Abstract
Objective
To correlate the chromatographic and computational method to calculate lipophilicity of selected ginger compounds and to observe the effects of log P on wound healing.
Methods
Mixtures of acetonitrile and water with acetonitrile content between 95% and 50% v/v in 5% increments were kept separately in 10 different chromatographic chambers, saturated with solvent for 2 h. Spots were observed under UV light at λ=254 nm p-anisaldehyde used as a spraying reagent. Theoretical calculation was done using the Alogps 2.1 online program at www.vcclab.org/lab/alogps. For percentage wound contraction, five groups of animal (mice) (25-30 g) of either sex were selected. Wound were created on dorsal surface of animals using toothed forceps, scalpel and pointed scissors. The wound areas were calculated using vernier caliper. After making wound mice were orally administered 35 mg/kg 6-shogoal, 6-gingerol, 8-gingerol and 10-gingerol respectively. Group E as the control group received tap water.
Results
The lipophilicity values determined in thin layer chromatography were correlated with the theoretically calculated various log P by linear regression analysis. Significant correlations were found between log P values calculated by software program and the experimental reversed-phase thin-layer chromatography data. Order of wound healing property of ginger compounds is directly dependent on lipophilicity i.e. more lipophilic compound has highest activity.
Conclusions
Experimentally determined lipophilicity (RMO) values were correlated with log P determined by software's and found satisfactory. Lipophilicity (RMO) is a useful parameter for the determination and prediction of biological activity of ginger compounds.
Keywords: lipophilicity, RP-TLC, RM0, Calculated partition coefficient, Wound healing
1. Introduction
Lipophilicity is the most important physicochemical properties frequently used parameter in quantitative structure-activity relationship (QSAR) analysis[1]. It is an important tool to describe pharmacodynamic, pharmacokinetic and toxic aspects of drug activity. The lipophilic nature of compounds has been defined in many ways. The most applied one is a partition coefficient, P, or its decimal logarithm, log P, which represents the tendency of a molecule to partition itself between organic and aqueous phase. The traditional shake-flask partition method between n-octanol and water is often substituted by chromatographic approaches [reversed-phase high performance liquid chromatography and reversed-phase thin-layer chromatography methods (RP-TLC)][2],[3]. Log P in the n-octanol–water system is a common measure of lipophilicity because of the similarity of this environment to biological membranes. However, other alternative approaches for measuring lipophilicity have also been developed such as chromatographic, artificial membranes, electro kinetic and partitioning between lipid and water phase approaches[4]. Lipophilicity is the most important physicochemical properties of compounds which involved in pharmacokinetic processes such as absorption, distribution, metabolism and excretion, as well as toxicity, usually referred to as ADMET[5]. Some of pharmaceutical industry publications have confirmed that poor oral absorption and pharmacokinetic properties are the main problems upset the potential drug claimants[6],[7]. The lipophilic ginger rhizome extracts have yielded the potentially active components, gingerols and shogaols[8] and the lipophilicity increases as their alkyl side chain increases in length from 10 to 16 carbons[9]. Among them, chromatographic methods are still widely used for determination of the lipophilicity of drug-like compounds[10],[11] and also has been found to offer a rapid method for the analysis of a large number compounds[11]. Ginger compounds have a variety of effects on the skin that may contribute to improved wound healing. Gingerol and shogaol in particular, is known to have anti-oxidant and anti-inflammatory properties[12], and has been reported to promote new blood vessel formation[13]. One of the recent experimental data suggests that a combination of curcumin and ginger extract might provide a novel approach to improving structure and function in skin and, concomitantly, reducing formation of non-healing wounds in “at-risk” skin[14]. Lipophilic drugs creating an effective dermal drug delivery system that simultaneously repairs the skin barrier and facilitates wound healing[15]. The aim of this study is the determination of the lipophilicity of a ginger compounds, by chromatographic and computational methods and to see the influence of lipophilicity on wound healing.
2. Materials and methods
2.1. Materials
Acetonitrile (HPLC-grade) was supplied by (Darmstadt, Germany), and water was obtained from our laboratory water still (DAFCO, Germany). TLC plates (5 cm×10 cm) RP-18 F254S (Merck, Darmstadt, Germany). Standard 6-shogaol (CAS No. 555-66-8), 6-gingerol (CAS No. 23513-14-6), 8-gingerol (CAS No. 23513-08-8) and 10-gingerol (CAS No. 23513-15-7) were obtained from Natural Remedies Bangalore Pvt. Ltd., India.
2.2. Methods
Mixtures of acetonitrile and water with acetonitrile content between 95% and 50% v/v in 5% increments were kept separately in 10 different chromatographic chambers, saturated with solvent for 2 h[16]. The tested compounds were dissolved in methanol (1 mg/ mL) and 10 µL samples of the solutions were separately spotted on the plates. After developing and drying the plates, the spots were observed under UV light at λ=254 nm after spraying p-anisaldehyde as spraying reagent. The Rf values are means from three independent determinations. RM values of tested compounds were calculated using equation (1):
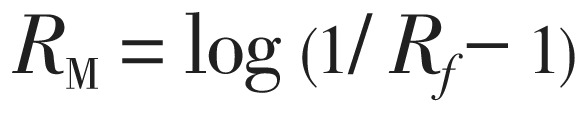 |
(1) |
The correlations between the RM values and concentration of organic solvent were calculated separately for each compound according to equation:
 |
(2) |
Where C is the concentration of acetonitrile in the mobile phase (% v/v).
Calculation of Log P: All the theoretical calculation was done using the Alogps 2.1 online program at Virtual Computational Chemistry Laboratory[17].
2.3. Excisions wound healing
2.3.1. Animals
The mice (25-30 g) of either sex were obtained from the experimental animal care centre, College of Pharmacy, Salman Bin Abdulaziz University, Al-Kharj. The animals were kept in animal house in standard condition of temperature [(22±2) °C] and relative humidity (55%) with 12 h light/dark condition. They were provided with Purina chow diet and drinking water ad libitum during the whole period of experiment. The experiments and procedures used were approved by the Ethical Committee of the College of Pharmacy, Salman Bin Abdulaziz University, Al-Kharj, KSA.
2.3.2. Wound
The excision wound model was used to monitor wound contraction and wound closure time. Five groups (n=5) of mice were used in the experiment. At the beginning of the experiment, the dorsal fur of each mouse was shaved with an electric clipper. After 24 h, all animals were anesthetized by 1 mL of intravenous ketamine hydrochloride (10 mg/kg body weight) and the shaved areas were sterilized with 70% alcoholic solution. A predetermined dorsal area (approximately 20 mm2) was excised using toothed forceps, scalpel and pointed scissors. A fresh surgical blade was used for the perpendicular cut in each animal and tension of skin was kept constant during the procedure.
2.3.3. Treatments
After the making wounds, all mice were randomly divided into five groups and colored with a non-toxic color. Group A, Group B, Group C, Group D were orally administered 35 mg/kg 6-shogoal, 6-gingerol, 8-gingerol, 10-gingerol respectively. Group E as the control group received tap water. All mice were monitored daily for 10 d. The wound areas were calculated using vernier caliper immediately after the wound excision and 10 d post wounding.
3. Results
The chemical structures of the ginger compounds are shown in Table 1. The lipophilicity (RMO) were obtained from Rf values (Table 2) by the following equations 1 and 2 and the RM value was calculated from the Rf value by the equation 1[18],[19].
Table 1. Name and structure of ginger compounds investigated.
| General name | IUPAC name | Structure |
| 6-Shogol | (4E)-1-(4-hydroxy-3-methoxyphenyl)dec-4-en-3-one | 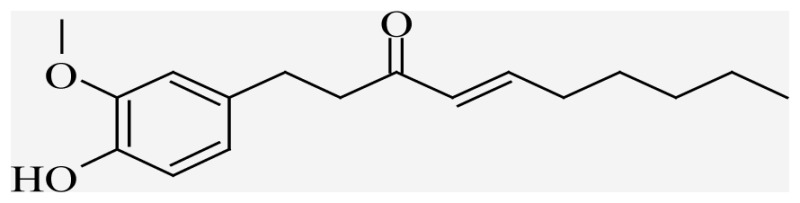 |
| 6-Gingerol | 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)decan-3-one | 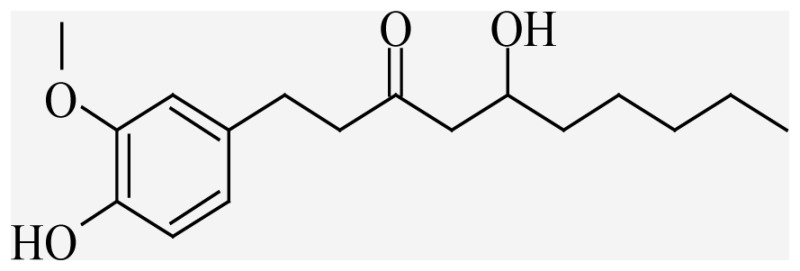 |
| 8-Gingerol | 5-hydroxy-1-(4-hydroxy-3- ethoxyphenyl)dodecan-3-one | 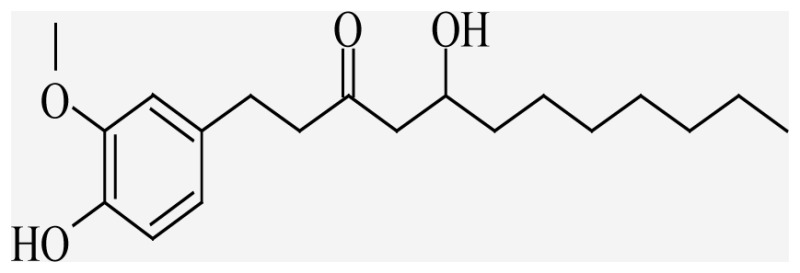 |
| 10-Gingerol | 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)tetradecan-3- one |  |
Table 2. Rf value of ginger compounds.
| Acetonitrile in mobile phase (%) |
Rf |
|||
| 6-Shogaol | 6-Gingerol | 8-Gingerol | 10-Gingerol | |
| 95 | 0.74 | 0.94 | 0.79 | 0.49 |
| 90 | 0.66 | 0.86 | 0.71 | 0.44 |
| 85 | 0.59 | 0.79 | 0.64 | 0.39 |
| 80 | 0.51 | 0.71 | 0.56 | 0.34 |
| 75 | 0.44 | 0.64 | 0.49 | 0.29 |
| 70 | 0.36 | 0.56 | 0.41 | 0.24 |
| 65 | 0.29 | 0.49 | 0.34 | 0.19 |
| 60 | 0.21 | 0.41 | 0.26 | 0.14 |
| 55 | 0.14 | 0.34 | 0.19 | 0.09 |
| 50 | 0.06 | 0.26 | 0.11 | 0.04 |
Lipophilicity value is obtained by the extrapolation to zero concentration of polar component in the Figure 1 drawn between RM and concentration of polar component in mobile phase.
Figure 1. Relationship between the extrapolated percentage organic solvent and RM.
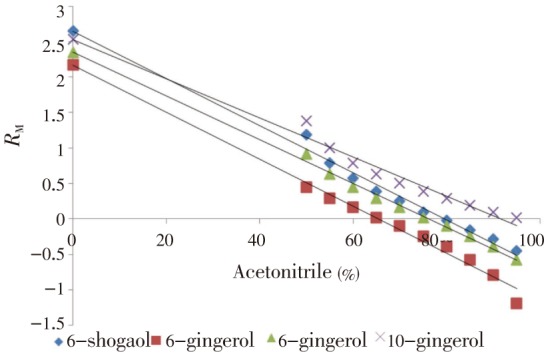
The RMO and b in the above equation 2 were represents intercepts and slope of the figure drawn between C and RM. The C in the equation 2 was the concentration of polar component in the mobile phase. The lipophilicity determined in the RP-TLC was being correlated with the theoretical partition coefficients (log P) of the compounds using ALOGPs, AC logp, miLogp, MLOGP, Kow-Win and XLOGP2 under the ALOGPS 2.1 program. The calculated log P values from above described computer programs are listed in Table 3. In general, the lowest log P values were obtained from the calculations made with MLOGP program. The difference between theoretical values derived from these programs was a consequence of a method of calculation of log P values.
Table 3. Various types of calculated log P for ginger compounds.
| Compounds | ALOGPs | AC logp | miLogp | MLOGP | KowWin | XLOGP2 |
| 6-Shogaol | 4.95 | 4.51 | 4.35 | 3.05 | 4.33 | 4.34 |
| 6-Gingerol | 3.45 | 3.85 | 3.22 | 2.32 | 2.72 | 3.04 |
| 8-Gingerol | 4.33 | 4.78 | 4.23 | 3.70 | 3.70 | 4.18 |
| 10-Gingerol | 5.59 | 5.71 | 5.24 | 4.16 | 4.68 | 5.31 |
The lipophilicity values determined in TLC were correlated with the above theoretically calculated various log P by linear regression analysis and as a result equation 3 to 8 are generated shown below:
 |
(3) |
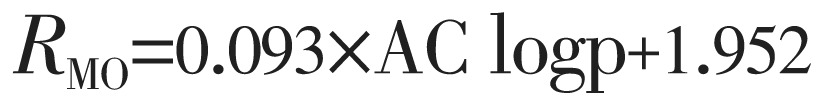 |
(4) |
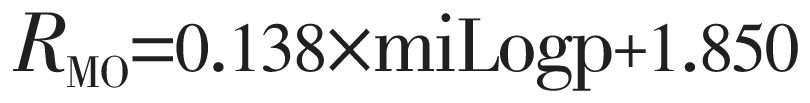 |
(5) |
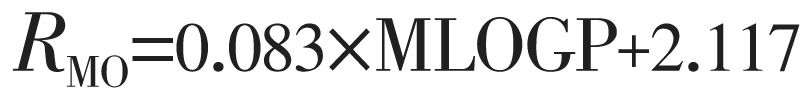 |
(6) |
 |
(7) |
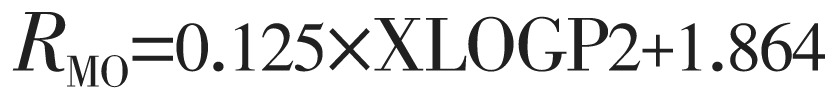 |
(8) |
RM values of the ginger compounds decreased linearly with increasing concentration of organic modifier (Acetonitrile) in the mobile phase. The dependence of RM on the concentration of acetonitrile in the mobile phase for ginger compounds is presented in Table 4 and Figure 1. The relative lipophilicity, expressed as RMO values, and statistical parameters for four ginger compounds under investigation are listed in Table 5. The lipophilicity parameters determined by RP-TLC and expressed as RMO values were in the range 2.170-2.640 (Table 5). The effects of lipophilicity (RMO) are compared with the wound healing activity of gingerol and shogaol presented in Table 6.
Table 4. RM value of ginger compounds.
| Acetonitrile in mobile phase (%) |
RM |
|||
| 6-Shogaol | 6-Gingerol | 8-Gingerol | 10-Gingerol | |
| 95 | -0.45 | -1.19 | -0.58 | 0.02 |
| 90 | -0.29 | -0.79 | -0.39 | 0.10 |
| 85 | -0.16 | -0.58 | -0.25 | 0.19 |
| 80 | -0.02 | -0.39 | -0.10 | 0.29 |
| 75 | 0.10 | -0.25 | 0.02 | 0.39 |
| 70 | 0.25 | -0.10 | 0.16 | 0.50 |
| 65 | 0.39 | 0.02 | 0.29 | 0.63 |
| 60 | 0.57 | 0.16 | 0.45 | 0.79 |
| 55 | 0.79 | 0.29 | 0.63 | 1.00 |
| 50 | 1.19 | 0.45 | 0.91 | 1.38 |
Table 5. The relative lipophilicity and statistical parameters for four ginger compounds.
| Compounds | RMO | b | r | r2 | s | F |
| 6-Shogaol | 2.640 | 0.033 | 0.984 | 0.991 | 0.096 | 12 |
| 6-Gingerol | 2.170 | 0.030 | 0.984 | 0.990 | 0.095 | 12 |
| 8-Gingerol | 2.342 | 0.031 | 0.995 | 0.997 | 0.049 | 12 |
| 10-Gingerol | 2.520 | 0.027 | 0.969 | 0.933 | 0.113 | 12 |
RMO: Relative lipophilicity, values of slopes: b, correlation coefficients: r, coefficient of determination: r2, Standard error of estimation: s, F-test of significance: F, n=10.
Table 6. Excision wound studies showing average reduction in wound size, when treated with compounds and control (n=5).
| Treatment | Average wound contraction (mm) |
Reduction wound size (%) | |
| 0-day | 10-day | ||
| Control | 20.04 | 15.62 | 19 |
| 6-Shogoal | 20.02 | 8.204 | 59 |
| 6-Gingerol | 20.18 | 11.45 | 43 |
| 8-Gingerol | 20.22 | 10.46 | 48 |
| 10 Gingerol | 20.08 | 9.44 | 53 |
The correlation coefficient and standard deviation of the equations 3–8 are in the Table 7. The comparison of correlation coefficient of equation 3–8 is shown in Figure 2 and it revealed that Kow-Win had higher correlation coefficient than the other theoretical calculated log P, hence theoretically calculated Kow-Win can be used instead of lipophilicity wherever applicable in quantitative structure-pharmacokinetics relationship (QSPR) and in turn QSAR study for the above ginger compounds under investigation.
Table 7. Correlation coefficient and standard deviation.
| Equation | Correlation coefficient | Standard deviation |
| Equation 3 | 0.72 | 0.165 |
| Equation 4 | 0.36 | 0.221 |
| Equation 5 | 0.58 | 0.193 |
| Equation 6 | 0.34 | 0.224 |
| Equation 7 | 0.79 | 0.144 |
| Equation 8 | 0.59 | 0.191 |
Figure 2. Comparison of the correlation coefficient of the equation 3–8.
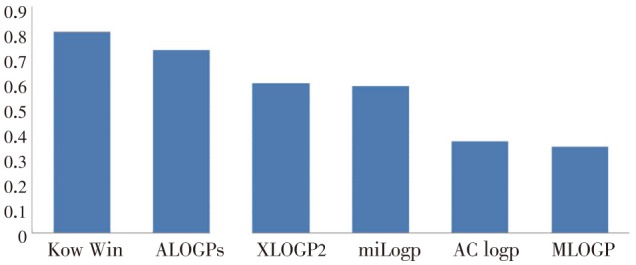
The order of wound healing activity of ginger compounds 6-shogaol>10-gingerol>8-gingerol>6-gingerol. The lipophilicity of compound has been compared with reduction in wound size (%). It is quite interesting to see that order of wound healing property of ginger compounds was directly dependent on lipophilicity i.e. more lipophilic compound has the highest activity (Table 5 and 6).
4. Discussion
A quantitative structure retention relationships could be obtained with the help of retention parameter (RMO). A higher RMO value indicates greater lipophilicity and depends on the different arrangements of substituents in a structure. Presence of aliphatic chain length and double bond greatly influences the difference of RMO amongst four ginger compounds in this experiment. Replacing OH group of 6-gingerol and inserting double bond the RMO was increased. Presence of additional –CH2– group in 10-gingerol makes their RMO (2.521) higher in comparison to 6-gingerol (RMO=2.170) and 8-gingerol (RMO=2.342) having less branching and no double bonds. Order of wound healing property is also directly proportional to their log P (RMO).On close examination of structure of ginger compounds it is also observed that double bond in aliphatic chain is more essential for the biological activity.
Experimentally determined RMO values depend on the concentration of organic modifier (acetonitrile) in the mobile phase and it is linearly dependent. Satisfactory correlation was obtained between retention constants and ALOGPs, AC logp, miLogp, MLOGP, Kow-Win and XLOGP2. RMO could be a useful parameter for the determination and prediction of QSPR and QSAR study of ginger compounds. Lipophilicity is most important determinant factor for any biological activity. In this particular experiment, more lipophilic ginger compound exhibiting higher wound healing activity.
Acknowledgments
The authors are thankful to the staff and College of Pharmacy, Salman Bin Abdul Aziz University, providing animal facilities for the present studies. This research is funded by grant from the deanship under Grant No. RGP-VPP-150 at King Saud University.
Comments
Background
Main objective of present study is to correlate the chromatographic and computational method to calculate lipophilicity of selected ginger compounds and to observe the effects of log P on wound healing. With the help of log P values we will generate QSAR data and their analysis, pharmacokinetics and pharmacodyanamics of a ginger compounds.
Research frontiers
The present study mainly described how lipophilicity influence the biological activity of a ginger compounds. Log P was generated by chromatographic (RP-TLC) and computational (Software) methods so as to access the effect of lipophilicity on wound healing.
Related reports
Comparison of predicted and experimental lipophilicity calculation and afterwards observation of their influence on biological activity were also reported for many organic synthesized compounds but not for the herbal originated compounds.
Innovations and breakthroughs
With known structure of herbal origin like ginger compounds of their lipophilicity to observe the impact of biological activity is quite interesting in the present research article.
Applications
Literature reveals that lipophilicity is an important physicochemical property of drugs. This research will be helpful for the scientists working on different plant constituents to identify lead molecules.
Peer review
The lipophilicity and wound healing activity of selected ginger compounds (6-shogaol 6-gingerol, 8-gingerol and 10-gingerol) has been investigated using chromatographic and computational methods, and percentage of wound contraction in experimental method was correlated to confirm the influence of log P on wound healing. Research reveals that lipophilicity could be a useful parameter for the determination and prediction of QSPR and QSAR study of ginger compounds.
Footnotes
Foundation Project: Supported by the deanship at King Saud University with Grant No. RGP-VPP-150.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Szymanski P, Skibinski R, Liszka M, Jargielo L, Mikiciuk-Olasik E, Komsta L. A TLC study of the lipophilicity of thirty-two acetylcholinesterase inhibitors—1,2,3,4-tetrahydroacridine and 2,3-dihydro-1H-cyclopenta[b]quinoline derivatives. Cent Eur J Chem. 2013;11:927–934. [Google Scholar]
- 2.Bajda M, Boryczka S, Wietrzyk J, Malawska B. Investigation of lipophilicity of anticancer-active thioquinoline derivatives. Biomed Chromatogr. 2007;21:123–131. doi: 10.1002/bmc.706. [DOI] [PubMed] [Google Scholar]
- 3.Kepczynska E, Obloza E, Stasiewicz UA, Bojarski J, Pyka A. Lipophilicity of thiobarbiturates determined by TLC. Acta Pol Pharm. 2007;64:295–302. [PubMed] [Google Scholar]
- 4.Hartmann T, Schmitt J. Lipophilicity-beyond octanol/water: a short comparison of modern technologies. Drug Discov Today Technol. 2004;1:431–439. doi: 10.1016/j.ddtec.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Biagi G, Recantini M, Barbaro A, Borea P. Lipophilicity estimation of drugs. Process Control Qual. 1997;10:129–149. [Google Scholar]
- 6.Vieth M, Siegel MG, Higgs RE, Watson IA, Robertson DH, Savin KA, et al. et al. Characteristic physical properties and structural fragments of marketed oral drugs. J Med Chem. 2004;47:224–232. doi: 10.1021/jm030267j. [DOI] [PubMed] [Google Scholar]
- 7.Leeson PD, Davis AM. Time-related differences in the physical property profiles of oral drugs. J Med Chem. 2004;47:6338–6348. doi: 10.1021/jm049717d. [DOI] [PubMed] [Google Scholar]
- 8.Haghighi M, Khalvat A, Toliat T, Jallaei S. Comparing the effects of ginger (Zingiber officinale) extract and ibuprofen on patients with osteoarthritis. Arch Iran Med. 2005;8:266–271. [Google Scholar]
- 9.Nurtjahja-Tjendraputra EA, Ammit J, Roufogalis BD, Tran VH, Duke CC. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res. 2003;111:259–265. doi: 10.1016/j.thromres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Waksmundzka-Hajnos MD, Matosiuk A, Petruczynik Kijkowska-Murak U. Determination of the lipophilicity of selected isoquinoline alkaloids by RP-TLC. Acta Chromatographica. 2008;20:563–573. [Google Scholar]
- 11.Jevrić L, Koprivica GB, Mišljenović NM, Jovanović BŽ. Chromatographic behavior and lipophilicity of s-triazine derivatives on silica gel impregnated with paraffin oil. APTEFF. 2010;41:159–168. [Google Scholar]
- 12.Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, et al. et al. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;15:2558–2567. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 13.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 14.Adam B, Muhammad N, Aslam K, Johnson J, James V. A combination of curcumin and ginger extract improves abrasion wound healing in corticosteroid-damaged hairless rat skin. Wound Repair Regen. 2009;17:360–365. doi: 10.1111/j.1524-475X.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie E, Xu R, Wang C, Pu Z, Liusup H, Zhou L, et al. et al. The effect of moist exposed burn ointment on maintaining a physiological moist environment in treating burn wound. Ann Burns Fire Disasters. 2002;15:166–167. [Google Scholar]
- 16.Malawska B, Kulig K, Buck A, Zbek P, Wieckowska A. The study of the lipophilicity of α-(4-phenylpiperazin-1-yl)-γ Phthalimidobutyramides using chromatographic and computational methods. Biomed Chromatogr. 2008;22:688–694. doi: 10.1002/bmc.982. [DOI] [PubMed] [Google Scholar]
- 17.Virtual Computational Chemistry Laboratory . bei Munich: Virtual Computational Chemistry Laboratory; 2005. ALOGPS 2.1. [Online] Available from: http://www.vcclab.org/lab/alogps/ [Accessed on 17th September, 2013]. [Google Scholar]
- 18.Sherma J. Thin-layer chromatography of pesticides- a review of applications for 2002-2004. Acta Chromatographica. 2005;15:5–30. [Google Scholar]
- 19.Tibor C, Gyula O. Relationship between lipophilicity and specific hydrophobic surface area of non-homologous series of synthetic dyes. Croat Chem Acta. 2000;73:293–303. [Google Scholar]


