Abstract
Background:
Invasive lobular breast cancer (ILC) and lobular carcinoma in situ (LCIS) are characterised by loss of E-cadherin expression. However germline CDH1 mutations are rare in cases of ILC with no family history of hereditary diffuse gastric cancer (HDGC) and have not been described in women with LCIS.
Methods:
We screened the CDH1 gene in 50 cases of bilateral LCIS/ILC using Sanger sequencing and MLPA.
Results:
Sanger sequencing revealed four pathogenic germline mutations, including a novel splicing mutation (c.48+1G>A). The remaining three (c.1465insC, c.1942G>T, c.2398delC) have been previously described. All four cases had bilateral LCIS +/− ILC and no family history of gastric cancer.
Conclusion:
CDH1 germline mutations have not been previously described in women with LCIS. We have shown that germline CDH1 mutations are associated with early onset of bilateral LCIS with or without ILC in women without a family history of gastric cancer. CDH1 mutation screening should be considered in women with early onset of bilateral LCIS/ILC with no family history of HDGC.
Keywords: bilateral, invasive lobular breast cancer, lobular carcinoma in situ, CDH1, E-cadherin, germline mutation
Invasive lobular breast cancer (ILC) accounts for 10–15% of invasive breast cancers and has distinct clinical and molecular characteristics compared with the more common invasive carcinoma of ductal/no special type (IDC). ILC is often associated with lobular carcinoma in situ (LCIS), a clinically undetectable form of non-invasive breast cancer. LCIS is typically found incidentally on biopsy and is being more commonly detected with the advent of screening mammography. ILC and LCIS are characterised by loss of expression of E-cadherin, which is due to a combination of somatic mutations, loss of heterozygosity and hypermethylation (Droufakou et al, 2001). Although LCIS shares many of the same genetic aberrations as ILC, suggesting that it is a precursor lesion, it is also a risk factor for developing invasive cancer in the contralateral breast (Fisher et al, 2004). Women who have had LCIS are 2.4 times more likely to develop invasive breast cancer compared with the general population (Chuba et al, 2005). These invasive cancers are not solely ILC, although there is an excess of ILC (23–88% of cases) (Fisher et al, 2004, Chuba et al, 2005). This together with the increased risk of contralateral breast cancer has led to debate as to whether LCIS should be regarded as a risk factor for breast cancer rather than a true precursor lesion.
Claus et al showed that LCIS was more likely to be bilateral rather than other histologic subtypes: 27% of LCIS cases in their series were bilateral compared with 5% of DCIS, 7% of ILC and 2% of IDC. In addition, cases of LCIS were significantly more likely to have a first-degree relative affected with breast cancer (23% of LCIS, 12% of DCIS, 11% of ILC and 12% of IDC). These findings suggest that LCIS is likely to have a genetic component, however only a minority of the LCIS cases in this series were both bilateral and reported a family history of breast cancer (Claus et al, 1993).
Germline CDH1 mutations were initially reported in patients with hereditary diffuse gastric cancer (HDGC) (Guilford et al, 1998). Approximately 30% of families with HDGC due to CDH1 germline mutations also include individuals with ILC (Pharoah et al, 2001; Brooks–Wilson et al, 2004; Suriano et al, 2005; Kaurah et al, 2007). However, germline CDH1 mutations in women with ILC that are present without a family history of HDGC appear to be rare. Of the 408 cases of LCIS/ILC with no family history of HDGC screened for CDH1 mutations and reported in the literature, only three germline mutations have been described, all in cases of ILC (Rahman et al, 2000; Masciari et al, 2007; Schrader et al, 2011; Xie et al, 2011) (Table 1). The cases in these studies were selected mainly on the basis of early onset disease or family history of ILC.
Table 1. Published breast cancer studies that screened the CDH1 gene for germline mutations in cases with no family history of gastric cancer.
| Studies | Phenotypes | Mutation carriers | Total cases |
|---|---|---|---|
| Current study |
Bilateral LCIS/ILC |
4 |
50 |
| |
FH of breast cancera |
2 |
27 |
| |
Early onset (<45 years) |
2 |
7 |
| |
Bilateral LCIS/ILC |
4 |
50 |
|
Rahman et al, 2000 |
LCIS |
0 |
65 |
| |
FH of breast cancer |
0 |
20 |
| |
Early onset (<45 years) |
0 |
Unknown |
| |
Bilateral LCIS |
0 |
17 |
|
Masciari et al, 2007 |
9 ILC/14 mixed pathology |
1 |
23 |
| |
FH of breast cancer |
1 |
19 |
| |
Early onset (<45 years) |
1 |
4 |
| |
Bilateral ILC |
Unknown |
Unknown |
|
Schrader et al, 2011 |
ILC |
0 |
318 |
| |
FH of breast cancer |
0 |
104 |
| |
Early onset (<45 years) |
0 |
214 |
| |
Bilateral ILC |
0 |
Unknown |
|
Xie et al, 2011 |
Familial ILCb |
2 |
2 |
| |
FH of breast cancer |
2 |
2 |
| |
Early onset (<45 years) |
1 |
1 |
| Bilateral ILC | 2 | 2 |
Abbreviations: ILC=invasive lobular breast cancer; LCIS=lobular carcinoma in situ.
Not confined to first-degree relative.
Index case only described, Family A, 5 ILC cases, 2 bilateral; Family B, 1 ILC.
In the current study, we aimed to assess the frequency of germline mutations in CDH1 in bilateral LCIS or ILC testing the hypothesis that cases of bilateral LCIS/ILC are more likely to have an inherited component to their disease. This hypothesis is supported by a recent retrospective study from France of all 165 index cases who had undergone CDH1 mutation screening in their region from 2006 to 2012. They identified 18 individuals with CDH1 mutations, three of which had bilateral ILC before the age of 50 prior to developing gastric cancer (Benusiglio et al, 2013).
Materials and methods
The GLACIER (Genetics of lobular carcinoma in situ in Europe) study, MREC 06/Q1702/64, has ascertained patients from throughout the UK with the aim of understanding genetic predisposition to LCIS and/or ILC. Any women aged 60 or less at the time of diagnosis, with a current or past history of LCIS (with or without invasive disease of any morphological subtype) or pure ILC were eligible. At the time of this analysis, 2210 cases of LCIS/ILC had been recruited from 97 UK hospitals and diagnosis was confirmed from local pathology reports in 1960 cases. Peripheral blood samples and formalin fixed paraffin embedded (FFPE) tissue blocks were collected from participants along with family history data and other risk factor information.
We identified 50 cases of bilateral LCIS/ILC from the GLACIER study of European ethnicity. Cases were considered bilateral if they had evidence of bilateral LCIS with or without invasive carcinoma, or pure ILC either synchronously or sequentially. All cases with FFPE tissue blocks (n=25) underwent histological review to confirm the diagnosis (SEP, AH). None had the histological diagnosis changed following the review.
Germline DNA was extracted from peripheral blood samples using the Nucleon product chemistry (Tepnel, Manchester, UK) and quantified using PicoGreen. The entire coding sequence and associated splice sites of the CDH1 gene were screened by Sanger sequencing using standard techniques. Exon flanking intronic primers were designed using Primer3 (http://frodo.wi.mit.edu/), DNA fragments amplified by PCR and PCR products were sequenced on the ABI 3730 Genetic Analyser (Applied Biosystems, San Francisco, CA, USA) using BDT V3.1. MLPA (Multiplex Ligation-dependent Probe Amplification) was performed to detect any large-scale deletions using the MRC Holland kit. DNA samples were amplified and PCR products were analysed on the ABI 3730 Genetic Analyser (Applied Biosystems) using TAMRA 500, as per the manufacturers instructions. Coffalyser (MRC Holland, Amsterdam, The Netherland) was used to call potential exonic deletions/rearrangements.
For statistical analysis, Fisher's exact test has been used for binary data and the Mann–Whitney U-test for continuous data (R v2.15.1).
Results
The characteristics of each bilateral case are summarised in Supplementary Table 1. The majority of patients had bilateral LCIS with or without invasive disease. One case had bilateral ILC with no LCIS and four had bilateral ILC with unilateral LCIS. The median age for pure bilateral LCIS was 49 years (range 44–56, IQR=5.5) and for LCIS with ILC was 51 years (range 36–60, IQR=6), Supplementary Table 2. The median age of diagnosis for all the 50 bilateral cases was 51 years (range 36–60, IQR=6), which was similar to the median age for the 1791 unilateral cases (51 years) of LCIS/ILC (all non-European cases excluded) collected through GLACIER (P=0.89, Mann–Whitney U-test). Family history of breast cancer (not confined to first-degree relative) was more frequent in bilateral cases (56%) than unilateral (41%) (P=0.042, Fisher's exact test). There was no excess of a family history of gastric cancer in patients with bilateral disease (10%) compared with unilateral (8%) (P=0.61 Fisher's exact test, Supplementary Table 3).
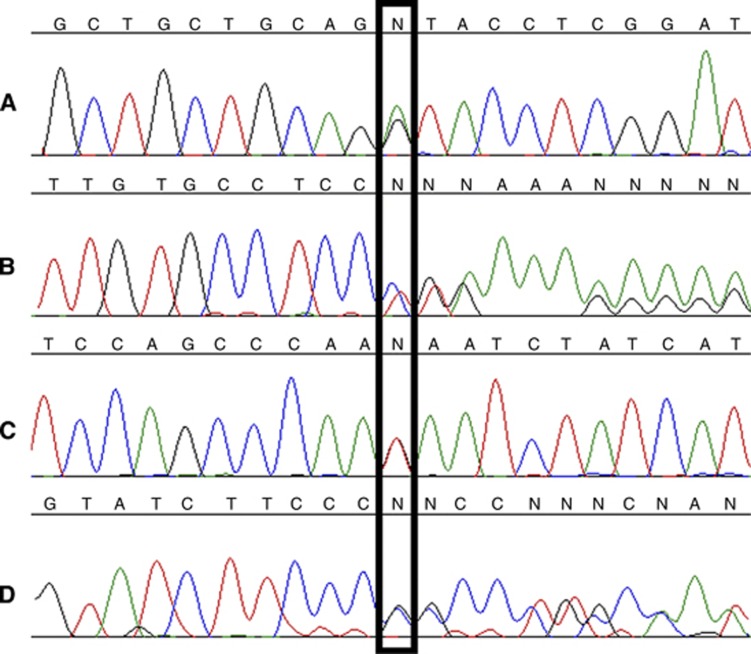
Four germline mutations were identified in four individuals: one donor splice-site mutation (c.48+1G>A), two frame-shift mutations (c.1465insC, c.2398delC) and a nonsense substitution (c.1942G>T), (Table 2, Figure 1). The c.48+1G>A mutation is novel, affecting the donor splice site of intron 1 and occurred in an individual with bilateral LCIS and ILC at the age of 51 with a family history of breast cancer. The other three mutations introduce a premature stop codon in exon 10, 13 and 15 respectively, leading to a protein lacking all or a part of the intracellular domain. These three mutations have been previously described as follows: c.1942G>T, a somatic mutation in colon cancer (Cancer Genome Atlas Network, 2012); c.1465insC, a germline mutation in diffuse gastric cancer (Barber et al, 2008); and c.2398delC, a founder mutation in four Newfoundland families with diffuse gastric and ILC (Kaurah et al, 2007). The c.1465insC mutation occurred in an individual with bilateral pure LCIS at the age of 46 whose mother had breast cancer at the age of 43. The remaining two carriers had bilateral LCIS and unilateral concurrent ILC with no family history of breast cancer. None of the four cases had a family history of gastric cancer. There was no evidence of exonic deletions in any of the remaining 46 cases without a germline mutation using MPLA.
Table 2. Pathogenic mutations found in four cases.
| Sample ID | Exon | Nucleotide substitution | Amino-acid substitution | Age of diagnosis | Pathology |
|---|---|---|---|---|---|
| BILAT-044 |
1 |
c.48+1G>A |
Donor splice site |
51 |
Bilateral LCIS, Bilateral ILC |
| BILAT-005 |
10 |
c.1465insC |
p.P489fs |
46 |
Bilateral LCIS, No ILC |
| BILAT-039 |
13 |
c.1942G>T |
p.E648X |
40 |
Bilateral LCIS, Unilateral ILC |
| BILAT-021 | 15 | c.2398delC | p.P799fs | 37 | Bilateral LCIS, Unilateral ILC |
Abbreviations: ILC=invasive lobular breast cancer; LCIS=lobular carcinoma in situ.
Figure 1.
Chromatograms of four germline mutations (A) c.48+1G>A, (B) c.1465insC, (C) c.1942G>T and (D) c.2398delC.
Using a control dataset (no personal history of breast cancer) from the King's College London exome sequencing database, we have found no truncating or splice-site mutations in CDH1 of 190 ethnicity matched individuals with no personal history of breast cancer (P=0.002, Fisher's exact test).
Discussion
Four (8%) of the bilateral cases in our cohort of LCIS/ILC were found to have a germline mutation in CDH1. All are predicted to be loss of function, with one being a splicing mutation and the remaining three being truncating mutations. Two have previously been shown to be pathogenic. The frequency of CDH1 mutations is much higher than previous studies of LCIS/ILC without a personal or family history of gastric cancer where only 0.7% of the sporadic or familial cases of LCIS/ILC without HDGC carry CDH1 mutations (P=0.003, Fisher's exact test), (Table 1). The median age of the mutation carriers at presentation was eight years lower than that for the other 46 bilateral cases (43 years vs 51 years). Interestingly only two cases had a family history of breast cancer with one having a first-degree relative with the disease (subtype unknown) and none had any family history of gastric cancer.
One of the limitations of our study is that family history is self-reported by the index case and we are therefore unable to ascertain what subtype of breast or gastric cancer the family members suffered from. It does not appear that there is an excess of personal or family history of gastric cancer in the GLACIER cohort when comparing it with the UK statistics produced by the Cancer Research UK, but we cannot be certain that diffuse gastric cancer is not overrepresented in our bilateral cases.
We have shown for the first time that CDH1 mutations predispose to LCIS, with 12.5% (1 out of 8) of bilateral pure LCIS and 11.5% (3 out of 26) of bilateral LCIS with ILC having CDH1 mutations in this study. Interestingly, none of the cases with bilateral LCIS and non-lobular invasive disease (11 cases) had CDH1 mutations, suggesting that the presence of a germline CDH1 mutation in bilateral LCIS predisposes to the development of ILC rather than IDC. In the study by Rahman et al, only 17 cases (26%) of the 65 cases of LCIS screened had bilateral disease, (Table 1), which may explain why no CDH1 mutations were detected.
None of our four mutation carriers satisfy the current criteria for CDH1 testing given the necessity to have a family HDGC in order to be eligible for screening (Table 3). Although they gave no family history of gastric cancer it is possible that they may develop diffuse gastric cancer (DGC) in the future, as Pharoah et al, 2001 showed that the estimated cumulative risk of gastric cancer is higher (83%) than that for breast cancer (39%) in women with CDH1 mutations. However, this was calculated using families with at least three cases of DGC and, as discussed by Pharoah et al, may not apply to individuals with a minimal family history, in whom the risks are likely to be lower (Pharoah et al, 2001).
Table 3. Current CDH1 testing criteria and potential new criteria.
| CDH1 testing criteria | Definition | Cases that meet criteria in this study | Number of cases with CDH1 mutation in this study |
|---|---|---|---|
| Current (Fitzgerald et al) |
2 GC cases in family, one confirmed DGC age <50 |
0 |
0 |
| |
3 confirmed DGC cases in 1st or 2nd degree relatives any age |
0 |
0 |
| |
DGC age <40 |
0 |
0 |
| |
Personal or family history of DGC and ILC, 1 dx <50 |
1 |
0 |
| New criterion suggested by Benusiglio et al |
Personal or family history of 2 ILC age <50a |
3 |
0 |
| New criterion suggested current study with different age thresholds |
Bilateral LCIS/ILC age <55 |
41 |
4 (10%) |
| |
Bilateral LCIS/ILC age <50 |
21 |
3 (14%) |
| Bilateral LCIS/ILC age <45 | 7 | 2 (29%) |
Abbreviations: DGC=diffuse gastric cancer; ILC=invasive lobular breast cancer; LCIS=lobular carcinoma in situ.
After exclusion of BRCA1/2 mutation.
Benusiglio et al suggested that women with a personal or family history of at least two ILC before the age of 50 should be offered CDH1 screening. However, none of the four carrier cases identified in our study would fulfil these criteria as they do not take into account bilateral LCIS. On the basis of our study, we recommend that this should be extended to include women with bilateral LCIS. Using an age threshold of less than 50 years would miss some CDH1 mutation carriers. Lowering the age threshold further to less than 45 years for mutation screening would result in 28% of eligible patients having a CDH1 mutation in this study, Table 3.
The current consensus guidelines recommend annual mammography and breast MRI starting at the age of 35 for CDH1 mutation carriers (Fitzgerald et al, 2010). This differs from the current UK screening recommendations for the BRCA1/2 carriers who are offered annual MRI surveillance, aged 30–49 years, and then annual mammography from the age of 50 and only continue MRI surveillance if there is evidence of a dense breast pattern (guidance.nice.org.uk/cg164). As MRI has been shown to have the lowest false negative rate in detecting ILC compared with mammography and ultrasound, and is routinely used for pre-operative work-up of ILC, it would seem prudent to continue MRI surveillance in CDH1 carriers rather than switch to mammography alone at the age of 50 (Boetes et al, 2004). In addition, two studies have shown a small benefit for MRI screening in LCIS over mammography (Port et al, 2007; Sung et al, 2011). CDH1 carriers may also benefit from chemoprevention as the NSABP Breast Cancer Prevention Trial (P-1) study showed that women with a history of LCIS who took tamoxifen had almost half the risk (relative risk=0.54) of developing an invasive cancer rather than women with a history of LCIS randomised to placebo (Fisher et al, 2005). As the penetrance of gastric cancer in CDH1 carriers with bilateral LCIS/ILC and no family history of DGC is not known, it is difficult to recommend prophylactic gastrectomy in these cases. Annual endoscopic surveillance would be a reasonable alternative until further data is available.
In conclusion, two studies including our own, have shown that CDH1 mutations in bilateral LCIS or ILC are more common than previously thought (Benusiglio et al, 2013). If further studies confirm these findings then, CDH1 testing could be offered to individuals with bilateral LCIS/ILC under the age of 50, enabling us to identify patients with CDH1 mutations who may benefit from regular breast MRI screening and endoscopic surveillance for diffuse gastric cancer.
Acknowledgments
This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London, the Breast Cancer Campaign, Cancer Research UK and the Rosetrees Trust.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Barber M, Murrell A, Ito Y, Maia AT, Hyland S, Oliveira C, Save V, Carneiro F, Paterson AL, Grehan N, Dwerryhouse S, Lao-Sirieix P, Caldas C, Fitzgerald RC. Mechanisms and sequelae of E-cadherin silencing in hereditary diffuse gastric cancer. J Pathol. 2008;216 (3:295–306. doi: 10.1002/path.2426. [DOI] [PubMed] [Google Scholar]
- Benusiglio PR, Malka D, Rouleau E, De Pauw A, Buecher B, Nogues C, Fourme E, Colas C, Coulet F, Warcoin M, Grandjouan S, Sezeur A, Laurent-Puig P, Moliere D, Tlemsani C, Di Maria M, Byrde V, Delaloge S, Blayau M, Caron O. CDH1 germline mutations and the hereditary diffuse gastric and lobular breast cancer syndrome: a multicentre study. J Med Genet. 2013;50 (7:486–489. doi: 10.1136/jmedgenet-2012-101472. [DOI] [PubMed] [Google Scholar]
- Boetes C, Veltman J, van Die L, Bult P, Wobbes T, Barentsz JO. The role of MRI in invasive lobular carcinoma. Breast Cancer Res Treat. 2004;86 (1:31–37. doi: 10.1023/B:BREA.0000032921.10481.dc. [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, Grehan N, Butterfield YS, Jeyes J, Schinas J, Bacani J, Kelsey M, Ferreira P, MacGillivray B, MacLeod P, Micek M, Ford J, Foulkes W, Australie K, Greenberg C, LaPointe M, Gilpin C, Nikkel S, Gilchrist D, Hughes R, Jackson CE, Monaghan KG, Oliveira MJ, Seruca R, Gallinger S, Caldas C, Huntsman D. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41 (7:508–517. doi: 10.1136/jmg.2004.018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487 (7407:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuba PJ, Hamre MR, Yap J, Severson RK, Lucas D, Shamsa F, Aref A. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol. 2005;23 (24:5534–5541. doi: 10.1200/JCO.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Claus EB, Risch N, Thompson WD, Carter D. Relationship between breast histopathology and family history of breast cancer. Cancer. 1993;71 (1:147–153. doi: 10.1002/1097-0142(19930101)71:1<147::aid-cncr2820710124>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Droufakou S, Deshmane V, Roylance R, Hanby A, Tomlinson I, Hart IR. Multiple ways of silencing E-cadherin gene expression in lobular carcinoma of the breast. Int J Cancer. 2001;92 (3:404–408. doi: 10.1002/ijc.1208. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97 (22:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- Fisher ER, Land SR, Fisher B, Mamounas E, Gilarski L, Wolmark N. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: twelve-year observations concerning lobular carcinoma in situ. Cancer. 2004;100 (2:238–244. doi: 10.1002/cncr.11883. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, Chung DC, Norton J, Ragunath K, Van Krieken JH, Dwerryhouse S, Caldas C, International Gastric Cancer Linkage, C. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47 (7:436–444. doi: 10.1136/jmg.2009.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392 (6674:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- Kaurah P, MacMillan A, Boyd N, Senz J, De Luca A, Chun N, Suriano G, Zaor S, Van Manen L, Gilpin C, Nikkel S, Connolly-Wilson M, Weissman S, Rubinstein WS, Sebold C, Greenstein R, Stroop J, Yim D, Panzini B, McKinnon W, Greenblatt M, Wirtzfeld D, Fontaine D, Coit D, Yoon S, Chung D, Lauwers G, Pizzuti A, Vaccaro C, Redal MA, Oliveira C, Tischkowitz M, Olschwang S, Gallinger S, Lynch H, Green J, Ford J, Pharoah P, Fernandez B, Huntsman D. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297 (21:2360–2372. doi: 10.1001/jama.297.21.2360. [DOI] [PubMed] [Google Scholar]
- Masciari S, Larsson N, Senz J, Boyd N, Kaurah P, Kandel MJ, Harris LN, Pinheiro HC, Troussard A, Miron P, Tung N, Oliveira C, Collins L, Schnitt S, Garber JE, Huntsman D. Germline E-cadherin mutations in familial lobular breast cancer. J Med Genet. 2007;44 (11:726–731. doi: 10.1136/jmg.2007.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharoah PD, Guilford P, Caldas C, International Gastric Cancer Linkage, C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121 (6:1348–1353. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14 (3:1051–1057. doi: 10.1245/s10434-006-9195-5. [DOI] [PubMed] [Google Scholar]
- Rahman N, Stone JG, Coleman G, Gusterson B, Seal S, Marossy A, Lakhani SR, Ward A, Nash A, McKinna A, A'Hern R, Stratton MR, Houlston RS. Lobular carcinoma in situ of the breast is not caused by constitutional mutations in the E-cadherin gene. Br J Cancer. 2000;82 (3:568–570. doi: 10.1054/bjoc.1999.0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader KA, Masciari S, Boyd N, Salamanca C, Senz J, Saunders DN, Yorida E, Maines-Bandiera S, Kaurah P, Tung N, Robson ME, Ryan PD, Olopade OI, Domchek SM, Ford J, Isaacs C, Brown P, Balmana J, Razzak AR, Miron P, Coffey K, Terry MB, John EM, Andrulis IL, Knight JA, O'Malley FP, Daly M, Bender P, kConFab. Moore R, Southey MC, Hopper JL, Garber JE, Huntsman DG. Germline mutations in CDH1 are infrequent in women with early-onset or familial lobular breast cancers. J Med Genet. 2011;48 (1:64–68. doi: 10.1136/jmg.2010.079814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JS, Malak SF, Bajaj P, Alis R, Dershaw DD, Morris EA. Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology. 2011;261 (2:414–420. doi: 10.1148/radiol.11110091. [DOI] [PubMed] [Google Scholar]
- Suriano G, Yew S, Ferreira P, Senz J, Kaurah P, Ford JM, Longacre TA, Norton JA, Chun N, Young S, Oliveira MJ, Macgillivray B, Rao A, Sears D, Jackson CE, Boyd J, Yee C, Deters C, Pai GS, Hammond LS, McGivern BJ, Medgyesy D, Sartz D, Arun B, Oelschlager BK, Upton MP, Neufeld-Kaiser W, Silva OE, Donenberg TR, Kooby DA, Sharma S, Jonsson BA, Gronberg H, Gallinger S, Seruca R, Lynch H, Huntsman DG. Characterization of a recurrent germ line mutation of the E-cadherin gene: implications for genetic testing and clinical management. Clin Cancer Res. 2005;11 (15:5401–5409. doi: 10.1158/1078-0432.CCR-05-0247. [DOI] [PubMed] [Google Scholar]
- Xie ZM, Li LS, Laquet C, Penault-Llorca F, Uhrhammer N, Xie XM, Bignon YJ. Germline mutations of the E-cadherin gene in families with inherited invasive lobular breast carcinoma but no diffuse gastric cancer. Cancer. 2011;117 (14:3112–3117. doi: 10.1002/cncr.25876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.