Abstract
In the risk assessment of chemical substances, aggregation of exposure to a substance from different sources via different pathways is not common practice. Focusing the exposure assessment on a substance from a single source can lead to a significant underestimation of the risk. To gain more insight on how to perform an aggregate exposure assessment, we applied a deterministic (tier 1) and a person-oriented probabilistic approach (tier 2) for exposure to the four most common parabens through personal care products in children between 0 and 3 years old. Following a deterministic approach, a worst-case exposure estimate is calculated for methyl-, ethyl-, propyl- and butylparaben. As an illustration for risk assessment, Margins of Exposure (MoE) are calculated. These are 991 and 4966 for methyl- and ethylparaben, and 8 and 10 for propyl- and butylparaben, respectively. In tier 2, more detailed information on product use has been obtained from a small survey on product use of consumers. A probabilistic exposure assessment is performed to estimate the variability and uncertainty of exposure in a population. Results show that the internal exposure for each paraben is below the level determined in tier 1. However, for propyl- and butylparaben, the percentile of the population with an exposure probability above the assumed “safe” MoE of 100, is 13% and 7%, respectively. In conclusion, a tier 1 approach can be performed using simple equations and default point estimates, and serves as a starting point for exposure and risk assessment. If refinement is warranted, the more data demanding person-oriented probabilistic approach should be used. This probabilistic approach results in a more realistic exposure estimate, including the uncertainty, and allows determining the main drivers of exposure. Furthermore, it allows to estimate the percentage of the population for which the exposure is likely to be above a specific value.
Keywords: aggregate, exposure, probabilistic, parabens, personal care products, children
INTRODUCTION
To evaluate the risk of adverse health effects from chemicals in the population, the toxicity of a substance at a certain dose (the hazard) as well as the exposure to a chemical need to be established. Many chemical substances in consumer products are used in multiple product categories, leading to multiple sources of exposure. In addition, a substance could enter the human body via multiple routes such as via the skin, the lungs or the gastrointestinal tract. In risk assessment, summation or aggregation of the exposure following different sources is not common practice. Especially when these sources are regulated under different legal frameworks, aggregate exposure is not taken into account. This may lead to a situation in which the risks of chemical exposure are significantly underestimated.1
In the WHO/IPCS framework, aggregate exposure is defined as exposure to the same substance from multiple sources, via multiple pathways and routes. It differs from cumulative exposure, which is defined as the total exposure of substances sharing the same mechanism of action.2 In several regulatory frameworks, the need to consider aggregate exposure is mentioned. However, for the execution no specific guidance document is available.2 Different methods and tools have been proposed to perform an aggregate exposure assessment, often in a tiered approach.3 This can range from a rough estimation of the maximal level of exposure for a population in orders of magnitude (designated here as tier 0) to deterministic modelling with conservative assumptions (tier 1), and ultimately to a more realistic estimation using (person-oriented) probabilistic methods (tier 2).2, 3, 4
In order to achieve a more complete estimate of the exposure, there is a need to better understand how to perform an aggregate exposure assessment. Here, we apply both a tier 1 and tier 2 approach for a case example on paraben exposure from personal care products to gain more insight into the feasibility and necessity of refining an aggregate exposure approach.
Parabens are currently used as preservatives in a wide variety of products. They are used in personal care products for adults and children, in consumer products such as dog shampoo, in pharmaceutical products such as antibiotics5 and they are used as food additives.6 In a 2005 publication, from a total exposure of 76 mg/kg bw/day to methyl- and propylparaben for adults, personal care product contribution has been estimated at 50 mg/kg bw/day, while pharmaceutical products contributed for 25 mg/kg bw/day and exposure via food was only 1 mg/kg bw/day.7 As personal care products provided the main source of exposure, the aggregate exposure assessment here is focused on these product types containing four linear paraben esters that are mostly used, namely methyl-, ethyl-, propyl- and butylparaben. The main exposure route is via dermal application of personal care products. A minor route is (accidental) oral exposure, as some toothpastes contain parabens.8 Inhalatory exposure is unlikely, as the vapour pressure of the four parabens is very low and no spray applications containing parabens have been identified.
Given the estrogenic effects of parabens found in immature rats and mice,5, 6, 9, 10, 11 and the potential severity of the effects during early human child development,12 we assessed the aggregate exposure for children between 0 and 3 years of age (hereafter designated as young children). This specific age group has also been identified by the Danish delegation of the Council of the European Union that has announced a ban on propyl- and butylparaben in personal care products for children aged <3 years. The ban was enforced in Denmark on 15 March 2011 after the publication of a report of the Danish Environmental Protection Agency (EPA) by Tonning et al.13 with an assessment done for 2-year-old children.
The hazard identification for parabens will not be discussed here as such. The results of the different tiers, however, are considered in light of no-observed adverse-effect levels (NOAELs). The NOAELs used are established in recent evaluations of the European Scientific Committee on Consumer Safety (SCCS) and the European Food and Safety Authority (EFSA). In the case of propylparaben for which there is no opinion document available, the NOAEL is based on a study by Oishi .9 The results of the evaluation will not be used to derive safe levels of exposure for parabens as this would require a more elaborate assessment of the hazard. Here, we focus on the aggregate exposure aspect of the risk assessment and how the outcome is influenced by the two different tiers.
MATERIALS AND METHODS
Tier 1: Deterministic Approach
Personal care products for young children that contain methyl-, ethyl-, propyl- or butylparaben have been identified;8 (Supplementary Information SI1, Table 1). For the exposure calculation per paraben, the following parameters are used: (1) the maximum amount of a paraben found in a product (in mg/kg product, Supplementary Information SI1, Table 2); (2) ConsExpo default use amounts of personal care products (in g of product) as reasonable worst-case estimates (Supplementary Information SI1, Table 3);14 and (3) ConsExpo defaults of frequency of use (events per day) as reasonable worst-case estimates (Supplementary Information SI1, Table 3).14
The Cosmetics Fact Sheet presents specific (default) estimations for bath oil, baby salve and toothpaste regarding the amount of product used by young children.14 For personal care products in the categories that involve application on body surface area (sunscreen, aftersun, body lotion, shower/bath soap), the default value for adults was extrapolated to children using a correction factor that accounts for the smaller total body surface area of children. For example, the amount of sunscreen applied by adults based on the Cosmetics Fact sheet is corrected by a factor of 0.27 (4800 cm2 for a child of 1.5 years old/17,500 cm2 for an adult based on table 16 and table 12 in reference Bremmer et al.,15 respectively). This is under the assumption that indeed less product is used for children than for adults. For liquid soap that is mainly used to wash hands, a factor of 0.29 is used (247.2 cm2 for children's hands of 1.5 years old/857.5 cm2 for adult- hands). The default factor for hair lotion, shampoo and two-inone shampoo were extrapolated using a factor of 0.66 (768 cm2 for head surface area of a child of 1.5 years old/1155 cm2 for adults).
Data on frequency of use and amount of baby wipes is scarce. The use frequency is estimated based on expert judgment to coincide with every change of a diaper resulting in on average five times a day one wipe. Commercially available wipes have an average weight of 5 g per wipe. As contact with the skin is not permanent and does not necessarily involve the full surface of the wipe, a retention factor is used. According to van Engelen et al.,16 0.5 ml of liquid from a wipe deposits on the skin per event. Taking 5 g of wipes used five times per day and a retention factor of 0.1, this results in a total amount on the skin of 2.5 g/day. The Research Institute for Fragrance Materials (RIFM) reports an exposure to 4 mg/cm2/day of a substance via wiping.17 When considering the surface area of application of a 1.5-year-old child of half the trunk (groin, buttocks and upper thighs: 1728 cm2), this results in an external exposure of 3 g of product per day,17 which is close to the 2.5 g/day that is used here (Supplementary Information SI1, Table 3).
For all rinse-off products like shampoo, two-in-one shampoo, liquid soap, shower/bathsoap and bath oil, a dilution factor is taken into account leading to a retention factor of 0.01.18 For leave-on products like sunscreen, aftersun, body lotion and baby salve, it is assumed that all product applied to the body stays in contact with the skin for a sufficient amount of time for parabens to be absorbed. Janjua et al.19 have reported maximum serum levels 3 h after dermal application of a cosmetic formulation containing 2% of butylparaben and 2% each of two different phthalates. Hair lotion is not rinsed-off, and it is estimated that skin contact takes place for 1/10 of the total amount. Therefore, a retention factor of 0.1 is used.18 The external exposure equation with refinement regarding retention as described above is:
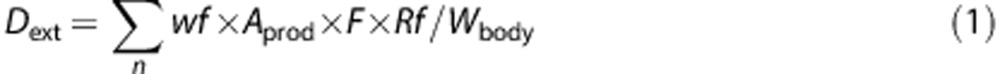 |
where Dext: external dose after the dermal and oral route (mg/kg bw/day); n: number of products; wf: weight fraction of the compound in the product (mg/kg); Aprod: amount of product applied (kg); F: frequency of use (events/day); Rf: retention factor; andWbody: average body weight of a 1.5-year-old child of 11.1 kg15 (kg bw).
The internal exposure calculation includes the dermal absorption of the unmetabolized paraben for all products, except toothpaste. Maximum values as determined in in vitro studies using human skin have been chosen (Table 1).
Table 1. Output tier 1: external and internal exposure to parabens and Margin of Exposure (MoE).
| Methyl | Ethyl | Propyl | Butyl | |
|---|---|---|---|---|
| External exposure (mg/kg bw/day) based on maximum amount including retention factors | 2.32 | 0.36 | 1.05 | 0.47 |
| Dermal absorption | 36%32 | 55%32 | 37%33 | 42%32 |
| Internal exposure (mg/kg bw/day) based on maximum amount including retention factors and dermal absorption | 1.01 | 0.20 | 0.41 | 0.20 |
| NOAEL (external and internal) (mg/kg bw/day) | 1000;2122 | 1000;2122 | 3.39 | 221 |
| MoE | 991 | 4966 | 8 | 10 |
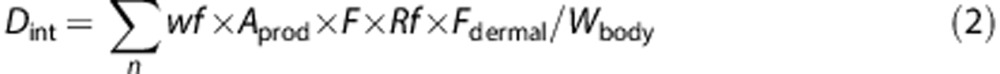 |
where Dint: internal dose after the dermal route (mg/kg bw/day) andFdermal: dermal absorption (fraction).
For toothpaste, in the absence of any data, the oral absorption is here assumed to be 1, so the internal exposure is the same as the external exposure. For all calculations, it is assumed that 100% of the products contain parabens. Calculations are done by hand according to the equations and a random part has been checked by running the calculations in ConsExpo 5.0 beta http://www.ConsExpo.com.
Tier 2: Person-Oriented Probabilistic Approach
Raw data on weight fraction measurements in 12 product types by the Dutch Food and Product Safety Authority (NVWA) in 2006 have been included.8 More detailed information on the type of product used, the amount of product and use frequency by young children has been obtained from a small pilot survey (Supplementary Information SI2). In this survey, we recruited Dutch parents of 28 children (13 girls and 15 boys) to participate in an online questionnaire (response rate of 21.5%). Respondents were asked to estimate product use amounts based on photographs showing three different amounts (Supplementary Information SI2, Figure 1). The survey included only those product types for which at least one product contained methyl-, ethyl-, propyl- and/or butylparaben (Supplementary Information SI1, Table 1). It is acknowledged that this pilot survey is only limited and cannot be taken to accurately represent the entire Dutch population. The responses are collected in an MS Access database. A model population of 1000 persons is constructed by repeated sampling from the data with product use data. This yielded a population for which the use frequency and the amount used per event is given for all the products considered. For all these persons, for a sequence of 56 days it is determined (based on the product use data) (1) whether a product is used on any particular day in the period and, if so, (2) how much of the product is being used. Combining each person in the modelled population with a product from the list of products in the product database (Supplementary Information SI2, Table 1), daily contact profiles for each person in the population are constructed. In summary, this contact profile contains information on how much of a product a person uses on a particular day and how much paraben this product contains. The different steps in the exposure calculation as described above are summarized in Figure 1.
Figure 1.
Structure and coupling of input provided by the survey and product use scenarios in the tier 2 person-oriented probabilistic approach.
For the exposure calculation, the oral absorption is set to 100%, and for the dermal absorption a distribution of 1–55% is used for each paraben. The distribution range represents the uncertainty in dermal absorption with the lowest reported number for unmetabolized paraben of 1%20 and the highest for ethylparaben from in vitro absorption studies with human skin. Summer is the relevant time period for the aggregate exposure assessment, because sunscreen and aftersun products are used in this period. Equations 1 and 2 are used to evaluate the aggregate exposure for all individuals and all days in the simulation. The aggregate exposure per day is determined by adding all exposures on the same day for one person and subsequently averaging the daily aggregate exposure for each individual. The result is a distribution of the daily average aggregate exposure for all persons in the population. Percentiles of this distribution can be compared against the NOAEL. It gives an indication on the fraction of the population with average exposures above a certain Margin of Exposure (MoE). All tier 2 calculations and Access data operations are performed in R modelling software. The access database and R files can be obtained from the authors upon request.
Uncertainty Analysis
The probabilistic evaluation of the aggregate population exposure takes account of the detailed information on the variability of exposure determinants. However, a number of factors remain uncertain. These include: (1) (age dependent) dermal absorption of parabens, (2) concentration of butylparaben in a product (due to analytical difficulties), (3) retention factors, (4) uncertainty in the specification of the amount of product used (using photographs), (5) uncertainty in the reporting of the frequency of use (in broad ranges such as between one and two times a week), and (6) the limited sample size of the survey. The potential effects of these uncertainties are assessed one by one. In the case of parameters 1 and 3, lower, most probable and upper bounds were estimated. Using these estimates, the population exposure was calculated in three different scenarios: the low, most probable and high-exposure scenarios. Uncertainty in parameters 4 and 5 results from specification of product use in broad ranges rather than precise numbers. The uncertainty due to this stratification was assessed quantitatively by a bootstrap simulation. The specified ranges for used amounts were repeatedly sampled. For each sample, the median and 97.5 percentile of the product use was assessed, giving an estimate of the variation in these percentiles. The uncertainty in the higher percentiles of the exposure distribution due to limited sample size of the survey was assessed by evaluating two scenarios: one that included all the data from the product use survey (lower bound), and the other by leaving out the data of the highest user in the survey (most probable and upper bound). This represents the uncertainty in whether this highest user accurately represents a high percentile of the population or rather is an outlier in the survey.
RESULTS
Tier 1
Methylparaben, which is used in the majority of personal care products, in the highest quantities leads to the highest external exposure of 2.32 mg/kg bw/day in young children. External exposure to propylparaben is around half the amount of methylparaben, while children are exposed to 0.36 mg/kg bw/day and 0.47 mg/kg bw/day ethyl- and butylparaben, respectively.
The internal exposure is calculated per product type by including the oral or dermal absorption fraction according to Equation 2 (Table 1). The highest reported paraben-specific dermal absorption fractions from human skin in vitro studies are chosen that range from 36% to 55%. Following oral exposure (ingestion of toothpaste), absorption is set to 100%. Of the four parabens, methylparaben has the highest aggregate internal exposure of 1.01 mg/kg bw/day. Aggregate internal exposure to propylparaben is estimated to be 0.41 mg/kg bw/day, while exposure to ethyl- and butylparaben equals 0.20 mg/kg bw/day.
Tier 2
A probabilistic approach is employed in tier 2 to refine the exposure assessment and to estimate the variation in the exposure of the population. Using a survey, data on (1) the type of personal care product used, (2) the amount of product used, (3) the frequency of use within the last 6 months as well as (4) the age of the child, and (5) gender has been obtained (Supplementary Information SI2, Table 1). On average, young children use six products from the list of 12 with a range from three (person 3) to eight (person 18 and 22) product types per person (Supplementary Information SI2, Table 1).
Tier 2 calculations resulted in cumulative probability plots for methyl- ethyl-, propyl- and butylparaben (Figures 2a–d). These figures show that, the entire modelled population is expected to have an internal exposure below the internal exposure level estimated by the tier 1 approach for every paraben. The probability of children in the population for whom the internal exposure level exceeds an assumed “safe” MoE (based on commonly applied safety factors for intra- and interspecies differences of 10 × 10) is estimated to be zero. Figures 2c and d shows that for propyl- and butylparaben, the percentile of the population with an exposure probability above the assumed “safe” MoE of 100 is 13% and 7% respectively.
Figure 2.
Cumulative probability plots with on the y axis the probability that a young child in the population is exposed and on the x axis the corresponding internal exposure level in mg/kg bw/day on a log scale for (a) methylparaben, (b) ethylparaben, (c) propylparaben and (d) butylparaben. The dashed line indicates the outcome of the exposure estimation in the first tier. The solid line indicates the NOAEL/100.
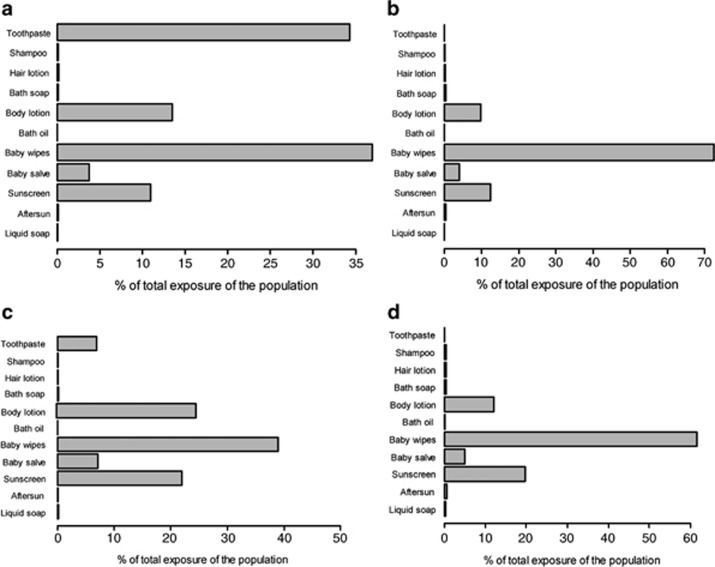
To identify drivers of exposure, the relative contribution of a certain product type can be calculated as a percentage of the total aggregated population exposure per paraben (Figures 3a–d). Baby wipes have the highest relative contribution for all parabens and can be assigned to a high total amount of product that is estimated to be used. For methylparaben, toothpaste has the second highest relative contribution. This can be explained by the high concentrations of methylparaben that are measured in toothpaste and the assumption that oral absorption is 100%. Leave-on products like body lotion, baby salve and sunscreen also show a high contribution. They contribute significantly due to the fact that the amount applied stays in contact with the skin. The other product types have either been reported to be hardly ever used or used in very small amounts or the product does not often contain one of the four parabens.
Figure 3.
Relative contribution of product types to the total aggregated internal exposure of (a) methylparaben, (b) ethylparaben, (c) propylparaben and (d) butylparaben for young children.
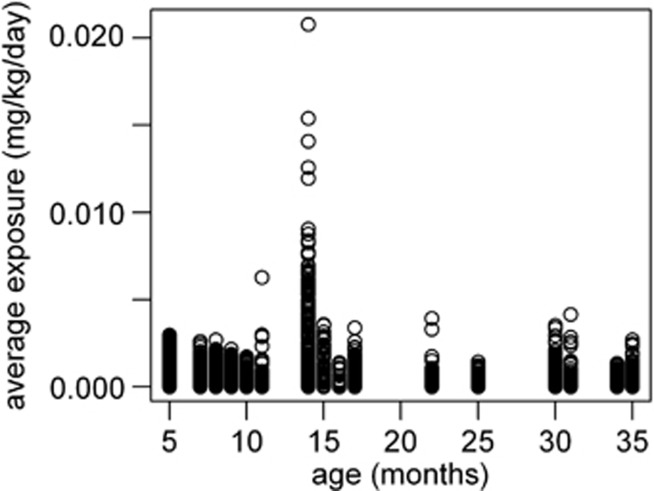
From the analysis of the age-dependent exposure for butylparaben (Figure 4) in combination with the data from the product use survey, it is observed that, at age 14 months, there is a single user that drives the highest percentiles of the population's exposure. The same profile is observed for the other three parabens (data not shown).
Figure 4.
Age-dependent aggregated internal exposure to butylparaben.
Uncertainty Analysis
The variation in the exposure assessment is due to both uncertainty (lack of knowledge) and natural variability in the input data. The potential effect of the uncertainties, with propylparaben as an example, has been assessed by performing an evaluation of the potential range of variation of exposure as a result of these uncertainties. For all uncertain parameters, the lower, most probable and upper bounds are estimated (Figure 5). The uncertainty analysis gives an estimate of the maximum range of uncertainty in each percentile of the population's exposure. This maximum uncertainty in the exposure assessment spans more than two orders of magnitude.
Figure 5.
Range of uncertainty for propylparaben exposure in young children. The dashed line indicates the outcome of the exposure estimation in the first tier. The solid line indicates the NOAEL/100 (assuming commonly applied safety factors for intraspecies and intraspecies 10 × 10 to result in a “safe” MoE).
DISCUSSION
Illustrative Risk Assessment
Following the tier 1 approach, the internal aggregate exposure levels of propyl- and butylparaben of 0.41 mg/kg bw/day and 0.20 mg/kg bw/day, respectively, approximate the worst-case internal exposure estimates reported by the Danish EPA (0.22 mg/kg bw/day for both).13 For methyl- and ethylparaben exposures of 1.01 mg/kg bw/day and 0.20 mg/kg bw/day, respectively, no comparative values are known in young children.
The interpretation of the tier 1 aggregate exposure assessment is best illustrated by using the outcome in a risk assessment. Here, the total internal exposure estimates are divided by specific NOAELs per paraben to obtain the MoE (Table 1). The NOAELs for methyl-, ethyl- and propylparaben are derived from studies following oral administration that show effects on male reproduction and hormone levels.10 The assumption is that there is 100% oral absorption in these studies. A NOAEL of 1000 mg/kg bw/day for methyl- and ethylparaben is chosen based on opinions from the SCCS21 and EFSA.22 For propylparaben, a NOAEL of 3.3 mg/kg bw/day is taken based on effects of sperm counts.9 The NOAEL of 2 mg/kg bw/day for butylparaben is derived after subcutaneous administration and based on effects on testis development,6 also assuming 100% absorption based on the SCCS opinion.21
For methyl-and ethylparaben, the MoE here is above the assumed “safe” MoE of 100 (based on commonly applied safety factors of 10 × 10 for intra- and interspecies differences), indicating that no further refinement or higher tier calculation is necessary. For propyl- and butylparaben, however, the MoE calculated is only 8 and 10, respectively, indicating that further evaluation of the exposure calculations is necessary.
When summing up the individual exposures for every product type that is used for children that contains methyl-, ethyl-, propyl- or butylparaben, this will likely lead to an unrealistically high exposure estimate. For example, it is highly unlikely that all 12 product types are used by one individual. In addition, not all products of the same product type contain paraben(s) to the same extent. It is conceivable that when performing a more realistic exposure assessment with further refinements, this might lead to a much higher MoE. The simple deterministic method is not suited to address this. Refinement is also difficult, as detailed data on the use of personal care products was initially unavailable, and it is unknown whether extrapolation from adult use by, for example, scaling the amount of product used to body surface area is appropriate.
The probabilistic approach used in tier 2 does not suffer from these drawbacks but requires a significantly higher data input. Following a small survey and construction of a daily contact profile, a probability distribution of paraben exposure in the modeled population is made. In the second tier assessment, it was estimated that no child in the modeled population is expected to be exposed to levels of methyl- and ethylparaben at or above the outcome of the tier 1 assessment (i.e., the calculated MoE is considered large enough). However, for propyl- and butylparaben, there is still a probability that some children in the population would be exposed to levels of propyl- and butylparaben exceeding the assumed “safe” MoE. The likelihood of this exposure to occur is determined by both uncertainty and variability in the assessment. A detailed uncertainty analysis is performed to discern this.
Uncertainty Analysis
The methods used in a first tier are not able to evaluate the degree of conservatism, as the contribution of information on variability and uncertainty is not explicitly accounted for. Variability is present in the body weight, age, weight percentage of parabens in a product and the number of products used. In tier 2, uncertainty in the dermal absorption is, to some extent, included in the initial estimation as a range of 1–55%. Still, the use of fixed absorption percentages rather than dose-dependent absorption rates may underestimate actual absorption in realistic low skin-loading conditions.23 In addition, dermal absorption may vary considerably with the location it is applied on the skin, the age of the child and from person to person. Overall, it seems that the range assumed in the exposure assessment may underestimate the actual internal exposure with a maximum factor of two (55% versus 100%), when other uncertain factors are not considered. The uncertainty is accounted for by setting the dermal absorption to a fixed level of 50% and performing the calculation. This step is then repeated with the dermal absorption set on 1% and on 100% as a minimum and maximum, respectively.
In addition, there is a level of uncertainty in the measured butylparaben concentration in some products. During measurements, the butylparaben peak in the chromatogram coincided with benzylparaben. Benzylparaben is used far less in personal care products according to the product labels while butylparaben is reported more frequently as an ingredient. The worst-case assumption is made that the whole amount reported is butylparaben. In doing so, the level of butylparaben in products that has been reported by Rastogi et al.24 of 0.07% is only exceeded three times out of 84 cases. Therefore, the influence of the uncertainty in this parameter is expected to be small and only slightly overestimates the exposure.
For the retention of parabens from dermal wipes, 10% was assumed. Actual data on transfer of solutions from wipes to skin is not available. To estimate the order of magnitude of the potential uncertainty, data on transfer of pesticides and fluorescent tracers from different surfaces are used as surrogate with ranges of transfer efficiencies of 0.1–20%,25 1–5%26 and 5–16%.27 The representativity of this data for the retention of solutions from dermal wipes is difficult to assess but assuming a possible range of 1–20% for the retention seems reasonable. From this point of view, the value of 10% adopted in the exposure assessment does not seem overly conservative. It is estimated that actual exposure to dermal wipes varies between 10 times lower and 2 times higher than used in the assessment.
Uncertainty due to stratification in the questionnaire results from the fact that respondents specified the amount and frequency of product use in broad ranges rather than precise numbers. The maximum variation in the median and 97.5 percentiles was 25–30% (data not shown). Compared with other sources of uncertainty, this is considered a minor contribution.
When plotting the exposure levels per person, it was observed that a specific child in the survey (14 months old) has a relatively high exposure. A larger sample size of the survey would give more insight whether this high-end user profile occurs more often in the population or is an isolated observation. The observation may be caused by bad reporting by the person filling out the survey, for example, when a question has been misinterpreted or difficulties exist in estimating use of amount of product from photographs and could then be regarded as an outlier. On the other hand, this observation could represent a high-end user that actually exists in the population.
Taken all of the above into account, the uncertainty in the tier 2 exposure assessment is estimated to span more than two orders of magnitude. However, this range represents an estimate of the maximum uncertainty, not taking into account the probability distribution of the uncertainty. Performing a two-dimensional probabilistic evaluation in which uncertainty and variability are separated and represented with probability distributions will lead to significantly smaller confidence intervals. Additionally, uncertainty in the exposure assessment for propyl- and butylparaben could be reduced by collecting more suitable data. Most prominently, this can be done by expanding the pilot survey on product use and obtaining more detailed information on the transfer of parabens from baby wipes that was identified as a main driver of the exposure.
Steps need to be taken before aggregate exposure can be assessed routinely. It would be useful to perform an extended personal care product user survey for children, in order to sketch the daily contact profile to multiple products and to get a complete characterization of exposure. Until now, large surveys into personal care product use have been performed only for adults.28, 29, 30 It is a considerable effort to obtain the data, but once established the survey could be used routinely for different exposure assessments. The data can be used not only to apply a probabilistic approach but can also be used to refine tier 1 assessments, by including co-use and non-use patterns as described for adult exposure to parabens from personal care products.31 As mentioned previously, based on Soni et al.,7 pharmaceutical products contributed as the second largest product group towards the total paraben exposure. More exposure data via these products would be needed to obtain an even more accurate aggregate estimate.
In conclusion, a tier 1 aggregate exposure approach can be performed using simple equations and default point estimates and serves as a starting point for exposure and risk assessment. If the outcome gives no reason for further refinement, the assessment is then finalized. In case a more detailed assessment is warranted, the person-oriented probabilistic modelling approach should be used. A tier 2 approach is more complex and data demanding but will lead to a more realistic and much more informative exposure assessment. When distributional data representative of the population is available, it is possible to determine the percentage of the population for which the exposure is above a specific value. It also allows determining the main drivers of the exposure and making a detailed analysis of the uncertainty in the exposure assessment, identifying key elements for future improvement of the assessment.
Acknowledgments
We thank Jacqueline Biesterbos (Radboud University Nijmegen Medical Centre, RUNMC) for providing the pictures for the survey and critically reading the manuscript, Remmelt van Dijk (NVWA) for making the data on paraben measurements in personal care products available for this approach and all respondents who have filled in the survey on personal care product use. This work was financially supported by order and for the account of Ministry of Health, Welfare, and Sports, The Netherlands (kennisvraag 5.1.11).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
Supplementary Material
References
- Schuur AG, Delmaar JE, van Engelen JGM.Geaggregeerde blootstelling. Gebruik in verschillende kaders en toekomstige mogelijkheden. National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2009. Report no. 3200150022009.
- Meek ME, Boobis AR, Crofton KM, Heinemeyer G, van Raaij MTM, Vickers C. Risk assessment of combined exposure to multiple chemicals: a WHO/IPCS framework. Regul Toxicol Pharmacol. 2011;60 (Suppl 1:S1–S14. doi: 10.1016/j.yrtph.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Delmaar JE, van Engelen JGM.Aggregating human exposure to chemicals. An overview of tools and methodologies. National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2006. Report no. 6307000122006.
- Arnold SF, Price PS. Modeling mixtures resulting from concurrent exposures to multiple sources. Toxicol Appl Pharmacol. 2007;223:121–124. doi: 10.1016/j.taap.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Hoberman AM, Schreur DK, Leazer T, Daston GP, Carthew P, Re T, et al. Lack of effect of butylparaben and methylparaben on the reproductive system in male rats. Birth Defects Res B Dev Reprod Toxicol. 2008;83:123–133. doi: 10.1002/bdrb.20153. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Turner KJ, Brown D, Sharpe RM. Effect of neonatal exposure to estrogenic compounds on development of the excurrent ducts of the rat testis through puberty to adulthood. Environ Health Perspect. 1999;107:397–405. doi: 10.1289/ehp.99107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens) Food Chem Toxicol. 2005;43:985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Netherlands Food and Consumer Product Safety Authority (NVWA) Cosmetische producten voor kinderen: Inventarisatie van de markt en de veiligheidsborging door producenten en importeurs2007
- Oishi S. Effects of propyl paraben on the male reproductive system. Food Chem Toxicol. 2002;40:1807–1813. doi: 10.1016/s0278-6915(02)00204-1. [DOI] [PubMed] [Google Scholar]
- Oishi S. Lack of spermatotoxic effects of methyl and ethyl esters of p-hydroxybenzoic acid in rats. Food Chem Toxicol. 2004;42:1845–1849. doi: 10.1016/j.fct.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Vo TT, Yoo YM, Choi KC, Jeung EB. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod Toxicol. 2010;29:306–316. doi: 10.1016/j.reprotox.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 2010;30:301–312. doi: 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Tonning K, Jacobsen E, Pedersen E, Strange M, Brunn Poulsen P, Moller L, et al. Survey and Health Assessment of the exposure of 2 year-olds to chemical substances in Consumer Products. Danish Ministry of the Environment, Copenhagen, Denmark, 2009, pp 1–327. . http://www.mst.dk/Publikationer/Publications/2009/10/978-87-92548-81-8.htm .
- Bremmer HJ, Prud'homme de Lodder LCH, van Engelen JGM.Cosmetics Fact Sheet. National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2006. Report no. 320104001/2006.
- Bremmer HJ, Prud'homme de Lodder LCH, van Engelen JGM.General Fact Sheet. National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2006. Report no. 320104002/2006.
- van Engelen JGM, Prud'homme de Lodder LCH.Non-food products: how to assess children's exposure? National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2007. Report no. 3200050052007.
- Research Institute for Fragrance Materials (RIFM) Dermal sensitization Quantitative Risk Assessment (QRA) for fragrance ingredients. Research Institute for Fragrance Materials: Bilthoven, The Netherlands, 2006.
- Scientific Committee on Consumer Safety (SCCS) The SCCS's notes of guidance for the testing of cosmetic ingredients and their safety evaluation2011
- Janjua NR, Mortensen GK, Andersson AM, Kongshoj B, Skakkebaek NE, Wulf HC. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ Sci Technol. 2007;41:5564–5570. doi: 10.1021/es0628755. [DOI] [PubMed] [Google Scholar]
- Andersen FA. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27 (Suppl 4:1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- Scientific Committee on Consumer Products (SCCP) Opinion on Parabens, COLIPA n° P822010
- European Food and Safety Authority (EFSA) Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to para hydroxybenzoates(E 214-219)2004
- Kissel JC. The mismeasure of dermal absorption. J Expo Sci Environ Epidemiol. 2011;21:302–309. doi: 10.1038/jes.2010.22. [DOI] [PubMed] [Google Scholar]
- Rastogi SC, Schouten A, de Kruijf N, Weijland JW. Contents of methyl-, ethyl-, propyl-, butyl- and benzylparaben in cosmetic products. Contact Dermat. 1995;32:28–30. doi: 10.1111/j.1600-0536.1995.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Hubal EA, Nishioka MG, Ivancic WA, Morara M, Egeghy PP. Comparing surface residue transfer efficiencies to hands using polar and nonpolar fluorescent tracers. Environ Sci Technol. 2008;42:934–939. doi: 10.1021/es071668h. [DOI] [PubMed] [Google Scholar]
- Camann D, Harding HJ, Geno PW, Agrawal SR.Comparison of Methods to Determine Dislodgeable Residue Transfer from FloorsEPA/600/R-96/089Office of Research and Development: Washington, DC, USA; 1996 [Google Scholar]
- Hsu JP, Camann DE, Schatterberg H, III, Wheeler B, Villalobos K, Garza M, et al. New dermal exposure sampling technique. EPA/AWMA International Symposium: Raleigh, NC, USA; 1990. Measurement of Toxic and Related Air Pollutants. [Google Scholar]
- Hall B, Tozer S, Safford B, Coroama M, Steiling W, Leneveu-Duchemin MC, et al. European consumer exposure to cosmetic products, a framework for conducting population exposure assessments. Food Chem Toxicol. 2007;45:2097–2108. doi: 10.1016/j.fct.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Hall B, Steiling W, Safford B, Coroama M, Tozer S, Firmani C, et al. European consumer exposure to cosmetic products, a framework for conducting population exposure assessments Part 2. Food Chem Toxicol. 2011;49:408–422. doi: 10.1016/j.fct.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Biesterbos JWH, Dudzina T, Delmaar JE, Bakker MI, Russel FG, von Goetz N, et al. Usage patterns of personal care products: important factors for exposure assessment Food Chem Toxicol 2013558–17.submitted. [DOI] [PubMed] [Google Scholar]
- Cowan-Ellsberry CE, Robison SH. Refining aggregate exposure: example using parabens. Regul Toxicol Pharmacol. 2009;55:321–329. doi: 10.1016/j.yrtph.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Cross SE, Roberts MS. The effect of occlusion on epidermal penetration of parabens from a commercial allergy test ointment, acetone and ethanol vehicles. J Invest Dermatol. 2000;115:914–918. doi: 10.1046/j.1523-1747.2000.00151.x. [DOI] [PubMed] [Google Scholar]
- Jewell C, Prusakiewicz JJ, Ackermann C, Payne N.A, Fate G, Voorman R, et al. Hydrolysis of a series of parabens by skin microsomes and cytosol from human and minipigs and in whole skin in short-term culture. Toxicol Appl Pharmacol. 2007;225:221–228. doi: 10.1016/j.taap.2007.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.