Abstract
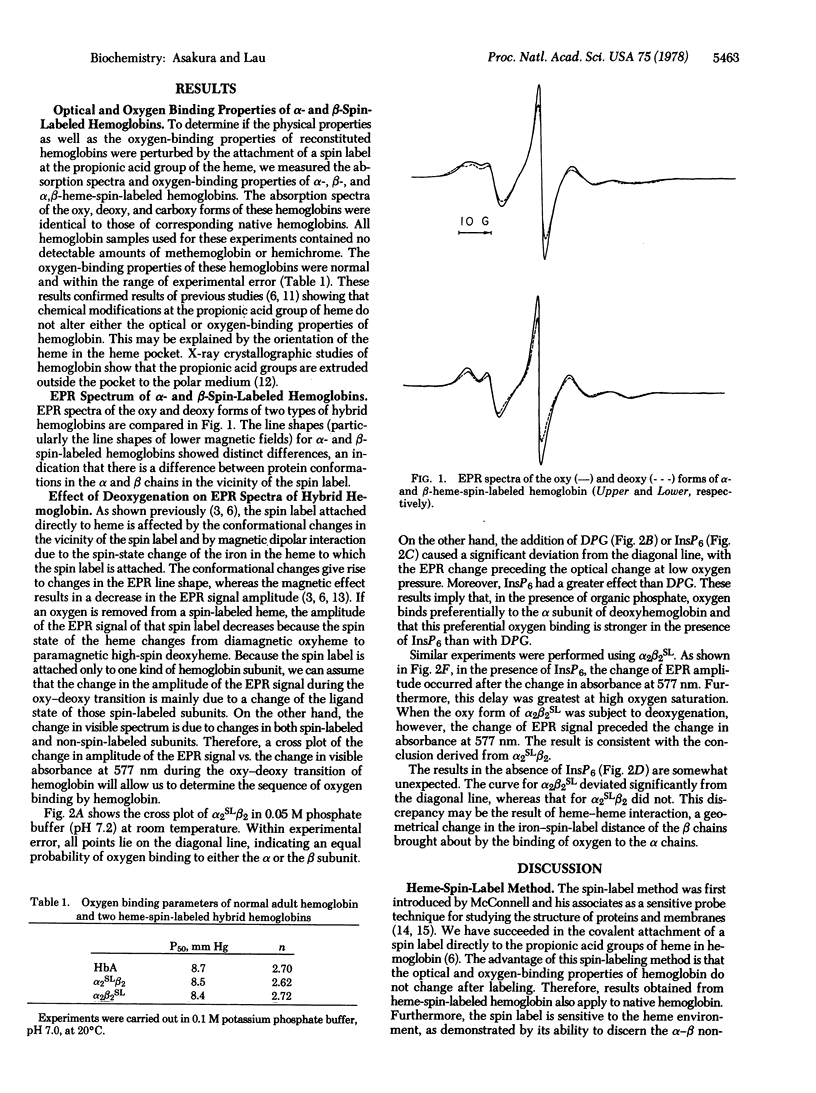
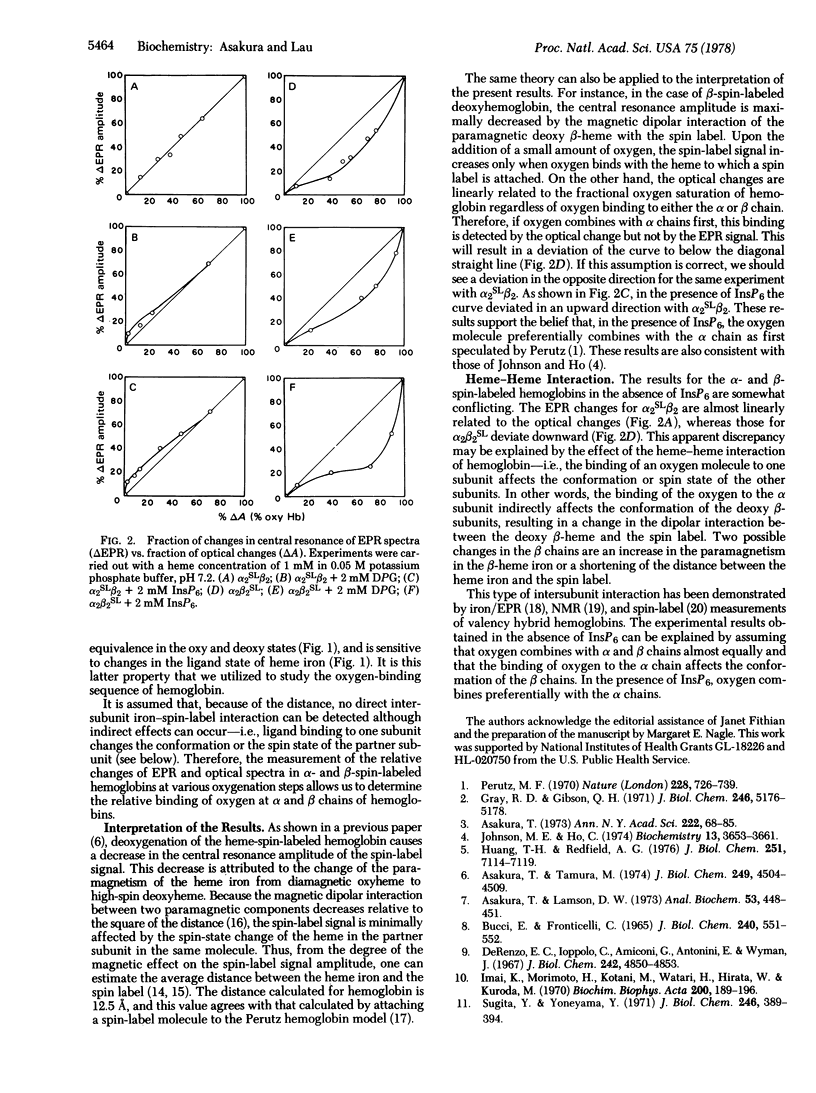
A nitroxide spin-label probe was attached directly to a propionic acid group of heme in either the α or the β chain of hemoglobin. The electron paramagnetic resonance (EPR) spectrum of the spin label is altered by the spin-state change of the heme iron to which the spin label is attached. These hybrid hemoglobins showed normal optical and functional properties, indicating that the attachment of the spin label did not perturb the function of hemoglobin. Upon deoxygenation of α-heme-spin-labeled hemoglobin, EPR signals changed proportionally with oxygen saturation (determined by measuring absorption spectra). This result indicates that there is no binding preference between the α and β chains of hemoglobin. However, the cross plot for the fraction of the EPR changes vs. the fraction of oxygen saturation deviated significantly from the diagonal straight line in response to the addition of 2,3-diphosphoglycerate and inositol hexaphosphate. The deviation indicated that the EPR change precedes the optical change at low oxygen tension. This result implies that, in the presence of organic phosphate, oxygen binds preferentially to the α subunit of deoxyhemoglobin. This conclusion was supported by the result obtained with β-heme-spin-labeled hemoglobin: the direction of the deviation for β-heme-spin-labeled hemoglobin in the presence of diphosphoglycerate and inositol hexaphosphate was opposite to that obtained for α-heme-spin-labeled hemoglobin. However, the curve deviated even in the absence of organic phosphate. This deviation for β-heme-spin-labeled hemoglobin can be explained by the intersubunit interaction of hemoglobin. From these results, it was concluded that, in the absence of organic phosphate, oxygen combines with the α and β chains with equal probability whereas, in the presence of organic phosphate, oxygen binds preferentially to the α chains of hemoglobin.
Keywords: hemoglobin subunits, spin-labeling, electron paramagnetic resonance, heme-heme interactions
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura T., Drott H. R. Evidence of heme-heme interaction in heme-spin-labeled hemoglobin. Biochem Biophys Res Commun. 1971 Sep;44(5):1199–1204. doi: 10.1016/s0006-291x(71)80213-9. [DOI] [PubMed] [Google Scholar]
- Asakura T. Heme-spin label studies of hemoglobin. I. Preparation and properties of heme-spin-labeled ferrihemoglobin. J Biol Chem. 1974 Jul 25;249(14):4495–4503. [PubMed] [Google Scholar]
- Asakura T. Heme-spin-label studies on human hemoglobin. Ann N Y Acad Sci. 1973 Dec 31;222:68–85. doi: 10.1111/j.1749-6632.1973.tb15253.x. [DOI] [PubMed] [Google Scholar]
- Asakura T., Lamson D. W. Preparation of protohemin monomethyl ester. Anal Biochem. 1973 Jun;53(2):448–451. doi: 10.1016/0003-2697(73)90093-6. [DOI] [PubMed] [Google Scholar]
- Asakura T., Leigh J. S., Jr, Drott H. R., Yonetani T., Chance B. Structural measurements in hemoprotiens: use of spin-labeled protoheme as a probe of heme environment. Proc Natl Acad Sci U S A. 1971 Apr;68(4):861–865. doi: 10.1073/pnas.68.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T., Tamura M. Heme-spin label studies of hemoglobin. II. Spin-labeled oxy- and deoxyhemoglobins. J Biol Chem. 1974 Jul 25;249(14):4504–4509. [PubMed] [Google Scholar]
- De Renzo E. C., Ioppolo C., Amiconi G., Antonini E., Wyman J. Properties of the alpha and beta chains of hemoglobin prepared from their mercuribenzoate derivatives by treatment with 1-dodecanethiol. J Biol Chem. 1967 Nov 10;242(21):4850–4853. [PubMed] [Google Scholar]
- Gray R. D., Gibson Q. H. The binding of carbon monoxide to and chains in tetrameric mammalian hemoglobin. J Biol Chem. 1971 Aug 25;246(16):5176–5178. [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Shimizu A., Morimoto H., Watari H. Changes in EPR spectra of M-type abnormal haemoglobins induced by deoxygenation and their implications for the haem-haem interaction. Biochim Biophys Acta. 1967 Oct 23;147(2):407–409. doi: 10.1016/0005-2795(67)90426-6. [DOI] [PubMed] [Google Scholar]
- Huang T. H., Redfield A. G. NMR study of relative oxygen binding to the alpha and beta subunits of human adult hemoglobin. J Biol Chem. 1976 Nov 25;251(22):7114–7119. [PubMed] [Google Scholar]
- Imai K., Morimoto H., Kotani M., Watari H., Hirata W. Studies on the function of abnormal hemoglobins. I. An improved method for automatic measurement of the oxygen equilibrium curve of hemoglobin. Biochim Biophys Acta. 1970 Feb 17;200(2):189–196. doi: 10.1016/0005-2795(70)90163-7. [DOI] [PubMed] [Google Scholar]
- Johnson M. E., Ho C. Effects of ligands and organic phosphates on functional properties of human adult hemoglobin. Biochemistry. 1974 Aug 27;13(18):3653–3661. doi: 10.1021/bi00715a005. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G. Observation of allosteric transition in hemoglobin. Biochem Biophys Res Commun. 1971 Jan 8;42(1):9–15. doi: 10.1016/0006-291x(71)90354-8. [DOI] [PubMed] [Google Scholar]
- Ohnishi S., Boeyens J. C., McConnell H. M. Spin-labeled hemoglobin crystals. Proc Natl Acad Sci U S A. 1966 Sep;56(3):809–813. doi: 10.1073/pnas.56.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Stone T. J., Buckman T., Nordio P. L., McConnell H. M. Spin-labeled biomolecules. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1010–1017. doi: 10.1073/pnas.54.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y., Yoneyama Y. Oxygen equilibrium of hemoglobins containing unnatural hemes. Effect of modification of heme carboxyl groups and side chains at positions 2 and 4. J Biol Chem. 1971 Jan 25;246(2):389–394. [PubMed] [Google Scholar]


