Abstract
Objective
To ascertain analgesic, antibacterial and central nervous system (CNS) depressant activities of ethyl acetate, dichloromethane and carbon tetrachloride fractions of methanol extract of Albizia procera (A. procera) leaves.
Methods
Leaves extracts of A. procera were tested for analgesic activity by acetic acid induced and formalin test method in mice. The in vitro antibacterial activity was performed by agar well diffusion method. CNS depressant activity was evaluated by hole cross and open field tests.
Results
All the extracts at 200 mg/kg exhibited significant (P<0.01) analgesic activity in acetic acid induced and formalin tests method in mice. Analgesic activity of the ethyl acetate fraction was almost same like as standard drug indomethacin in acetic acid induced method. The CNS depressant activity of the extracts at 500 mg/kg was comparable to the positive control diazepam as determined by hole cross and open field test method. The extracts exhibited moderate antimicrobial activity against all the tested microorganisms (Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, Esherichia coli, Shigella soneii, Shigella boydii) at concentration of 0.8 mg/disc. The measured diameter of zone of inhibition for the extracts was within the range of 7 to 12 mm which was less than the standard kanamycin (16-24 mm).
Conclusions
It is concluded that all the extracts possess potential analgesic and CNS depressants activity. This study also showed that different fractions of methanol extract could be potential sources of new antimicrobial agents.
Keywords: Analgesic, CNS depressants, Antimicrobial, Albizia procera leaves
1. Introduction
All plant species contain chemical compounds which can be poisonous, medicinal and nutritional[1]. A wide range of analgesics, antibiotics and central nervous system (CNS) depressants from both natural and synthetic origin have been proposed for use in the treatment of various human diseases. It has been suggested that compounds from synthetic origin have shown toxic effects like liver damage, mutagenesis. Emerging new infectious diseases, development of drug resistance in human pathogens against commonly used antibiotics has also been necessitated a search for new antimicrobial substances from other sources including plants[2]. In order to manage this problem, a continuous effort to find new medicament from plant origin has greatly increased in recent years. Albizia procera (A. procera) (Family: Fabaceae) is a tree widely distributed from India and Myanmar through Southeast Asia to Papua New Guinea and Northern Australia. Leaves are said to be insecticidal[3]. In folk medicine, bark is used for fish poison. Leaves are poulticed onto ulcers in India. Bark is also considered useful in pregnancy and stomachache and is given with salt as a medicine for water buffalo[4]. The ethanolic extracts of bark showed significant anti-HIV-1 integrase activity[5]. The bark and leaf extracts of A. procera showed potent DPPH scavenging activity[6],[7]. Considering the biomedical importance of A. procera, the present study was aimed to study the pharmacological potential especially the analgesic, antibacterial and CNS depressant activities of different fractions of methanol extracts of A. procera leaves.
2. Materials and methods
2.1. Collection of plant material
Fresh leaves of the plants were collected in May, 2012 and identified by Botany Department, Rajshahi University, Bangladesh. A voucher specimen with accession No. 3798 has been deposited in Bangladesh National Herbarium, Dhaka, Bangladesh.
2.2. Preparation of plant extracts
The leaves were left to dry under shade, grinded and extracted with methanol by cold maceration for 7 d at room temperature. The extract was then filtered off through a cotton plug and finally through filter paper. The filtrate was concentrated using vacuum rotary evaporator at 50 °C. The concentrated methanol extract was partitioned successively with dichloromethane, carbon tetrachloride and ethyl acetate. The percentage yields of extracts were calculated as petroleum ether 2.3% w/w, ethyl acetate 5.58% w/w and methanolic 13.13% w/w. All the extracts were stored in a refrigerator for further use.
2.3. Experimental animals
The experiment of analgesic activity was conducted on Long-Evans rats of both sexes, aged 4-5 weeks, weighting about 20-25 g. The mice were purchased from the animal research branch of the International Center for Diarrheal Disease and Research, Bangladesh. Before initiating the experiment, the mice were kept in standard environmental conditions [temperature: (23.0±2.0) °C, relative humidity: 55%-65% and 12 h light/12 h dark cycle] and had free access to feed and water ad libitum. The animals were acclimatized to laboratory condition for 1 week prior to experiments. All protocols for animal experiment were approved by the Institutional Animal Ethical Committee.
2.4. Analgesic activity
2.4.1. Acetic acid induced writhing method
The analgesic activity of the samples was assessed using acetic acid-induced writhing model in mice described by Nwafor et al[8]. The rats were divided into five groups (Group-I to Group-V) of four animals each. Group I (control group) received normal saline intraperitonealy and Group II (positive control) was given indomethacin orally at concentration of 10 mg/kg. Group III, IV and V received dichloromethane fraction (DCMF), carbon tetrachloride fraction (CTCF) and ethyl acetate fraction (EAF) extracts at 200 mg/kg orally. After 30 min, the writhing inducing chemical, acetic acid (0.7%) was injected intraperitoneally to each of the animals of all the groups to create pain sensation. After an interval of 5 min, the rats were observed for specific contraction of body referred to as ‘writhing’ and the number of writhing for the next 10 min were counted for each rat[9]. The number of writhing in each treated group was compared to that of a control group (positive control) and the percent inhibition (% analgesic activity) was calculated by the following formula:
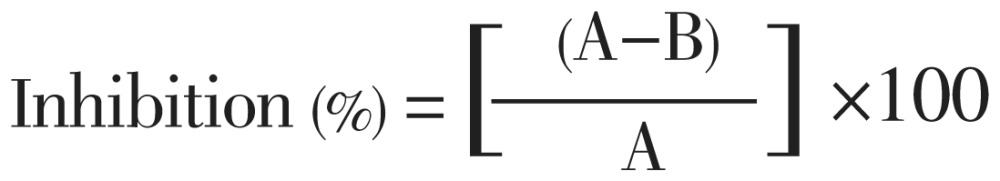 |
Where, A refers to average number of writhing of control per group; and B is average number of writhing of test per group.
2.4.2. Formalin test method
The analgesic activity was also determined using the formalin test according to the method described Okokon and Nwafor[10]. The rats were divided into five groups as for acetic acid induced writhling test. About 20 µL of 5% formalin was injected subcutaneously into the right hind paw of rats. The time (in seconds) spent in licking the paw and the biting responses of the injected paw were taken as an indicator of pain response. The rats were observed for 30 min after the injection of formalin, and the amount of time spent licking the injected hind paw was recorded. The first 5 min post formalin injection is referred to as the early phase and the period between 15 and 30 min as the late phase. Extracts (200 mg/kg, orally) and indomethacin (10 mg/kg, i.p.) were administered 30 min prior to formalin injection. Control animals received 10 mL/kg of distilled water orally. The percent inhibition (% licking activity) is calculated by the following formula:
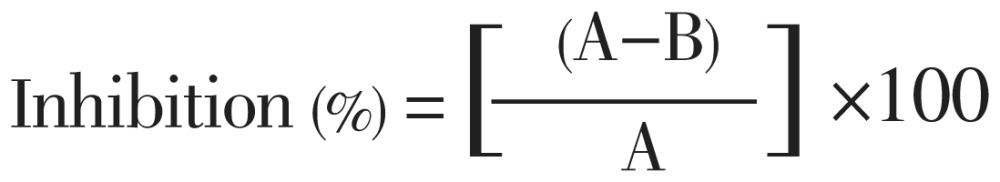 |
Where, A refers to average number of licking of control per group; and B is average number of licking of test per group.
2.5. CNS depressant activity
2.5.1. Hole cross test
The method was followed as described by Javid et al[11]. A cage with a fixed steel partition in the middle position having a size of 30 cm×20 cm×14 cm was taken. A hole of 3 cm diameter was made at a height of 7.5 cm in the midpoint of the cage. The rats were divided into control, standard control and test group. The test groups received EAF, DCMF and CTCF of methanolic extracts at the dose of 500 mg/kg body weight orally whereas control group received vehicle (1% Tween 80 in water) at 10 mL/kg body weight orally and standard group received diazepam at a dose of 1 mg/kg body weight orally. The number of passages of a rat through the hole from one chamber to the other was counted for a period of 3 min at 0, 30, 60, 90 and 120 min after oral administration of test drugs.
2.5.2. Open field method
This experiment was carried out according to the technique described by Gupta et al[12]. The test is based on behavioral responses to stay away from brightly illuminated areas. The animals were divided into control, standard and test group. The test groups received EAF, DCMF and CTCF of methanolic extract at a dose of 500 mg/kg body weight orally whereas control group receive vehicle (1% Tween 80 in water) at 10 mL/kg body weight orally and standard group received diazepam at the dose of 1 mg/kg body weight orally.
2.6. Antimicrobial susceptibility test
The disc diffusion method was used to test antimicrobial activity against 13 bacteria[13]. Solutions of known concentration (500 mg/mL) of the test samples were made by dissolving measured amount of the samples in calculated volume of solvents. Dried and sterilized filter paper discs (6 mm diameter) were then impregnated with known amounts of the test substances using micropipette. Discs containing the test material were placed on nutrient agar medium uniformly seeded with the pathogenic test microorganisms. Standard antibiotic discs (kanamycin: 30 µg/disc) and blank discs (impregnated with solvents) were used as a positive and negative control. These plates were then kept at low temperature (4 °C) for 1 h to allow diffusion. There was a gradual change in concentration in the media surrounding discs. The plates were then incubated at 37 °C for 12 h to allow growth of the organisms. The test materials having antibacterial activity inhibited the growth of the microorganisms and a clear, distinct zone of inhibition was visualized surrounding the medium. The antibacterial activity of the test agent was determined by measuring the diameter of zone of inhibition expressed in millimeter. The experiment was carried out three times and the mean of the reading is required. The antibacterial activity of different fractions of methanol extract of leaves of A. procera was determined at a concentration of 800 µg/disc.
2.7. Statistical analysis
The results were expressed as mean±SD. All statistical comparisons were made by Dennett's test after conducting one-way ANOVA. The level of significance was considered to be at P<0.05 and P<0.01.
3. Results
3.1. Analgesic activity
In acetic acid induced writhing test, the three fractions of methanol extract of A. procera leaves significantly suppress the frequency of acetic acid-induced writhing in rats after oral administration. At 200 mg/kg body weight, EAF, DCMF and CTCF showed 79.5%, 75% and 70.83% writhing inhibition, respectively. At 10 mg/kg body weight, the standard drug indomethacin shows 81.25% writhing inhibition. Among the three fractions, EAF showed the highest writhing inhibition which was very close to the standard drug used in the experiment. Table 1 shows the analgesic activity of standard and different fractions of methanol extract of A. procera leaves.
Table 1. Analgesic activity of different extracts of A. procera leaves on acetic acid induced writhings in mice.
| Group | Treatment | Dose | Writhing (%) | Inhibition (%) |
| Group-I (Control) | 1% Tween 80 | 0.1 mL/g | 12.000±2.160 | - |
| Group-II (Standard) | Indomethacin | 10 mg/kg | 2.250±0.957b | 81.25 |
| Group-III (Extract) | EAF | 200 mg/kg | 2.460±0.869b | 79.50 |
| Group-IV (Extract) | DCMF | 200 mg/kg | 3.000±0.816b | 75.00 |
| Group-V (Extract) | CTCF | 200 mg/kg | 3.500±0.577b | 70.83 |
Values are presented as mean±SD. EAF: ethyl acetate fraction; DCMF: dichloromethane fraction; CTCF: carbon tetrachloride fraction; aP<0.05, bP<0.01 when compared to castor oil control.
3.2. Formalin test method
The results of analgesic activity determined by formalin test method are shown in Table 2. In this test, the different fractions of methanolic extract of A. procera leaves (100 and 200 mg/kg, p.o.) also significantly suppressed the licking activity in either phase of the formalin-induced pain in rats in a dose dependant manner. At 200 mg/kg of body weight, ethyl acetate, dichloromethane and carbon tetrachloride showed 33.33%, 17.73% and 19.14% licking inhibition respectively. The standard drug (indomethacin) used in the experiment showed 53.19% licking inhibition.
Table 2. Formalin induced licking response of of different extracts of A. procera leaves.
| Group | Treatment | Dose | Early phase (0 to 5 min) |
Late Phase (0 to 5 min) |
||
| No. of licking | Inhibition (%) | No. of licking | Inhibition (%) | |||
| Group-I (Control) | Distilled water | 10 mL/kg | 46 | - | - | - |
| Group-II (standard) | Indomethacin | 10 mg/kg | 16.750±2.753b | 53.19 | 7.750±3.304b | 83.15 |
| Group-III (Extract) | EAF | 200 mg/kg | 23.500±4.654a | 33.33 | 7.000±2.160b | 84.78 |
| Group-IV(Extract) | DCMF | 200 mg/kg | 29.000±5.354 | 17.73 | 8.000±4.242b | 82.61 |
| Group-V (Extract) | CTCF | 200 mg/kg | 28.500±6.557 | 19.14 | 5.250±2.217 | 88.58 |
Values are presented as mean±SD. EAF: ethyl acetate fraction; DCMF: dichloromethane fraction; CTCF: carbon tetrachloride fraction; aP<0.05, bP<0.01 when compared to castor oil control.
3.3. Hole cross test
Table 3 shows the effect of different extracts (EAF, DCMF, CTCF) on the locomotion activity. The locomotor activity lowering effect was manifested at the 2nd observation (30 min) period and was sustained up to the 5th observation period (120 min) for extracts. The extracts significantly diminished the locomotion activity which was comparable with standard diazepam. At 500 mg/kg, the extracts EAF, DCMF and CTCF showed depressant activity of 3.25±1.89, 2.25±2.06 and 2.50±0.57, respectively whereas the standard drug chlorpromazine showed 1.75±0.95 at dose of 1 mg/kg.
Table 3. CNS depressant activity of different fraction of A. procera leaves by hole cross test in rats.
| Group | Mean hole cross (no.) before and after drug administration |
||||
| 0 min | 30 min | 60 min | 90 min | 120 min | |
| Control | 11.750±1.708 | 12.750±1.707 | 14.250±1.707 | 9.25±4.57 | 12.50±1.29 |
| Standard | 10.000±1.633 | 5.250±1.500 | 4.250±1.892b | 3.00±1.41a | 1.75±0.95b |
| EAF | 8.500±1.291a | 6.500±1.290a | 5.000±0.816b | 3.75±0.95a | 3.25±1.89b |
| CTCF | 9.750±1.708 | 5.500±1.291b | 6.500±1.290b | 3.75±1.70a | 2.25±2.06b |
| DCMF | 10.750±1.893 | 6.500±0.577b | 5.000±1.414b | 3.50±2.08a | 2.50±0.57 |
Values are presented as mean±SD. EAF: ethyl acetate fraction; DCMF: dichloromethane fraction; CTCF: carbon tetrachloride fraction; aP<0.05, bP<0.01 when compared to control.
3.4. Open field test
Open field test was carried out to determine the depressive action of the test drugs on CNS in rats. The extracts showed a noticeable decrease in locomotion in the test animals from the second observation period to last study period at dose level (500 mg/kg body weight). The results are shown in Table 4. The effect observed was increasing with time and a noticeable result was found at 120 min of test sample administration. Test animals were showing significant decrease in number of movement in the dosages of 500 mg/kg (EAF: 5.50±2.65, CTCF: 6.25±1.71 and DCMF: 16.25±7.27) respectively, as compared to 52.00±5.35 in the control group and 5.250±0.957 in the standard group) at 120 min of administration of the extract.
Table 4. CNS depressant activity of different fractions of A. procera leaves by open field test in rats.
| Group | Mean movements (no.) before and after drug administration |
||||
| 0 min | 30 min | 60 min | 90 min | 120 min | |
| Control | 57.750±9.673 | 51.50±2.38 | 50.75±5.61 | 51.75±4.03 | 52.000±5.354 |
| Standard | 51.250±6.396a | 23.75±4.03b | 13.50±3.41b | 10.75±2.75b | 5.250±0.957b |
| EAF | 47.750±6.849 | 25.00±2.16b | 16.00±2.82b | 11.25±3.50b | 5.500±2.645b |
| CTCF | 59.000±10.645 | 46.25±8.30 | 21.25±6.50b | 14.00±2.82b | 6.250±1.707b |
| DCMF | 56.750±2.986 | 47.00±4.96 | 34.00±6.37a | 19.75±4.64b | 16.250±7.270b |
Values are presented as mean±SD. EAF: ethyl acetate fraction; DCMF: dichloromethane fraction; CTCF: carbon tetrachloride fraction; aP<0.05, bP<0.01 when compared to control.
3.5. Antimicrobial activity
Antibacterial activities were carried out against two Gram-positive [Staphylococcus aureus (S. aureus), Bacillus cereus] and four Gram-negative [Pseudomonas aeruginosa, Esherichia coli (E. coli), Shigella soneii, Shigella boydii] bacteria. Aactivities of the CTCF, DCMF and EAF of the A. procera leaves obtained by the disc diffusion method against these organisms are shown in (Table 5). CTCF exhibited higher degree of antimicrobial activity against the Gram-positive bacteria as compared with EAF and DCMF. On the other hand, DCMF was the most active against all the gram-negative bacteria except E. coli.
Table 5. Antimicrobial activities of the CTCF, DCMF and EAF extracts of A. procera leaves.
| Bacteria | Diameter of the zone of inhibition (mm) |
||||
| CTCF 800 µg/disc | DCMF 800 µg/disc | EAF 800 µg/disc | Kanamycin 30 µg/disc | ||
| Gram-positive | S. aureus | 12 | 7 | 10 | 22 |
| B. cereus | 10 | 6 | 8 | 23 | |
| Gram-negative | P. aeruginosa | 11 | 10 | 9 | 25 |
| E. coli | 8 | 8 | 10 | 16 | |
| S. soneii | 9 | 11 | 10 | 21 | |
| S. boydii | 9 | 10 | 8 | 24 | |
4. Discussion
Medicinal plants have drawn the great attention as a sources of commercial drugs or as lead compounds in drug development. The drugs which are used presently for the management of pain and inflammatory conditions are either narcotics or non narcotics (NSAIDS), and have known toxic and lethal effects[14]. On the contrary, herbal medicines with good absorption, less toxicity, and easy availability have been used since ancient times[15]. It is therefore essential that efforts be made to introduce new medicinal plants to develop cheaper, and the acetic acid induced abdominal constriction method is a sensitive method for the evaluation of peripheral antinociceptive activity[16]. This response was believed to be mediated by the prostaglandin, peritoneal mast cells, and acid sensing ion channels pathways[17]. Our results indicated that the extracts could significantly reduce the number of writhing, showing potential anti-nociceptive action and the mechanism by which they exert their analgesic effect probably by inhibiting synthesis or action of any of the above pathway. In order to confirm whether the antinociceptive action was central or peripheral, the extracts were also examined using formalin test method, which was generally considered a central action. The test consists of two different phases: early phase where the pain began due to the direct stimulation of the sensory nerve fibers by the direct action of formalin, and in the late phase, pain induced due to different inflammatory mediators, such as histamine, prostaglandins, serotonin and bradykinins[18]. Central analgesic drugs like narcotics, inhibited equally both phases, while peripherally acting drugs, such as steroids (hydrocortisone and dexamethasone) and NSAIDs (indomethacine), suppresses mainly the late phase[19]. The results obtained here indicated that the extracts inhibited late phase mechanisms of pain, suggesting that the plant extract may act as steroids and NSAIDs. It is also reported that the inhibition of pain may also due to the presence of phenolic constituents[20] which may be due to the similar type of constituents present in the extracts of A. procera leaves. However, the exact mechanism of this action has not been investigated here.
Locomotor activity refers to an increase in alertness and decrease in locomotor activity considered as sedative effect. In this study, locomotor activity measured by hole cross and open field tests, showed that the extract significantly decreased locomotor activity which indicates it has CNS depressant activity. Diazepam, which was used to induce sleep in this study, believed to act at specific binding sites that are closely linked to γ-aminobutyric acid (GABA) receptors, the binding of benzodiazepines enhancing GABA-ergic transmission. It has been reported that many flavonoids and neuroactive steroids were found to be ligands for the GABA receptors in the central nervous system; that can act as benzodiazepine-like molecules[16]. Preliminary phytochemical studies revealed the presence of glycosides, flavonoids, tannin etc. in methanol extract of A. procera leaves. So, it is probable that flavonoids present in the extracts may responsible for its CNS depressant activity.
A number of studies have raised the necessity of developing alternative antimicrobial drugs[21],[22]. Plant antimicrobials would appear to be an excellent choice. It has been shown previously that methanol extract of A. procera stem bark exhibit a potent antimicrobial activity against Bacillus subtilis, S. aureus, Staphylococcus epidermidis, and Enterococcus faecalis[23]. No previous report on the antibacterial activity of the leaves of A. procera could be found in the literature. Use of leaves extract in the current study demonstrated that the leaf also produces antibacterial compounds against Gram-positive and Gram-negative bacteria.
To the best of our knowledge, this is the first report of analgesic, CNS depressant and antibacterial activity of A. procera leaves. On the basis of results obtained from the above investigation, we can conclude that the extracts of A. procera leaves have neuropharmacological activity as evident by CNS depressant activity. Central depressant activity along with strong analgesic effect may complement to each other. It may be useful as CNS depressant and analgesic agent in clinical conditions. But present work was a preliminary effort which will require further detailed advanced investigation including characterization of active compounds and requires pre-formulation studies for development of a potential dosage form.
Acknowledgments
The authors gratefully acknowledge Botanical garden, Rajshahi University, for providing the plant material. This study was partially funded to MMK by the National Science and Technology (NST), Government of the People's Republic of Bangladesh (Grant No. 39.012.002.01.03.018.2012/256).
Comments
Background
A wide range of synthetic drugs has been proposed for use in the treatment of pain, CNS deficiencies and infectious diseases. Mostly, synthetic drugs have toxic effects, side effects and facing drug resistance against human pathogens. Mostly plant herbal drugs are less toxic, cause no or less side effects compare to synthetic drugs. Hence, there is need of less toxic drugs and broad spectrum antibiotics.
Research frontiers
The present research depicts analgesic, CNS depressant, antimicrobial activity of three fractions (EAF, DCMF and CTCF) of methanol extract of A. procera leaves. Analgesic activity assessed using acetic acid-induced writhing model in mice and formalin induced paw licking, CNS depressant activity assessed using hole cross test and open field method, antibacterial activity assessed by disc diffusion method. All three fractions (EAF, DCMF and CTCF) of methanol extract of A. procera leaves showed good analgesic, CNS depressant and antimicrobial activity.
Related reports
The acetic acid induced writhing is a sensitive method for the evaluation of peripheral antinociceptive activity. This response was believed to be mediated by the prostaglandin, peritoneal mast cells, and acid sensing ion channels pathways. Formalin induced paw licking test based on central action. It consisted two phases, early phase (stimulation of the sensory nerve fibers) and late phase (inflammatory mediators, like histamine, prostaglandins, serotonin and bradykinins). Locomotor activity test (hole cross test and open field test) is very benifecial to evaluate CNS depressant activity. It has been shown previously that methanol extract of A. procera stem bark exhibited a potent antimicrobial activity against Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus faecalis.
Innovations and breakthroughs
This is the first report of analgesic, CNS depressant and antibacterial activity of A. procera leaves. Central depressant activity along with good analgesic effect may complement to each other. It may be useful as CNS depressant and analgesic agent in clinical conditions.
Applications
From the literature survey, it has been found that A. procera is safe to humans. This scientific study supports the use of this plant as analgesics, CNS depressant and antimicrobials.
Peer review
This is a valuable research work in which authors have demonstrated the analgesic, CNS depressant and antimicrobial activities of three fractions (EAF, DCMF and CTCF) of methanol extract of A. procera leaves by using different models in rodants. The plant decreased writhes and paw licking in analgesic models, decreased locomator activity in CNS depressant models and decreased microbial growth in disc diffusion method. All models were promising its analgesic, CNS depressant and antimicrobial activities.
Footnotes
Foundation Project: Supported by the National Science and Technology (NST), Government of the People's Republic of Bangladesh (Grant No. 39.012.002.01.03.018.2012/256).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Chōmchalao N, Henle HV. Medicinal and aromatic plants in Asia: breeding and improvement. Bangkok, Thailand: RAPA Publication; 1993. [Google Scholar]
- 2.Bhattacharjee I, Chatterjee SK, Ghosh A, Chandra G. Antibacterial activities of some plant extracts used in Indian traditional folk medicine. Asian Pac J Trop Biomed. 2011;1(Suppl 2):S165–S169. [Google Scholar]
- 3.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 4.Bunluepuech K, Tewtrakul S. Anti-HIV-1 integrase activity of Thai medicinal plants in longevity preparations. Songklanakarin J Sci Technol. 2011;33:693–697. [Google Scholar]
- 5.Kirtikar KR, Basu BD. Indian medicinal plants. Dehra Dun, India: Bishen Singh Mahendra Pal Singh; 1975. p. p. 894. [Google Scholar]
- 6.Yutana P, Patcharin N, Narumon S. Antioxidant activity and xanthine oxidase inhibitor from Thai medicinal plants used for tonic and longevity. Bangkok, Thailand: Kasetsart University Annual Conference; 2009. [Google Scholar]
- 7.Khatoon M, Islam E, Islam R, Rahman AA, Alam AH, Khondkar P, et al. et al. Estimation of total phenol content and in vitro antioxidant activity of Albizia procera leaves. BMC Res Notes. 2013;6:121. doi: 10.1186/1756-0500-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nwafor PA, Nwajiobi N, Uko IE, Obot JS. Analgesic and anti-inflammatory activities of an ethanol extract of Smilax krausiana leaf in mice. Afr J Biomed Res. 2010;13:141–148. [Google Scholar]
- 9.Yaro AH, Magaji MG, Danjuma NM, Malamu S, Isah A. Studies on analgesic and anti-inflammatory activities of Cissampelos mucronata Linn. A. Rich in laboratory animals. Int J Pure Appl Sci. 2008;2:111–117. [Google Scholar]
- 10.Okokon JE, Nwafor PA. Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak J Pharm Sci. 2010;23(4):385–392. [PubMed] [Google Scholar]
- 11.Hussain J, Ur Rehman N, Hussain H, Al-Harrasi A, Ali L, Rizvi TS. Analgesic, anti-inflammatory, and CNS depressant activities of new constituents of Nepeta clarkei. Fitoterapia. 2012;83(3):593–598. doi: 10.1016/j.fitote.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Bhosale U, Yegnanarayan R, Prachi P, Zambare M, Somani RS. Study of CNS depressant and behavioral activity of an ethanol extract of Achyranthes aspera (Chirchita) in mouse model. Ann Neurosci. 2011;18:44–47. doi: 10.5214/ans.0972.7531.1118204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfoze NL, Kumar Y, Myrboh B, Bhagobaty RK, Joshi SR. In vitro antibacterial activity of alkaloid extract from stem bark of Mahonia manipurensis Takeda. J Med Plants Res. 2009;5:859–861. [Google Scholar]
- 14.Park JH, Son KH, Kim SW, Chang HW, Bae K, Kang SS, et al. et al. Anti-inflammatory activity of Synurus deltoids. Phytother Res. 2004;18:930–933. doi: 10.1002/ptr.1595. [DOI] [PubMed] [Google Scholar]
- 15.Li RW, Mayers SP, Leach DN, Lin GD, Leach G. A cross-cultural study: anti-inflammatory activity of Australian and Chinese plants. J Ethanopharmacol. 2003;85:25–32. doi: 10.1016/s0378-8741(02)00336-7. [DOI] [PubMed] [Google Scholar]
- 16.Dal Molin MM, Silva S, Alves DR, Quintão NL, Delle Monache F, Cechinel Filho V, et al. et al. Phytochemical analysis and antinociceptive properties of the seeds of Garcinia achachairu. Arch Pharm Res. 2012;35:623–631. doi: 10.1007/s12272-012-0405-3. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, et al. et al. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol. 2000;387:111–118. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- 18.Voilley N. Acid-sensing ion channels (ASICs): new targets for the analgesic effects of non-steroid anti-inflammatory drugs (NSAIDs) Curr Drug Targets Inflamm Allergy. 2004;3:71–79. doi: 10.2174/1568010043483980. [DOI] [PubMed] [Google Scholar]
- 19.Kumar BS, Lakshman K, Jayaveera KK, Shekar DS, Vivek C. Antinociceptive and antipyretic activities of Amaranthus viridis Linn. in different experimental models. Arch Biol Sci. 2010;62:397–402. [PMC free article] [PubMed] [Google Scholar]
- 20.Annegowda HV, Gooi TS, Awang SH, Alias NA, Mordi MN, Ramanathan S, et al. et al. Evaluation of analgesic and antioxidant potency of various extracts of Cinnamomum iners Bark. Int J Pharmacol. 2012;8:198–203. [Google Scholar]
- 21.Poole K. Mechanisms of bacterial biocide and antibiotic resistance. Symp Ser Soc Appl Microbiol. 2002;92:55S–64S. [PubMed] [Google Scholar]
- 22.Abreu AC, McBain AJ, Simões M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat Prod Rep. 2012;29:1007–1021. doi: 10.1039/c2np20035j. [DOI] [PubMed] [Google Scholar]
- 23.Duraipandiyan V, Ayyanar M, Ignacimuthu S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement Altern Med. 2006;6:35. doi: 10.1186/1472-6882-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]


