Abstract
Background
The importance of sialic acid binding adhesin (sabA) as a new outer membrane protein in gastroduodenal diseases has been recognized. The prevalence rate of sabA gene varies in different geographic areas.
Objectives
The aim of this study was to determine the frequency of sabA gene in Helicobacter pylori (H. pylori) strains isolated from different clinical outcomes in Tehran, Iran.
Patients and Methods
The study included 120 patients with dyspeptic symptoms admitted to the endoscopy suite of gastroenterology section of Firouzgar University Hospital, Tehran, Iran from March to August 2011. Gastric biopsy specimens were evaluated for the presence of H. pylori using standard microbiological method and polymerase chain reaction (PCR) assay. The sabA genopositive was determined by PCR in H. pylori strains.
Results
H. pylori isolates were recovered from 82 patients with duodenal ulcer (DU; n = 17), gastric ulcer (GU; n = 15), gastric cancer (GC; n = 13), and gastritis (G; n = 37). The frequency of sabA gene in H. pylori strains was 100% in gastric cancer, 86.7% in gastric ulcer, and 83.3% in both gastritis and duodenal ulcer.
Conclusions
This is a report on the prevalence of sabA gene in H. pylori isolated from different gastric patients in Iran. The results showed a high prevalence of sabA in our clinical H. pylori isolates.
Keywords: SabA protein, Helicobacter pylori; Gene Frequency; Gastric Ulcer
1. Background
Helicobacter pylori as a gram negative, curved, microaerophilic and motile organism with multiple polar flagella is the most common cause of gastric diseases including gastritis, duodenal and gastric ulcer, gastric atrophy, adenocarcinoma, and mucosa associated lymphoid tissue (MALT) (1). H. pylori infection is a major health problem in many parts of the world, occurring in 40 - 50% and 80 - 90% of the population in developed and developing countries respectively (2).
Several studies have shown that host and bacterial factors can play essential role in the appearance of clinical and pathologic complications. Among the bacterial factors, the ability of bacteria to bind to gastric epithelial cells is a crucial step for a successful infection, because it protects the bacteria from cleaning mechanisms such as fluid flow or pouring the mucous layers (3-7).
H. pylori has a wide range of adhesion components to bind to different carbohydrates. Sialic acid binding adhesin (sabA) plays a critical role in the initial colonization of H. pylori, and this molecule has an important role in the establishment of persistent infection and chronic inflammation, which causes tissue damage (8, 9).
sabA, a 70-kDa (651-aa protein) outer membrane protein, is essential for attachment and activation of human cells via sialyl-Lewis x/a antigens (sLex and sLea) (10).
2. Objectives
The clinical prevalence of the H. pylorisabA genotype has not yet been determined in clinical isolates in Iran. In this study, we investigated the presence of sabA gene in Iranian clinical H. pylori isolates with different clinical outcomes.
3. Patients and Methods
3.1. Study Population
One hundred twenty patients with dyspeptic symptoms were enrolled in the endoscopy suite of gastroenterology section of Firouzgar University Hospital, Tehran, Iran from March to August 2011. All patients were given informed consent for gastroscopic biopsy samples, which were obtained from the antrum.
3.2. Detection of H. pylori and the Presence of sabA Gene
Two gastric biopsy specimens were recovered from patients with gastrointestinal disease. One biopsy was evaluated for rapid urease test (RUT), and the other was sent to laboratory for DNA extraction. DNA was extracted using the DNeasy blood & tissue kit (Qiagen Germany). The sample was considered H. pylori positive when a 411bp fragment of urease A gene (ureA) was amplified and RUT made positive. For the H. pylori -positive samples, the presence of sabA in H. pylori clinical isolates was analyzed by PCR using two primer pairs. PCR primers are listed in Table 1. Amplification was performed as previously described ( 11 - 13 ).The PCR products were analyzed by 1% agarose gel electrophoresis with ethidium bromide staining.
Table 1. The Primers used for the Amplification of ureA andsabAin This Study .
3.3. Data analysis
Fisher’s exact test was used for analyzing categorical data. A P-value < 0.05 was considered statistically significant.
4. Results
From 120 patients, H. pylori isolates were recovered from 82 patients with duodenal ulcer (DU; n = 17), gastric ulcer (GU; n = 15), gastric cancer (GC; n = 13), and gastritis (G; n=37). The mean age of the patients (50 male and 32 female) was 46 years. The presence of sabA gene was examined in all 82 of the H. pylori -infected patients with gastrointestinal diseases. The strains were considered sabA positive if they had one or two fragments of the gene. Two PCR assays yielded different positive rates for the sabA gene in 82 H. pylori isolates as shown in Table 2.
Table 2. Detection ofsabAGene by PCR in 82H. pyloriClinical Isolates .
| Primer pair 1 | Primer pair 2 | Number |
|---|---|---|
| + | + | 60 |
| _ | + | 7 |
| + | _ | 4 |
| _ | _ | 11 |
Seventy one strains (86.6%) had positive results for sabA . Table 3 shows the frequency of sabA gene among different clinical outcomes. The sabA genotype was detected in all patients with gastric cancer (100%), in 86.7% of patients with gastric ulcer, and in 83.3% of patients with gastritis and duodenal ulcer.
Table 3. The Prevalence ofsabAin the Different Disease Groups .
| Gene | Clinical outcomes | |||
|---|---|---|---|---|
| DU | GU | GC | G | |
| PositivesabA | 15 | 13 | 13 | 30 |
| SabANegative | 3 | 2 | 0 | 6 |
| Total | 18 | 15 | 13 | 36 |
Figure 1 and 2 show the results of sabA gene amplification in some strains of H. pylori using primers pair 1 and 2 respectively.
Figure 1. Amplification of sabA Using Primer Pair 1 in Representative Strains of H. pylori.
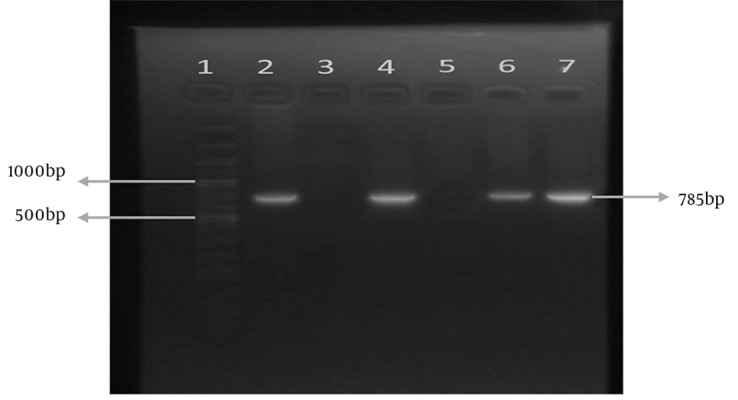
Lane 1 is the 100 bp marker. Lanes 2 and 3 are positive and negative control strains respectively. Lanes 4 to 7 are representative clinical H. pylori strains.
Figure 2. Amplification of sabA Using Primer Pair 2 in Representative Strains of H. pylori.
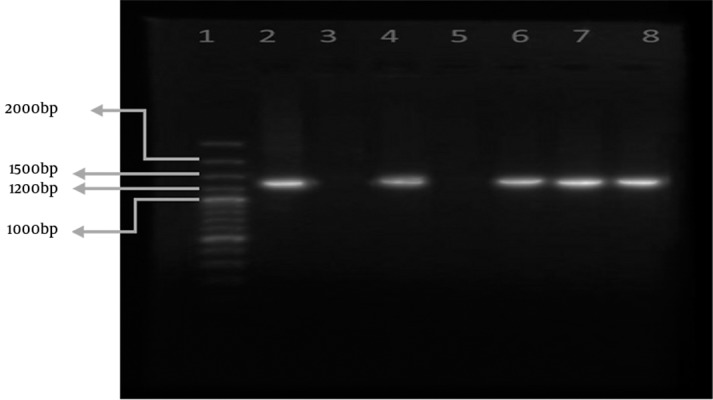
Lane 1 is the 100 bp marker. Lanes 2 and 3 are positive and negative control strains respectively. Lanes 4 to 8 are representative clinical H. pylori strains.
5. Discussion
The adherence of H. pylori to gastric epithelial cells has a crucial role in the specific tropism and pathogenicity of the organism in the human gastric epithelium and is a key step in creating a successful infection in gastric mucosa (3, 14-16). sabA is an important adhesion molecule in H. pylori which adheres to the host gastric epithelium (17).
In a previous study performed on 200 H. pylori-infected patients, including 120 from Colombia and 80 from the United States, the prevalence rates of sabA-positive isolates were 44% in duodenal ulcer, 66% in gastritis, and 70% in gastric cancer (18). In a study in Taiwan, 80% (116 of 145) of H. pylori strains had positive results for sabA (19). In two other studies the frequency of sabA-positive isolates was reported 86% (37 of 43) and 93% (89/96) in French and Dutch respectively (20, 21). Moreover, the prevalence of sabA gene was 23.6% in Iran (22).
When compared to previous studies, our results showed that the sabA gene has high frequency in Iranian clinical isolates (86.6% (71 of 82)). The frequency of sabA in different clinical outcomes was 100% in gastric cancer, 86.7% in gastric ulcer, and 83.3% in both gastritis and duodenal ulcer. However no significant association was seen between the prevalence of sabA genotype and clinical outcomes (P > 0.05).
Two PCR assays produced different positive rates for the sabA gene in H. pylori isolates, with the 81.7% and 78% using primer pairs 1 and 2 respectively; indicating the possibility of sequence diversity in the sabA gene (12).
In conclusion, this is a report on the prevalence of sabA gene in H. pylori isolated from different gastric patients in Iran. More studies in other parts of country with a higher sample size are necessary to be performed to obtain a complete evaluation on the prevalence of sabA gene in Iran. Further tests should be performed to determine the exact role of sabA in H. pylori infection.
Acknowledgments
None declared.
Footnotes
Implication for health policy/practice/research/medical education:
No specific implication has been declared by author.
Authors’ Contribution:
None declared.
Financial Disclosure:
None declared.
Funding/Support:
None declared.
References
- 1.Marshall B, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. The Lancet. 1984;323(8390):1311–5. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Bittencourt PFS, Rocha GA, Penna FJ, Queiroz DMM. Úlcera péptica gastroduodenal e infecção pelo Helicobacter pylori na criança e adolescente. Jornal de Pediatria. 2006;82:325–34. doi: 10.2223/JPED.1529. [DOI] [PubMed] [Google Scholar]
- 3.Hessey SJ, Spencer J, Wyatt JI, Sobala G, Rathbone BJ, Axon AT, et al. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990;31(2):134–8. doi: 10.1136/gut.31.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logan RP. Adherence of Helicobacter pylori. Aliment Pharmacol Ther. 1996;10 Suppl 1:3–15. doi: 10.1046/j.1365-2036.1996.22164001.x. [DOI] [PubMed] [Google Scholar]
- 5.Odenbreit S. Adherence properties of Helicobacter pylori: impact on pathogenesis and adaptation to the host. Int J Med Microbiol. 2005;295(5):317–24. doi: 10.1016/j.ijmm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Segal ED, Falkow S, Tompkins LS. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci U S A. 1996;93(3):1259–64. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen S, Moss SF. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009;282(1):1–8. doi: 10.1016/j.canlet.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aspholm M, Olfat FO, Norden J, Sonden B, Lundberg C, Sjostrom R, et al. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathog. 2006;2(10) doi: 10.1371/journal.ppat.0020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ota H, Nakayama J, Momose M, Hayama M, Akamatsu T, Katsuyama T, et al. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. 1998;433(5):419–26. doi: 10.1007/s004280050269. [DOI] [PubMed] [Google Scholar]
- 10.Mahdavi J, Sonden B, Hurtig M, Olfat FO, Forsberg L, Roche N, et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297(5581):573–8. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podzorski RP, Podzorski DS, Wuerth A, Tolia V. Analysis of the vacA, cagA, cagE, iceA, and babA2 genes in Helicobacter pylori from sixty-one pediatric patients from the Midwestern United States. Diagnostic microbiology and infectious disease. 2003;46(2):83–8. doi: 10.1016/s0732-8893(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 12.Shao L, Takeda H, Fukui T, Mabe K, Han J, Kawata S, et al. Genetic diversity of the Helicobacter pylori sialic acid-binding adhesin (sabA) gene. Biosci Trends. 2010;4(5):249–53. [PubMed] [Google Scholar]
- 13.Yanai A, Maeda S, Hikiba Y, Shibata W, Ohmae T, Hirata Y, et al. Clinical relevance of Helicobacter pylori sabA genotype in Japanese clinical isolates. J Gastroenterol Hepatol. 2007;22(12):2228–32. doi: 10.1111/j.1440-1746.2007.04831.x. [DOI] [PubMed] [Google Scholar]
- 14.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55(10):2111–5. [PubMed] [Google Scholar]
- 15.Hamlet* ‡ A, Thoreson‡ AC, Nilsson§ O, Svennerholm‡ AM, Olbe* L. Duodenal Helicobacter pylori infection differs in cagA genotype between asymptomatic subjects and patients with duodenal ulcers. Gastroenterology. 1999;116(2):259–268. doi: 10.1016/s0016-5085(99)70121-6. [DOI] [PubMed] [Google Scholar]
- 16.Magalhães A, Reis CA. Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors. Braz J Med Biol Res. 2010;43:611–8. doi: 10.1590/s0100-879x2010007500049. [DOI] [PubMed] [Google Scholar]
- 17.Yamaoka Y. Increasing evidence of the role of Helicobacter pylori SabA in the pathogenesis of gastroduodenal disease. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaoka Y, Ojo O, Fujimoto S, Odenbreit S, Haas R, Gutierrez O, et al. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006;55(6):775–81. doi: 10.1136/gut.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheu BS, Odenbreit S, Hung KH, Liu CP, Sheu SM, Yang HB, et al. Interaction between host gastric Sialyl-Lewis X and H. pylori SabA enhances H. pylori density in patients lacking gastric Lewis B antigen. Am J Gastroenterol. 2006;101(1):36–44. doi: 10.1111/j.1572-0241.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 20.de Jonge R, Pot RG, Loffeld RJ, van Vliet AH, Kuipers EJ, Kusters JG. The functional status of the Helicobacter pylori sabB adhesin gene as a putative marker for disease outcome. Helicobacter. 2004;9(2):158–64. doi: 10.1111/j.1083-4389.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 21.Lehours P, Menard A, Dupouy S, Bergey B, Richy F, Zerbib F, et al. Evaluation of the association of nine Helicobacter pylori virulence factors with strains involved in low-grade gastric mucosa-associated lymphoid tissue lymphoma. Infect Immun. 2004;72(2):880–8. doi: 10.1128/IAI.72.2.880-888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goudarzi H, Rezaee H, Rafizadeh M, Taghavi A. Determination of the Status of Helicobacter pylori sabA Gene in Relation to Clinical Findings. J Med Bacteriol. 2012;1(1) [Google Scholar]


