Abstract
Background
Neoadjuvant chemoradiotherapy (NACRT) for esophageal squamous cell carcinoma (ESCC) is beneficial in the setting of a complete pathological response. Rad51 expression affects both chemo- and radiosensitivity in many cancers; however, its role in ESCC is unclear.
Methods
Rad51 expression was investigated by immunohistochemical staining with resected specimens in 89 ESCC patients who underwent surgery without preoperative therapy. The association with Rad51 and clinicopathological factors was assessed. The expression of Rad51 was also investigated in pretreatment biopsy specimens in 39 ESCC patients who underwent surgery after NACRT and compared with the pathological response to NACRT.
Results
Lymph node metastasis was more frequently observed in Rad51-positive cases than negative cases (58.5 vs. 30.6 %, P = 0.0168) in patients treated with surgery alone. Disease-specific survival was decreased in Rad51-positive cases compared to Rad51-negative cases (5 year survival: 79.6 vs. 59.3 %, P = 0.0324). In NACRT patients, completed pathological responses were more frequently observed in Rad51-negative cases than in Rad51-positive cases (68.8 vs. 46.5 %, P = 0.0171).
Conclusions
Rad51 expression in ESCC was associated with lymph node metastasis and poor survival. Additionally, Rad51 expression in pretreatment biopsy specimens was a predictive factor for the response to NACRT.
Electronic supplementary material
The online version of this article (doi:10.1245/s10434-013-3220-2) contains supplementary material, which is available to authorized users.
Esophageal cancer, unlike other gastrointestinal malignancies, is extremely difficult to control with surgery alone.1 Neoadjuvant chemoradiotherapy (NACRT), frequently with cisplatin, is an important treatment strategy for advanced esophageal squamous cell carcinoma (ESCC); however, the clinical usefulness of NACRT for potentially resectable esophageal cancer remains controversial. In multiple meta-analyses, neoadjuvant treatment has been demonstrated to be superior to primary surgery in terms of local tumor control and disease-free survival.2–4 Other reports, however, have not demonstrated NACRT plus surgery to be superior to surgery alone.5–7 NACRT for esophageal cancer may also increase the risk of perioperative complications.8,9 Therefore, identification of molecular markers that predict the response to NACRT could potentially reduce perioperative complications by improving patient selection.
One predictive factor for chemoradiotherapy response in a variety of human cancers is Rad51.10,11 Rad51 is a key factor in homologous recombination.12 Overexpression of Rad51 decreases radiation sensitivity and confers resistance to DNA cross-linking agents such as cisplatin.13,14 We therefore hypothesized that Rad51 expression would predict the response to NACRT in ESCC.
The aims of this study were to evaluate the significance of Rad51 in ESCC and to correlate Rad51 expression in the pretreatment biopsy ESCC specimens with NACRT response.
Materials and Methods
Patients
Between July 1997 and March 2006, 89 patients (pT1–3, pN0–1, M0) with ESCC underwent esophagectomy without neoadjuvant therapy at the Department of Surgery and Science, Kyushu University Hospital, Fukuoka, Japan. The 78 male patients and 11 female patients ranged in age from 38 to 90 years (mean 63.3 years). Using the resected specimens from these patients, we evaluated the significance of the overexpression of Rad51 in ESCC.
For the NACRT group, patients were treated between 2003 and 2008. Thirty-nine patients (cT1–4, N0–1, M0) with ESCC underwent NACRT followed by esophagectomy: 22 patients at the Department of Surgery and Science, Kyushu University Hospital, and 17 patients at National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan. For NACRT, 30–42 Gy of radiation was administered preoperatively to the primary tumor and metastatic lymph nodes. The chemotherapy regimen consisted of low-dose cisplatin and 5-fluorouracil (5-FU) (cisplatin: 5 mg/m2/day, 5-FU: 250 mg/m2/day, administered on weekdays, repeated every 3–4 weeks). Using pretreatment biopsy specimens in these patients, we compared the effectiveness of NACRT with the expression of Rad51.
Immunohistochemistry
All surgically resected tumor specimens and biopsy specimens were fixed with 10 % formalin and embedded in paraffin. Four-micrometer sections were deparaffinized with xylene and rehydrated in a series of ethanols. Heat-induced epitope retrieval was performed in 0.1 M NaOH-citrate buffer (pH 7.0) for Rad51 immunostaining, and the samples were heated in an autoclave at 121 °C for 15 min. Endogenous peroxidase was blocked at room temperature using 3 % hydrogen peroxide in methanol for 30 min. After blocking with normal goat serum, slides were incubated with mouse monoclonal antibody against Rad51 (MS-988-P, NeoMarkers, Fremont, CA) using a 1:100 dilution of primary antibody at 4 °C overnight. After washing, the sections were treated for 60 min at room temperature with goat–anti-mouse immunoglobulin. Staining for Rad51 was completed using the streptavidin–biotin–peroxidase complex method with diaminobenzidine as a chromogen, and the slides were counterstained with hematoxylin. Positive staining was defined as a minimum of 10 % of the cancer cell nuclei showing positive nuclear staining.15 The tumors were staged according to the International Union Against Cancer’s tumor, node, metastasis (TNM) classification.16 A pathological complete response (pCR) was defined as no evidence of viable cancer cells in the primary regions; a pathological nonresponse was defined as viable cancer cells still observed.
Assessment of Rad51 Staining in ESCC
Immunohistochemical staining was assessed for 89 samples from patients without preoperative therapy and 39 samples from patients who had undergone NACRT. Staining was scored under a light microscope by a pathologist (Nakashima Y) who was unaware of the clinical, pathological, and follow-up data. The concept of positive-cell index (PCI), indicating the proportion of positively stained tumor cells, was adopted for the analyses in this study. Rad51-positive staining was defined by identifying the optimal cutoff point. Qiao et al.15 have previously reported the optimal threshold required to separate prognostically. For this procedure, a PCI of 10 % was identified as the optimal cutoff. Cases whose immunohistochemistry (IHC) scores were less than 10 % were called “low-level expressers,” whereas those with IHC scores greater than 10 % were called “high-level expressers.” High-level expressers were defined as Rad51-positive staining cases, and low-level expressers were defined as negative staining cases.
In order to evaluate the heterogeneity of staining, we evaluated the area of Rad51 staining by dividing the tumor nest into three equal parts: the shallow level, the middle level, and the deep level of the invasive cancer.17 Homogenous staining was defined by identical staining patterns in all three parts of the tumor. If the staining pattern was homogenous, the Rad51 status in the biopsy specimen was considered to reflect the results in the surgical specimen.
Statistical Analysis
The differences in distribution frequencies among the groups were evaluated using Fisher’s exact test or an unpaired t-test. The survival curves were plotted according to the Kaplan–Meier method and any differences were analyzed using the log-rank test. A multivariate analysis with Cox proportional hazards model was adopted to clarify the independent prognostic factors. Differences were considered to be significant if the P value was less than 0.05.
Results
Rad51 Expression in the Resected Specimens and Clinicopathological Factors in the Patients Who Underwent Surgery Without Preoperative Therapy
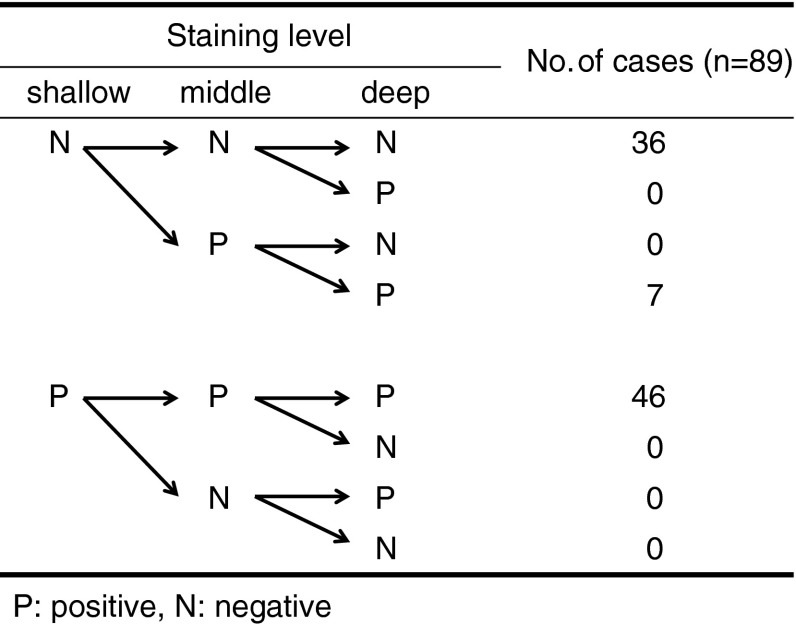
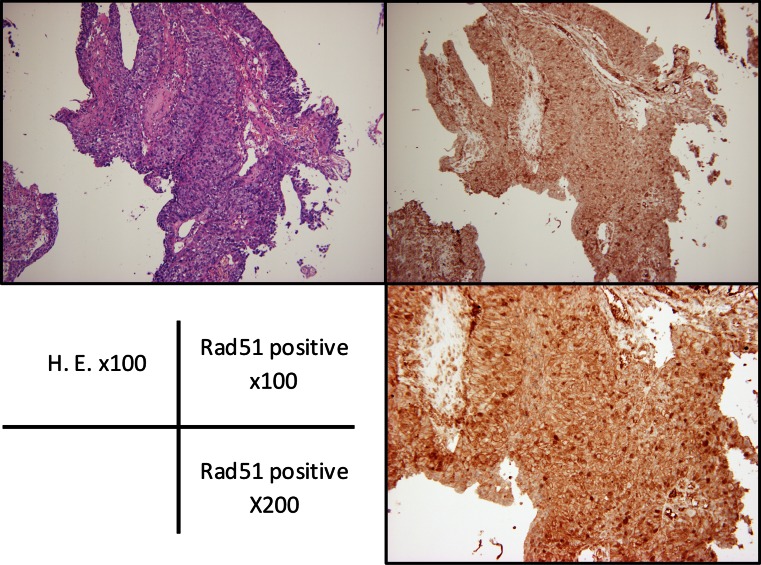
Positive staining of Rad51 was observed in 53 (59.6 %) of 89 cases (Fig. 1). The patterns of Rad51 staining were homogenous in almost all specimens (Fig. 2). Additionally, 46 of 53 (86.7 %) cases without NACRT presented diffuse staining patterns from the shallow to deep levels of the tumor nest, with the expression pattern of Rad51 appearing homogenous. In the seven cases exhibiting heterogeneous staining patterns, there was no expression of Rad51 in the shallow level, while positive expression was observed in the middle and deep levels of the tumor.
Fig. 1.
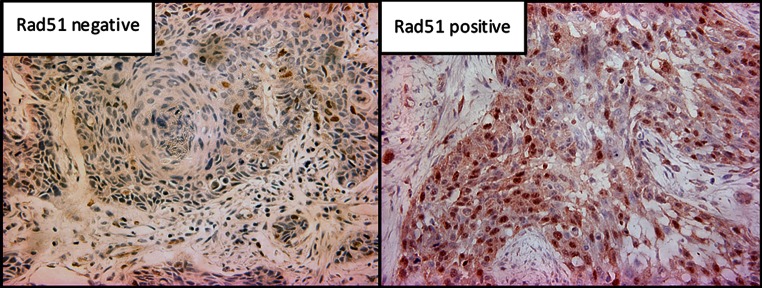
Immunohistochemistry for the detection of Rad51 in the resected specimens by esophagectomy without preoperative therapy (original magnification, ×200). Immunohistochemistry for Rad51 in ESCC resected specimens without preoperative therapy. Positive staining of Rad51 was present in 53 (59.6 %) cases and negative staining in 36 (40.4 %)
Fig. 2.
Rad51 staining patterns in surgical specimens. In 46 (86.7 %) cases of Rad51-positive staining in surgical specimens, a homogenous staining pattern was observed. In the seven cases with heterogeneous staining patterns, there was no expression of Rad51 in the shallow level, while positive expression was observed in the middle and deep levels of the tumor
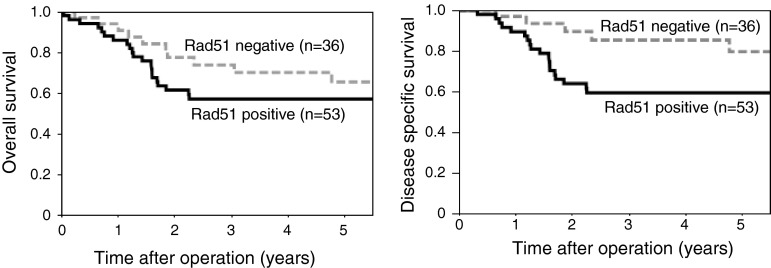
There was a significant association between Rad51 expression and lymph node metastasis with node positive cases numbering 11 (30.6 %) and 31 (58.5 %) in the Rad51 negative and positive groups, respectively (Table 1, P = 0.0168). There were no significant associations between Rad51 expression and age, gender, or tumor location. There was no significant association between Rad51 expression and overall survival (Fig. 3; 5-year survival rates: 57.0 versus 65.4 %, for positive and negative, respectively; P = 0.1768). Rad51-positive cases had significantly poorer disease-specific survival compared to Rad51-negative cases (Fig. 3; 5-year disease-specific survival rates: 59.3 versus 79.6 % for positive and negative, respectively; P = 0.0324). In Rad51-negative cases, recurrence was less frequent than in Rad51-positive cases (Table 1; 9 of 36 cases versus 27 of 53 cases; P = 0.0171). In multivariate analysis, Rad51 was not an independent prognostic factor (Table 2, P = 0.5287), while lymph node metastasis was an independent prognostic factor (P = 0.0051).
Table 1.
Rad51 expression in the resected specimen and clinicopathological factors in patients who underwent surgery without preoperative therapy
| Factor | Rad51 negative | Rad51 positive | P value |
|---|---|---|---|
| (n = 36) | (n = 53) | ||
| Age (year) | 62.3 ± 10.1 | 64.1 ± 9.9 | 0.5193 |
| Sex | 0.5144 | ||
| Male | 33 (91.7 %) | 45 (84.9 %) | |
| Female | 3 (8.3 %) | 8 (15.1 %) | |
| Differentiation of ESCC | 0.0999 | ||
| Well | 8 (22.2 %) | 15 (28.3 %) | |
| Moderate | 24 (66.7 %) | 24 (45.3 %) | |
| Poor | 4 (11.1 %) | 14 (26.4 %) | |
| Location | 0.1083 | ||
| Upper | 3 (8.4 %) | 8 (15.1 %) | |
| Middle | 21 (58.3 %) | 19 (35.9 %) | |
| Lower | 12 (33.3 %) | 26 (49.1 %) | |
| Depth of invasion | 0.1246 | ||
| pT 1, 2 | 25 (69.4 %) | 27 (50.9 %) | |
| pT 3 | 11 (30.6 %) | 26 (49.1 %) | |
| Lymph node metastasis | 0.0168 | ||
| pN 0 | 25 (69.4 %) | 22 (41.5 %) | |
| pN 1 | 11 (30.6 %) | 31 (58.5 %) | |
| Lymphatic involvement | 0.2794 | ||
| Negative | 22 (61.1 %) | 25 (47.2 %) | |
| Positive | 14 (38.9 %) | 28 (52.8 %) | |
| Vascular involvement | 1.0000 | ||
| Negative | 25 (69.4 %) | 36 (67.9 %) | |
| Positive | 11 (30.6 %) | 17 (32.1 %) | |
| pStage | 0.0905 | ||
| I, II | 30 (83.3 %) | 35 (66.0 %) | |
| III | 6 (16.7 %) | 18 (34.0 %) | |
| Recurrence | 0.0171 | ||
| Negative | 27 (75.0 %) | 26 (49.1 %) | |
| Positive | 9 (25.0 %) | 27 (50.9 %) |
ESCC esophageal squamous cell carcinoma
Fig. 3.
Survival for patients without preoperative chemoradiotherapy. Comparison of the 5-year OS rate and DSS rate between Rad51 positive and negative staining groups in ESCC without preoperative therapy. The difference in DSS was statistically significant (P = 0.0324)
Table 2.
Multivariate analysis of Rad51 expression in the without NACRT
| Factor | Object | Control | Odds ratio | 95 % confidence interval | P value |
|---|---|---|---|---|---|
| Depth of invasion | T3/T4 | T1/T2 | 0.92 | 0.77–2.12 | 0.3376 |
| Lymph node metastasis | Positive | Negative | 7.85 | 1.27–3.74 | 0.0051 |
| Distant metastasis | Positive | Negative | 0.05 | 0.19–4.16 | 0.8251 |
| Recurrence | Positive | Negative | 3.39 | 0.96–2.88 | 0.0654 |
| Rad51 | Positive | Negative | 0.40 | 0.73–1.89 | 0.5287 |
NACRT neoadjuvant chemoradiotherapy
Rad51 Expression in the Biopsy Specimens and Clinicopathological Factors in the Patients Who Underwent Surgery after NACRT
Negative staining of Rad51 was observed in 12 (30.8 %) of 39 cases, while 27 cases were positive (69.2 %, Fig. 4). With respect to the efficacy of NACRT, seven of 39 cases (17.9 %) were histologically pCR. There were no significant associations between demographic and clinical factors, depth of invasion, lymph node metastasis, or TNM clinical stage (Table 3). Rad51 expression significantly predicted a response to NACRT; five (41.7 %) of 12 Rad51 negative cases were classified as pCR, while seven cases (58.3 %) were non-pCR patients. Twenty-five of 27 Rad51 positive cases (92.6 %) were non-pCR to NACRT with only two Rad51 positive cases (7.4 %) being classified as pCR (Table 4, P = 0.0197).
Fig. 4.
Immunohistochemistry for Rad51 in the biopsy specimens before preoperative therapy. Positive staining was present in 27 (69.2 %) cases, and 12 (30.8 %) cases were negative
Table 3.
Rad51 expression and clinical factors in patients with NACRT
| Factor | Rad51 negative | Rad51 positive | P value |
|---|---|---|---|
| (n = 12 %) | (n = 27 %) | ||
| Age (year) | 58.8 ± 12.3 | 62.5 ± 7.9 | 0.3071 |
| Sex | 0.6536 | ||
| Male | 9 (75.0 %) | 23 (85.2 %) | |
| Female | 3 (25.0 %) | 4 (14.8 %) | |
| Differentiation of ESCC | 0.6641 | ||
| Well | 2 (16.7 %) | 8 (29.6 %) | |
| Moderate | 8 (66.6 %) | 16 (59.3 %) | |
| Poor | 2 (16.7 %) | 3 (11.1 %) | |
| Location | 0.1850 | ||
| Upper | 3 (25.0 %) | 10 (37.0 %) | |
| Middle | 4 (33.3 %) | 13 (48.2 %) | |
| Lower | 5 (41.7 %) | 4 (14.8 %) | |
| Depth of invasion | 1.0000 | ||
| cT 1, 2 | 2 (16.7 %) | 4 (14.8 %) | |
| cT 3 | 10 (83.3 %) | 23 (84.6 %) | |
| Lymph node metastasis | 0.2694 | ||
| cN 0 | 2 (16.7 %) | 11 (40.7 %) | |
| cN 1 | 10 (83.3 %) | 16 (59.3 %) | |
| cStage | 0.6450 | ||
| I, II | 1 (8.3 %) | 5 (18.5 %) | |
| III | 11 (91.7 %) | 22 (81.5 %) |
NACRT neoadjuvant chemoradiotherapy, ESCC esophageal squamous cell carcinoma
Table 4.
Relationship of Rad51 expression and the responses of NACRT
| Efficacy of NACRT | Expression of Rad51 | P value | |
|---|---|---|---|
| Negative (n = 12) | Positive (n = 27) | ||
| Non-pCR | 7 (58.3 %) | 25 (92.6 %) | 0.0197 |
| pCR | 5 (41.7 %) | 2 (7.4 %) | |
NACRT neoadjuvant chemoradiotherapy, pCR pathological complete response
Discussion
Overexpression of Rad51 has been observed in several cancers and may be involved in either the initiation or the progression of tumorigenesis.18,19 In non-small cell lung cancer (NSCLC), overexpression of Rad51 is related to decreased survival and increased tumor cell survival.15 Overexpression of Rad51 has been reported to correlate with histological grading of sporadic invasive ductal breast cancer, and is more frequently observed in advanced prostate cancer.18,19 These results suggest a relationship between Rad51 overexpression and more aggressive tumor behavior.
In ESCC, the significance of Rad51 overexpression is still unclear. In this study, high expression of Rad51 was associated with lymph node metastases in cases of esophageal cancer in which the patients had not undergone NACRT. However, the mechanism through which Rad51 expression affects the migratory ability of cancer cells has not been elucidated. Using canine adenocarcinoma metastatic models, it was demonstrated that Rad51 mRNA overexpression could be observed in metastatic lymph nodes.20 However, the details of the metastatic mechanisms mediating these effects are still unclear. In breast cancer, pancreatic cancer, soft tissue sarcoma, and non–small cell lung cancer, Rad51 overexpression was associated with poor prognoses, suggesting that Rad51 overexpression may enhance genetic instability and maintain DNA damage at a tolerable level to permit cell survival.15,18,21,22 The relationship between overexpression of double-stranded break (DSB) repair genes and the ability of tumor cells to undergo migration has not yet been elucidated. XRCC3, a DSB repair gene, was reported to be associated with increased tumor cell migration in breast cancer cells.23 Considering our results, which demonstrated that ESCC specimens with Rad51 overexpression often exhibited lymph node metastasis, further studies are needed to investigate the role of Rad51 using ESCC cell lines. On the basis of data from a tissue microarray, Li et al.24 reported that Rad51 was an independent prognostic factor in ESCC. In this study, Rad51 expression was investigated by conventional IHC using surgical resection and biopsy specimens. It is possible to investigate the entire cancer area using surgical and biopsy specimens by IHC; however, histological observation of the tumor nest is limited in tissue microarray analysis. Thus, differences in the evaluation methods for Rad51 expression may explain the inconsistencies between our study and previous studies.
In our study, some population bias was observed between the groups with and without NACRT; the NACRT group included more advanced cases of ESCC than the group without NACRT. Because there was a discrepancy in the association between lymph node metastasis and Rad51 expression in the groups with and without NACRT, we also analyzed Rad51 expression and clinicopathological factors, matching the staging of subjects. When limited to Stage I/II cases or Stage III cases, there were no significant relationships between lymph node metastasis and Rad51 expression, both in patients with and without NACRT, suggesting that the population bias resulted in variations in the association between lymph node metastasis and Rad51 expression (data not shown). Additionally, differences in staging methods, i.e. that patients’ backgrounds were based on pathological staging in the without NACRT group but on clinical staging in the with NACRT group, could explain the discrepancy in the association between lymph node metastasis and Rad51 expression in the three groups.
Our data indicated that Rad51 expression status in biopsy specimens could be a predictive factor for treatment efficacy of NACRT in ESCC. Because the Rad51 staining pattern was homogenous in the majority of cases, the expression pattern of biopsy specimens was considered to reflect the Rad51 expression status in the whole tumor nest. In assessment of the HER2 status of gastric cancer biopsies, the concordance rate of diagnosis between biopsy and surgical specimens was reported to be over 70 %.25,26 In this study, the concordance of Rad51-positive staining was 86.7 %, suggesting that Rad51 IHC results in biopsy specimens are useful as a predictive tool of Rad51-positive staining in surgical specimens, similar to the usefulness of HER2 status in biopsies of gastric cancer specimens.
Our data suggested that Rad51 is a useful predictive tool for NACRT in ESCC. However, Rad51 expression incompletely predicted the efficacy of NACRT, implying that the pathway is multifactorial. Thus, further studies are required in order to elucidate other mechanisms and markers that would allow us to predict the efficacy of NACRT.
DNA double strand breaks (DSBs), if unrepaired, are lethal to the tumor cell. Radiation and cisplatin trigger apoptosis in tumor cells by creating genetic instability through a DSBs mechanism.27 Rad51 plays an important role in the repair of DSBs through homologous recombination, thereby decreasing sensitivity to radiation and cisplatin. Radiation and CDDP inhibits cellular growth by inducing DNA DSBs.28–30 Cells can use DNA repair machinery to respond to the DNA damage. The levels of DNA repair proteins correlate with resistance to radiation and anticancer drugs in human cancer cell lines.12,31 Two pathways, homologous recombination and nonhomologous end joining, are used to repair DNA DSBs, and Rad51 is involved in the former process, homologous recombination. Recent evidence suggests that homologous recombination is involved in the repair of DNA DSBs generated by radiation and CDDP.14,32,33 Cancer cells may become resistant to radiation and CDDP by increasing the activity of homologous recombination repair machinery.34 On the other hand, 5-FU, an antimetabolic drug, exerts its antitumor effects through suppression of both DNA and RNA synthesis, pathways separate from CDDP or radiation. Because there are few studies of the direct relationship between DSB repair and 5-FU, further investigations with ESCC cells are required to elucidate the role of Rad51 in 5-FU sensitivity.35
In recent studies, down-regulation of Rad51 has been demonstrated to increase therapeutic sensitivities. In NSCLC, Tsai et al. reported that down-regulation of Rad51 using specific Rad51 small interfering RNA significantly increased cytotoxicity.36 Chan et al. 37 reported that down-regulation of Rad51 decreased homologous recombination, and increased sensitivity to the DNA cross-linking agents mitomycin C and cisplatin. Down-regulation of homologous recombination could result in low-fidelity DNA repair and have significant implications for response to therapy and genetic instability. Cancer cells may become resistant to cisplatin by increasing the activity of homologous recombination repair via Rad51 over expression. Down-regulation of Rad51 in ESCC may represent a novel therapeutic strategy to increase sensitivity for radiation and cisplatin chemotherapy.
In ESCC, radiation and cisplatin are the mainstays of treatment.38–42 NACRT with cisplatin results in significant down staging and induces pCR.43,44 A pCR to NACRT is critical for improving the survival of patients with ESCC.45 NACRT may, however, increase the incidence of postoperative complications.46 Thus, patient selection should identify those patients unlikely to benefit from NACRT. Although several other predictive factors have been reported, clear molecular prognostic factors are still needed to reduce the frequency of perioperative complications.47–49 On the basis of our results, Rad51 has great potential and warrants further investigation.
Considering our results, NACRT using a cisplatin/5-FU protocol is likely to fail when Rad51 overexpression is observed on a biopsy specimen. Use of NACRT for these patients may unnecessarily increase the rate of perioperative complications as there is no nonsurgical alternative to esophagectomy. In the current study, docetaxel, cisplatin and fluorouracil combination chemotherapy has been demonstrated to have activity in advanced and recurrent ESCC.50 Cetuximab may also have activity in ESCC.51,52 Rad51 targeted therapies may represent another novel therapeutic strategy.
In conclusion, Rad51 expression may predict NACRT response in ESCC. Rad51 expression may serve as a means by which to select patients for NACRT, thereby minimizing perioperative complications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgment
We thank Akio Nagaoka for assisting with the preparation of the document.
Disclosure
The authors declare no conflict of interest.
Contributor Information
Tomonori Nakanoko, Phone: +81-92-642-5466, FAX: +81-92-642-5482, Email: nakanoko@surg2.med.kyushu-u.ac.jp.
Hiroshi Saeki, Email: h-saeki@surg2.med.kyushu-u.ac.jp.
References
- 1.Morita M, Yoshida R, Ikeda K, et al. Advances in esophageal cancer surgery in Japan: an analysis of 1,000 consecutive patients treated at a single institute. Surgery. 2008;143:499–508. doi: 10.1016/j.surg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Kaklamanos IG, Walker GR, Ferry K, et al. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol. 2003;10:754–761. doi: 10.1245/ASO.2003.03.078. [DOI] [PubMed] [Google Scholar]
- 3.Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538–543. doi: 10.1016/S0002-9610(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 4.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 5.Urba S, Orringer M, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 6.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomized controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 7.Greer SE, Goodney PP, Sutton JE, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery. 2005;137:172–177. doi: 10.1016/j.surg.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Barnett SA, Rizk NP. Randomized clinical trials in esophageal carcinoma. Surg Oncol Clin N Am. 2010;19:59–80. doi: 10.1016/j.soc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Avendano CE, Flume PA, Silvesri GA, et al. Pulmonary complications after esophagectomy. Ann Thorac Surg. 2002;73:922–926. doi: 10.1016/S0003-4975(01)03584-6. [DOI] [PubMed] [Google Scholar]
- 10.Vispé S, Cazaux C, Lesca C, et al. Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Res. 1998;26:2859–2864. doi: 10.1093/nar/26.12.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slupianek A, Schmutte C, Tombline G, et al. BCR/ABL regulates mammalian RecA homologs, resulting in drug resistance. Mol Cell. 2001;8:795–806. doi: 10.1016/S1097-2765(01)00357-4. [DOI] [PubMed] [Google Scholar]
- 12.Baumann P, West SC. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci. 1998;23:247–251. doi: 10.1016/S0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 13.Henning W, Stürzbecher HW. Homologous recombination and cell cycle checkpoints: Rad51 in tumour progression and therapy resistance. Toxicology. 2003;193:91–109. doi: 10.1016/S0300-483X(03)00291-9. [DOI] [PubMed] [Google Scholar]
- 14.Aloyz R, Xu ZY, Bello V, et al. Regulation of cisplatin resistance and homologous recombinational repair by the TFIIH subunit XPD. Cancer Res. 2002;62:5457–5462. [PubMed] [Google Scholar]
- 15.Qiao GB, Wu YL, Yang XN, et al. High-level expression of Rad51 is an independent prognostic marker of survival in non-small-cell lung cancer patients. Br J Cancer. 2005;93:137–143. doi: 10.1038/sj.bjc.6602665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. Oxford: Wiley–Blackwell; 2010. [Google Scholar]
- 17.Kuwano H, Saeki H, Kawaguchi H, et al. Proliferative activity of cancer cells in front and center areas of carcinoma in situ and invasive sites of esophageal squamous-cell carcinoma. Int J Cancer. 1998;78:149–152. doi: 10.1002/(SICI)1097-0215(19981005)78:2<149::AID-IJC4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Maacke H, Opitz S, Jost K, et al. Over-expression of wild-type Rad51 correlates with histological grading of invasive ductal breast cancer. Int J Cancer. 2000;88:907–913. doi: 10.1002/1097-0215(20001215)88:6<907::AID-IJC11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Mitra A, Jameson C, Barbachano Y, et al. Overexpression of RAD51 occurs in aggressive prostatic cancer. Histopathology. 2009;55:696–704. doi: 10.1111/j.1365-2559.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klopfleisch R, Gruber AD. Increased expression of BRCA2 and RAD51 in lymph node metastases of canine mammary adenocarcinomas. Vet Pathol. 2009;46:416–422. doi: 10.1354/vp.08-VP-0212-K-FL. [DOI] [PubMed] [Google Scholar]
- 21.Maacke H, Jost K, Opitz S, et al. DNA repair and recombination factor Rad51 is over-expressed in human pancreatic adenocarcinoma. Oncogene. 2000;19:2791–2795. doi: 10.1038/sj.onc.1203578. [DOI] [PubMed] [Google Scholar]
- 22.Collis SJ, Tighe A, Scott SD, et al. Ribozyme minigenemediated RAD51 down-regulation increases radiosensitivity of human prostate cancer cells. Nucleic Acids Res. 2001;29:1534–1538. doi: 10.1093/nar/29.7.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Marignac VL, Rodrigue A, Davidson D, et al. The effect of a DNA repair gene on cellular invasiveness: XRCC3 over-expression in breast cancer cells. PLoS One. 2011;24(6):e16394. doi: 10.1371/journal.pone.0016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Yu H, Luo RZ, et al. Elevated expression of Rad51 is correlated with decreased survival in resectable esophageal squamous cell carcinoma. J Surg Oncol. 2011;104:617–622. doi: 10.1002/jso.22018. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 26.Pirrelli M, Caruso ML, Di Maggio M, et al. Are biopsy specimens predictive of HER2 status in gastric cancer patients? Dig Dis Sci. 2013;58:397–404. doi: 10.1007/s10620-012-2357-3. [DOI] [PubMed] [Google Scholar]
- 27.Miyagawa K. Clinical relevance of the homologous recombination machinery in cancer therapy. Cancer Sci. 2008;99:187–194. doi: 10.1111/j.1349-7006.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts JJ, Kotsaki-Kovatsi VP. Potentiation of sulphur mustard or cisplatin-induced toxicity by caffeine in Chinese hamster cells correlates with formation of DNA double-strand breaks during replication on a damaged template. Mutat Res. 1986;165:207–220. doi: 10.1016/0167-8817(86)90056-8. [DOI] [PubMed] [Google Scholar]
- 29.Kanno S, Hyodo M, Suzuki K, Ohkido M. Effect of DNAdamaging agents on DNA replication and cell-cycle progression of cultured mouse mammary carcinoma cells. Jpn J Cancer Res. 1985;76:289–296. [PubMed] [Google Scholar]
- 30.Zdraveski ZZ, Mello JA, Marinus MG, Essigmann JM. Multiple pathways of recombination define cellular responses to cisplatin. Chem Biol. 2000;7:39–50. doi: 10.1016/S1074-5521(00)00064-8. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z, Chen ZP, Malapesta A, et al. DNA repair protein level vis-à-vis anticancer drug resistance in the human tumor cell lines of the National Cancer Institute drug screening program. Anticancer Drugs. 2002;13:511–519. doi: 10.1097/00001813-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Husain A, He G, Venkatraman ES, Spriggs DR. BRCA1 upregulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II) Cancer Res. 1998;58:1120–1123. [PubMed] [Google Scholar]
- 33.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 34.Raderschall E, Stout K, Freier S, Suckow V, Schweiger S, Haaf T. Elevated levels of Rad51 recombination protein in tumor cells. Cancer Res. 2002;62:219–225. [PubMed] [Google Scholar]
- 35.El-Awady RA, Saleh EM, Dahm-Daphi J. Targeting DNA double-strand break repair: Is it the right way for sensitizing cells to 5-fluorouracil? Anticancer Drugs. 2010;21:277–287. doi: 10.1097/CAD.0b013e328334b0ae. [DOI] [PubMed] [Google Scholar]
- 36.Tsai MS, Kuo YH, Chiu YF, et al. Down-regulation of rad51 expression overcomes drug resistance to gemcitabine in human non-small-cell lung cancer cells. J Pharmacol Exp Ther. 2010;335:830–840. doi: 10.1124/jpet.110.173146. [DOI] [PubMed] [Google Scholar]
- 37.Chan N, Koritzinsky M, Zhao H, et al. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 2008;68:605–614. doi: 10.1158/0008-5472.CAN-07-5472. [DOI] [PubMed] [Google Scholar]
- 38.Engstron PF, Lavin PT, Klaassen DJ. Phase II evaluation of mitomycin and cisplatin in advanced esophageal carcinoma. Cancer Treat Rep. 1983;67:713–715. [PubMed] [Google Scholar]
- 39.Hilgenberg AD, Carey RW, Wilkins EW, Jr, et al. Preoperative chemotherapy, surgical resection, and selective postoperative therapy for squamous cell carcinoma of the esophagus. Ann Thorac Surg. 1988;45:357–363. doi: 10.1016/S0003-4975(98)90004-2. [DOI] [PubMed] [Google Scholar]
- 40.Ajani JA, Ryan B, Rich TA, et al. Prolonged chemotherapy for localized squamous carcinoma of the oesophagus. Eur J Cancer. 1992;28:880–884. doi: 10.1016/0959-8049(92)90140-W. [DOI] [PubMed] [Google Scholar]
- 41.Wong RK, Malthaner RA, Zuraw L, et al. Combined modality radiotherapy and chemotherapy in nonsurgical management of localized carcinoma of the esophagus: a practice guideline. Int J Radiat Oncol Phys. 2003;55:930–942. doi: 10.1016/S0360-3016(02)04278-5. [DOI] [PubMed] [Google Scholar]
- 42.Rebecca WO, Richard MA. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2003;CD002092. [DOI] [PubMed]
- 43.Law S, Fok M, Chow S, et al. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114:210–217. doi: 10.1016/S0022-5223(97)70147-8. [DOI] [PubMed] [Google Scholar]
- 44.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 45.Saeki H, Morita M, Nakashima Y, et al. Neoadjuvant chemotherapy for clinical stage II–III esophageal squamous cell carcinoma. Anticancer Res. 2011;31:3073–3077. [PubMed] [Google Scholar]
- 46.Morita M, Masuda T, Okada S, et al. Preoperative chemotherapy for esophageal cancer: factors associated with clinical response and postoperative complications. Anticancer Res. 2009;29:2555–2562. [PubMed] [Google Scholar]
- 47.Sarbia M, Ott N, Pühringer-Oppermann F, et al. The predictive value of molecular markers (p53, EGFR, ATM, CHK2) in multimodally treated squamous cell carcinoma of the esophagus. Br J Cancer. 2007;97:1404–1508. doi: 10.1038/sj.bjc.6604037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duong C, Greenawalt DM, Kowalczyk A, et al. Pretreatment gene expression profiles can be used to predict response to neoadjuvant chemoradiohterapy in esophageal cancer. Ann Surg Oncol. 2007;14:3602–3609. doi: 10.1245/s10434-007-9550-1. [DOI] [PubMed] [Google Scholar]
- 49.Ishida M, Morita M, Saeki H, et al. Expression of p53 and p21 and the clinical response for hyperthermochemoradiotherapy in patients with squamous cell carcinoma of the esophagus. Anticancer Res. 2007;27:3501–3506. [PubMed] [Google Scholar]
- 50.Yamasaki M, Miyata H, Tanaka K, et al. Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology. 2011;80:307–313. doi: 10.1159/000329806. [DOI] [PubMed] [Google Scholar]
- 51.Kawaguchi Y, Kono K, Mimura K, et al. Targeting EGFR and HER-2 with cetuximab- and trastuzumab-mediated immunotherapy in oesophageal squamous cell carcinoma. Br J Cancer. 2007;97:494–501. doi: 10.1038/sj.bjc.6603885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Vita F, Orditura M, Martinelli E, et al. A multicenter phase II study of indication chemotherapy with FOLFOX-4 and cetuximab followed by radiation and cetuximab in locally advanced oesophageal cancer. Br J Cancer. 2011;104:427–432. doi: 10.1038/sj.bjc.6606093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.