Abstract
Background:
The endothelin axis has been shown to have a pivotal role in several human malignancies. The aim of this study was to clarify the clinical importance of endothelin receptor type B (ETBR) in human oesophageal squamous cell carcinoma (OSCC).
Methods:
We evaluated ETBR expression in 107 patients with OSCC by immunohistochemistry. Microvessel density (MVD) and lymphatic vessel density were assessed by CD31 and D2-40 immunostaining, respectively. Furthermore, CD4, CD8, and CD45RO+ tumour-infiltrating lymphocytes (TILs) were immunohistochemically analysed.
Results:
Sixty-one (57%) cases showed high expression of ETBR. Endothelin receptor type B expression was correlated with several clinicopathological factors including tumour differentiation, tumour depth, and lymph node metastasis. The overall and disease-specific survival rates were significantly lower in patients with high ETBR expression than patients with low expression. Furthermore, multivariate analysis revealed that ETBR status was an independent prognostic factor for patient survival. Mechanistic analysis indicated that MVD was significantly higher in tumour tissues with high ETBR expression compared with those with low expression, suggesting that angiogenesis may be a key mechanism in tumour progression and metastasis of OSCC mediated by ETBR expression. By contrast, there were no significant correlations between TILs and ETBR expression.
Conclusion:
Endothelin receptor type B has a pivotal role in oesophageal cancer and may be therapeutic target for this intractable malignancy.
Keywords: endothelin B receptor, oesophageal cancer, angiogenesis
Oesophageal cancer is highly aggressive and is the sixth leading cause of cancer deaths in the world (Parkin et al, 2005). Although several advancements in the treatment including chemotherapy and radiotherapy have been achieved, the prognosis of patients remains poor. Even in curatively resected patients, the 5-year survival rate is below 50% after surgery (Courrech Staal et al, 2009). In addition, most cases are diagnosed at an advanced stage with lymphatic and hematogenous dissemination (Okines et al, 2010). Therefore, to elucidate the underlying mechanisms of tumour progression, and to identify new biomarkers and therapeutic targets for oesophageal cancer are critically important to improve patients' prognosis.
The endothelial cell-derived peptide endothelin (ET) was discovered as a potent vasoconstrictor in 1988 (Yanagisawa et al, 1988). The ET family includes three 21-amino-acid peptides, ET-1, ET-2 and ET-3, which bind to two G-protein-coupled receptors, ET receptor type A (ETAR) and ET receptor type B (ETBR) (Levin, 1995). The system of these three ET peptides and two receptors is referred to as the ET axis. Extensive studies have revealed the various roles of ET system in cardiovascular and renal disorders (Tomobe et al, 1988; Lehrke et al, 2001; Feldstein and Romero, 2007; Iglarz and Clozel, 2007). Furthermore, the ET axis has also been shown to have significant roles in a variety of human malignancies including ovarian, prostate, cervical, breast, colorectal, lung cancers, and melanoma (Nelson et al, 2003). Endothelin axis has been revealed to regulate tumour growth and metastasis via various mechanisms including cell proliferation, angiogenesis, antiapoptotic activity, and immune modulation (Spinella et al, 2002; Nelson et al, 2003; Wulfing et al, 2004; Herrmann et al, 2006; Bagnato et al, 2008; Buckanovich et al, 2008). Whilst upregulation of ET-1 expression has been consistently reported in various malignancies, the different expressions and functions of its receptors ETAR and ETBR have been shown in distinct tumours. Thus, each receptor has unique roles and its function may be dependent on cancer cell type. Furthermore, selective antagonists for each receptor as well as dual ETAR/ETBR antagonist have been widely investigated, and some of them were evaluated in clinical trials (Bagnato et al, 2008).
A few previous studies have addressed the role of ET axis in oesophageal cancer (Ishibashi et al, 2003; Jiao et al, 2007, 2008). These studies have shown that tissue or circulating serum expression of ET has a significant prognostic value in oesophageal squamous cell carcinoma (OSCC). A study has also shown that a dual receptor antagonist inhibited migration of human oesophageal cancer cells in vitro (Jiao et al, 2007). Furthermore, a recent study has identified aberrant promoter methylation of EDNRB gene, which encodes ETBR in humans, in OSCC (Zhao et al, 2009). Although these studies have suggested a potential role of ETBR in OSCC, little is known about its clinical importance. In this study, we evaluated tumour ETBR expression to investigate its clinical significance in OSCC.
Materials and Methods
Patients
We examined 107 patients with OSCC. The patients underwent surgery including subtotal oesophagectomy with dissection of regional lymph nodes at Nara Medical University Hospital between November 1995 and May 2007. None of them have received preoperative treatment, such as radiation or chemotherapy. The patients' median age was 61 years (range, 42–75). Postoperative follow-up data were obtained from all patients. The pathologic features of the specimens were classified based on the 7th edition of the pathological tumour-node-metastasis classification of the International Union against Cancer. Documented informed consent was obtained from individual patients for use of their tissue samples and clinical record.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded tissues were cut into 5-μm sections, deparaffinised and rehydrated in a graded series of ethanol. Immunohistochemical staining for ETBR was performed with a Dako Envision kit (DAKO Cytomation, Tokyo, Japan). Antigen retrieval was performed by heating tissue sections using a target retrieval solution, pH 9.0 (DAKO). Then, the samples were incubated for 5 min in peroxidase blocking solution (DAKO) to inhibit endogenous peroxidase and washed thrice in fresh phosphate-buffered saline (PBS), each of 5 min duration. The anti-human ETBR antibody (LS-A54, MBL, Woburn, MA, USA) diluted 1 : 50 with Antibody Diluent (DAKO) was added and incubated at 37 °C for 30 min. After the sections were washed thrice in PBS, each of 5 min duration, a subsequent reaction was carried out using second antibodies (DAKO) at 37 °C for 30 min. The sections were then washed thrice in PBS and the colour was displayed subsequently with diaminobenzidine (DAKO) for approximately 5 min and rinsed in distilled water. Sections were counterstained with haematoxylin, dehydrated in ethanol, cleared in xylene and coverslipped. Furthermore, for the evaluation of tumour angiogenesis and tumour-infiltrating lymphocytes (TILs), immunohistochemical stainings for CD31, D2-40, CD4, CD8, and CD45RO were carried out by the same method as above. Monoclonal antibodies were purchased from Abcam (Tokyo, Japan); anti-CD31 (JC/70A), anti-D240 (D2-40), anti-CD4 (mAb51312), anti-CD8 (144B), and anti-CD45RO (UCH-L1).
Extraction of total RNAs and real-time reverse transcriptase PCR analysis
Total RNA was isolated using RNAspin Mini (GE Healthcare, Tokyo, Japan) and the first-strand cDNA was synthesised from 1 μg RNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), according to the instructions of the manufacturer. Real-time quantitative PCR analysis was carried out using an ABI Prism 7700 sequence detector system (Applied Biosystems). All primer/probe sets were purchased from Applied Biosystems. PCR was carried out using the TaqMan Universal PCR Master Mix (Applied Biosystems) using 1 μl of cDNA in a 20 μl final reaction volume. The PCR thermal cycle conditions were as follows: initial step at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The expression level of the housekeeping gene β2-microglobulin was measured as an internal reference with a standard curve to determine the integrity of template RNA for all specimens. The ratio of the mRNA level of each gene was calculated as follows: (absolute copy number of each gene)/(absolute copy number of β2-microglobulin).
Evaluation of ETBR expression
All cases were classified into two groups according to the per cent of positively stained cells of ETBR. The evaluation was performed by authorised pathologists who had no knowledge of the patients' clinical status and outcome. Low ETBR immunoreactivity was defined as staining of <50% of tumour cells, whereas high ETBR immunoreactivity was defined as staining of ⩾50% of tumour cells.
Evaluation of microvessel density and lymphatic vessel density
Vessel count was assessed by light microscopy in areas of the tumour containing the highest numbers of capillaries. The highly vascular areas were identified at low power ( × 40 magnification). After six areas of the most abundant positively stained vessels were identified, a vessel count was performed on a × 200 field, and the average count of six fields was determined as the microvessel density or lymphatic vessel density, respectively, as previously described (Weidner et al, 1991; Ikeda et al, 1999; Matsumoto et al, 2007).
Evaluation of TILs
We selected five areas with the most abundant positively stained cells in each tissue under × 40 magnification. We then counted these cells and calculated the mean number of each sample stained by anti-CD4, CD8, and CD45RO antibody, respectively.
Statistical analysis
Comparisons among the clinical and pathological features were evaluated using χ2 and Fisher's exact tests. Statistical significance between two groups of parametric data was evaluated using a unpaired Student's t-test. Survival curves were estimated using the Kaplan–Meier method, and the significances of differences between survival curves were determined using the log-rank test. Multivariate comparisons of survival distributions were made using Cox proportional hazard models. A P-value of<0.05 was considered to indicate statistically significance.
Results
Endothelin receptor type B expression in human OSCC
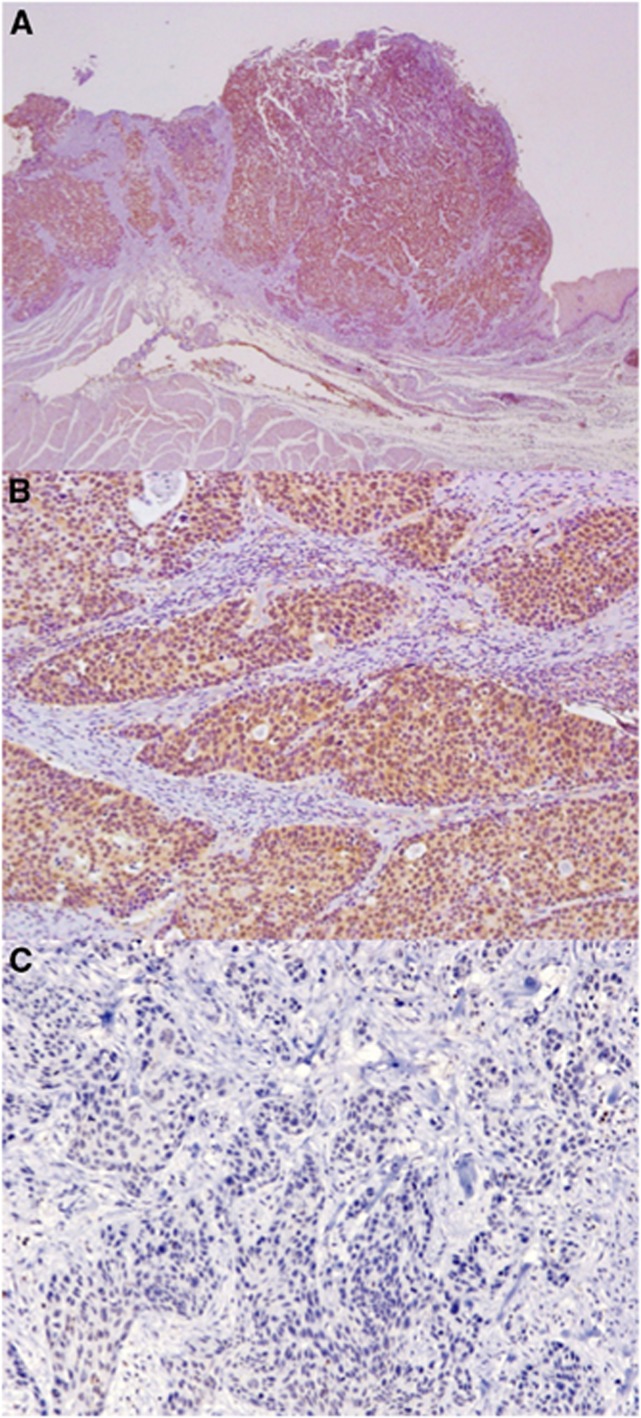
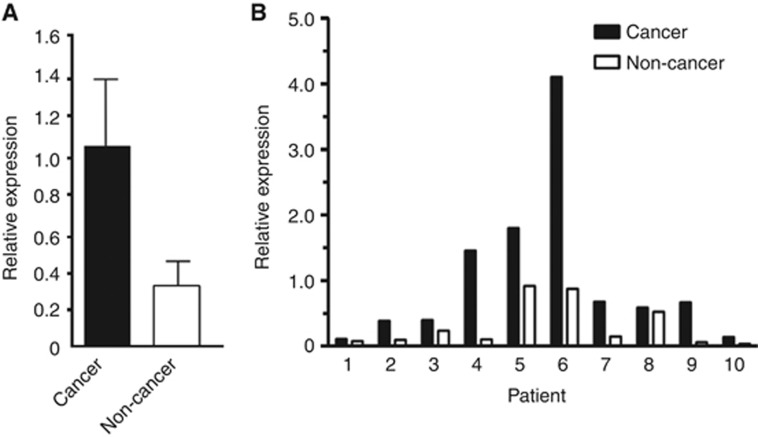
We first examined the expression of ETBR on 107 OSCC tissues by immunohistochemistry. Sixty-one (57.0%) cases showed high expression of ETBR. Endothelin receptor type B expression was identified in the cytoplasm of carcinoma cells (Figure 1). We then compared the relative expression of ETBR between oesophageal cancer and non-cancer tissues using available frozen tissues by quantitative real-time PCR analysis. The ETBR expression in oesophageal carcinoma tissues was significantly higher than in non-carcinoma tissues (P=0.036; Figure 2A). Furthermore, the ETBR expression of cancer tissue was consistently higher than that of non-cancer tissue in each individual patient with OSCC (Figure 2B). These data suggested that ETBR might have some role in OSCC and may be a potential therapeutic target.
Figure 1.
Expression of ETBR in oesophageal squamous cell carcinoma. Representative case of positive expression of ETBR. (A) Original magnification, × 20. (B) Original magnification, × 100. (C) Representative case of negative expression of ETBR. Original magnification, × 100.
Figure 2.
Comparison of ETBR expression between cancer and non-cancer tissue of the oesophagus. (A) The cumulative ETBR expression in cancer tissue was significantly higher compared with that in non-cancer tissue as determined by real-time PCR (n=10 of each, P=0.036). (B) The expression in cancer tissue is consistently higher than that in non-cancer tissue of individual oesophageal cancer patients.
Correlation between ETBR expression and clinicopathological findings
To clarify the clinical significance of ETBR in OSCC, we compared ETBR expression with clinicopathological features. As a result, there was no significant correlation between ETBR expression and several clinicopathological variables including gender, age, and distant metastatic status (Table 1). In contrast, ETBR expression was significantly correlated with tumour differentiation, tumour depth, lymph node metastasis, lymphatic invasion, venous invasion, and tumour stage (P=0.013, 0.041, 0.037, 0.002, 0.019, and 0.036, respectively). Moreover, larger tumour and metastatic lymph nodes were found more often in patients with high ETBR expression (P=0.012 and 0.016, respectively). Thus, ETBR expression in OSCC may have a critical role in tumour growth and metastasis.
Table 1. Relationship between ETBR expression and clinicopathological characteristics in oesophageal sequamous cell carcinoma.
| |
|
ETBR expression |
|
|
|---|---|---|---|---|
| Characteristics | Total (n=107) | High (n=61) | Low (n=46) | P-value |
|
Gender | ||||
| Male | 88 | 48 | 40 | 0.315 |
| Female |
19 |
13 |
6 |
|
| Age (years) (mean±s.d.) |
|
61.1±7.01 |
61.0±7.66 |
0.948 |
|
Diffrentiation | ||||
| Well | 39 | 16 | 23 | 0.013 |
| Moderate | 52 | 37 | 15 | |
| Poor |
16 |
8 |
8 |
|
|
Tumour depth | ||||
| pT1 | 23 | 9 | 14 | 0.041 |
| pT2 | 21 | 9 | 12 | |
| pT3 | 58 | 39 | 19 | |
|
pT4 |
5 |
4 |
1 |
|
|
Lymph node metastasis | ||||
| pN0 | 37 | 16 | 21 | 0.037 |
|
pN1 |
70 |
45 |
25 |
|
|
Distant metastasis | ||||
| M0 | 106 | 61 | 45 | 0.431 |
| M1 |
1 |
0 |
1 |
|
|
Lymphatic invasion | ||||
| Negative | 20 | 5 | 15 | 0.002 |
| Positive |
87 |
56 |
31 |
|
|
Venous invasion | ||||
| Negative | 60 | 28 | 32 | 0.019 |
| Positive |
47 |
33 |
14 |
|
|
Tumour stage | ||||
| I | 16 | 6 | 10 | 0.036 |
| II | 32 | 14 | 18 | |
| III | 42 | 29 | 13 | |
| IV |
17 |
12 |
5 |
|
| Tumour size (mm) (mean±s.d.) |
|
43.7±18.9 |
33.5±17.6 |
0.012 |
| Number of lymph node metastasis (mean±s.d.) | 4.94±7.15 | 1.90±3.40 | 0.016 | |
Prognostic value of ETBR expression in OSCC
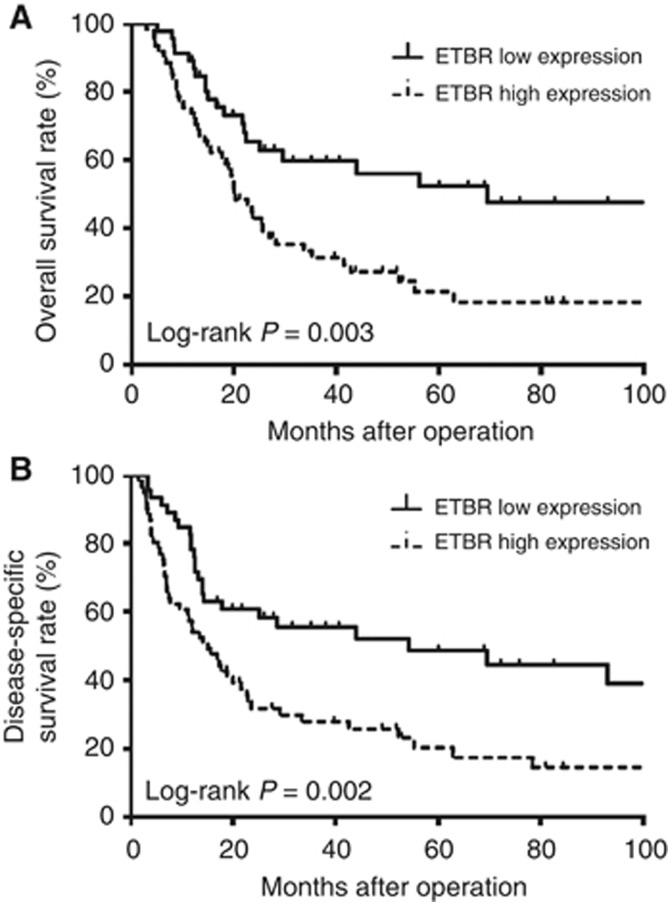
Next, we examined the prognostic importance of ETBR expression in patients with OSCC. The overall and disease-specific survival rates were significantly lower in patients with high ETBR expression than in those with low expression (P=0.003 and 0.002; Figure 3). The 5-year overall survival rate for ETBR high-expression patients was 56.2% and for low-expression patients was 20.1%. In this study, gender, tumour status, lymph node metastasis and venous invasion were also found to have a prognostic value for patient survival of OSCC. To determine independent variables among these prognostic factors, we performed a multivariate analysis using Cox proportional hazard models. The analysis revealed that, in addition to gender and lymph node metastasis, ETBR expression was one of the independent prognostic factors for the patients with OSCC (Table 2). Taken together, ETBR is functional and has a significant role in OSCC.
Figure 3.
Prognosis of oesophageal cancer patients according to tumour ETBR expression status. (A) Overall survival rate (P=0.003). (B) Disease-specific survival rate (P=0.002).
Table 2. Univariate and multivariate analysis for overall survival in 107 patients with oesophageal sequamous cell carcinoma.
| |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Characteristics | P-value | HR | 95% CI | P-value |
| Gender (female vs male) |
0.017 |
2.967 |
1.274–6.944 |
0.012 |
| Tumour depth (T1 vs T2–4) |
0.003 |
1.693 |
1.112–2.577 |
0.014 |
| Lymph node metastasis (N0 vs N1) |
<0.001 |
2.165 |
1.119–4.186 |
0.022 |
| Venous invasion (negative vs positive) |
0.015 |
0.725 |
0.400–1.312 |
0.287 |
| Tumour size |
0.014 |
0.999 |
0.981–1.018 |
0.948 |
| ETBR expression (low vs high) | 0.003 | 2.104 | 1.108–3.996 | 0.023 |
Abbreviations: CI=confidence interval; ETBR=endothelin receptor type B; HR=hazard ratio.
Association of ETBR expression with tumour angiogenesis and lymphangiogenesis
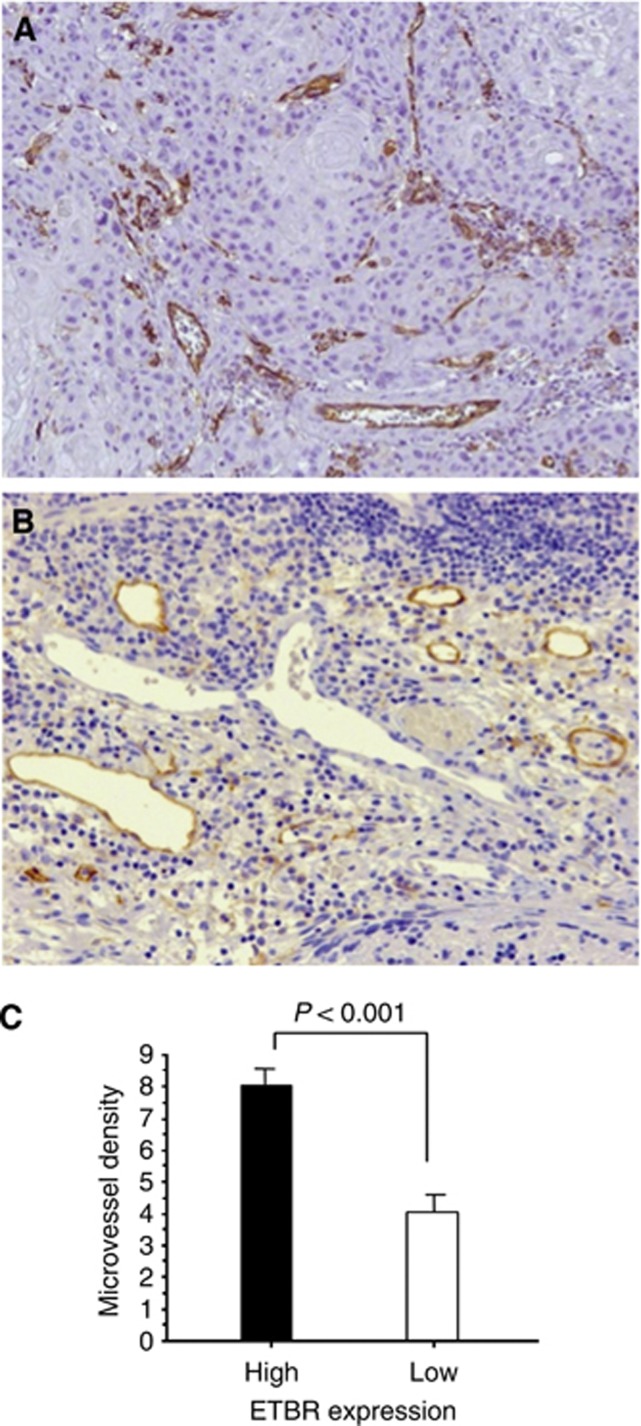
To investigate the underlying mechanisms of ETBR expression in OSCC, we examined tumour angiogenesis and lymphangiogenesis. To this end, we performed immunohistochemical staining of CD31 and D2-40 in the same tissues of OSCC in which ETBR expression was evaluated (Figures 4A and B). We found that microvessel density was significantly higher in tissues with high ETBR expression compared with those with low expression (8.04±4.31 and 4.03±3.99; P<0.001; Figure 4C). On the other hand, there was no significant correlation between tumour ETBR expression and lymphatic vessel density (high ETBR group and low group; 2.96±2.54 and 4.02±2.70, respectively). We further analysed the postoperative recurrence pattern. In this series, a total of 61 patients had recurrence during the follow-up period. Thirty-eight patients had hematogenous metastasis including 12 in the liver, 21 in the lung, and eight in the bone. Furthermore, 40 patients had lymphatic metastasis. There were significant correlations of ETBR expression with hematogenous and lymphatic metastasis (P=0.042 and 0.025, respectively). On the other hand, neither microvessel density nor lymphatic vessel density correlated with each metastatic pattern. Taken together, angiogenesis might have some roles in tumour progression rather than metastasis in relation to ETBR.
Figure 4.
Association of tumour angiogenesis with ETBR expression. (A) CD31 expression was detected in endothelial cells of tumour vasculature (original magnification, × 200). (B) Immunohistochemistry for D2-40 showing lymphatic channels with staining of endothelium (original magnification, × 200). (C) Microvessel density (MVD) was significantly higher in tissues with high ETBR expression compared with those with low expression.
Association between ETBR expression and tumour-infiltrating lymphocytes
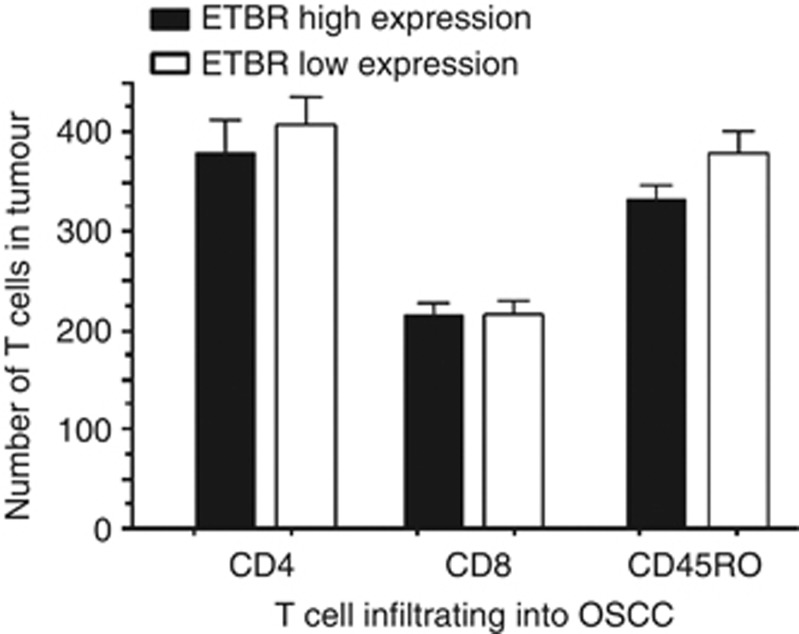
Finally, we investigated the immunomodulatory function of ETBR in OSCC. We performed immunohistochemical analysis of TILs including CD4, CD8, and CD45RO. As a result, there were no significant correlations of tumour ETBR expression with TILs in any T-cell subsets (Figure 5).
Figure 5.
Association of tumour-infiltrating lymphocytes with ETBR expression. There were no significant correlations of tumour ETBR expression with the number of tumour-infiltrating lymphocytes in CD4, CD8, and CD45RO, respectively.
Discussion
Accumulating evidence demonstrates that ET axis has significant roles in tumours by a number of complex mechanisms including cell survival, proliferation, migration, invasion, epithelial–mesenchymal transition, methylation, angiogenesis, and immune modulation (Carducci et al, 2003; Nelson et al, 2003; Nelson, 2003; Eltze et al, 2007; Bagnato et al, 2008). Furthermore, several clinical studies have shown that ET-1 and its two receptors, ETAR and ETBR, are overexpressed in various actual human cancer tissues (Bagnato et al, 2008). Although a few studies have also reported a potential role of ET-1 in human oesophageal cancer, the data are very limited. As a basic research has suggested a potential involvement of ETBR in OSCC, this study focused on the clinical significance of ETBR expression in OSCC tissues (Zhao et al, 2009). As a result, we found several important findings. First, ETBR was highly expressed in cancer tissues compared with non-cancer tissues. Similar results were also reported in several other tumours (Wulfing et al, 2004; Eltze et al, 2007). Second, there were significant associations of ETBR expression with some clinicopathological features including tumour differentiation, tumour size, lymph node metastasis, and venous invasion. Thus, data suggest that ETBR expression in OSCC may have an important role in both tumour progression and metastasis. Third, most importantly, ETBR expression has a significant prognostic value in OSCC. The patients with high ETBR expression had a worse prognosis in overall and disease-specific survival compared with patients with low expression. To our knowledge, this is the first report to demonstrate that ETBR is an independent prognostic marker for human oesophageal cancer. Previous studies have also demonstrated that tumour ETBR expression was a negative prognostic marker in other cancers (Shen et al, 2011). On the other hand, ETBR gene expression was reported as a positive prognostic marker in renal cell carcinoma (Wuttig et al, 2012). Therefore, the function of ETBR may be dependent on tumour type.
Next, we investigated the underlying mechanisms in ETBR expression of OSCC. We first focused on angiogenesis that has a key role in tumour growth and metastasis. ET-1 has been shown to promote angiogenesis both directly and indirectly by inducing endothelial cell survival, proliferation, invasion, and upregulating VEGF production in the vasculature through ETBR (Salani et al, 2000; Kandalaft et al, 2009). In this study, we found a significant positive correlation of ETBR expression with angiogenesis evaluated by counting intratumour microvessel density. This is consistent with previous data in different tumours (Wulfing et al, 2004). Although several studies have demonstrated that angiogenesis has a significant role and prognostic value in OSCC, the mechanisms to control angiogenesis are not fully elucidated (Tanigawa et al, 1997; Igarashi et al, 1998; Kitadai et al, 1998). Furthermore, our data also indicated that there was a significant association between ETBR expression and venous invasion. Taken together, tumour ETBR expression may promote neovascularisation and enhance venous invasion. In addition, ETBR has been shown to have a critical role in tumour lymphangiogenesis (Spinella et al, 2009). However, we had no significant correlation between tumour ETBR expression and lymphangiogenesis determined by D2-40 immunostaining. Therefore, lymphangiogenesis may not be a key mechanism in tumour progression mediated by ETBR in OSCC.
Then, we evaluated the association of ETBR with tumour immunity. Previous studies have shown that ET-1 axis also has a unique role to regulate immune response in tumour environment (Grimshaw et al, 2004; Guruli et al, 2004; Buckanovich et al, 2008). Endothelin-1 axis modulates the activation, differentiation and trafficking of tumour-infiltrating immune cells. Furthermore, ETBR has a crucial role in lymphocyte homing, and overexpression of endothelial ETBR in tumours prevent T-cell homing. We have recently reported that TILs, especially CD45RO-expressing memory T cells, have significant prognostic value in OSCC (Enomoto et al, 2012). Therefore, we hypothesised that ETBR may regulate TILs in OSCC. To clarify this possibility, we evaluated the association of tumour-expressing ETBR with TILs including CD4, CD8, and CD45RO by immunohistochemistry. As a result, we found no significant correlations between them. Thus, our data suggest that ETBR may have little role in modulating immune response in the progression and metastasis in OSCC. However, further studies are required to completely rule out the immunological role of ETBR in OSCC.
On the basis of diverse functions and involvement of ET axis in a variety of tumours, several agents targeting ET axis have been extensively investigated (Bagnato et al, 2008; Kandalaft et al, 2009). Furthermore, some ET antagonists have been clinically evaluated (Bagnato et al, 2011). Although most of them are selective ETAR antagonists, the ETBR antagonist is also available for clinical use and expected to be of clinical benefit. Compared with other cancers, there were only a few therapeutic agents clinically available in OSCC. In addition, OSCC is generally difficult to treat and cure by nonsurgical antitumour treatments. Therefore, combination therapy of ETBR antagonist with other antitumour agents may be desirable for this intractable tumour. Our data indicates a significant relationship between ETBR and angiogenesis. Therefore, combination of ETBR with anti-angiogeneic treatment may exert a synergistic effect. As anti-angiogenic treatment including anti-VEGF monoclonal antibody is widely used in clinical cancer therapy, this therapeutic strategy can be relatively easily tested. However, there are limitations in this study and further studies are essential before clinical application. The number of samples evaluated for this study is relatively small. In fact, we have treated more patients with oesophageal cancer in the study period. Owing to some reasons including sample limitations, we randomly chose samples of 107 patients and investigated. Therefore, a large-scale and careful evaluation for ETBR is critically important to confirm reproducibility of our data.
In conclusion, we have shown that ETBR is expressed in human oesophageal cancer and has a significant prognostic value. This study may provide a rationale for developing a novel cancer therapy targeting ETBR for this fatal malignant disease. However, more experimental and clinical evidence is needed to prove the significance of ETBR as a new therapeutic target.
Acknowledgments
This work was supported by the following grants: grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (M Sho).
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Bagnato A, Loizidou M, Pflug BR, Curwen J, Growcott J. Role of the endothelin axis and its antagonists in the treatment of cancer. Br J Pharmacol. 2011;163 (2:220–233. doi: 10.1111/j.1476-5381.2011.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato A, Spinella F, Rosano L. The endothelin axis in cancer: the promise and the challenges of molecularly targeted therapy. Can J Physiol Pharmacol. 2008;86 (8:473–484. doi: 10.1139/Y08-058. [DOI] [PubMed] [Google Scholar]
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O'Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14 (1:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, Schulman CC, Nabulsi AA, Humerickhouse RA, Weinberg MA, Schmitt JL, Nelson JB. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21 (4:679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- Courrech Staal EF, van Coevorden F, Cats A, Aleman BM, van Velthuysen ML, Boot H, Peeters MJ, van Sandick JW. Outcome of low-volume surgery for esophageal cancer in a high-volume referral center. Ann Surg Oncol. 2009;16 (12:3219–3226. doi: 10.1245/s10434-009-0700-5. [DOI] [PubMed] [Google Scholar]
- Eltze E, Bertolin M, Korsching E, Wulfing P, Maggino T, Lelle R. Expression and prognostic relevance of endothelin-B receptor in vulvar cancer. Oncol Rep. 2007;18 (2:305–311. [PubMed] [Google Scholar]
- Enomoto K, Sho M, Wakatsuki K, Takayama T, Matsumoto S, Nakamura S, Akahori T, Tanaka T, Migita K, Ito M, Nakajima Y. Prognostic importance of tumour-infiltrating memory T cells in oesophageal squamous cell carcinoma. Clin Exp Immunol. 2012;168 (2:186–191. doi: 10.1111/j.1365-2249.2012.04565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein C, Romero C. Role of endothelins in hypertension. Am J Ther. 2007;14 (2:147–153. doi: 10.1097/01.pap.0000249912.02763.65. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ, Hagemann T, Ayhan A, Gillett CE, Binder C, Balkwill FR. A role for endothelin-2 and its receptors in breast tumor cell invasion. Cancer Res. 2004;64 (7:2461–2468. doi: 10.1158/0008-5472.can-03-1069. [DOI] [PubMed] [Google Scholar]
- Guruli G, Pflug BR, Pecher S, Makarenkova V, Shurin MR, Nelson JB. Function and survival of dendritic cells depend on endothelin-1 and endothelin receptor autocrine loops. Blood. 2004;104 (7:2107–2115. doi: 10.1182/blood-2003-10-3559. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Bogemann M, Bierer S, Eltze E, Hertle L, Wulfing C. The endothelin axis in urologic tumors: mechanisms of tumor biology and therapeutic implications. Expert Rev Anticancer Ther. 2006;6 (1:73–81. doi: 10.1586/14737140.6.1.73. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Dhar DK, Kubota H, Yamamoto A, El-Assal O, Nagasue N. The prognostic significance of microvessel density and thymidine phosphorylase expression in squamous cell carcinoma of the esophagus. Cancer. 1998;82 (7:1225–1232. doi: 10.1002/(sici)1097-0142(19980401)82:7<1225::aid-cncr3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Iglarz M, Clozel M. Mechanisms of ET-1-induced endothelial dysfunction. J Cardiovasc Pharmacol. 2007;50 (6:621–628. doi: 10.1097/FJC.0b013e31813c6cc3. [DOI] [PubMed] [Google Scholar]
- Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H, Miyake M. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79 (9–10:1553–1563. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y, Hanyu N, Nakada K, Suzuki Y, Yamamoto T, Takahashi T, Kawasaki N, Kawakami M, Matsushima M, Urashima M. Endothelin protein expression as a significant prognostic factor in oesophageal squamous cell carcinoma. Eur J Cancer. 2003;39 (10:1409–1415. doi: 10.1016/s0959-8049(03)00318-6. [DOI] [PubMed] [Google Scholar]
- Jiao W, Xu J, Zheng J, Shen Y, Lin L, Li J. Elevation of circulating big endothelin-1: an independent prognostic factor for tumor recurrence and survival in patients with esophageal squamous cell carcinoma. BMC Cancer. 2008;8:334. doi: 10.1186/1471-2407-8-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao WJ, Xu J, Pan H, Wang TY, Shen Y. Effect of endothelin-1 in esophageal squamous cell carcinoma invasion and its correlation with cathepsin B. World J Gastroenterol. 2007;13 (29:4002–4005. doi: 10.3748/wjg.v13.i29.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandalaft LE, Facciabene A, Buckanovich RJ, Coukos G. Endothelin B receptor, a new target in cancer immune therapy. Clin Cancer Res. 2009;15 (14:4521–4528. doi: 10.1158/1078-0432.CCR-08-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitadai Y, Haruma K, Tokutomi T, Tanaka S, Sumii K, Carvalho M, Kuwabara M, Yoshida K, Hirai T, Kajiyama G, Tahara E. Significance of vessel count and vascular endothelial growth factor in human esophageal carcinomas. Clin Cancer Res. 1998;4 (9:2195–2200. [PubMed] [Google Scholar]
- Lehrke I, Waldherr R, Ritz E, Wagner J. Renal endothelin-1 and endothelin receptor type B expression in glomerular diseases with proteinuria. J Am Soc Nephrol. 2001;12 (11:2321–2329. doi: 10.1681/ASN.V12112321. [DOI] [PubMed] [Google Scholar]
- Levin ER. Endothelins. N Engl J Med. 1995;333 (6:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Yamada Y, Narikiyo M, Ueno M, Tamaki H, Miki K, Wakatsuki K, Enomoto K, Yokotani T, Nakajima Y. Prognostic significance of platelet-derived growth factor-BB expression in human esophageal squamous cell carcinomas. Anticancer Res. 2007;27 (4B:2409–2414. [PubMed] [Google Scholar]
- Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3 (2:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- Nelson JB.2003Endothelin inhibition: novel therapy for prostate cancer J Urol 170(6 Pt 2S65–S67.discussion S67-68). [DOI] [PubMed] [Google Scholar]
- Okines A, Sharma B, Cunningham D. Perioperative management of esophageal cancer. Nat Rev Clin Oncol. 2010;7 (4:231–238. doi: 10.1038/nrclinonc.2010.20. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55 (2:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Salani D, Taraboletti G, Rosano L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157 (5:1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Yang L, Yuan X. Endothelin B receptor expression in human astrocytoma: association with clinicopathological variables and survival outcomes. Int J Neurosci. 2011;121 (11:626–631. doi: 10.3109/00207454.2011.602808. [DOI] [PubMed] [Google Scholar]
- Spinella F, Garrafa E, Di Castro V, Rosano L, Nicotra MR, Caruso A, Natali PG, Bagnato A. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res. 2009;69 (6:2669–2676. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem. 2002;277 (31:27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- Tanigawa N, Matsumura M, Amaya H, Kitaoka A, Shimomatsuya T, Lu C, Muraoka R, Tanaka T. Tumor vascularity correlates with the prognosis of patients with esophageal squamous cell carcinoma. Cancer. 1997;79 (2:220–225. [PubMed] [Google Scholar]
- Tomobe Y, Miyauchi T, Saito A, Yanagisawa M, Kimura S, Goto K, Masaki T. Effects of endothelin on the renal artery from spontaneously hypertensive and Wistar Kyoto rats. Eur J Pharmacol. 1988;152 (3:373–374. doi: 10.1016/0014-2999(88)90736-4. [DOI] [PubMed] [Google Scholar]
- Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324 (1:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Wulfing P, Kersting C, Tio J, Fischer RJ, Wulfing C, Poremba C, Diallo R, Bocker W, Kiesel L. Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res. 2004;10 (7:2393–2400. doi: 10.1158/1078-0432.ccr-03-0115. [DOI] [PubMed] [Google Scholar]
- Wuttig D, Zastrow S, Fussel S, Toma MI, Meinhardt M, Kalman K, Junker K, Sanjmyatav J, Boll K, Hackermuller J, Rolle A, Grimm MO, Wirth MP. CD31, EDNRB and TSPAN7 are promising prognostic markers in clear-cell renal cell carcinoma revealed by genome-wide expression analyses of primary tumors and metastases. Int J Cancer. 2012;131 (5:E693–E704. doi: 10.1002/ijc.27419. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332 (6163:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zhao BJ, Sun DG, Zhang M, Tan SN, Ma X. Identification of aberrant promoter methylation of EDNRB gene in esophageal squamous cell carcinoma. Dis Esophagus. 2009;22 (1:55–61. doi: 10.1111/j.1442-2050.2008.00848.x. [DOI] [PubMed] [Google Scholar]