Dayton WR. Effect of transforming growth factor β-1 on ovine satellite cell proliferation and fusion. J Cell Physiol. 1991;146(3):435–441 [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Foster W, Deasy BM, et al. Transforming growth factor-β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164(3):1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu J, Li Y, Shen W, et al. Relationships between transforming growth factor-β1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem. 2007;282(35):25852–25863 [DOI] [PubMed] [Google Scholar]
- 29. Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008;283(28):19371–19378 [DOI] [PMC free article] [PubMed] [Google Scholar]