Abstract
Objective
To study the phytochemical and biological properties (antioxidant, anthelmintic and thrombolytic) of methanolic extracts of Enhydra fluctuans Lour., a plant belonging to the Asteraceae family.
Methods
The phytochemical evaluation was carried out by qualitative analysis. In vitro antioxidant activity of extract was studied using free radical scavenging assay, ability of reduction, total phenol and total flavonoid contents determination assays. The anthelmintic activity was determined using paralysis and death time of Pheretima posthuma (earthworm) and thrombolytic activity by clot disruption assay.
Results
The phytochemical evaluation showed significant presence of flavonoids, triterpenes, carbohydrate, reducing sugars, saponins, phenols, diterpenes, protein and tannin. The antioxidant activity was found significant [IC50=(135.20±0.56) µg/mL] as compared to ascorbic acid [(130.00±0.76) µg/mL]. The reducing power was increased with concentration. Total phenol and total flavonoid contents were (153.08±0.38) mg/mL and (172.04±0.56) mg/mL respectively. The paralysis and death time of earthworms for different concentrations of extract were determined and compared with albendazole. The results showed that 10 mg/mL of the crude extract had similar effect with albendazole. Additionally, the crude extract showed a concentration depended relationship with its anthelmintic property. The clot lysis activity of crude extract was compared to the standard streptokinase's clot lysis (40.13%) activity and found significant (31%).
Conclusions
The study proves that the crude methanolic extract of Enhydra fluctuans Lour. has significant antioxidant, anthelmintic and thrombolytic activity containing wide range of phytochemicals.
Keywords: Enhydra fluctuans Lour., Phytochemicals, Antioxidant, DPPH radical scavenging, Pheretima posthuma, Streptokinase
1. Introduction
Nature has been a supply of medicative agents for thousands of years and a formidable variety of recent medications were isolated from natural sources, several of that supported their use in ancient medication. In sight of this, our attention has been targeted significantly to Enhydra fluctuans Lour. (E. fluctuans) (Family: Asteraceae), native name (helencha), edible semi aquatic nonwoody vegetable plant with separate leaves. It is native to India, Bangladesh, Burma, Srilanka and a number of other places in South East Asia[1]. This plant may be a prostate, spreading, annual herb. The stems are somewhat fleshy, thirty centimeters or a lot of long, branched development at the lower nodes, and somewhat bushy. The leaves are sessile, linear-oblong, three to five centimeters long, pointed or blunt at the tip, sometimes truncate at the bottom[1]. The leaves are slightly bitter, cure inflammation, skin diseases and small pox. It possesses biological value and its fuel extract has been reportable to own analgesic, cytotoxic, antimicrobial, hepatoprotective, hypotensive, CNS depressant, antidiarrheal activity[2]–[7].
The role of free radical reactions in disease pathology is well established. The free radicals are responsible for not only in support of aging but also many age-related diseases[8]. Free radical damage within cells has been connected to a range of disorders including cancer, arthritis, atherosclerosis, Alzheimer's disease, and diabetes[9]–[11]. There has been some proof to suggest that free radicals and some reactive nitrogen species trigger and increase cell death mechanisms such as apoptosis and in extreme cases necrosis[12],[13]. Scientists recommend that antioxidant can reduce the activity of free radicals including their so called side effects and thus increase the cell survival times effectively[14].
Thrombosis is the fundamental patho-physiological process that underlies the acute coronary disorders which are the main causes of morbidity and mortality in developed countries. Portal vein thrombosis frequently caused by thrombus formation in vein leads to the constricting of portal vein followed by portal hypertension. Cerebral epithelial duct occlusion may be a common disorder that in the midst of vital morbidity and mortality. Clot buster medication like tissue proteinase, alteplase (Activase), reteplase (Retavase), tenecteplase (TNKase), streptokinase, urokinase etc. play an important role in the management of patients with cerebral epithelial duct occlusion[11].
Helminthiasis, a macro-parasitic disease, is observed in humans and animals which reflects serious social and economic problems throughout the world, especially in the third world countries. In this disease, a part of the body is infested with parasitic worms like roundworms (nematodes), tapeworms (cestodes) or flukes (trematodes)[15]. In the medical field, helminthes have been a matter of concern for centuries and they still cause considerable problems to human and other animals. World Health Organization estimates that about two billion of people throughout the world are affected by parasitic worm infection and the reason for it is associated with poor management practices and inadequate control measures[16]. Although numerous advances were made in understanding the mode of transmission and the treatment of the helminthes during the last few decades, there is still no potential product which can control specific helminthes[17].
However, indiscriminate use of several anthelmintic, antioxidant, clot buster medication has emerged problems, leading to the development of resistance as well as chemical residue and toxicity problems[18]. For these reasons, phytochemical screening of medicinal plants for their antioxidant, anthelmintic and clot lysis activity has become a matter of great scientific interest though synthetic chemicals are extensively used in modern clinical practices worldwide[19]. On the contrary, our traditional system of medicine and folklore are using the whole medicinal plant or a part of it for the treatment of all types of disease successfully including antibacterial, anthelmintic, anti-inflammatory etc. since the time immemorial[20]. This is because the traditional medicines act as an easily available and effective source of medicines to people with a broad spectrum of action like high percentage of cure with single therapeutic dose, cost effective and free from toxicity[21].
Thus the native use of E. fluctuans as medicament prompted us to research the phytochemical analysis, antioxidant, anthelmintic and clot buster activity of E. fluctuans that has not been explored so far. The methanolic extracts of E. fluctuans were evaluated for phytoconstituents, total phenolic content, total flavonoid content, the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity, ability of reduction, the anthelmintic and clot lysis activity in the present study.
2. Materials and methods
2.1. Chemicals
Lyophilized S-Kinase™ (streptokinase) vial (1 500 000 IU) was purchased from Popular Pharmaceuticals Ltd., Bangladesh; Batch No: VEH 09. DPPH (1, 1-diphenyl 2-picryl hydrazyl), trichloroacetic acid, gallic acid, ferric chloride and quercetin were obtained from Sigma Chemical Co. Ltd, (St. Louis, MO, USA). Ascorbic acid was obtained from SD Fine Chem. Ltd., India. All other chemicals and reagents were of analytical grade.
2.2. Plant materials
Whole plant of E. fluctuans was collected from Lakshmipur district, Bangladesh in July 2012. After collection whole plant were thoroughly washed with water. The plant was identified and authenticated by taxonomist of Bangladesh National Herbarium, Mirpur, Dhaka, Bangladesh (accession number-37925). The collected plant parts were separated carefully. The separated samples were then dried at room temperature in the shade and away from direct sunlight for 5 d and finally kept in hot air oven for 3 d.
2.3. Preparation of crude extract
After drying, the total plants were coarsely fine-grained (120 g) and extracted by dissolving with methanol (500 mL) for 15 d concomitant occasional shaking and stirring. The sediments were filtered and also the filtrates were dried at 40 °C during a water bathtub. The solvent was utterly removed by filtering with Whatman paper (Bibby RE200, Sterilin Ltd., UK) and obtained dried crude extract was used for experiment.
2.4. Phytochemical screening
Testing of various chemical compounds within the extract, represents the preliminary phytochemical studies. Little amount of methanolic extracts of E. fluctuans was subjected to preliminary quantitative phytochemical investigation for detection of phytochemicals like alkaloids, carbohydrates, viscous glycosides, phytosterols, proteins, flavonoids, tannins, saponins, phenols, terpenes etc. exploiting the quality ways[22]–[24].
2.5. Antioxidant activity
2.5.1. DPPH radical scavenging activity
The free radical scavenging activity of the extract, based on the scavenging activity of the stable DPPH free radical, was analyzed by the method described by Braca et al[25]. About 2.0 mL of a methanol solution of the extract at different concentration (500 to 0.977 µg/mL) were mixed with 3.0 mL of a DPPH methanol solution (20 µg/mL). After 30 min reaction period at room temperature in dark place, the absorbance was measured at 517 nm against methanol as blank by UV spectrophotometer. The percentage inhibition activity was calculated from
Inhibition (%)=[(A0-A1)/A0]×100
Where, A0 is the absorbance of the control, and A1 is the absorbance of the extract/standard. Then the inhibition curves were prepared and IC50 values were calculated. BHT was used as positive control.
2.5.2. Reducing power
Reducing power of crude extract was analyzed by the method described at Srinivas et al[26]. The different concentrations of extract (125, 250, 500 and 1 000 µg/mL) in 1 mL of distilled water were mixed with phosphate buffer (2.5 mL, 0.2 mol/L, pH 6.6) and potassium ferricyanide- K3Fe(CN)6 (2.5 mL, 1% w/v). The mixture was incubated at 50 °C for 20 min. A portion (2.5 mL) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3 000 r/min for 10 min. The upper layer of the solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1% w/v) and the absorbance was measured at 700 nm. The increased absorbance of the reaction mixture indicates increased reducing power. Ascorbic acid was used as the reference and phosphate buffer (pH 6.6) was used as blank solution.
2.5.3. Total phenol content
Total phenolic content of methanolic extract of E. fluctuans was measured applying the method involving Folin-Ciocalteu reagent as oxidizing agent and gallic acid as standard[27],[28]. Different gallic acid solutions were prepared having a concentration ranging from 50 µg/mL to 0 µg/mL. A volume of 2.5 mL of Folin-Ciocalteau reagent (diluted 10 times with water) and 2.0 mL of Na2CO3 (7.5% w/v) solution was added to 0.5 ml of gallic acid solution. The mixture was incubated for 20 min at room temperature. After 20 min, the absorbance was measured at 760 nm. After plotting the absorbance in ordinate against the concentration in abscissa, a linear relationship was found which was used as a standard curve for the determination of the total phenolic content of the test samples. In 0.5 mL of extract solution (conc. 2 mg/mL), 2.5 mL of Folin-Ciocalteu reagent (diluted 10 times with water) and 2.0 mL of Na2CO3 (7.5% w/v) solution was added. The mixture was incubated for 20 min at room temperature. After 20 min, the absorbance was measured at 760 nm by UV-spectrophotometer and using the standard curve prepared from gallic acid solution with different concentration, the total phenolic content of the sample was determined. The phenolic contents of the sample were expressed as mg of (gallic acid equivalent)/g of the extractive.
2.5.4. Total flavonoids content
The total flavonoids content was determined following a method demonstrated by Kumaran et al. using quercetin as a reference compound[29]. A volume of 1 mL of the plant extract in methanol (200 µg/mL) was mixed with 1 mL aluminium trichloride in methanol (20 mg/mL) and a drop of acetic acid, and then diluted with methanol to 25 mL. The absorbance was measured at 415 nm after 40 min. Blank samples were prepared from 1 mL of plant extract and a drop of acetic acid, and then diluted to 25 mL with methanol. The total flavonoid content was determined using a standard curve of quercetin (12.5-100 µg/mL) and expressed as mg of quercetin equivalent (QE/mg of extract).
2.6. Anthelmintic activity
The anthelmintic assay was carried out as per the method of Malvankar et al. with minor modifications[30]. Adult earthworms (Pheretima posthuma) were used to study the anthelmintic activity due to its anatomical and physiological resemblance with the intestinal roundworm parasite of human beings. Because of availability of earthworms, they are widely used as effective tools for anthelmintic study[31]. After collection, earthworms were washed with saline water. Different concentrations of test sample (10-80 mg/mL) were prepared. About 150 mg of albendazole was measured by weighing machine and dissolved in 10 mL water to make a concentration of 15 mg/mL standard solution. A control group was established with distilled water to ensure that the test was a validate one. Earthworms were divided into eleven groups each containing three earthworms. Five groups were used to the five concentrations of methanolic extract of E. fluctuans One group was applied to reference standard and another to control group. For each concentration, triplets (three Petri dishes) were used, each Petri dish containing equal sized earthworm. Continuous observation was made to notice any physical change in the earthworms. The time of paralysis was recorded when no movement of any sort could be observed except the worms were shaken vigorously. Time for death of worms were recorded after ascertaining that the worms neither moved when shaken vigorously nor when dipped in warm water at 50 °C.
2.7. Thrombolytic activity
In vitro clot lysis activity of E. fluctuans was carried out according to the method illustrated by Dewan and Das with minor modification[32]. In the commercially available lyophilised streptokinase vial (1 500 000 IU) 5 mL sterile distilled water was added and mixed properly. This suspension was used as a stock solution from which appropriate dilution was made. Five milliliter of venous blood was drawn from the healthy volunteers (n=10) without the history of oral contraceptive or anticoagulant therapy and was distributed (0.5 mL/tube) to each 10 previously weighed sterile micro centrifuge tube and incubated at 37 °C for 45 min to form the clot. After the formation of clot, serum was completely removed from the tubes (carried out without disturbing the clot formed) and each tube having clot was again weighed to determine the weight of the clot.
Clot weight = weight of clot containing tube - weight of tube alone.
Each micro-centrifuge tube containing clot was properly labeled and 100 µL of the plant extract with various concentrations (2, 4, 6, 8 and 10 mg/mL respectively) was added to the tubes accordingly. As a positive control, 100 µL of streptokinase and as a negative non thrombolytic control, 100 µL of sterilized distilled water were separately added to the control tubes numbered. Then all the tubes were incubated again at 37 °C for 90 min and observed for clot lysis. After incubation, the obtained fluid was removed from the tubes and they were again weighed to observe the difference in weight after clot disruption. At last, difference obtained in weight was calculated and the result was expressed as percentage of clot lysis following the underneath equation.
Clot lysis (%) = (weight of released clot/clot weight)×100
2.8. Statistical analysis
All the results obtained by in vitro experiment were expressed as mean±SEM of three measurements following paired t-test analysis where P<0.05 was considered as statistically significant.
3. Results
The phytochemical screening of methanolic extract of E. fluctuans indicated the qualitative presence of flavonoids, triterpenes, carbohydrate, reducing sugar, saponins, phenols, diterpenes, proteins and tanins (Table 1).
Table 1. Phytochemical constituents identified in the methanolic extract of E. fluctuans.
| Phytochemicals | Methanolic extract of E. fluctuans |
| Alkaloid | - |
| Flavonoids | + |
| Triterpenes | + |
| Cardiac glycoside | - |
| Carbohydrate | + |
| Reducing sugars | + |
| Saponins | + |
| Phenols | + |
| Diterpenes | + |
| Proteins | + |
| Tanin | + |
| Phytosterols | - |
+: Presence, -: Absence.
The DPPH free radical scavenging activity depends on the ability of DPPH, a stable free radical, to be decolorized in the presence of antioxidants[33]. DPPH radical scavenging activity of E. fluctuans was found to increase with increasing concentration of the extract (Figure 1). IC50 value of DPPH scavenging activity was (135.20±0.56) µg/mL as compared to ascorbic acid (130.00±0.76 µg/mL). The extract also demonstrated significant reducing power which was found to increase with the increasing concentration (Figure 2). During the determination of total phenol and flavonoid content, it was observed that the amount of phenols and flavonoids was significant. Total phenol and total flavonoid contents were (153.08±0.38) mg/mL and (172.04±0.56) mg/mL respectively. The methods and results for determination of antioxidant activity were compared with other studies[34]–[36].
Figure 1. The free radical scavenging activity of E. fluctuans and ascorbic acid by DPPH.
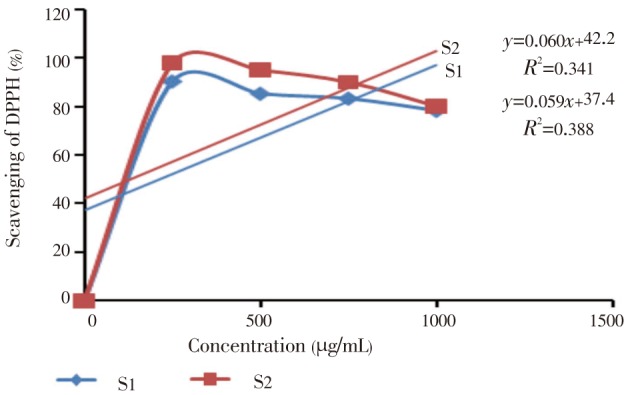
S1=%Scavenging of E. fluctuans extract, S2=%Scavenging of ascorbic acid. The results are expressed as mean±SEM of three consecutive experiments.
Figure 2. Reducing capacity of E. fluctuans extract.
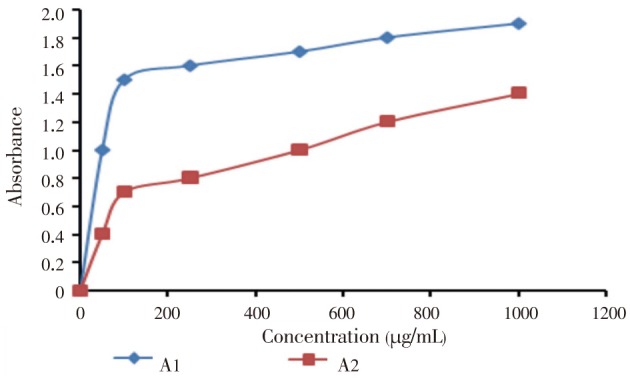
A1=Absorbance of ascorbic acid, A2=Absorbance of extract. The results are expressed as mean±SEM of three consecutive experiments.
The anthelmintic activity of methanolic extract of E. fluctuans on earthworms (Pheretima posthuma) was determined by different concentration of extract compared with standard (albendazole) and negative control. Crude methanol extracts of E. fluctuans inhibited earthworms in a significant dose-dependent manner (Figure 3). The paralysis time of earthworms for extract at different concentrations, including 10 mg/mL, 20 mg/mL, 40 mg/mL, 60 mg/mL and 80 mg/mL was 33.66, 32.33, 29.00, 23.33 and 21.33 min respectively, whereas death time was 70.33, 67.66, 63.66, 36.66 and 33.33 min respectively compared with albendazole (35.33 min and 71.33 min respectively).
Figure 3. Paralysis and death time of methanolic extract of E. fluctuans.
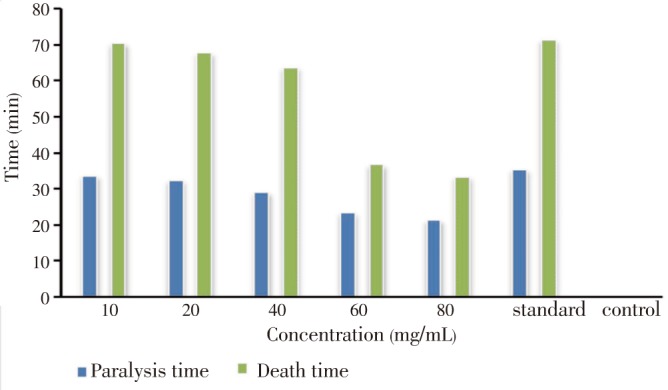
The results are expressed as mean±SEM of three measurements.
The clot lysis activity of methanolic extract of E. fluctuans increased with the increase of concentration as compared to standard streptokinase's clot lysis (40.13%) activity. The concentration 10 mg/mL methanolic extract of E. fluctuans exhibited the highest (31%) thrombolytic activity (Figure 4). The percentage (%) of clot lysis was significant (P<0.05) when compared with control.
Figure 4. Clot lysis activity of different concentrate E. fluctuans.
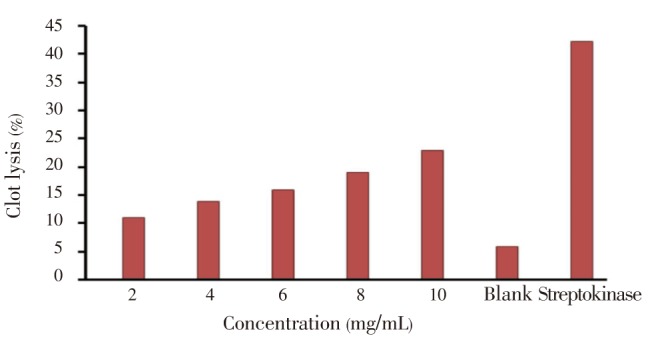
The results are mean±SEM of three consecutive experiments.
4. Discussion
The phytochemical screening of methanolic extracts of E. fluctuans shows the presence of flavonoids, triterpenes, saccharide, reducing sugar, saponins, phenols, diterpenes, proteins and tanins. The biological activities of this plant may be due to the presence of those various groups of chemical compounds[37],[38].
Antioxidants are useful to cut back and forestall injury from radical reactions by donating electrons that neutralize the unconventional while not forming another. For example, vitamin C will lose associate degree negatron to a radical and stay stable itself by passing its unstable negatron round the inhibitor molecule[39]. The methanolic extract of E. fluctuans has been tested for the determination of inhibitor activity. The reducing activity of a compound depends on the presence of reductors that has been exhibited antioxidative activity by breaking the radical chain, donating a chemical element atom. The screening of the inhibitor activity of this plant has proven its capability to scavenge the radical (DPPH) at low concentration, which may be due to the presence of phenol and alternative phytoconstituents in its crude extract.
The anthelmintic activity shows that methanolic extracts of E. fluctuans possess potent anthelmintic activity in dose dependent manner. The activity shown by this extract contains a very significant importance. The extract of E. fluctuans shows the highest activity that is nearly adequate to the consequences shown by albendazole solution. Albendazole acting by inhibiting fibre bundle transmission in worm could also be by acting like GABA; the repressive neurochemical in nematodes. This permits host body to simply take away out the harmful organisms. The time taken for the induction of dysfunction and death in each albendazole and E. fluctuans was nearly same. The extent of activity shown by crude extracts was found to be dose dependent.
Platelets play a significant role in the formation of clot by adhering to the broken regions of the epithelial tissue surface. Most thrombolytic agents work by regulating the enzyme plasminogen, which cuts the cross-linked fibrin mesh. This makes the clot soluble and leads to further proteolysis by other enzymes, and thus causes blood flow over occluded blood vessels. Thus thrombolytic agents are useful for the treatment of myocardial infarction, thromboembolic strokes, deep vein thrombosis etc[40]. The comparison of the positive management (streptokinase) with negative management clearly showed that clot dissolution didn't occur when water was added to the clot. Having the results of the positive management, we tend to compare five totally different concentrations of the test sample within the same manner with the negative management and discovered vital clot lysis activity. It was conjointly discovered from the study that the proportion of clot lysis is proportional to the concentration. Since phytochemical analysis showed that the crude extract contains flavonoids, triterpenes, carbohydrate, reducing sugar, saponins, phenols, diterpenes, proteins and tanins; it can be predicted that these phytoconstituents could also be liable for its clot lysis activity[37],[38].
Taking into consideration all of the findings from the current study, it will build a general comment that the methanolic extracts of E. fluctuans has significant antioxidant, anthelmintic and thrombolytic activity with wide range of phytoconstituents[41]. Since, this study was conducted by crude extract, any advanced studies ought to be allotted for compound isolation and it's necessary to look at that compounds are literally answerable for specific effects.
Acknowledgments
The authors are thankful to Professor Dr. A.K.M. Saifuddin, Chairman, Dr. S.K.M. Azizul Islam, Associate Professor and the technical and non-technical staffs of the Department of Physiology, Biochemistry and Pharmacology, Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Chittagong, Bangladesh for their kind co-operation and research facility. The authors are also thankful to all the teachers and staffs of the Department of Pharmacy, Noakhali Science and Technology University, Noakhali, Bangladesh for their valuable support. This work was supported by the Department of Physiology, Biochemistry and Pharmacology, Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Chittagong, Bangladesh (Grant No. RFLBC/MSC/res. sund/5004/3096).
Comments
Background
Herbs contain various phytochemical constituents and biological properties that used to treat numerous diseases of human and animals without any side effects. As such E. fluctuans is an indigenous herbs in Bangladesh used as vegetables but it is not probably unexplored as medicinal plants till this study.
Research frontiers
The present research work depicts various properties of E. fluctuans, native name (helencha). The present study revealed various photochemical constituents in this herb and showed antioxidant, anthelmintics and thrombolytic activities in vitro. These properties could be tested in vivo animal model in future.
Related reports
Extract of E. fluctuans possesses high antioxidant properties which increased with the increasing concentration of extract. Paralysis and death of earth worm were revealed based on concentration of extract. Moreover, properties of thrombolytic activity would be an indication in reduction of myocardial infarction, thrombo-embolic strokes, and deep vein thrombosis in vivo study.
Innovations and breakthroughs
E. fluctuans is an available herbs in Bangladesh generally used as vegetables. In the present study, the authors have studied the phytochemical constituents and their action as antioxidant, anthelmintics and thrombolytic activity in vitro.
Applications
From the literature survey it has been found that E. fluctuans is safe for human health. This scientific study supports and suggests the use of this plant as vegetables would be beneficial for health.
Peer review
This is a valuable research work in which authors have demonstrated three important activities of E. fluctuans, antioxidant, anthelmintics and thrombolytic activities. In general, people eat this herb as vegetables. Therefore, the findings of this study will encourage people to use this as medicinal plants in future.
Footnotes
Foundation Project: Supported by the Department of Physiology, Biochemistry and Pharmacology, Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Chittagong, Bangladesh (Grant No. RFLDC/MSC/res. sund/5004/3096).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ramjan A, Mustahsan B, Mahadi H, Masudur Rahman DS, Emran A. Enhydra fluctuans Lour: a review. Res J Pharm Technol. 2013;6(9):927–929. [Google Scholar]
- 2.Swain PK, Dinda SC, Nayak DP, Kar B, Patro VJ. Antioxidant activity of Enhydra fluctuans Lour. aerial parts. J Phytotherapy Pharmacol. 2012;1:23–34. [Google Scholar]
- 3.Amin MR, Mondol R, Habib MR, Hossain MT. Antimicrobial and cytotoxic activity of three bitter plants Enhydra fluctuans, Andrographis peniculata and Clerodendrum viscosum. Adv Pharm Bull. 2012;2(2):207–211. doi: 10.5681/apb.2012.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sannigrahi S, Mazumder UK, Mondal A, Pal D, Mishra SL, Roy S. Flavonoids of Enhydra fluctuans exhibit anticancer activity against Ehrlich's ascites carcinoma in mice. Nat Prod Commun. 2010;5(8):1239–1242. [PubMed] [Google Scholar]
- 5.Roy SK, Mazumder UK, Islam A. Pharmacological evaluation of Enhydra fluctuans aerial parts for central nervous system depressant activity. Pharmacologyonline. 2011;1:632–643. [Google Scholar]
- 6.Uddin SJ, Ferdous MM, Rouf R. Evaluation of anti-diarrheal activity of Enhydra fluctuans. J Med Sc. 2011;5(4):324–327. [Google Scholar]
- 7.Bhakta J, Majumdar P, Munekage Y. Antimicrobial efficacies of methanol extract of Asteracantha longifolia, Ipomoea aquatica and Enhydra fluctuans against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Micrococcus luteus. Internet J Altern Med. 2008;7(2):125–135. [Google Scholar]
- 8.Harman D. Origin and evolution of the free radical theory of aging: a brief personal history 1954-2009. Biogerontology. 2009;10(6):773–781. doi: 10.1007/s10522-009-9234-2. [DOI] [PubMed] [Google Scholar]
- 9.Clancy D, Birdsall J. Flies, worms and the free radical theory of ageing. Ageing Res Rev. 2013;12(1):404–412. doi: 10.1016/j.arr.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Karthikeyan R, Manivasagam T, Anantharaman P, Balasubramanian T, Somasundaram ST. Chemopreventive effect of Padina boergesenii extracts on ferric nitrilotriacetate (Fe- NTA)-induced oxidative damage in Wistar rats. J Appl Phycol. 2011;23:257–263. [Google Scholar]
- 11.Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9(4):746–757. doi: 10.1111/j.1600-6143.2008.02541.x. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee S, Lardinois O, Bhattacharjee S, Tucker J, Corbett J, Deterding L, et al. et al. Oxidative stress induces protein and DNA radical formation in follicular dendritic cells of the germinal center and modulates its cell death patterns in late sepsis. Free Radic Biol Med. 2011;50(8):988–999. doi: 10.1016/j.freeradbiomed.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepard B. Birmingham, Alabama: UAB News; 2011. Antioxidant may prevent alcohol-induced liver disease. [Online] Available from: http://www.uab.edu/news/latest/item/1181-antioxidant-may-prevent-alcohol-inducedliver-disease . [Accessed on 25th December, 2013] [Google Scholar]
- 14.Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int J Cancer. 2008;123(6):1227–1239. doi: 10.1002/ijc.23754. [DOI] [PubMed] [Google Scholar]
- 15.Rafi KP, Karthikeyan M, Kannan M, Rajasekar S. Anthelmintic activity of Nerium olender flower extract in Indian adult earthworm. J Nat Prod Plant Resour. 2011;1(4):40–46. [Google Scholar]
- 16.Gaikwad SA, Kale AA, Jadhav BG, Deshpande NR, Salvekar JP. Anthelmintic activity of Cassia auriculata L. extracts - in vitro study. J Nat Prod Plant Resour. 2011;1(2):62–66. [Google Scholar]
- 17.Dama GY, Tare HL, Gore MS, Deore SR, Bidkar JS. Comparative helmintholytic potential of extracts obtained from Cymbopogon citratus and Wrightia tinctoria leaves. Int J Pharm Bio Sci. 2011;2(1):321–327. [Google Scholar]
- 18.Iqbal Z, Nadeem QK, Khan MN, Akhtar MS, Waraich FN. In-vitro anthelmintic activity of Allium sativum, Zingiber officinale, Cucurbita mexicana, Ficus religiosa. Int J Agric Biol. 2001;3(4):454–457. [Google Scholar]
- 19.Eguale T, Tadesse D, Giday M. In vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus. J Ethnopharmacol. 2011;137(1):108–113. doi: 10.1016/j.jep.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 20.Rastogi T, Bhutda V, Moon K, Aswar PB, Khadabad SS. Comparative studies on anthelmintic activity of Moringa oleifera and Vitex negundo. Asian J Res Chem. 2009;2(2):181–182. [Google Scholar]
- 21.Yadav P, Singh R. A review on anthelmintic drugs and their future scope. Int J Pharm Pharm Sci. 2011;3(3):17–21. [Google Scholar]
- 22.Mbaebie BO, Edeoga HO, Afolayan AJ. Phytochemical analysis and antioxidants activities of aqueous stem bark extract of Schotia latifolia Jacq. Asian Pac J Trop Biomed. 2012;2(2):118–124. doi: 10.1016/S2221-1691(11)60204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govindasamy C, Srinivasan R. In vitro antibacterial activity and phytochemical analysis of Catharanthus roseus (Linn.) G. Don. Asian Pac J Trop Biomed. 2012;2(Suppl 1):S155–S158. [Google Scholar]
- 24.Basma AA, Zakaria Z, Latha LY, Sasidharan S. Antioxidants activity and phytochemical screening of the methanol extracts of Euphoria hirta L. Asian Pac J Trop Med. 2011;4(5):386–390. doi: 10.1016/S1995-7645(11)60109-0. [DOI] [PubMed] [Google Scholar]
- 25.Braca A, de Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia tarapotensis. J Nat Prod. 2001;64(7):892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 26.Srinivas K, Jimoh FO, Grierson DS, Afolayan AJ. Antioxidant activity of two steroid alkaloids extracted from Solanum aculeastrum. J Pharmacol Toxicol. 2007;2:160–167. [Google Scholar]
- 27.Demiray S, Pintado ME, Castro PM. Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. World Acad Sci Eng Tech. 2009;54:312–317. [Google Scholar]
- 28.Majhenič L, Škerget M, Knez Z. Antioxidant and antimicrobial activity of guarana seed extracts. Food Chem. 2007;104(3):1258–1268. [Google Scholar]
- 29.Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci Technol. 2007;40:344–352. [Google Scholar]
- 30.Malvankar PR. Anthelmintic activity of water extracts of Trikatu churna and its individual ingredients on Indian earthworms. Int J Pharm Bio Sci. 2012;3(2):374–378. [Google Scholar]
- 31.Szewezuk VD, Mongelli ER, Pomilio AB. Antiparasitic activity of Melia azadirach growing in Argentina. Mol Med Chem. 2003;1:54–57. [Google Scholar]
- 32.Dewan SM, Das A. Investigation of in vitro thrombolytic potential and phytochemical nature of Crinum latifolium L. leaves growing in coastal region of Bangladesh. Int J Bio Pharm Res. 2013;4(1):1–7. [Google Scholar]
- 33.Kumarasamy Y, Byres M, Cox PJ, Jaspars M, Nahar L, Sarker SD. Screening seeds of some Scottish plants for free radical scavenging activity. Phytother Res. 2007;21(7):615–621. doi: 10.1002/ptr.2129. [DOI] [PubMed] [Google Scholar]
- 34.Basma AA, Zakaria Z, Latha LY, Sasidharan S. Antioxidants activity and phytochemical screening of the methanol extracts of Euphoria hirta L. Asian Pac J Trop Med. 2011;4(5):386–390. doi: 10.1016/S1995-7645(11)60109-0. [DOI] [PubMed] [Google Scholar]
- 35.Kumar RS, Rajkapoor B, Perumal P. Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac J Trop Biomed. 2012;2:256–261. doi: 10.1016/S2221-1691(12)60019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sreedhar SA. Hsp70 confines tumor progression of rat histiocytoma and impedes the cytotoxicity induced by natural killer cells and peritoneal macrophages. Asian Pac J Trop Med. 2010;3(4):302–309. [Google Scholar]
- 37.Vital PG, Rivera WL. Antimicrobial activity, cytotoxicity, and phytochemical screening of Voacanga globosa (Blanco) Merr. leaf extract (Apocynaceae) Asian Pac J Trop Med. 2011;4(10):824–828. doi: 10.1016/S1995-7645(11)60202-2. [DOI] [PubMed] [Google Scholar]
- 38.Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities and synthesis. Angew Chem Int Ed Engl. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 39.Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, et al. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent and aif-mediated cell death. Cell Metab. 2008;8(3):237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Ali MS, Amin MR, Kamal CM, Hossain MA, Hossain ME. In vitro antioxidant, cytotoxic, thrombolytic activities and phytochemical evaluation of methanol extract of the A. philippense L. leaves. Asian Pac J Trop Biomed. 2013;3(6):464–469. doi: 10.1016/S2221-1691(13)60097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vital PG, Rivera WL. Antimicrobial activity, cytotoxicity, and phytochemical screening of Voacanga globosa (Blanco) Merr. leaf extract (Apocynaceae) Asian Pac J Trop Med. 2011;4(10):824–828. doi: 10.1016/S1995-7645(11)60202-2. [DOI] [PubMed] [Google Scholar]


