Abstract
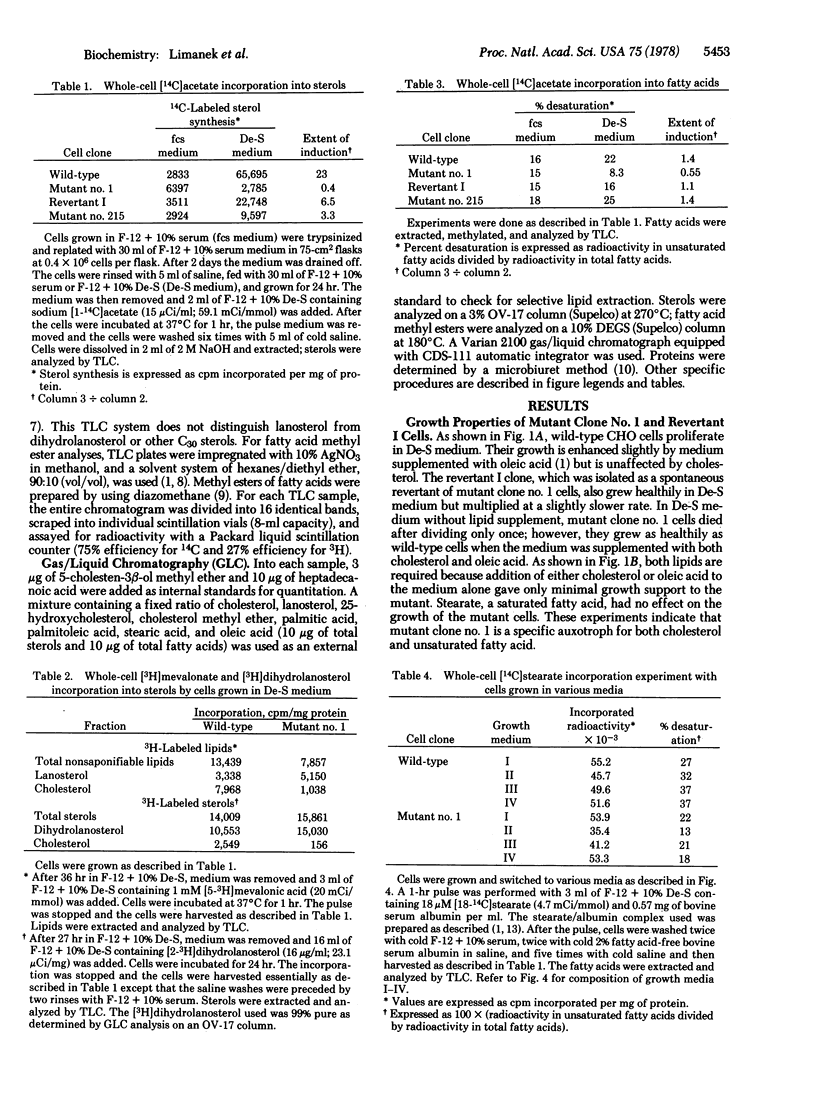
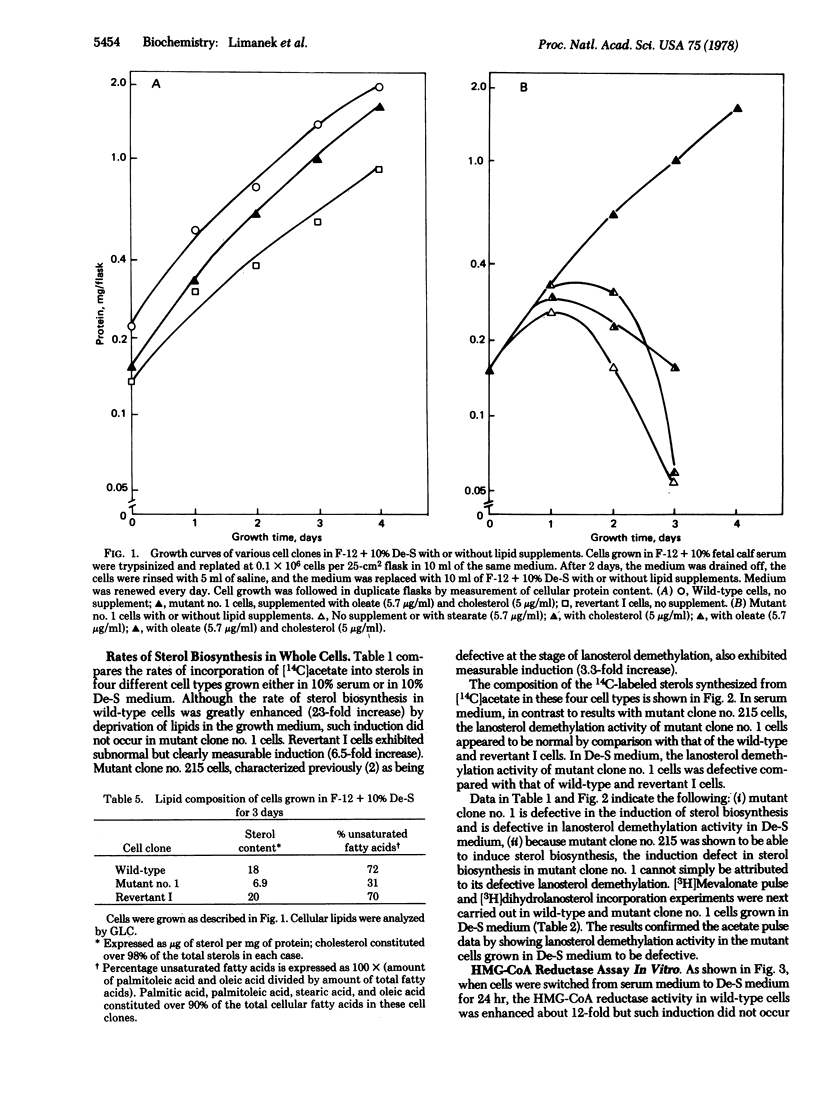
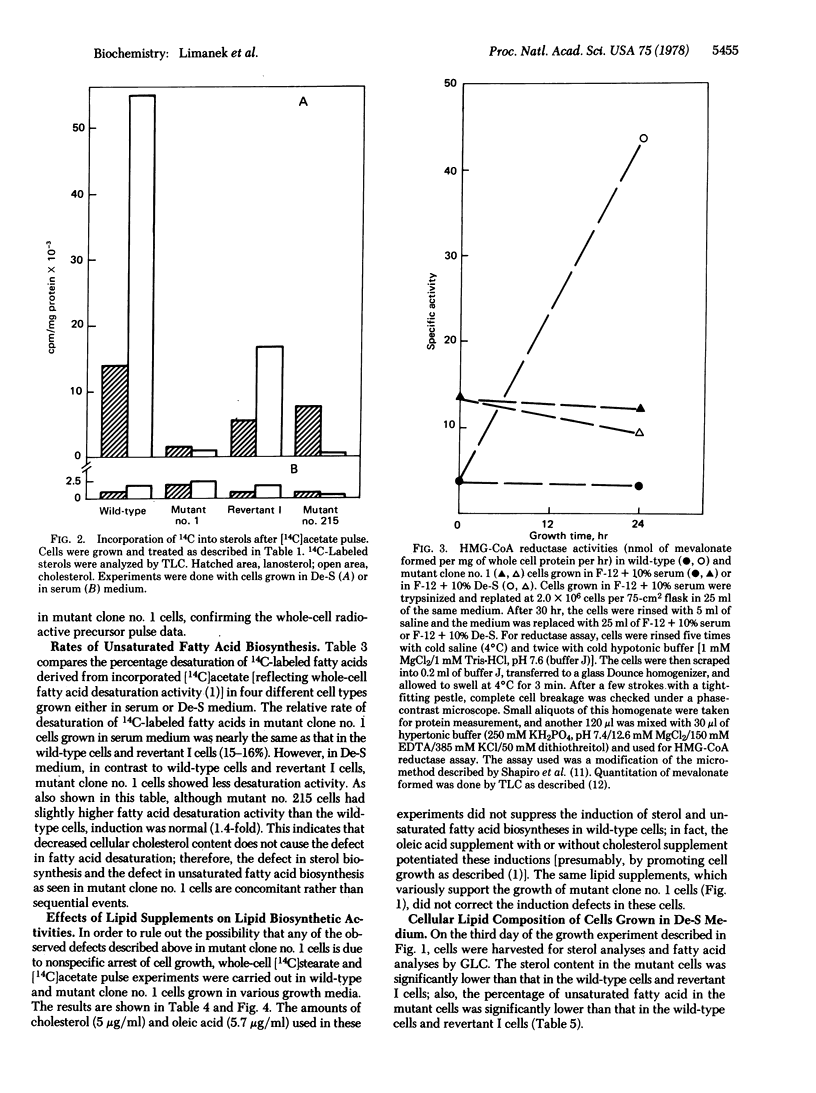
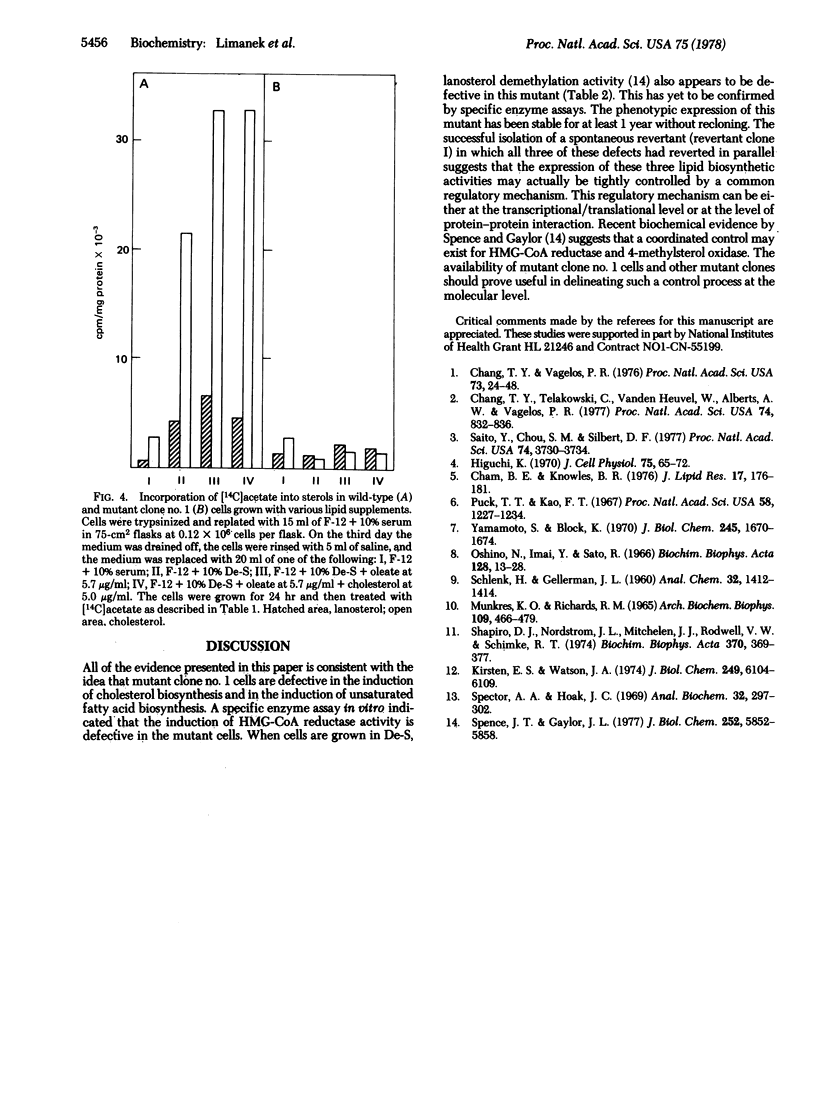
A mutant requiring both cholesterol and oleate for growth has been isolated from mutagenized Chinese hamster ovary cells. By comparison with wild-type cells, sterol and unsaturated fatty acid biosynthetic activities in the mutant cells grown in fetal calf serum medium appear to be nearly intact. However, whole-cell radioactive acetate, mevalonate, dihydrolanosterol, and stearate incorporation studies show that sterol synthesis from acetate, lanosterol demethylation, and fatty acid desaturation are defective in the mutant cells grown in delipidated serum medium. In vitro enzyme assays with crude cell extracts demonstrated that beta-hydroxy-beta-methylglutaryl-coenzyme A reductase is not induced in the mutant. These experiments were substantiated by gas/liquid chromatographic analyses which showed the sterol content and the percentage unsaturated fatty acids in mutant cells to be drastically reduced when the cells are grown in delipidated serum medium. A spontaneous revertant exhibiting prototrophic growth in lipid-free medium has been isolated from 50 X 10(6) mutant cells. All three defects in this revertant reverted back in parallel, which suggests that these three biosynthetic activities may be controlled by a common regulatory mechanism.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cham B. E., Knowles B. R. A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res. 1976 Mar;17(2):176–181. [PubMed] [Google Scholar]
- Chang T. Y., Telakowski C., Heuvel W. V., Alberts A. W., Vagelos P. R. Isolation and partial characterization of a cholesterol-requiring mutant of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):832–836. doi: 10.1073/pnas.74.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. Y., Vagelos P. R. Isolation and characterization of an unsaturated fatty acid-requiring mutant of cultured mammalian cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):24–28. doi: 10.1073/pnas.73.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K. An improved chemically defined culture medium for strain L mouse cells based on growth responses to graded levels of nutrients including iron and zinc ions. J Cell Physiol. 1970 Feb;75(1):65–72. doi: 10.1002/jcp.1040750108. [DOI] [PubMed] [Google Scholar]
- Kirsten E. S., Watson J. A. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in hepatoma tissue culture cells by serum lipoproteins. J Biol Chem. 1974 Oct 10;249(19):6104–6109. [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Oshino N., Imai Y., Sato R. Electron-transfer mechanism associated with fatty acid desaturation catalyzed by liver microsomes. Biochim Biophys Acta. 1966 Oct 17;128(1):13–27. doi: 10.1016/0926-6593(66)90137-8. [DOI] [PubMed] [Google Scholar]
- Puck T. T., Kao F. T. Genetics of somatic mammalian cells. V. Treatment with 5-bromodeoxyuridine and visible light for isolation of nutritionally deficient mutants. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1227–1234. doi: 10.1073/pnas.58.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Chou S. M., Silbert D. F. Animal cell mutants defective in sterol metabolism: a specific selection procedure and partial characterization of defects. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3730–3734. doi: 10.1073/pnas.74.9.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Nordstrom J. L., Mitschelen J. J., Rodwell V. W., Schimke R. T. Micro assay for 3-hydroxy-3-methylglutaryl-CoA reductase in rat liver and in L-cell fibroblasts. Biochim Biophys Acta. 1974 Dec 29;370(2):369–377. doi: 10.1016/0005-2744(74)90098-9. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Hoak J. C. An improved method for the addition of long-chain free fatty acid to protein solutions. Anal Biochem. 1969 Nov;32(2):297–302. doi: 10.1016/0003-2697(69)90089-x. [DOI] [PubMed] [Google Scholar]
- Spence J. T., Gaylor J. L. Investigation of regulation of microsomal hydroxymethylglutaryl coenzyme A reductase and methyl sterol oxidase of cholesterol biosynthesis. J Biol Chem. 1977 Aug 25;252(16):5852–5858. [PubMed] [Google Scholar]
- Yamamoto S., Bloch K. Studies on squalene epoxidase of rat liver. J Biol Chem. 1970 Apr 10;245(7):1670–1674. [PubMed] [Google Scholar]


