Abstract
Objective
To investigate the ameliorative role of Tetrapleura tetraptera (Schum and Thonn) Taub (T. tetraptera) leaf in hyperglycemia with associated conditions like oxidative stress, kidney damage and disorders in lipid metabolism.
Methods
Five groups of five rats each intraperitoneally received the following treatment schedules for 7 d: untreated normal control, untreated alloxan-diabetic control, diabetic treated with glibenclamide, normal rats treated with extract (50 mg/kg) and diabetic rats treated with the extract. Evaluations were made for fasting blood sugar, body weight changes, malondialdehyde, aspartate aminotransferase, alanine aminotransferase, bilirubin, superoxide dismutase, catalase, lipid profile, packed cell volume, hemoglobin, urea and creatinine in all the rats.
Results
Whereas the untreated diabetic rats showed a significant decrease (P<0.05) in packed cell volume, superoxide dismutase, catalase and high-density lipoprotein-cholesterol with a concomitant increase in the levels of malondialdehyde, fasting blood sugar, aspartate aminotransferase, alanine aminotransferase, bilirubin, urea and creatinine, administration of methanolic extract of T. tetraptera leaf or glibenclamide alleviated these altered parameters in the treated rats.
Conclusions
Methanolic extract of T. tetraptera leaves possesses a potent capacity for treatment of diabetes and the accompanying complications, including oxidative stress and hyperlipidemia.
Keywords: Antidiabetic, Tetrapleura tetraptera, Alloxan-induced diabetes, Antioxidant, Hepatoprotection, Disease chemoprevention
1. Introduction
Many herbal products have been prescribed for the management of diabetes mellitus in ancient and recent literature[1]. Herbal medicines are popularly used remedies for many diseases by a vast majority of the world's population, because allopathic medicines have large number of side-effects. Thus, herbal formulations have attained widespread acceptability as therapeutic agents in many developing countries as anti-diabetics, hepatoprotective and lipid lowering agents[2]–[4]. Whereas a number of plants used in folk medicine have indeed been scientifically confirmed to possess such activity, many others, including Tetrapleura tetraptera (Schum and Thonn) Taub (T. tetraptera) used in the traditional medicine by the Igala people of north central Nigeria, are yet to be validated.
T. tetraptera, a popular medicinal plant belonging to the family Fabaceae (formerly Leguminosae: Mimosoideae), is commonly known as “Aridan” or “Aidan” among the Yoruba ethnic group of South Western Nigeria, “Abogolo” among the Igala people of north central Nigeria, and “Dawo” among the Hausa people of Northern Nigeria. It is generally found in the lowland forest of many tropical African countries, and known to have fruit that consists of fleshy pulp and small, brownish-black seeds, a characteristic fragrant and pungent aromatic odor[5]. It is used as a popular seasoning spice in Southern and Eastern Nigeria[6], and its fruit is used for the management of convulsions, leprosy, inflammation, rheumatism, flatulence, jaundice and fevers, as well as the management and control of adult-onset type 2 diabetes mellitus[5],[6]. Previous studies in our laboratory showed that the methanolic extract of the leaf of this plant has antioxidant effect on carbon tetrachloride-induced hepatotoxicity[7], while the aqueous fruit extract has been shown to possess hypoglycemic properties[8]. However, information on the scientific validation of the leaves of T. tetraptera for management of diabetes and the associated complications appears to be lacking. Therefore, this study was undertaken to investigate the ameliorative role of T. tetraptera leaves in hyperglycemia with associated conditions like oxidative stress, kidney damage and disorders in lipid metabolism.
2. Materials and methods
2.1. Sample collection and identification
The leaves of T. tetraptera were collected from Emere in Ankpa Local Government Area of Kogi State, Nigeria in August, 2010. The plant was identified at the Herbarium Unit of the Nigerian Institute for Pharmaceutical Research and Development, Idu, Abuja, Nigeria, where a voucher number 5881 was assigned.
2.2. Sample preparation and extraction
The leaves of T. tetraptera were dried in the laboratory at room temperature, following initial drying in the morning sun for 4 h on the first day to arrest enzymatic deterioration, before pulverization using laboratory mortar and pestle. Pulverized material (35 g) was placed in the thimble of Soxhlet extractor and extracted first using petroleum ether (300 mL) for 8 h each (to remove the lipid content) and then methanol (300 mL), three times for 5 h each. The methanol extracts were combined and dried in vacuo at 45 °C using a rotary evaporator (Büchi Labortechnik AG, Switzerland)[2],[9],[10].
2.3. Animal management
Wistar strain of male albino rats with body weight ranging from 160-200 g were used for the study. The rats were bred in the Animal House of Department of Biochemistry, Ahmadu Bello University, Zaria. The rats were fed on pelleted commercial growers mash (Vital Feeds, Jos, Nigeria). They were kept at room temperature and maintained ad libitum on tap water and growers mash (ECWA Feeds, Bukuru-Jos, Plateau State, Nigeria), except in the last 12-15 h before termination of the experiment. The animals were housed in plastic cages under conditions of 12 h light/12 h dark cycle at 25 °C. The rats were weighed prior to commencement and termination of the experiment[2],[9],[11].
2.4. Induction of diabetes and experimental grouping
Diabetes mellitus was induced in rats by intraperitoneal injection of freshly prepared alloxan monohydrate solution dissolved in normal saline (120 mg/kg), followed 48 h later by a booster dose of 100 mg/kg. After the booster dose for 3 d, fasting blood glucose was measured and rats with blood glucose levels above 140 mg/dL were considered diabetic and included in the study.
Five groups of five rats each were allocated to the following intraperitoneal treatment schedule for 7 d: untreated normal control, untreated alloxan-diabetic control, diabetic rats treated with glibenclamide, normal rats treated with extract and diabetic rats treated with the extract. The extract was administered at a dose of 50 mg/kg, while the standard glibenclamide was administered at a dose of 1 mg/kg.
2.5. Collection of blood sample and blood glucose determination
Blood samples were drawn from tail tip of each rat fasted overnight on Day 0 and Day 7 (i.e. one week) for estimation of fasting blood glucose, following which the body weight measurement was taken. Blood glucose estimation was done by one touch electronic glucometer using glucose test strips. On Day 7, after blood was collected for fasting blood sugar determination, animals were placed under mild chloroform anesthesia before sacrifice[12].
2.6. Animal sacrifice
All rats were sacrificed 24 h following last administration of drug or T. tetraptera extract. Animals were sacrificed under chloroform anesthesia and whole blood was collected and allowed to stand for 2 h before collection of serum for assay for biochemical parameters. All serum samples were kept in Eppendorf tubes and stored at -20 °C. The organs were immediately harvested, rapidly rinsed in ice-cold normal saline and stored at -20 °C for analysis of malondialdehyde (MDA) as indicator of lipid peroxidation[2],[9],[11].
2.7. Tissue homogenization
The whole liver, kidneys and heart from each rat were removed after sacrificing the animal, and were rinsed in normal saline and immediately stored in deep freezer. Tissues were homogenized in 10 parts in ice-cold potassium phosphate buffer (pH 7.4) using mortar and pestle. The homogenate was centrifuged at 3 000 ×g for 15 min and the supernatant was collected. Protein concentration of the sample was determined by Biuret method using bovine serum albumin as standard[2],[9],[11].
2.8. Assay for lipid peroxidation
Lipid peroxidation was determined as thiobarbituric acid-reactive substances as described by Torres et al. based on the principle that peroxide intermediates generated released MDA upon cleavage[13]. This compound reacted with thiobarbituric acid to form a coloured complex that was measured at 535 nm. one milliliter of 14% trichloroacetic acid was measured into a test tube, 1 mL thiobarbituric acid (0.67%) was added and 50 µL of the tissue homogenate was added. The mixture was incubated at 80 °C for 30 min in a water bath, and then allowed to cool rapidly in ice for 5 min, followed by centrifugation at 3000 rpm for 10 min. MDA was measured colorimetrically at 535 nm and the level of lipid peroxidation was calculated using the molar extinction coefficient of MDA[2],[9],[11].
2.9. Determination of the activity of endogenous antioxidant enzymes
The ability of the extracts to boost the capacity of antioxidant enzymes was evaluated by determining the activity of two endogenous antioxidant enzymes, namely, catalase (CAT) and superoxide dismutase (SOD) as follows.
2.9.1. Catalase
CAT activity was measured using the procedure reported by Abei Briefly[14]. A total of 10 µL of serum was added to test tube containing 2.8 mL of 50 mmol/L phosphate buffer (pH 7.0). The reaction was initiated by adding 0.1 mL of fresh 30 mmol/L H2O2 and the decomposition rate of H2O2 was measured at 240 nm for 5 min on a spectrophotometer (Jenway 640 UV/Vis). A molar extinction coefficient of 0.041 1 mmol/L/cm was used to calculate CAT activity.
2.9.2. Superoxide dismutase
SOD activity was evaluated according to the method described by Martin et al[15]. Exactly 920 µL of assay buffer was added into clean test tube containing 40 µL of sample, mixed and incubated for 2 min at 25 °C, following which 40 µL of hematoxylin solution was added, mixed quickly and the absorbance was measured immediately at 560 nm.
2.10. Determination of liver function parameters
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined colorimetrically at 546 nm using Randox assay kits based on the principle first described by Reitman and Frankel[16]. Also, using the Randox kit, the colorimetric assay method for conjugated bilirubin employed involved reaction with diazotized sulphanilic acid in alkaline medium to form a blue complex, while total bilirubin was determined in the presence of caffeine, which releases albumin-bound bilirubin that then reacts with diazotized sulphanilic acid[17].
2.11. Determination of packed cell volume
Whole blood samples were collected into heparinized capillary tubes, filled up to about 2/3 the length, sealed with plasticine and centrifuged at 3 000 r/min for 10 min. The packed cell volume (PCV) was determined using hematocrit reader, and the hemoglobin concentration was calculated from the PCV values[18].
2.12. Determination of total and high-density lipoprotein (HDL)-cholesterol
The method of Roeschlau and Gruber was applied using assay kits (Randox Laboratories Ltd., UK) to determine the total serum cholesterol spectrophotometrically at 546 nm following enzymatic hydrolysis and oxidation[19]. The HDL-cholesterol was determined using assay kits (Randox Laboratories Ltd., UK) after low density lipoprotein (LDL and VLDL) and chylomicron fractions were precipitated quantitatively by the addition of phosphotungstic acid in the presence of magnesium ions. After centrifugation, the cholesterol concentration in the HDL fraction in the supernatant was assayed colorimetrically at 540 nm[19].
2.13. Statistical analysis
The results obtained were statistically analyzed using analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT) to separate the means with significant differences. The level of significance was set at P<0.05.
3. Results
The diabetic groups treated with either the methanolic extract of T. tetraptera leaf or the standard anti-diabetic drug, glibenclamide showed significant percentage change (P<0.05) in the fasting blood sugar level (30.15% and 47.56%, respectively) compared to the diabetic (0.59%) and normal controls (2.30%) between Day 0 and Day 7 (Figure 1).
Figure 1. Percentage change in FBS in alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
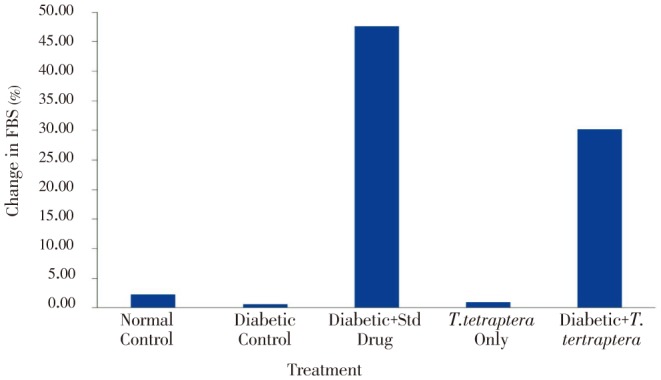
FBS: fasting blood sugar.
Whereas other groups, including the diabetic group treated with either the methanolic extract of T. tetraptera leaves or the standard drug experienced positive growth, which were increases in absolute weight, the untreated diabetic group experienced more than 11% decrease in weight (Figure 2). However, no statistical difference (P>0.05) was observed in the percentage organ to body weight ratio for the liver, kidney and heart between the treated groups and the control groups (Figure 3). Figure 4 shows AST and ALT activities while Table 1 shows the bilirubin levels in the serum of treated animals and control. There was a statistically significant (P<0.05) elevation in the activities of the liver enzymes, AST and ALT, as well as in the levels of total and direct bilirubin in the diabetic control rats compared to normal control group, but treatment with the methanolic extract of T. tetraptera leaves (50 mg/kg) significantly prevented the elevation of these indices of liver function in the diabetic rats.
Figure 2. Percentage change in weight in alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
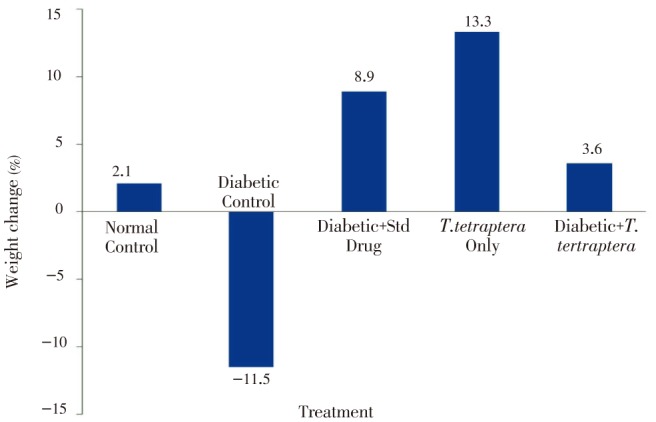
Figure 3. Percentage of organ and body weight ratio in alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
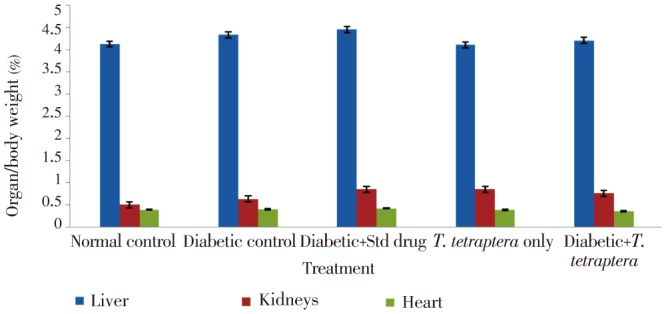
Figure 4. Activities of AST and ALT in alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
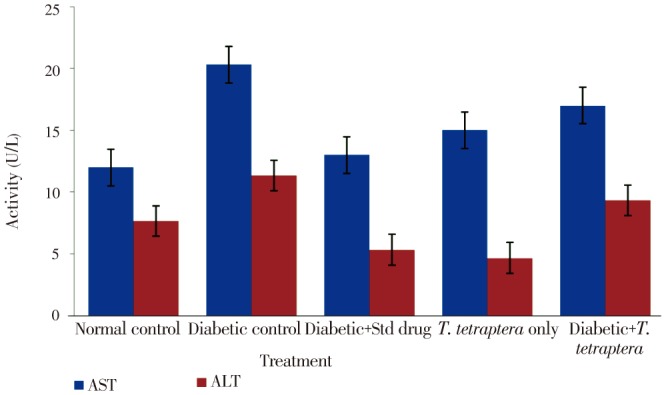
Table 1. Total and direct bilirubin levels in alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
| Treatment | Total bilirubin (µmol/L) | Direct bilirubin (µmol/L) |
| Normal control | 2.46±0.07a | 6.56±0.12ab |
| Diabetic control | 8.01±0.18b | 10.20±0.35b |
| Diabetic+Std drug | 6.16±0.16 b | 7.20±0.25ab |
| T. tetraptera only | 5.55±0.12ab | 5.38±0.13a |
| Diabetic+T. tetraptera | 6.16±0.16b | 8.20±0.21ab |
Data with different superscript letters along the same column are statistically different (P<0.05).
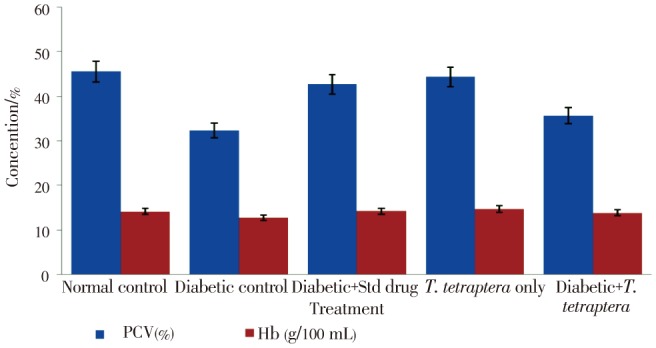
It was also observed that the T. tetraptera leaf extract was more effective in managing these conditions than the standard drug, glibenclamide at the recommended dose of 1 mg/kg. There was also a significant (P<0.05) reduction in PCV in diabetic rats compared to the control (Figure 5), but extract treatment significantly improved the condition to a level that was not statistically different (P>0.05) from that of the normal group. The standard drug exhibited more potency in improving hematological parameters than the T. tetraptera extract. In general, there was no statistically significant (P>0.05) difference observed in levels of hemoglobin concentration between the groups (Figure 5).
Figure 5. Levels of some hematological parameters in alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
The levels of MDA in the liver, kidneys and heart are shown in Table 2. Compared with the untreated control, the highest significant elevation in the levels of MDA was found in the organs of the diabetic group of rats with the kidneys. However, extract treatment significantly (P<0.05) lowered MDA levels in all the treated groups.
Table 2. MDA levels in major organs of alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
| Treatment | TBARS (nmoL/mg protein) |
||
| Liver | Kidney | Heart | |
| Normal control | 0.02±0.01a | 0.12±0.01a | 0.06±0.00a |
| Diabetic control | 0.18±0.01c | 0.23±0.01c | 0.18±0.01c |
| Diabetic+Std drug | 0.08±0.00b | 0.14±0.00ab | 0.07±0.04a |
| T. tetraptera only | 0.11±0.01bc | 0.11±0.01a | 0.07±0.00a |
| Diabetic+T. tetraptera | 0.10±0.01b | 0.17±0.01b | 0.13±0.01c |
Data with different superscript letters along the same column are statistically different (P<0.05); TBARS=thiobarbituric acid reactive substances.
Tables 3 and 4 present the activities of the endogenous antioxidant enzymes in the major organs of the control and treated animals.
Table 3. SOD activity in major organs of alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
| Treatment | SOD activity (µ/mg protein) |
||
| Liver | Kidney | Heart | |
| Normal control | 39.27±1.13bc | 23.01±1.10b | 26.99±1.10b |
| Diabetic control | 25.81±0.95a | 17.04±0.66a | 21.04±1.02a |
| Diabetic+Std drug | 30.20±2.04ab | 21.84±0.91b | 25.46±1.20b |
| T. tetraptera only | 42.08±1.50c | 30.94±1.62c | 35.04±2.01c |
| Diabetic+T. tetraptera | 34.03±2.12bc | 21.50±1.15b | 25.02±1.25b |
Data with different superscript letters along the same column are statistically different (P<0.05).
Table 4. CAT activity in major organs of alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
| Treatment | CAT activity (µ/mg protein) |
||
| Liver | Kidney | Heart | |
| Normal control | 16.16±0.95c | 13.79±0.46bc | 14.09±0.55c |
| Diabetic control | 11.72±0.40a | 9.15±0.39a | 9.19±0.36a |
| Diabetic+Std drug | 16.74±0.43bc | 16.01±0.56bc | 12.25±0.44ab |
| T. tetraptera only | 21.11±0.94c | 21.56±0.68c | 14.09±0.55c |
| Diabetic+T. tetraptera | 17.76±0.92b | 13.72±0.52b | 12.25±0.44b |
Data with different superscript letters along the same column are statistically different (P<0.05).
In all organs considered, there was a significant (P<0.05) reduction in activity of CAT and SOD in diabetic rats when compared to the non-diabetic control rats. Treatment of the diabetic rats with the methanolic extract of T. tetraptera leaves was able to restore the activities of the enzymes in all the organs to the normal range, except in the heart. It was worthy to note that SOD activity was generally higher in the heart than kidney tissues.
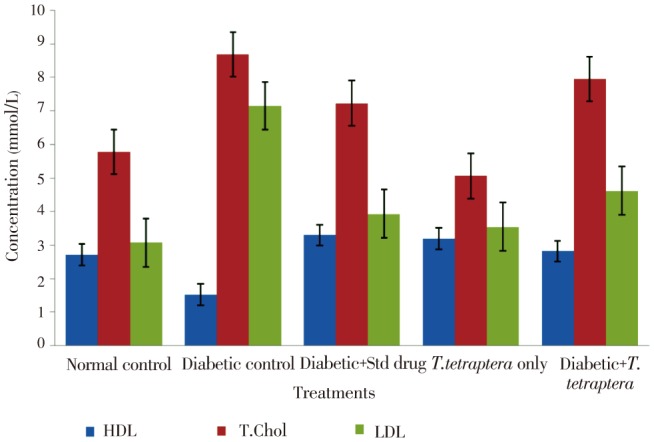
There was a significant (P<0.05) elevation in total cholesterol level with concomitant lowered HDL level in diabetic rats compared to the non-diabetic control (Figure 6), but in diabetic control groups treated with T. tetraptera, the HDL level was raised to a level that was statistically not different from that of the normal control. In all these lipid parameters. There was no statistically significant difference between the group treated with the extract and that treated with the standard control, glibenclamide (Figure 6).
Figure 6. Lipid profile in alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
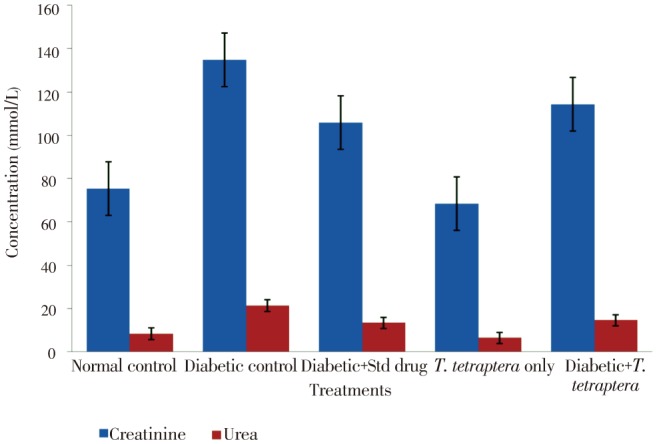
Diabetic condition caused a doubling in the level of urea and a significant (P<0.05) elevation in the concentration of creatinine compared to that of the normal control, but treatment with the methanolic extract of T. tetraptera leaves reduced the level to a value insignificantly different from that of the normal control group (Figure 7). Even in non-diabetic control, extract administration significantly (P<0.05) reduced the serum urea and, to a large extent, the creatinine levels.
Figure 7. Levels of some renal function parameters in alloxan-induced diabetic rats treated with methanolic extract of T. tetraptera leaves (50 mg/kg) for 7 d.
4. Discussion
4.1. Effect on weight changes and fasting blood sugar
Diabetes is now recognized as one of the major killer diseases and a leading cause of death, claiming many lives world over[1]. Oral hypoglycemic agents, especially the sulphonylureas and biguanides, have been commonly used in the management of the disease, especially type II diabetes, although they have serious side effects. Consequently, attention has been focused on the use of plants and herbal remedies believed to be safer and devoid of serious side effects as alternatives in the treatment of diabetes[2],[4],[20]. The significant low blood sugar level in diabetic rats by administration of the methanolic extract of T. tetraptera leaf confirms that it could serve as a more accessible and affordable alternative to orthodox antidiabetics.
The positive weight change observed in the diabetic animals treated with the extract or the standard drug clearly suggested that T. tetraptera might be useful in managing dysfunctional lipid metabolism, which is one of the undesirable side effects encountered when treating diabetics with sulphonylureas[20]. The non-significant changes in organ to weight ratio between the treated and normal control suggested that administration of methanol extract of T. tetraptera leaves had little or no toxic effect in the treated animals. The T. tetraptera extract apparently decreased plasma glucose levels in diabetic rats than the standard drug, glibenclamide at the recommended dose seems to suggest that T. tetraptera has the capacity to reduce the risk of other complications associated with people suffering with diabetes[21], and hence validates its local use as a hypoglycemic agent by the Igala people of north central Nigeria.
4.2. Effect of T. tetraptera leaf extract on hepatocellular and oxidative damage
Damage to hepatic tissues mainly releases the enzymes AST and ALT from the hepatic cells into the plasma, and, hence, elevation of plasma activities of these enzymes is considered a reliable indicator of liver damage, since these enzymes are intracellular[22]. That treatment of alloxan-diabetic rats with methanolic extract of T. tetraptera leaves significantly (P<0.05) reduced their elevated levels of AST and ALT activities, suggesting that the plant contains substances (polyphenols) that are capable of protecting and alleviating liver damage. Also, in diabetic rats treated with methanolic extract of T. tetraptera leaves, the levels of PCV and hemoglobin concentration were greatly improved, while the elevations in the levels of total and direct bilirubin were significantly reduced. The results suggest that T. tetraptera leaf extract contains compounds possessing antioxidant properties, judging from its effect on MDA level, which may provide oxidative stability for membranes of red blood cells and hepatic tissues.
Lipid peroxidation is considered to be a primary mechanism of cell membrane destruction by free radicals because MDA conjugates with amino group of proteins to form intra- and inter-molecular cross-links that inactivate the membrane-bound enzymes and receptors[23]. Hence, the administration of methanolic extract of T. tetraptera leaves significantly (P<0.05) reduced the level of MDA in diabetic rats, which strongly suggests the ability of the plant and its constituents to prevent oxidative damage, probably by interfering with the free radical-induced processes, leading to intra- and inter-molecular cross-linkages.
Superoxide anion radical (O2.-), hydrogen peroxide (H2O2) and hydroxyl radical (OH*), which are the major reactive oxygen species, generated during oxidative stress are known to decrease insulin receptor substrate and tyrosine phosphorylation, and, thus, the activity of phosphatidyl inositol-3-kinase leads to insulin resistance, a major feature of type 2 diabetes[24]. Fortunately, aerobic cells are endowed with extensive antioxidant defense mechanisms, including low-molecular weight scavengers such as reduced glutathione, ascorbic acid, vitamin E and endogenous antioxidant enzyme system such as CAT, SOD and glutathione peroxidase. In this investigation, diabetes-induced depletion in the activities of SOD and CAT, possibly due to less availability of NADPH or gradual decrease in erythrocyte CAT activity through excessive generation of O2** that inactivates the enzyme, was significantly ameliorated by administration of the methanolic extract of T. tetraptera leaves, suggesting that the plant contains antioxidant phytochemicals such as vitamin E and polyphenols that can significantly boost the activity of these endogenous antioxidant enzymes[24],[25].
4.3. Effect of T. tetraptera leaf extract on lipid profile and renal function parameters
The low plasma total cholesterol and the significant increase in HDL-cholesterol level in the treated animals compared with the untreated diabetic rats clearly demonstrates the presence of hypolipidemic agents in the methanol extract of T. tetraptera leaves. The ability of the extract to manage dyslipidemia is a potential beneficial effect on cardiovascular disorders, an associated diabetic complication that is a major cause of death among diabetics[26],[27]. The diabetic condition caused a significant (P<0.05) increase in plasma creatinine and urea levels which could be a sign of impaired renal function that was restored to the normal range through treatment with methanolic extract of T. tetraptera leaves[27]. This finding demonstrates the therapeutic importance of the plant in the management of chronic glomerular disease, one of the complications of diabetes that is associated with elevated levels of blood urinary nitrogen and creatinine[28]. Further studies are required to identify and elucidate the exact constituents in T. tetraptera leaves that are responsible for the observed pharmacological and therapeutic activities.
The present study validates the use of T. tetraptera leaf in Igala traditional medicine and demonstrates that the methanol extract of the plant contains phytochemicals that exhibits very impressive potency and promise in the management of diabetes and its complications.
Acknowledgments
The scientific work of SEA is supported by Material Grant No. V-8151/04084 from Alexander von Humboldt of Germany, to whom we express our profound appreciation.
Comments
Background
Diabetes mellitus is a debilitating disease, which is a leading cause of death in the world. Measures aimed at improving the current management of the disease, and its complications, through herbal interventions are pertinent and highly promising. Some studies conducted earlier have shown that extracts of T. tetraptera possess antioxidant and hypoglycaemic activities, indicating that the extracts may be of value in the management of diabetes mellitus and its associated complications.
Research frontiers
The study was carried out in order to determine the effect of methanol extract of the leaves of T. tetraptera on hyperglycaemia and some markers of oxidative stress in rats induced with diabetes mellitus. The extract reduced tissue concentrations of biomarkers of oxidative stress, including malondialdehyde, increased catalase and superoxide dismutase activities, and alleviated other complications associated with diabetes, without any side-effect on the kidneys and liver in rats. It was a promising search for natural, less toxic and yet readily available phytochemical compounds that may be used for the management of the disease. The work has demonstrated that the extract contains potent antioxidants and hypoglycaemic agents. If isolated, the active constituents in the extract may be added to the arsenal of drugs currently used to treat diabetes.
Related reports
The results of the study agree with the findings of previous studies (Aladesanmi, 2007; Odesanmi et al., 2011) that extracts of the plant contain chemical constituents, possessing potent activities which are of value in the treatment of diseases.
Innovations and breakthroughs
There is paucity of information on the use of the extract of the plant, T. tetraptera in the treatment of diabetes mellitus. Studies on the alleviating role of the leaf extract of plants, including T. tetraptera in complications associated with diabetes are currently lacking in the available literature. The present study has shown that the extract may be used not only in the therapy of diabetes, but also in the management of complications due to the disease, including oxidative stress and hyperlipidaemia.
Applications
The biochemical parameters measured in the present study are important markers of oxidative stress and liver function. They may be used to determine the responses of diabetic individuals with or without complications to phytomedicine. The plant extract may be a cheap and readily available source of compounds possessing active principles in the management of diabetes mellitus.
Peer review
This is an interesting study carried out by the authors on the effect of methanol leaf extract of T. tetraptera on diabetes mellitus. The study has shown that the extract contains potent chemical constituents, which exert antioxidant and hypoglycaemic effects. The extract may be beneficial in the treatment of diabetes and its complications, including hyperlipidaemia.
Footnotes
Foundation Project: The work was supported by Alexander von Humboldt of Germany (Grant No. V-8151/04084).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.World Health Organization . Geneva: World Health Organization; 2010. Global status report on non-communicable diseases 2010. [Online] Available from: http://www.who.int/nmh/publications/ncd_report_full_en.pdf. [Accessed on 30th August, 2012]. [Google Scholar]
- 2.Atawodi SE. Evaluation of the hypoglycemic, hypolipidemic and antioxidant effects of methanolic extract of “Ata-ofa” polyherbal tea (A-Polyherbal) in alloxan-induced diabetic rats. Drug Invention Today. 2011;3(11):270–276. [Google Scholar]
- 3.Madhu V, Swamy TN. Ethnomedicine against jaundice used by Gond Tribes of Adilabad District, Andhra Pradesh, India. Ethnobotanical Leaflets. 2010;14:687–693. [Google Scholar]
- 4.Abolfathi AA, Mohajeri D, Rezaie A, Nazeri M. Protective effects of green tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2012 doi: 10.1155/2012/740671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aladesanmi JA. Tetrapleura tetraptera: molluscicidal activity and chemical constituents. Afr J Tradit Complement Altern Med. 2007;4(1):23–36. [PMC free article] [PubMed] [Google Scholar]
- 6.Odesanmi OS, Lawal RA, Ojokuku SA. Effects of ethanolic extract of Tetrapleura tetraptera fruit on serum lipid profile and kidney function in male Dutch-white rabbits. Nig Q J Hosp Med. 2011;21(4):299–302. [PubMed] [Google Scholar]
- 7.Atawodi SE, Adekunle OO, Bala I. Antioxidant, organ protective and ameliorative properties of methanol extract of Anogeissus leiocarpus stem bark against carbon tetrachloride-induced liver injury. Int J Pharm Sci Res. 2011;2(6):1443–1448. [Google Scholar]
- 8.Lekana-Douki JB, Liabagui SL, Bongui JB, Zatra R, Lebibi J, Toure-Ndouo FS. In vitro antiplasmodial activity of crude extracts of Tetrapleura tetraptera and Copaifera religiosa. BMC Res Notes. 2011;4:506. doi: 10.1186/1756-0500-4-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atawodi SE, Atawodi JC, Idakwo GA, Pfundstein B, Haubner R, Wurtele G, et al. et al. Polyphenol composition and antioxidant potential of Hibiscus esculentus fruits cultivated in Nigeria. J Med Food. 2009;12(6):1316–1320. doi: 10.1089/jmf.2008.0211. [DOI] [PubMed] [Google Scholar]
- 10.Atawodi SE, Atawodi JC, Idakwo GA, Pfundstein B, Haubner R, Wurtele G, et al. et al. Polyphenol composition and antioxidant potential of Hibiscus esculentus fruits cultivated in Nigeria. J Med Food. 2009;12(6):1316–1320. doi: 10.1089/jmf.2008.0211. [DOI] [PubMed] [Google Scholar]
- 11.Asuku O, Atawodi SE, Onyike E. Antioxidant, hepatoprotective, and ameliorative effects of methanolic extract of leaves of Grewia mollis Juss. on carbon tetrachloride treated albino rats. J Med Food. 2012;15(1):83–88. doi: 10.1089/jmf.2010.0285. [DOI] [PubMed] [Google Scholar]
- 12.Jones CP, Carver S, Kendall LV. Evaluation of common anesthetic and analgesic techniques for tail biopsy in mice. J Am Assoc Lab Anim Sci. 2012;51(6):808–814. [PMC free article] [PubMed] [Google Scholar]
- 13.Torres SH, De Sanctis JB, de L Briceno M, Hernandez N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of type II diabetic patients. J Endocrinol. 2004;181(3):419–427. doi: 10.1677/joe.0.1810419. [DOI] [PubMed] [Google Scholar]
- 14.Abei H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1974. pp. 673–684. [Google Scholar]
- 15.Martin JP, Jr, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys. 1987;255(2):329–336. doi: 10.1016/0003-9861(87)90400-0. [DOI] [PubMed] [Google Scholar]
- 16.Reitman S, Frankel SA. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Jenrassik L, Groff P. Quantitative determination of total and direct bilirubin. Biochem Z. 1938;297:81–85. [Google Scholar]
- 18.Tondon R, Verma A, Pandey P, Chaudhary R. Quality evaluation of four hemoglobin screening methods in a blood donor setting along with their comparative cost analysis in an Indian scenario. Asian J Transfus Sci. 2009;3(2):66–69. doi: 10.4103/0973-6247.53874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 20.Seino S, Takahashi H, Takahashi T, Shibasaki T. Treating diabetes today: a matter of selectivity of sulphonylureas. Diabetes Obes Metab. 2012;14(Suppl 1):9–13. doi: 10.1111/j.1463-1326.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 21.Mima A, Hiraoka-Yamomoto J, Li Q, Kitada M, Li C, Geraldes P, et al. et al. Protective effects of glp-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes. 2012;61(11):2967–2979. doi: 10.2337/db11-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver diseases. Exp Diabetes Res. 2012;2012:145754. doi: 10.1155/2012/145754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen S, Chakraborty R, Sridhar C, Reddy YS, De B. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospects. Int J Pharm Sci Rev Res. 2010;3(1):91–100. [Google Scholar]
- 24.Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JP. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2(11):1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catalá A. Lipid peroxidation modifies the picture of membranes from the “fluid mosaic model” to the “lipid whisker model”. Biochimie. 2012;94(1):101–109. doi: 10.1016/j.biochi.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Valli G, Giardina VE. Benefits, adverse effects and drug interactions of herbal therapies with cardiovascular effects. J Am Coll Cardiol. 2002;39(7):1083–1095. doi: 10.1016/s0735-1097(02)01749-7. [DOI] [PubMed] [Google Scholar]
- 27.Zhou W, Chai H, Lin HP, Lumsden BA, Yao Q, Chen C. Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovasc Drug Rev. 2004;22(4):309–319. doi: 10.1111/j.1527-3466.2004.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal SK, Chandra B. Vascular complications in diabetic kidney disease patients. Clin Queries: Nephrol. 2012;1(2):178–182. [Google Scholar]


