Abstract
Background
HIV-1 incidence estimates and correlates of HIV-1 acquisition in African men who have sex with men are largely unknown.
Methods
Since 2005, HIV-1-uninfected men who reported sex with men and women (MSMW) or sex with men exclusively (MSME) were followed at scheduled visits for collection of behavioural and clinical examination data and plasma for HIV-1 testing. Urethral or rectal secretions were collected from symptomatic men to screen for gonorrhoea. Poisson regression methods were used to estimate adjusted incidence rate ratios (aIRR) to explore associations between risk factors and incident HIV-1 infection. Plasma viral loads (PVL) were assessed over two years following seroconversion.
Results
Overall HIV-1 incidence in 449 men was 8.6 (95% confidence interval [CI]: 6.7–11.0) per 100 person-years (py). Incidence was 5.8 (95% CI: 4.2–7.9) per 100 py among MSMW, and 35.2 (95% CI: 23.8–52.1) per 100 py among MSME. Unprotected sex, receptive anal intercourse, exclusive sex with men, group sex, and gonorrhoea in the past 6 months were strongly associated with HIV-1 acquisition, adjusted for confounders. PVL in seroconverters was >4 log10 copies/mL at 230 (73.4%) of 313 visits in MSMW and 153 (75.0%) of 204 visits in MSME.
Conclusions
HIV-1 incidence is very high among MSM in coastal Kenya, and many seroconverters maintain high PVL for up to two years after infection. Effective HIV-1 prevention interventions, including treatment as prevention, are urgently needed in this population.
Keywords: HIV-1 incidence, MSM, gonorrhoea, anal intercourse, viral load, sexually transmitted infection, group sex, Africa
Introduction
Adult male same-sex behaviour is criminalised in Kenya, as in many other countries in Africa, and is highly stigmatised in African society. Front-line health workers are often ill-prepared to provide care and appropriate risk reduction counselling to men who have sex with men (MSM).1 Despite the prevailing belief among researchers in the recent past that anal intercourse between men was not epidemiologically significant to the African HIV-1 epidemic,2 a growing body of evidence documents populations of MSM with high HIV-1 prevalence in many African countries.3,4,5,6
A recent systematic review of 57 HIV-1 incidence studies in sub-Saharan Africa over the period 1987–2008 identified no cohort studies involving MSM participants or including anal sex as a risk factor for HIV-1 acquisition.7 The highest reported HIV-1 incidence rates in Africa occurred in the 1990s among urban female sex workers, with over 16 infections per 100 person years (py) of follow-up.7 Young people, individuals in concurrent or multiple partnerships, and individuals with a current or recent sexually transmitted infection (STI) were consistently identified as being at risk for HIV-1 infection. A recent global review of HIV-1 incidence estimates in MSM over the period 1995–2010 identified studies from 15 countries only, including 2 reports from Asia, and a preliminary report from Africa (present study).6 Price et al reported in 2012 an HIV-1 incidence of 6.8 per 100 py for MSM in Kenya over the period 2005–2008, including data from Nairobi and coastal Kenya.8
Despite recent advances in the recognition of MSM in Africa, research of HIV-1 risk in this group to date has been based almost exclusively upon HIV-1 prevalence studies,6 and has not included a distinction between MSM who reported sex with men and women, and MSM who reported sex with men only. Earlier, we reported that the high prevalence of HIV-1 in Kenyan MSM is probably due to unprotected receptive anal intercourse (RAI).9 In the present study, we present estimates of HIV-1 incidence, correlates of HIV-1 acquisition among Kenyan MSM during 6 years follow-up, and viral load measurements in the two years following seroconversion.
Methods
Study population
In July 2005, a prospective study of men and women considered to be at high risk for HIV-1 acquisition was initiated in a research clinic in Mtwapa town, approximately 20 km north of Mombasa. Adults aged 18–49 years were eligible if they met any of the following criteria: HIV-1 negative and reporting any of transactional sex work, a recent sexually transmitted infection (STI), multiple sexual partners, sex with an HIV-1-infected partner, or anal sex during the 3 months before enrolment.9 The initial focus of recruitment was on female sex workers and high-risk heterosexual men. Men who reported anal intercourse with another man in the preceding 3 months became the focus of recruitment in late 2005. In March 2008, a second research clinic in Kilifi town, approximately 40 km north of Mtwapa, started to enrol additional MSM. While MSM could only be engaged for HIV-1 prevention, care and research in the context of providing services to all high-risk men and women, the population for the current study is limited to MSM.
Recruitment
MSM were recruited at two walk-in VCT-centres adjacent to the research clinics.9 In addition, identification and recruitment of MSM was conducted by a team of 10–15 peer mobilisers, who approached individuals via personal networks and at social venues.9,10 Recruitment activities encompassed a region of coastal Kenya extending from Malindi in the north (60 km from Kilifi) to Mombasa in the south. Meetings were held with local stake-holders to enlist support for the ongoing research and prevent misunderstanding in the target communities.11 Written informed consent was obtained from all study participants. The study was approved by the ethical review board at the Kenya Medical Research Institute.
Behavioural assessment and clinical procedures
Detailed procedures are described elsewhere.12, 13 Briefly, enrolment and follow-up assessments included: face-to-face interviews using standardized questionnaires to ascertain recent behaviour and genitourinary symptoms;13 HIV-1 testing; and a physical examination, including genital examination.12 Urethral swabs were collected if urethral symptoms were reported or urethral discharge was clinically apparent. Proctoscopy and rectal screens were offered to men who reported recent RAI and who also had anorectal pain, rectal discharge or bleeding. Persons who tested HIV-1 seronegative at initial screening were enrolled and attended either monthly (when RAI was reported) or quarterly behavioural, clinical and laboratory follow-up visits using the same procedures. Those who had at least one follow-up sample available for testing through April 2011 were analysed in this study. Subjects were considered lost to follow up when they had not reported to the clinic for a period of 6 months since their last study visit.
Treatment of minor illnesses and risk reduction counselling
At all visits, risk reduction counselling, condoms, and water-based lubricants were provided. Participants with genital symptoms were provided syndromic treatment, and laboratory-diagnosed infections were treated following Kenyan Ministry of Health guidelines. After July 2010, standard treatment for gonorrhoea was changed from norfloxacin to cefixime, due to evidence of increasing resistance of Neisseria gonorrhoeae to fluoroquinolones.14
Screening for sexually transmitted infections
HIV-1 testing was performed using two rapid test kits (Determine, Abbott Laboratories, Abbott Park, Illinois, USA; Unigold, Trinity Biotech plc, Bray, Ireland) in parallel. Discordant rapid HIV-1 test results were resolved using an ELISA test (Genetic System HIV-1/2 plus O EIA, Bio-Rad Laboratories, Redmond, Washington, USA). All HIV-1 negative samples were tested for p24 antigen (Vironostika HIV-1 p24 ELISA, Biomérieux, Ltd, France), and pre-and post- seroconversion plasma samples were tested for HIV-1 RNA level (Amplicor Monitor vs. 1.5, Roche), with a positive result defined as >400 copies/mL.
The estimated date of HIV-1 infection (EDI) was calculated as follows:15 10 days before the sample collection date when the sample had a positive HIV-1 RNA level and negative p24 antigen and HIV-1 serology; 14 days before a positive p24 antigen test (with or without a positive HIV-1 RNA level), or the mid-point between a previously negative and subsequently positive HIV-1 serologic test, in the absence of either a positive HIV-1 RNA level or p24 antigen test. When volunteers had a positive p24 antigen test, or documented HIV-1 seroconversion they were invited to enrol in an acute HIV-1 infection study with frequent blood sample collection for HIV-1 plasma viral load (PVL) and CD4 T-cell assessments in the first 3 months following EDI and quarterly collections from 3 months up to two years post EDI.
Gonococcal infection was defined as the detection of Gram-negative, intracellular diplococci consistent with Neisseria gonorrhoeae in urethral or rectal secretions,12 and recent gonorrhoea as urethral or rectal gonococcal infection within a 6-month window.16
Prevalent syphilis infection was diagnosed by a positive rapid plasma reagin (RPR) titre confirmed by Treponema pallidum haemagglutination assay (TPHA). Incident syphilis was defined as a four-fold increase in RPR titre confirmed by TPHA. Serological testing for HSV-2 was performed on archived samples (HerpeSelect®-2, Focus Diagnostics, Cypress, California, USA) as described elsewhere.17 Briefly, all participants had their enrolment plasma sample screened for HSV-2 antibodies. Volunteers who had a negative HSV-2 serology at enrolment had their last follow-up sample tested to define HSV-2 serostatus at the end of the study. For participants who were HSV-2 seropositive at their last visit, intervening samples were tested to determine seroconversion timing. For those participants who seroconverted for both HSV-2 and HIV-1, timing of HSV-2 seroconversion was confirmed using Western Blot testing.18 HSV-2 serostatus for each participant was defined as seronegative, prevalent at enrollment, or incident during follow-up.
Data management and analysis
Questionnaire, clinical, and laboratory data were entered into a secure database. Individual and aggregate data were subjected to routine accuracy checks by research staff and periodic review by study monitors. Data cleaning, recoding and analysis were conducted using Stata version 11 (StataCorp, College Station, Texas).
Socio-demographic data were collected at enrolment. At each visit, men were asked to report whether they had been sexually active with men, women, or both. As in our previous work,9 this variable was aggregated across all study visits and used to classify MSM into two risk groups for analysis: (1) men reporting sex with men exclusively (MSME), and (2) men reporting sex with both men and women (MSMW). Reported sexual intercourse and condom use in the week before each visit were used to define three outcomes: ‘abstinent’ where sexual activity was not reported, ‘100% condom use’ where sexual activity occurred and condoms were used for all reported sex acts (defined as either vaginal or anal intercourse), and ‘unprotected’ where sexually activity occurred but condoms were not used for all sex acts.19 Other time-dependent sexual risk behaviour variables recorded at each visit included receipt of payment for sex, payment for sex, participation in group sex (defined as sex with more than person at the same time), anal intercourse (whether receptive or insertive), and rape. Oral sex was not assessed. Non-sexual behaviour variables included use of alcohol (in month prior to visit) and use of intravenous drugs since the last visit. Iatrogenic exposure to HIV-1 was based on any medical injection or blood transfusion since the last visit.
Two seroconverters had no behavioural and clinical data collected in the 3-month period prior to seroconversion, and were excluded from analysis for lack of precision. Associations between categorical variables were tested using Pearson’s Chi-squared or Fisher’s exact tests as appropriate. Associations between non-normally distributed continuous and categorical variables were assessed using the Wilcoxon ranksum test or Spearman rank correlation. Reported p values are two-sided.
HIV-1 incidence rates were expressed as incidence per 100 py. Incidence rates for risk groups were compared by log rank test. We evaluated potential risk factors for HIV-1 incidence as either fixed covariates (e.g., circumcision status) or time-varying covariates (e.g., transactional sex in previous 3 months). Incidence rate ratios (IRRs) were used to describe associations between covariates and estimated HIV-1 incidence. A population-averaged multivariable Poisson model with robust variance estimates was used to estimate adjusted incidence rate ratios (aIRRs). Factors identified a priori (e.g., age) and factors associated with prevalent or incident HIV-1 with p≤0.1 were included in the initial multivariable model. To reduce the number of predictors, only those factors significant at p<0.10 in the initial multivariable model were retained in the final multivariable model.
Results
Between July 2005 and April 2011, 449 HIV-1 negative MSM contributed at least one follow-up visit (Table 1). Of these, 372 (82.9%) were MSMW and 77 (17.2%) MSME. Baseline socio-demographic characteristics differed by age (a larger proportion of MSME were between 18–24 years), marital status (only 2 MSME were currently married) and personal assets; MSME reported possession of a greater number of material assets than MSMW.
Table 1.
Demographic, behavioural and other characteristics of MSM at enrolment
Characteristics and behaviours of 449 at-risk men enrolled in a cohort study of HIV-1 risks, Kilifi, Kenya, 2005–2011
| Total MSM N=449 (% or median) | MSMW N=372 (% or median) | MSME N=77 (% or median) | P-value | |
|---|---|---|---|---|
| Age group (yrs) | 0.004 | |||
| 18–24 | 217 (48.3) | 167 (44.9) | 50 (64.9) | |
| 25–34 | 180 (40.1) | 157 (42.2) | 23 (29.9) | |
| >34 | 52 (11.6) | 48 (12.9) | 4 (5.2) | |
| Education | 0.1 | |||
| Primary or none | 248 (55.2) | 206 (55.4) | 42 (54.6) | |
| Secondary | 163 (36.3) | 139 (37.4) | 24 (31.2) | |
| Higher/Tertiary | 38 (8.5) | 27 (7.3) | 11 (14.3) | |
| Currently married | 77 (17.2) | 75 (20.2) | 2 (2.6) | < 0.001 |
| Employment | ||||
| None | 210 (46.8) | 173 (46.5) | 37 (48.1) | 0.6 |
| Self | 170 (37.9) | 144 (38.7) | 26 (33.8) | |
| Formal | 69 (15.4) | 55 (14.8) | 14 (18.2) | |
| Personal assets | ||||
| Private piped water | 56 (12.5) | 38 (10.2) | 18 (23.4) | 0.001 |
| Own/private toilet/latrine | 138 (30.7) | 104 (28.0) | 34 (44.2) | 0.005 |
| Radio | 300 (66.8) | 249 (66.9) | 51 (66.2) | 0.9 |
| Electricity | 207 (46.1) | 162 (43.6) | 45 (58.4) | 0.02 |
| Television | 120 (26.7) | 97 (26.1) | 23 (29.9) | 0.5 |
| Cell/mobile phone | 154 (34.3) | 120 (32.3) | 34 (44.2) | 0.05 |
| Median asset score (IQR) | 2 assets (1–3) | 2 assets (1–3) | 3 assets (1–4) | 0.006 |
| Sexual exposure and protection with condoms past week | 0.4 | |||
| No sexual activity | 101 (22.5) | 84 (22.6) | 17(22.1) | |
| Only protected sex | 82 (18.3) | 72 (19.4) | 10 (13.0) | |
| Any unprotected sex | 266 (59.3) | 216 (58.1) | 50 (64.9) | |
| Sexual activity past week1 | ||||
| Number of sex partners (Median [IQR]) | 2 (1 – 3) | 2 (1 – 3) | 1 (1 – 3) | 0.8 |
| Number of sex acts (Median [IQR]) | 3 (1 – 6) | 3 (1 – 6) | 3 (1 – 6) | 0.8 |
| 100% condom use past week | 82 (23.6) | 72 (25.1) | 10 (16.7) | 0.2 |
| Group sex2 | 73 (16.3) | 62 (16.7) | 11 (14.3) | 0.6 |
| Been raped2 | 10 (2.2) | 9 (2.4) | 1 (1.3) | 0.5 |
| Insertive anal intercourse2 | 321 (71.5) | 297 (79.8) | 24 (31.2) | <0.001 |
| Receptive anal intercourse2 | 298 (66.4) | 229 (61.6) | 69 (89.6) | <0.001 |
| Received payment for sex2 | 314 (69.9) | 255 (68.6) | 59 (76.6) | 0.2 |
| Paid for sex2 | 154 (34.3) | 144 (38.7) | 10 (13.0) | <0.001 |
| Alcohol use (in past month) | 289 (64.4) | 244 (65.6) | 45 (58.4) | 0.2 |
| Intravenous drug use2 | 9 (2.0) | 9 (2.4) | 0 (0.0) | 0.2 |
| Medical injection2 | 55 (12.2) | 47 (12.6) | 8 (10.4) | 0.6 |
| Urethral gonorrhoea3 | 9 (2.0) | 9 (2.4) | 0 (0.0) | 0. 2 |
| Rectal gonorrhoea3 | 2 (0.4) | 1 (0.3) | 1 (1.2) | 0.5 |
| Genital sores2 | 50 (11.1) | 41 (11.0) | 9 (11.7) | 0.9 |
| HSV-2 seropositive | 84 (18.7) | 68 (18.3) | 16 (20.8) | 0.6 |
| Syphilis | 7 (1.6) | 6 (1.6) | 1 (1.3) | 0.8 |
| Circumcised | 420 (93.5) | 349 (93.8) | 71 (92.2) | 0.6 |
Among those reporting having sex
In previous three months
Gonorrhoea based on Gram staining of swabs collected from 235 volunteers who had urethral or rectal symptoms or discharge on examination
Sexual risk behaviour and STI prevalence at enrolment
Participants reported a median of 2 sex partners (IQR: 1–3) and 3 sex acts (IQR: 1–6) in the last week; 23.6% of sex acts were protected (Table 1). This was similar between MSMW and MSME. Group sex was commonly reported by both MSMW and MSME, rape and intravenous drug use were rare, and the majority of men used alcohol. As our data revealed previously, MSMW reported more often insertive anal intercourse (IAI), and MSME more often RAI. Over two thirds of participants reported being paid for sex (no difference between MSMW and MSME), while over a third paid for sex themselves (38.7% of MSMW vs.13.0% of MSME, p<0.001). Recent urethral and rectal gonorrhoea was diagnosed in 2.0% and 0.4% of all men, respectively. Prevalence of genital sores was 11.1%; syphilis 1.6%, and HSV-2 seropositivity 18.7%.
HIV-1 incidence estimates and correlates of infection
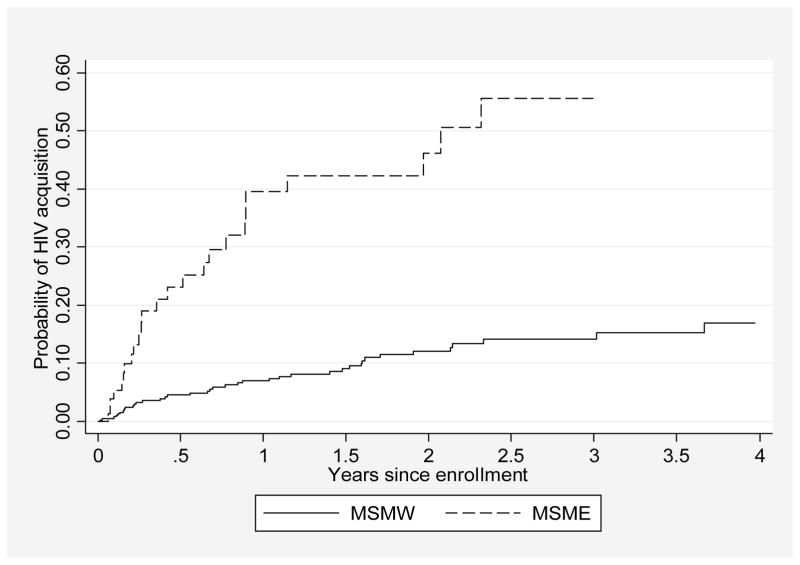
The 449 participants contributed 743.9 py, with median follow-up times of 21.0 months (IQR: 8.6–37.4) for MSMW and 4.6 months (IQR: 2.5–16.5, p<0.001) for MSME. Sixty-four men acquired HIV-1 during follow up, for an estimated HIV-1 incidence of 8.6 (95% CI: 6.7–11.0) per 100 py (Table 2). HIV-1 incidence was 5.8 (95% CI: 4.2–7.9) per 100 py among MSMW and 35.2 (95% CI: 23.8–52.1) per 100 py among MSME (p<0.001, Figure 1). Loss to follow-up was 25.7 (95% CI: 22.3–29.6) per 100 py; 23.9 (95% CI: 20.5–27.9) per 100 py among MSMW and 42.2 (29.5–60.4) per 100 py among MSME (log-rank test, p=0.009). In univariate analysis, HIV-1 acquisition was associated at p≤0.10 with reporting exclusive sex with men, younger age, being single, having unprotected sex, having been paid for sex, not having paid for sex, IAI, RAI, group sex, having been raped, genital sores and a recent laboratory-diagnosed gonorrhoea infection (details in Table 2). In multivariable analysis, exclusive sex with men (aIRR 3.7, 95% CI: 2.1–6.5), recent unprotected sex (aIRR 2.1, 95% CI: 1.1–4.1], relative to no recent sexual activity), RAI (aIRR 9.7, 95% CI: 3.8–25.1), group sex (aIRR 1.9, 95% CI: 1.0–3.4), and gonorrhoea infection in the past 6 months (aIRR 14.7, 95% CI: 8.3–26.0) were strongly associated with HIV-1 acquisition, after adjustment for age, marital status, transactional sex, IAI, having been raped, and genital sores.
Table 2.
Risk Factors for HIV-1 acquisition in MSM
Risk factors for HIV-1 acquisition in men, Kilifi, Kenya, 2005–2011
| HIV-1 incidence cases/py | Incidence/100 py (95% CI) | Unadjusted analysis | Adjusted Analysis | |||
|---|---|---|---|---|---|---|
| IRR (95% CI) | p-value | IRR (95% CI) | p-value | |||
| All Men (n = 449) | 64/743.9 | 8.6 (6.7 – 11.0) | ||||
| Sex with men and women (MSMW) Sex with men exclusively (MSME) |
39/673.2 39/70.7 |
5.8 (4.2–7.9) 35.4 (23.9–52.3) |
Reference 6.1 (3.5–10.7) |
<0.001 | Reference 3.7 (2.1–6.5) |
<0.001 |
| Age group (yrs) | 0.02 | 0.2 | ||||
| 18–24 | 33/275.9 | 12.0 (8.5 – 16.8) | 7.5 (1.8 – 32.0) | 3.7 (0.9 – 15.5) | ||
| 25–34 | 29/342.1 | 8.5 (5.9– 12.2) | 5.3 (1.2 – 22.8) | 2.8 (0.7 – 12.3) | ||
| Above 34 | 2/125.9 | 1.6 (0.4 – 6.3) | Reference | Reference | ||
| Education | 0.4 | |||||
| Primary or none | 32/433.1 | 7.4 (5.2 – 10.4) | Reference | |||
| Secondary | 27/255.6 | 10.6 (7.2 – 15.4) | 1.4 (0.8 – 2.4) | |||
| Higher/Tertiary | 5/55.2 | 9.1 (3.8 – 21.8) | 1.2 (0.5 – 3.4) | |||
| Ever married | 0.03 | NI2 | ||||
| No | 59/602.0 | 9.8 (7.6 – 12.6) | Reference | |||
| Yes | 5/141.9 | 3.5 (1.5 – 8.5) | 0.4 (0.1 – 0.9) | |||
| Employment | 0.1 | |||||
| None | 29/357.5 | 8.1 (5.6 – 11.7) | Reference | |||
| Self | 19/273.9 | 6.9 (4.4 – 10.9) | 0.9 (0.5 – 1.6) | |||
| Formal | 16/112.5 | 14.2 (8.7 – 23.2) | 1.7 (0.9 – 3.3) | |||
| Sexual exposure and protection past week | 0.06 | 0.05 | ||||
| No sexual activity | 13/239.6 | 5.4 (3.2 – 9.3) | Reference | Reference | ||
| Only protected sex | 17/212.0 | 8.0 (5.0 – 12.9) | 1.5 (0.7 – 3.1) | 1.2 (0.6 – 2.6) | ||
| Any unprotected sex | 34/290.5 | 11.7 (8.4 – 16.4) | 2.2 (1.1 – 4.1) | 2.1 (1.1 – 4.1) | ||
| Received payment for sex1 | 0.003 | NI2 | ||||
| No | 16/328.5 | 4.9 (3.0 – 7.9) | Reference | |||
| Yes | 48/415.4 | 11.6 (8.7 – 15.3) | 2.4 (1.3 – 4.2) | |||
| Paid for sex1 | 0.004 | NI2 | ||||
| No | 56/520.3 | 10.8 (8.3 – 14.0) | Reference | |||
| Yes | 8/223.6 | 3.6 (1.8 – 7.2) | 0.3 (0.2 – 0.7) | |||
| Insertive anal intercourse1 | 0.003 | NI2 | ||||
| No | 37/286.2 | 12.9 (9.4 – 17.8) | Reference | |||
| Yes | 27/457.7 | 5.9 (4.0 – 8.6) | 0.5 (0.3 – 0.8) | |||
| Receptive anal intercourse1 | <0.001 | <0.001 | ||||
| No | 5/414.1 | 1.2 (0.5 – 2.9) | Reference | Reference | ||
| Yes | 59/329.8 | 17.9 (13.9 – 23.1) | 14.7 (5.9 – 36.8) | 9.7 (3.8 – 25.1) | ||
| Alcohol use1 | 0.4 | |||||
| No | 22/290.6 | 7.6 (5.0 – 11.5) | Reference | |||
| Yes | 42/453.3 | 9.3 (6.8 – 12.5) | 1.2 (0.7 – 2.1) | |||
| Group sex1 | <0.001 | 0.04 | ||||
| No | 47/672.7 | 7.0 (5.2 – 9.3) | Reference | Reference | ||
| Yes | 17/71.2 | 23.9 (14.9 – 38.4) | 3.4 (1.9 – 6.0) | 1.9 (1.0– 3.4) | ||
| Used intravenous drugs1 | 0.2 | |||||
| No | 63/740.9 | 8.5 (6.6 – 10.9) | Reference | |||
| Yes | 1/3.0 | 33.9 (4.8 – 240.3) | 4.0 (0.5 – 34.7) | |||
| Been raped1 | 0.008 | NI2 | ||||
| No | 61/737.2 | 8.3 (6.4 – 10.6) | Reference | |||
| Yes | 3/6.7 | 44.6 (14.4 – 138.4) | 5.4 (1.6 – 17.5) | |||
| Had medical injection1 | 0.3 | |||||
| No | 60/715.8 | 8.4 (6.5 – 10.8) | Reference | |||
| Yes | 4/28.1 | 14.2 (5.3 – 37.9) | 1.7 (0.6 – 4.7) | |||
| Gonorrhoea infection within past 6 months | <0.001 | <0.001 | ||||
| No | 58/738.9 | 7.8 (6.1 – 10.2) | Reference | Reference | ||
| Yes | 6/5.0 | 119.2 (53.7 – 266.1) | 15.1 (7.7 – 29.7) | 14.7 (8.3 – 26.0) | ||
| Genital Sores1 | 0.006 | NI2 | ||||
| No | 58/721.9 | 8.0 (6.2 – 10.4) | Reference | |||
| Yes | 6/22.0 | 27.2 (12.2 – 60.6) | 3.4 (1.4 – 7.9) | |||
| HSV-2 status | 0.4 | |||||
| Sero-negative at all study visits | 45/567.9 | 7.9 (5.9 – 10.6) | Reference | |||
| Prevalent positive at enrolment | 16/138.4 | 11.6 (7.1 – 18.9) | 1. 5 (0.8 – 2.6) | |||
| Incident positive during follow up | 3/37.7 | 8.0 (2.6 – 24.7) | 1.0 (0.3 – 3.3) | |||
| Incident syphilis | ||||||
| Negative | 64/741.7 | 8.6 (6.8 – 11.0) | ||||
| Positive | 0/2.2 | – | ||||
| Circumcision status | 0.9 | |||||
| No | 4/43.8 | 9.1 (3.4 – 24.3) | Reference | |||
| Yes | 60/700.1 | 8.6 (6.7 – 11.0) | 0.9 (0.3 – 2.8) | |||
Since last visit (monthly or quarterly)
NI = Not included in final model. Only factors significant at p<0.10 in the initial multivariable model (data not shown) were retained in the final multivariable model
Figure 1.
Cumulative probability of HIV-1 acquisition in men who have sex with men exclusively (MSME) and men who have sex with men and women (MSMW) by year since cohort enrolment, Coastal Kenya, 2005–2011.
HSV-2 acquisition
Of the 64 men who seroconverted for HIV-1, 45 (70%) were HSV-2 seronegative throughout the observation period, 16 (25%) were HSV-2 seropositive at enrolment, and 3 (5%) had a change of HSV-2 antibody status consistent with HSV-2 sero-conversion. Of these 3 men, two had an incident HSV-2 infection approximately 5 months before, and one just before or at HIV-1 acquisition. Thirty-seven men seroconverted for HSV-2 in the absence of HIV-1 acquisition.
Gonorrhoea acquisition
A total of 42 men acquired a urethral or rectal gonorrhoea infection during follow up. The estimated incidence of gonococcal infection was 5.9 (95% CI: 4.4–8.0) per 100 py overall. Incidence was 5.6 (95% CI: 3.9–8.3) per 100 py for MSMW, and 9.3 (95% CI: 4.2–20.7) per 100 py for MSME (p=0.6). Incident gonoccocal infections occurred in the 6 months prior to HIV-1 acquisition in 6 men, including one that coincided with the timing of HIV-1 acquisition. The median time from the incident gonorrhoea infection to HIV-1 acquisition in these 6 men was 57 days (IQR: 22 – 128).
Post-infection viral dynamics
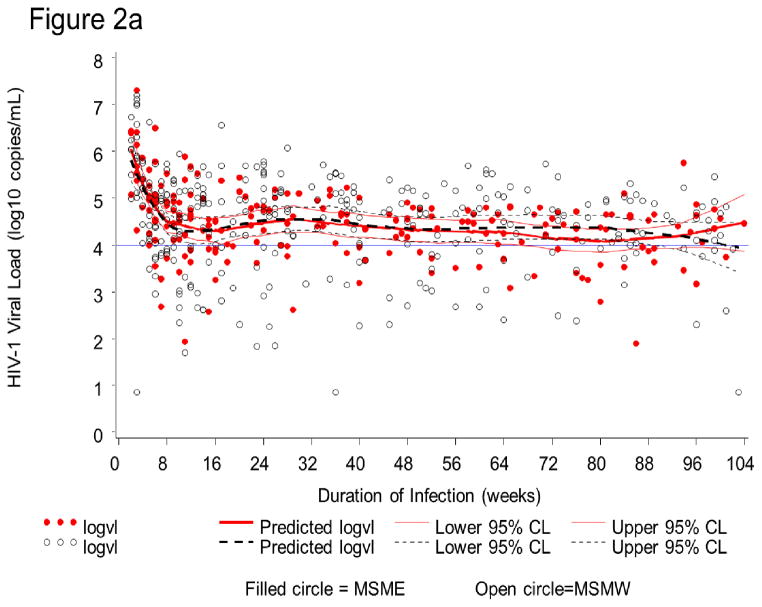
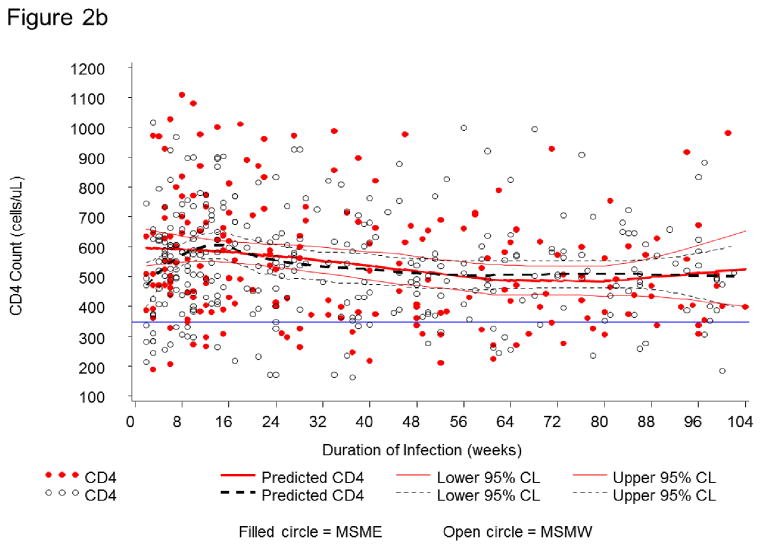
HIV-1 RNA dynamics were assessed in 63 seroconverters. In the first two years (104 weeks) after infection, 517 PVL measurements were obtained. PVL was >4 log10 copies/mL at 230 (73.4%) of 313 visits in MSMW and 153 (75.0%) of 204 visits in MSME (Figure 2a). At corresponding time-points for which CD4 T-cell counts measurements were available (n=504), CD4 T-cell counts were <350 cells/μL, the current threshold for ART-initiation in Kenya, at only 66 visits (13.1%) (Figure 2b).
Figure 2.
Figure 2a. HIV-1 plasma viral load (PVL) dynamics in 63 Kenyan seroconverters. In the first two years (104 weeks) after infection, PVL measurements are presented for men who have sex with men exclusively (MSME= filled circle) and men who have sex with men and women (MSMW = open circle). All visits with PVL (N = 517) are shown. Visits with high PVL (>4.0 log10) are above the horizontal line. Solid and dotted curves correspond to mean predicted PVL and 95% boundaries for MSME (204 person-visits) and MSMW (313 person-visits), respectively.
Figure 2b. CD4+ T-cell (CD4) counts in 63 Kenyan seroconverters. In the first two years (104 weeks) after infection, CD4 counts are presented for MSME (filled circle) and MSMW (open circle). Among all visits (N = 504) shown, those with low CD4 count (<350 cells/mL) (n = 66 or 13.1%) are below the horizontal line. Solid and dotted trajectories correspond to mean predicted CD4 count and 95% boundaries for MSME and MSMW, respectively.
Discussion
We documented very high HIV-1 incidences among a large group of MSM in Coastal Kenya. Among MSME, over one third acquired HIV-1 within one year of study enrolment, while HIV-1 incidence in MSMW (82.9% of our cohort) was 5.8 per 100 py. These rates are among the highest currently documented in Africa.6,7 Previous research in this cohort has demonstrated that many MSM have concurrent sexual partnerships.20 Most sex partners of MSM in this cohort were Kenyan,12,20 and HIV-1 isolates from a subgroup of MSM in this cohort were similar to isolates from the general population in Kenya.21 Among MSM who seroconverted during follow-up, PVL remained high (>4 log10 copies/mL) in the two-year period following infection. Because CD4 T cell counts in these seroconverters remained above the threshold for antiretroviral therapy (ART) initiation in Kenya, these men posed a significant risk of HIV-1 transmission to their sexual partners during the study period.
The very high HIV-1 incidence among MSM from Coastal Kenya may be the result of studying an inter-connected network of adults with ongoing high-risk sexual activity. Interestingly, the estimated HIV-1 incidence in MSM who participated in a study in Nairobi during 2007–08 was similar (9.1 per 100 py) to our estimate among Coastal MSM (8.6 per 100 py).8 Given that up to 40% of new HIV-1 infections are likely transmitted by individuals in the early phase of HIV-1 infection (within 6 months following the estimated date of infection),22 interventions aiming to reduce HIV-1 acquisition and transmission among MSM in Kenya should include frequent, targeted HIV testing and linkage to care, with a strong focus on effective biomedical interventions such as pre-exposure prophylaxis (PrEP) and early ART.23 Such interventions will benefit both MSM and the wider, primarily heterosexual population who are connected to Kenyan MSM through their sexual networks.
We identified several risk factors that were independently associated with HIV-1 acquisition in this population. Men who reported unprotected sex, or group sex had a two-fold higher risk, exclusive sex with men had a 4-fold higher risk, RAI had a 10-fold higher risk, and those who had a laboratory-diagnosed gonorrhoea infection in the past 6 months, had a 15-fold higher risk, respectively, of acquiring HIV-1 when compared to men who did not report these risk factors, after adjustment for potentially confounding factors. The risk estimate of RAI is similar to those from previous reports comparing the risks of RAI to those of penile-vaginal intercourse.24,25 MSME reported significantly less IAI and more RAI than MSMW at study enrolment. MSME in our cohort were younger, better off financially than MSMW, and extremely vulnerable to acquiring HIV-1. These MSME, who have also been referred to as ‘queens’ in Kenya,9 experience very strong societal rejection and may face greater barriers than MSMW to accessing medical services, including ART. Such barriers may impair their ability to attain virologic suppression, relative to MSMW.
Unprotected sex derived from a composite variable based on the number of sex partners, number of sex acts, and number of sex acts protected by a condom in the week prior to the assessment. Targeting any of these components for behaviour change intervention is important in itself, but may not be a sufficient strategy for reducing HIV transmission among MSM.26,27 Very few studies have found group sex to be practiced in African settings,28,29 and our study is the first to document that group sex is an independent risk factor for HIV-1 acquisition in African MSM. Anecdotally, participants who reported group sex said that unprotected sex is common during these encounters, which frequently happens when alcohol is taken in excess. Our data did not assess characteristics of group sex or situations in which group sex commonly occur. That one of six men reported it at study enrolment suggests that this practice is relatively common. Risk reduction counselling targeting MSM needs to explore if men participate in group sex and provide counselling about the high risk of HIV-1 and STI acquisition and transmission due to these encounters.
Gonorrhoea incidence was 5.6 per 100 py in MSMW and 9.3 per 100 py in MSME based on detection of gram-negative, intracellular organisms compatible with Neisseria gonorrhoeae in Gram-stained urethral or rectal secretions collected from symptomatic men. Our estimated incidence is likely to be an underestimate since the microscopic method used for detection is relatively insensitive.30 We have recently reported a relatively high prevalence of asymptomatic infections and of quinolone-resistant gonorrhoea in this population.14,30 Persisting inflammation of urethra or rectum due to unsuccessfully treated or undetected infections may have contributed to the increased risk for HIV-1 acquisition we observed.31 Of note, the World Health Organization’s new guidelines for STI management in MSM and transgender people recommend that asymptomatic MSM who report RAI in the past 6 months and have multiple partners or a partner with a STI should receive treatment for gonorrhoea and chlamydia.32 Most MSM in this study would have been eligible to receive presumptive treatment for gonorrhoea and chlamydia infection at cohort enrolment per the new WHO recommendation. A recent study conducted in our MSM cohort revealed that for every 7 MSM meeting WHO criteria for presumptive treatment, 1 asymptomatic anorectal infection (detected by NAAT screening) would have been treated.33
In the “EXPLORE” study involving almost 4300 HIV-1-seronegative MSM with an HSV-2 prevalence of 20.3% and HSV-2 incidence of 1.9 (95% CI: 1.6–2.2) per 100 py, both incident and prevalent HSV-2-infections increased the risk of HIV-1 acquisition.18 HIV-1 acquisition has been strongly associated with incident HSV-2 infection among heterosexual African men,34 and with self-reported genital ulcer disease in a recent analysis of over 300 Kenyan MSM (a subset of the present study).8 We have previously reported that HIV-1acquisition was associated with HSV-2 acquisition in this population.17 However, prevalent HSV-2 was not a risk factor for HIV-1 acquisition in this study, and only 3 of 64 incident HSV-2 infections occurred before or around the time of HIV-1 acquisition. Because women in the same population have a much higher prevalence of HSV-2 (50.8% vs. 20.0% in men),17 it is possible that men in our study population acquired HSV-2 infection primarily from female partners and HIV-1 infection primarily from male partners, through unprotected RAI.
This study has a number of limitations. First, MSM populations have been mobilised through peer educators who frequently reported sex work. As such, MSM enrolled in this study likely do not represent the wider and largely hidden non-sex worker MSM population in Coastal Kenya. Second, our data was collected through face-to-face interview, which may be less sensitive than other methods such as audio computer-assisted self-interview (ACASI) for reporting sensitive information.13 Third, STI screening was targeted at symptomatic individuals and employed a relatively insensitive methodology due to budget constraints. Finally, as this is the first ongoing longitudinal study of MSM in Kenya, men’s willingness to participate was likely impacted by the need for MSM participants to protect their safety and privacy, especially as the most vulnerable men, MSME, had the highest loss to follow-up. Notwithstanding these limitations, our study demonstrates that MSM can be engaged to participate in longitudinal research in Kenya.
In summary, while cross-sectional studies in volving MSM in Africa have shown very high HIV-1 prevalence among MSME relative to MSMW,6,9,35 this is the first study that has estimated HIV-1 acquisition risks among MSMW and MSME together. Our findings suggest that unprotected sex, RAI, exclusive sex with men, group sex, and co-infection with gonorrhoea are the primary biological drivers of the HIV-1 epidemic among MSM from coastal Kenya. While some risk factors are potentially modifiable and require both structural and targeted behaviour and public health interventions, the very high HIV-1 incidence and sustained high viral loads in the majority of MSMW and MSME for up to two years following seroconversion highlight the urgent need for both PrEP and early-ART interventions in this population.
Acknowledgments
We thank the International AIDS Vaccine Initiative (IAVI) for supporting the high-risk cohort studies in Kilifi, and staff in the HIV/STI project at the Kenya Medical Research Institute in Kilifi for their commitment to serving MSM. We are also grateful for support and guidance provided by the KEMRI-Wellcome Trust Research Programme to carry out research with stigmatised and vulnerable populations. We thank James Tang and Wei Song, at the University of Alabama for their kind assistance of the analysis of RNA viral load and CD4,-T cell dynamics in 63 sero-converters, and Larry Corey, Rhoda Morrow, and Anne Cent, for kindly testing our seroconverter samples by HSV-2 Western blot. HSV-2 testing received support from the University of Washington Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757). We are grateful for comments by two anonymous AIDS’ reviewers on an earlier version of this manuscript. This work was made possible in part by the generous support of the American people through the United States Agency for International Development (USAID). The contents are the responsibility of the study authors and do not necessarily reflect the views of USAID, the NIH, or the United States Government. This report was published with permission from KEMRI.
Footnotes
Presented in part at the 18th Conference on Retroviruses and Opportunistic infections (#1042), Boston, Massachusetts, 2011
Conflicts of interest
We declare that we have no conflicts of interest
Author Contributions
Eduard Sanders designed the HIV-1 incidence cohort study, performed data analysis and wrote the first draft of the manuscript.
Haile Selassie Okuku performed data acquisition and the full data analysis.
Adrian Smith performed initial data analysis, and manuscript editing
Mary Mwangome performed behaviour and clinical data collections
Elizabeth Wahome performed data acquisition and quality assurance
Gregory Fegan assisted with and advised on data analysis, and manuscript editing
Norbert Peshu supported engagement of local stake holders
Elisabeth M. van der Elst supported engagement of local stake holders
Matthew A Price designed the HIV-1 incidence cohort study and manuscript editing
R. Scott McClelland designed the HIV-1 incidence cohort study, and manuscript editing
Susan M. Graham designed the HIV-1 incidence cohort study, data analysis and critical revision of the manuscript
References
- 1.Sanders EJ, Wahome E, Mwangome M, Thiong’o AN, Okuku HS, Price MA, et al. Most adults seek urgent healthcare when acquiring HIV-1 and are frequently treated for malaria in coastal Kenya. AIDS. 2011;25:1219–24. doi: 10.1097/QAD.0b013e3283474ed5. [DOI] [PubMed] [Google Scholar]
- 2.Smith AD, Tapsoba P, Peshu N, Sanders EJ, Jaffe HW. Men who have sex with men and HIV/AIDS in sub-Saharan Africa. Lancet. 2009;374:416–22. doi: 10.1016/S0140-6736(09)61118-1. [DOI] [PubMed] [Google Scholar]
- 3.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med. 2007;4:e339. doi: 10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caceres CF, Konda K, Segura ER, Lyerla R. Epidemiology of male same-sex behaviour and associated sexual health indicators in low- and middle-income countries: 2003–2007 estimates. Sex Transm Infect. 2008;84 (Suppl 1):i49–i56. doi: 10.1136/sti.2008.030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyrer C, Baral SD, Walker D, Wirtz AL, Johns B, Sifakis F. The expanding epidemics of HIV type 1 among men who have sex with men in low- and middle-income countries: diversity and consistency. Epidemiol Rev. 2010;32:137–51. doi: 10.1093/epirev/mxq011. [DOI] [PubMed] [Google Scholar]
- 6.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380:367–77. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braunstein SL, van de Wijgert JH, Nash D. HIV incidence in sub-Saharan Africa: a review of available data with implications for surveillance and prevention planning. AIDS Rev. 2009;11:140–56. [PubMed] [Google Scholar]
- 8.Price M, Rida W, Mwangome M, Mutua G, Middelkoop K, Roux S, et al. Identifying at-risk populations in Kenya and South Africa: HIV incidence in cohorts of men who report sex with men, sex workers, and youth. JAIDS. 2012;59:185–93. doi: 10.1097/QAI.0b013e31823d8693. [DOI] [PubMed] [Google Scholar]
- 9.Sanders EJ, Graham SM, Okuku HS, van der Elst EM, Muhaari A, Davies A, et al. HIV-1 infection in high risk men who have sex with men in Mombasa, Kenya. AIDS. 2007;21:2513–20. doi: 10.1097/QAD.0b013e3282f2704a. [DOI] [PubMed] [Google Scholar]
- 10.Geibel S, van der Elst EM, King’ola N, Luchters S, Davies A, Getambu EM, et al. ‘Are you on the market?’: a capture-recapture enumeration of men who sell sex to men in and around Mombasa, Kenya. AIDS. 2007;21:1349–54. doi: 10.1097/QAD.0b013e328017f843. [DOI] [PubMed] [Google Scholar]
- 11.Tindana PO, Singh JA, Tracy CS, Upshur RE, Daar AS, Singer PA, et al. Grand challenges in global health: community engagement in research in developing countries. PLoS Med. 2007;4:e273. doi: 10.1371/journal.pmed.0040273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grijsen ML, Graham SM, Mwangome M, Githua P, Mutimba S, Wamuyu L, et al. Screening for genital and anorectal sexually transmitted infections in HIV prevention trials in Africa. Sex Transm Infect. 2008;84:364–70. doi: 10.1136/sti.2007.028852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Elst EM, Okuku HS, Nakamya P, Muhaari A, Davies A, McClelland RS, et al. Is audio computer-assisted self-interview (ACASI) useful in risk behaviour assessment of female and male sex workers, Mombasa, Kenya? PLoS One. 2009;4:e5340. doi: 10.1371/journal.pone.0005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan S, Thiong’o AN, Macharia M, Wamuyu L, Mwarumba S, Mvera B, et al. High prevalence of quinolone resistance in Neisseria gonorrhoeae in coastal Kenya. Sex Transm Infect. 2011;87:231. doi: 10.1136/sti.2010.048777. [DOI] [PubMed] [Google Scholar]
- 15.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 16.Jansen IA, Geskus RB, Davidovich U, Jurriaans S, Coutinho RA, Prins M, et al. Ongoing HIV-1 transmission among men who have sex with men in Amsterdam: a 25-year prospective cohort study. AIDS. 2011;25:493–501. doi: 10.1097/QAD.0b013e328342fbe9. [DOI] [PubMed] [Google Scholar]
- 17.Okuku HS, Sanders EJ, Nyiro J, Ngetsa C, Ohuma E, McClelland RS, et al. Factors associated with herpes simplex virus type 2 incidence in a cohort of human immunodeficiency virus type 1-seronegative Kenyan men and women reporting high-risk sexual behavior. Sex Transm Dis. 201;38:837–44. doi: 10.1097/OLQ.0b013e31821a6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown EL, Wald A, Hughes JP, Morrow RA, Krantz E, Mayer K, et al. High risk of human immunodeficiency virus in men who have sex with men with herpes simplex virus type 2 in the EXPLORE study. Am J Epidemiol. 2006;164:733–41. doi: 10.1093/aje/kwj270. [DOI] [PubMed] [Google Scholar]
- 19.McClelland RS, Graham SM, Richardson BA, Peshu N, Masese LN, Wanje GH, et al. Treatment with antiretroviral therapy is not associated with increased sexual risk behavior in Kenyan female sex workers. AIDS. 2010;24:891–7. doi: 10.1097/QAD.0b013e32833616c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith AD, Ferguson A, Kowuar D, van der Elst E, Agwanda C, Muhaari A, et al. Role versatility and female partnerships among MSM sex workers in Mombasa, Kenya. Paper presented at the 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 21.Tovanabutra S, Sanders EJ, Graham SM, Mwangome M, Peshu N, McClelland RS, et al. Evaluation of HIV type 1 strains in men having sex with men and in female sex workers in Mombasa, Kenya. AIDS Res Hum Retroviruses. 2010;26:123–31. doi: 10.1089/aid.2009.0115. [DOI] [PubMed] [Google Scholar]
- 22.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–68. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan P, Carballo-Dieguez A, Coates T, Goodreau S, McGowan I, Sanders EJ, et al. Successes and challenges of HIV Prevention fin men who have sex with men. Lancet. 2012;380:388–399. doi: 10.1016/S0140-6736(12)60955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comparison of female to male and male to female transmission of HIV in 563 stable couples. European Study Group on Heterosexual Transmission of HIV. BMJ. 1992;304:809–13. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29:38–43. doi: 10.1097/00007435-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372:669–84. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson WD, Hedges LV, Ramirez G, Semaan S, Norman LR, Sogolow E, et al. HIV prevention research for men who have sex with men: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2002;30 (Suppl 1):S118–29. [PubMed] [Google Scholar]
- 28.Dietrich J, Khunwane M, Laher F, de Bruyn G, Sikkema KJ, Gray G. “Group sex” parties and other risk patterns: A qualitative study about the perceptions of sexual behaviors and attitudes of adolescents in Soweto, South Africa. Vulnerable Child Youth Stud. 2011;11:244–254. doi: 10.1080/17450128.2011.597796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston LG, Holman A, Dahoma M, Miller LA, Kim E, Mussa M, et al. HIV risk and the overlap of injecting drug use and high-risk sexual behaviours among men who have sex with men in Zanzibar (Unguja), Tanzania. Int J Drug Policy. 2010;21:485–92. doi: 10.1016/j.drugpo.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Sanders EJ, Thiong’o AN, Okuku HS, Mwambi J, Priddy F, Shafi J, et al. High prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections among HIV-1 negative men who have sex with men in coastal Kenya. Sex Transm Infect. 2010;86:440–1. doi: 10.1136/sti.2010.043224. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537–43. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 32.WHO. Prevention and treatment of HIV and other Sexually Transmitted Infections among Men who have Sex with Men and Transgender People. World Health Organization; Switserland: 2011. pp. 1–86. [PubMed] [Google Scholar]
- 33.Okuku HS, Wahome E, Duncan S, Thiongo’ A, Mwambi J, Shafi J, et al. Evaluation of presumptive treatment recommendation for asymptomatic anorectal gonorrhoea and chlamydia infections in at-risk MSM in Kenya. IAS Conference; Washington DC. July 21–26, 2012. [Google Scholar]
- 34.Tobian AA, Ssempijja V, Kigozi G, et al. Incident HIV and herpes simplex virus type 2 infection among men in Rakai, Uganda. AIDS. 2009;23:1589–94. doi: 10.1097/QAD.0b013e32832d4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lane T, Raymond HF, Dladla S, Rasethe J, Struthers H, McFarland W, et al. High HIV Prevalence Among Men Who have Sex with Men in Soweto, South Africa: Results from the Soweto Men’s Study. AIDS Behav. 2011;15:626–34. doi: 10.1007/s10461-009-9598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]