Abstract
Background:
Female breast cancer patients with a BRCA1/2 mutation have an increased risk of contralateral breast cancer. We investigated the effect of rapid genetic counselling and testing (RGCT) on choice of surgery.
Methods:
Newly diagnosed breast cancer patients with at least a 10% risk of a BRCA1/2 mutation were randomised to an intervention group (offer of RGCT) or a control group (usual care; ratio 2 : 1). Primary study outcomes were uptake of direct bilateral mastectomy (BLM) and delayed contralateral prophylactic mastectomy (CPM).
Results:
Between 2008 and 2010, we recruited 265 women. On the basis of intention-to-treat analyses, no significant group differences were observed in percentage of patients opting for a direct BLM (14.6% for the RGCT group vs 9.2% for the control group; odds ratio (OR) 2.31; confidence interval (CI) 0.92–5.81; P=0.08) or for a delayed CPM (4.5% for the RGCT group vs 5.7% for the control group; OR 0.89; CI 0.27–2.90; P=0.84). Per-protocol analysis indicated that patients who received DNA test results before surgery (59 out of 178 women in the RGCT group) opted for direct BLM significantly more often than patients who received usual care (22% vs 9.2% OR 3.09, CI 1.15–8.31, P=0.03).
Interpretation:
Although the large majority of patients in the intervention group underwent rapid genetic counselling, only a minority received DNA test results before surgery. This may explain why offering RGCT yielded only marginally significant differences in uptake of BLM. As patients who received DNA test results before surgery were more likely to undergo BLM, we hypothesise that when DNA test results are made routinely available pre-surgery, they will have a more significant role in surgical treatment decisions.
Keywords: breast cancer, BRCA1, BRCA2, rapid genetic counselling and testing, contralateral prophylactic mastectomy
A substantial minority of breast cancer patients are at risk of having a hereditary form of the disease because of their age at diagnosis and/or family history, and therefore have an indication for genetic counselling and testing (GCT). In a study by Schlich-Bakker et al (2008), ∼15% of newly diagnosed breast cancer patients was eligible for GCT. Of all breast cancer patients, 2–5% carry a BRCA1 or BRCA2 gene mutation (Turnbull and Hodgson, 2005; Fackenthal and Olopade, 2007; Schlich-Bakker et al, 2008; Koumpis et al, 2011). Breast cancer patients carrying a BRCA1/2 mutation have an increased risk of up to 60% of developing a second primary breast cancer and up to a 60% increased risk of developing ovarian cancer (Graeser et al, 2009; Malone et al, 2010; van der Kolk et al, 2010). Because of these increased risks, some women opt for preventive surgery in addition to their breast cancer treatment (Schwartz et al, 2004; Metcalfe et al, 2008). Bilateral mastectomy (BLM) and delayed contralateral prophylactic mastectomy (CPM) reduce significantly the risk of developing contralateral breast cancer, although there is inconsistent evidence of the effect on breast cancer-specific and overall mortality (van Sprundel et al, 2005; Herrinton et al, 2005; Lostumbo et al, 2010).
The timing of preventive surgery may depend, in part, on the timing of genetic testing and patients' age (Evans et al, 2009). Women who receive information on their carrier status before or around the time of their breast cancer diagnosis can incorporate this knowledge into decisions on primary surgical treatment (Schwartz et al, 2004; Trainer et al, 2010). If proven to be a BRCA1/2 mutation carrier, these women may opt for an immediate BLM. This would spare them an additional surgery and radiotherapy (Reavey and McCarthy, 2008).
In current practice, breast cancer patients with a high risk of having hereditary breast cancer usually receive GCT after completing their breast cancer treatment (Ardern-Jones et al, 2005; Schwartz et al, 2005; Meiser et al, 2008; Van Riel et al, 2010). However, it is now possible to generate DNA test results quite rapidly. In the United States, where commercial DNA testing is available, test results are available within 2 weeks. In countries of Western Europe, where DNA testing is typically carried out on a non-commercial basis, test results are available within 4 weeks, if necessary. This provides a window of opportunity for DNA test results to guide the choice of surgery.
We performed a randomized controlled trial to determine the uptake of rapid GCT (RGCT) among newly diagnosed, high-risk breast cancer patients, the impact of the offer of RGCT on the choice of (primary and delayed) surgical treatment (specifically on the uptake of direct BLM), and the impact of the offer of RGCT on psychological distress. This latter outcome will be addressed in a subsequent report. We hypothesised that women who undergo RGCT will choose significantly more often for a direct BLM or delayed CPM than women who receive usual care.
Materials and methods
Trial design and participants
Participants in this multicentre, randomised controlled trial were recruited from one university medical centre, one specialized oncology hospital, and ten community hospitals in the Netherlands between November 2008 and December 2010. Eligible women were newly diagnosed with primary breast cancer (invasive or DCIS) and had not yet undergone surgical treatment. They had to have at least ∼10% risk of carrying a BRCA1 or BRCA2 gene mutation according to Dutch guidelines (The Netherlands Foundation for the Detection of Hereditary Tumours (STOET), the Dutch Society of Clinical Genetics (VKGN), 2005; Comprehensive Cancer Center, The Netherlands (IKNL), 2008). The Supplementary Appendix provides the full set of inclusion criteria.
Women were excluded if they were younger than 18 years, had evidence of distant metastatic disease, lacked basic fluency in Dutch, or had previously undergone genetic testing for the BRCA1/2 genes. The study was approved by the institutional review board of all participating hospitals and all participants gave written informed consent before participating.
Randomisation and masking
Two-third of patients were allocated to the intervention group and one-third to the usual care control group. Randomisation was computerised and stratified by hospital, blocked per nine cases (Piantadosi, 1997; Wevers et al, 2011). Given the nature of the intervention, masking of participants (surgeons and patients) to treatment allocation was not possible.
Procedures
Detailed procedures of the trial have been reported previously (Wevers et al, 2011). Briefly, all patients were recruited as soon as possible after their breast cancer was diagnosed. Patients in the intervention group (offer of RGCT) received an appointment with a clinical geneticist within 5 working days after recruitment. DNA testing was offered to all of these women, but the rapidity with which the test results were made available varied. Rapid DNA testing (results available within 4 weeks) was offered to women whose surgery was scheduled to take place soon and whose genetic status could influence the choice of primary therapy (breast-conserving therapy or (bilateral) mastectomy). Semi-rapid DNA testing (results available between 4 weeks and 4 months) was offered to women who had more time until surgery (e.g., because of neo-adjuvant chemotherapeutic treatment) or who did not want to delay their scheduled, primary surgery. Finally, routine DNA testing (results available after 4 or more months) was offered to women whose treatment decisions would not be influenced by carrier status. Hence, all patients received rapid genetic counselling, with the option of rapid DNA testing. As the Dutch quality of care guidelines prescribe surgical treatment within 3 weeks of diagnosis, in the most favourable situation, RGCT would require a delay of 1–2 weeks if the patient would want to wait for test results (The National Breast cancer Organisation The Netherlands (NABON) and Comprehensive Cancer Center The Netherlands (VIKC), 2008). All participating hospitals agreed to provide such a delay, if requested.
Patients in the control group received usual care. They could be referred to a clinical geneticist by their treating physician at any time, including pre-surgically. However, as in current practice, if GCT was offered, this was typically after the primary treatment had been completed.
All participants were asked to complete questionnaires at three points in time: before randomisation by telephone interview, and at 6 and 12 months follow-up by mail. Details of the study procedures and questionnaire content have been published elsewhere (Wevers et al, 2011). As the questionnaires assessed primarily psychosocial issues, they will not be discussed further here.
Sociodemographic and clinical data
Patients' sociodemographic characteristics were obtained via the baseline questionnaire. Clinical information, including tumour and treatment characteristics, were abstracted from the medical records.
Primary outcomes were the uptake of RGCT, the percentage of women choosing for a direct BLM, and the percentage of women choosing for a delayed CPM. Delayed CPM was defined as removal of the contralateral breast if the first surgery was a mastectomy, or removal of both breasts if the first surgery was a lumpectomy, within 1 year after diagnosis.
Statistical analyses
Comparisons of background characteristics of the intervention and control groups were performed using a Student's t-test or a χ2-test. The primary statistical analysis was conducted on an intention-to-treat basis. Multiple logistic regression analysis was used to test group differences in the uptake of BLM and delayed CPM. As a significant difference between groups was observed in the percentage of women with a current diagnosis of bilateral breast cancer, this variable was used as a covariate throughout the analysis. A detailed power analysis is presented elsewhere (Wevers et al, 2011).
Additional subgroup analyses included only women with unilateral breast cancer. This was done because a BLM in women with bilateral breast cancer may have a bilaterally therapeutic intent, in contrast to the preventive intention in women with unilateral breast cancer. As no statistically significant differences in background characteristics were observed between the intervention and the control group when restricted to this subset of women, group differences in the uptake of BLM and delayed CPM were analysed with the χ2-statistic.
Finally, although the trial protocol did not specify that the results had to be made available before surgery, we performed a per-protocol analysis to compare the uptake of BLM among women in the RGCT group who actually received their DNA results before surgery with the control group. We used multiple logistic regression analysis with education as a covariate to adjust for group differences on this latter variable, as in this subset of women there was a significant difference between groups on education.
Outcomes are expressed as group differences in the percentage of patients undergoing a BLM or a delayed CPM, as well as odds ratios (ORs) and confidence intervals (CIs). A two-tailed P-value of <0.05 was considered statistically significant.
For all analyses, SPSS Statistics version 20 (IBM SPSS Statistics Corp., Armonk, NY, USA) was used.
Role of the funding source
The financial sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Participants
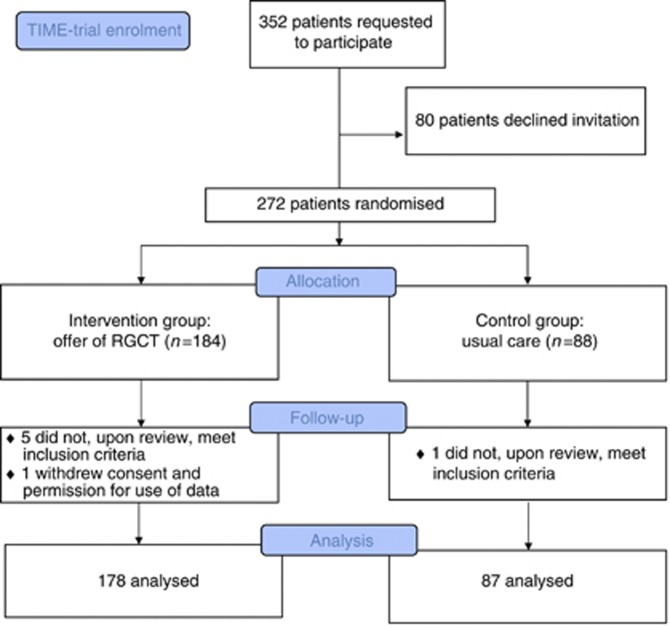
Between November 2008 and December 2010, we invited 352 women to participate in the study, of whom 80 declined and one withdrew consent at follow-up. In addition, six patients, who were randomly assigned, were subsequently determined not to have met inclusion criteria. In total, 265 women were included in the current analysis, of whom 178 were allocated to the intervention group and 87 to the control group (Figure 1). Of all participants, 129 (48.7%) were recruited in the university medical centre or specialised oncology hospital and 136 (51.3%) in community hospitals. The median time between breast cancer diagnosis and completing the baseline questionnaire, followed by randomisation, was 8 days (range=0–71 days, including women undergoing neo-adjuvant chemotherapy or requesting a second opinion).
Figure 1.
Study profile/CONSORT diagram.
The only baseline characteristic for which, unexpectedly, a significant between group difference was observed (Table 1) was percentage of patients diagnosed with bilateral breast cancer (2.8% and 9.2% in the intervention and control group, respectively; P=0.02).
Table 1. Characteristics of study participants.
| Intervention group N=178 | Control group N=87 | P-value | |
|---|---|---|---|
| Age at diagnosis (years) | Mean 44.9 (11.4 s.d.) | Mean 44.8 (11.2 s.d.) | 0.93 |
| |
Range 19–79 |
Range 25–72 |
|
|
Marital status |
|
|
0.30 |
| Single/widow/divorced | 36 (20.2%) | 13 (14.9%) | |
| Partner/married |
142 (79.8%) |
74 (85.1%) |
|
|
Patient's country of birth |
|
|
0.52 |
| The Netherlands | 162 (91%) | 77 (88.5%) | |
| Other |
16 (9%) |
10 (11.5%) |
|
|
Occupational status |
|
|
0.05 |
| Employed | 128 (71.9%) | 72 (82.8%) | |
| Not employed (including retired, student, volunteer and homemaker) |
50 (28·1%) |
15 (17.2%) |
|
| Children (yes) |
130 (73%) |
64 (74%) |
0.93 |
|
Education (n) |
|
|
0.31 |
| Primary/middle school | 41 (23.0%) | 22 (25.3%) | |
| High school | 42 (23.6%) | 27 (31.0%) | |
| College/university | 92 (51.7%) | 38 (43.7%) | |
| Other |
3 (1.7%) |
0 (0%) |
|
|
Laterality of current breast cancer |
|
|
0.02 |
| Unilateral | 173 (97.2%) | 79 (90.8%) | |
| Bilateral |
5 (2.8%) |
8 (9.2%) |
|
|
Prior history of breast cancer |
|
|
0.50 |
| Ipsilateral | 2 (1.1%) | 0 (0%) | |
| Contralateral | 12 (6.7%) | 9 (10.3%) | |
| None |
164 (92.1%) |
78 (89.7%) |
|
|
Tumour stage |
|
|
0.79 |
| Stage 0 (DCIS) | 10 (5.6%) | 7 (8.0%) | |
| Stage 1 | 65 (36.5%) | 35 (40.2%) | |
| Stage 2 | 78 (43.8%) | 36 (41.4%) | |
| Stage 3 | 20 (11.2%) | 8 (9.2%) | |
| Missing | 5 (2.8%) | 1 (1.1%) |
Abbreviation: DCIS=ductal carcinoma in situ.
Genetic counselling and testing
All but one woman in the intervention group underwent genetic counselling within 1 year after diagnosis. The median time between randomisation and the first genetic counselling consultation was 4 days (range=0–374 days). Six women (3.4%) did not have a DNA test. One hundred seventy-one women in the intervention group underwent genetic testing, of whom 71 (39.9% of 178) had a rapid DNA test (median time from the first counselling consultation to the test result disclosure=36 days, range=15–82 days), 38 (21.3%) had a semi-rapid DNA test (median time=61 days, range=26–113 days), and 50 (28.1%) had a routine DNA test (median time=137.5 days, range=36–302 days). Fifty-nine women (33%) actually received their results before the first surgery. Twelve women (3.7%) underwent DNA testing at a subsequent consultation. Seventeen (9.9%) pathogenic mutations were found (eight BRCA1 mutations and nine BRCA2 mutations).
In the control group, 62 women (71.3%) underwent genetic counselling within 1 year after diagnosis and 54 women had DNA testing. Ten of these patients requested rapid genetic testing and five mutations (9.3%) were found, all of them being BRCA1. Further results of GCT are described in Table 2.
Table 2. Results of genetic counselling and testing of study participants.
| Intervention group N=178 | Control group N=87 | |
|---|---|---|
| Had genetic counselling before 1st surgery |
161 (90.4%) |
20 (23.0%) |
| Had genetic counselling <1 year follow-up |
177 (99.4%) |
62 (71.3%) |
|
Requested timing of DNA test results | ||
| Rapid (<4 weeks) | 71 (39.9%) | 10 (11.5%) |
| Semi-rapid (4 weeks–4 months) | 38 (21.3%) | 7 (8.0%) |
| Routine (⩾4 months) | 50 (28.1%) | 36 (41.4%) |
| No DNA test at 1st counselling session but at following counselling session | 12 (6.7%) | 1 (1.1%) |
| Genetic counselling without DNA test | 6 (3.4%) | 8 (9.2%) |
| No genetic counselling or testing |
1 (0.6%) |
25 (28.7%) |
|
DNA test resulta | ||
| No pathogenic mutation (total) | 154 (90.1%) | 49 (90.7%) |
| Inconclusive | 146 (85.4%) | 44 (81.5%) |
| Non-carrier of familial mutation | 0 (0%) | 1 (1.9%) |
| Unclassified variant | 8 (4.7%) | 4 (7.4%) |
| BRCA1 mutation | 8 (4.7%) | 5 (9.3%) |
| BRCA2 mutation | 9 (5.2%) | 0 (0%) |
Abbreviation: RGCT=rapid genetic counselling and testing.
n and % based on participants who had a DNA test (171 and 54 in RGCT and control group, respectively).
Women reported various reasons for not undergoing DNA testing. Some women wanted more time to consider this option. Others had relatives who had previously undergone testing without finding a mutation, and thus felt less compelled to do so themselves. Still, other women chose not to undergo testing because, although they themselves had developed breast cancer at a relatively young age (fulfilling criteria for testing eligibility), they did not have any relatives affected with breast or ovarian cancer.
Surgical treatment
Intention-to-treat analyses
Results of the intention-to-treat analyses indicated that patients who were routinely offered RGCT opted for a direct BLM more often than women in the control group, but this difference did not reach conventional levels of statistical significance (14.6% for the RGCT group vs 9.2% for the control group, OR 2.31, CI 0.92–5.81, P=0.08; Table 3). No significant difference was observed between the RGCT and the control group in the percentage of women undergoing delayed CPM (4.5% vs 5.7%, OR 0.89, CI 0.27–2.90, P=0.84). Only 1 of the 17 women who did not have genetic counselling before surgery (Table 2) opted for a direct BLM.
Table 3. Primary and delayed surgery and adjuvant therapy of study participants.
| |
Intervention group
N=178 |
Control group
N=87 |
P-value |
|---|---|---|---|
| Type of primary breast cancer surgery | |||
| Unilateral breast-conserving surgery | 76 (42.7%) | 39 (44.8%) | |
| Bilateral breast-conserving surgery | 0 (0%) | 4 (4.6%) | |
| Unilateral mastectomy | 76 (42.7%) | 35 (40.2%) | |
| Bilateral mastectomy | 26 (14.6%) | 8 (9.2%) | |
| Breast conserving on one side with a contralateral mastectomy |
0 (0%) |
1 (1.1%) |
|
| Delayed CPM |
8 (4.5%) |
5 (5.7%) |
|
| Neo-adjuvant chemotherapy | 39 (22%) | 18 (21%) | 0.80 |
| Adjuvant chemotherapy |
74 (42%) |
40 (46%) |
0.52 |
| Radiotherapy |
111 (62.3%) |
51 (58.6%) |
0.56 |
| Prophylactic salpingo-oophorectomy within 1 year follow-up |
28 (15.7%) |
9 (10.3%) |
0.24 |
| Time in days between diagnosis and primary surgery (median, range) | 36 (6–218) | ||
| Rapid test | 36 (8–197) | ||
| Semi-rapid test | 114.5 (8–242) | ||
| Routine test | 33 (9–146) | ||
Abbreviation: CPM=contralateral prophylactic mastectomy.
We found no significant differences in the percentages of direct BLM and delayed CPM between patients treated in community hospitals vs the university medical centre and specialised oncology hospital (data not shown).
Subgroup analysis of women with unilateral breast cancer
In total, 231 patients had a current diagnosis of unilateral breast cancer without a prior history of breast cancer (161 and 70 in the intervention and the control group, respectively). Among this subset of women, no significant differences were observed between the RGCT and the control group in direct BLM (only 3.2% more often, 19 out of 161 (11.8%) for the RGCT group vs 6 out of 70 (8.6%) for the control group, OR 1.43, CI 0.54–3.74, P=0.47), or in delayed CPM (7 out of 161 (4.3%) vs 4 out of 70 (5.7%), OR 0.75, CI 0.21–2.65, P=0.66).
Per-protocol analysis: women who received DNA test results before surgery
One-third of the women in the RGCT group (n=59) actually received their DNA results before undergoing breast surgery. These women opted significantly more often for a direct BLM than did women in the control group (22% vs 9.2%, respectively; OR 3.09, CI 1.15–8.31, P=0.03). Seven of these 59 women had a BRCA1/2 mutation, of whom 5 (71.4%) opted for a direct BLM. Of the 52 patients without a pathogenic mutation, 8 (15.4%) had a direct BLM.
Choice of surgery in carriers
Of the 22 carriers, 9 received DNA test results before primary surgery (7 women in the RGCT group as described above and 2 women in the control group). Six of these nine women had a direct BLM. Of the remaining 13 women who received their DNA test results after their primary surgery, 1 had a direct BLM and 4 had a delayed CPM.
Discussion
To the best of our knowledge, ours is the first randomised controlled trial to assess the effect of RGCT on choice of surgical treatment among newly diagnosed breast cancer patients at increased risk of carrying a BRCA1/2 gene mutation. We searched the PubMed database up to 30 January 2013, for randomised clinical trials, using the terms ‘breast cancer', ‘genetic counselling', ‘genetic testing', and ‘newly diagnosed'. Additional keywords and related terms were used to maximise the sensitivity. A few studies have been published on treatment decisions of women who had GCT shortly after diagnosis, but not in the setting of an RCT. Furthermore, these women were often self-referred, and the timing of GCT was not always the same (Weitzel et al, 2003; Schwartz et al, 2004, 2005; Stolier et al, 2004; Evans et al, 2005; Stolier and Corsetti, 2005).
We observed a higher albeit statistically non-significant (P=0.08) percentage of direct BLM's in the RGCT group than in the control group. The fact that a relatively high percentage (71.3%) of women in the control group received genetic counselling within 1 year of diagnosis cannot explain the lack of significant group difference in direct BLM, as most of these women received counselling after their primary surgery. Importantly, only one-third of the women in the RGCT group received their DNA test results before surgery. Although the results need to be interpreted with caution, the per-protocol analysis suggests that, if all women were to have received their DNA test results before their surgery, RGCT would have had a statistically significant impact on the frequency of direct BLM.
Only one-third of women in the RGCT group received their DNA test results in a time frame that could optimally contribute to the choice of surgery. Although this percentage is relatively low, it reflects the complex situation that is created by offering RGCT to all eligible breast cancer patients. Part of that clinical reality is that women who are offered the opportunity to undergo rapid genetic counselling can decline to do so (which was seldom the case here), and that women who are offered the opportunity to undergo rapid DNA testing may decide that the speed with which the results are made available is not critical to their decision-making process. In our study, some women had already decided which type of surgery to undergo based on their family history and their physician's advice (Katz et al, 2005; Yi et al, 2010). In such cases, it was not necessary to await the DNA test results before proceeding with surgery. In other cases, women had a strong preference for breast-conserving surgery, and thus were willing to accept, at least initially, the 10% risk of being a BRCA1/2 gene mutation carrier. In those cases, it was still possible that the DNA test results could inform decisions regarding adjuvant radiotherapy (i.e., whether to follow through with breast-conserving therapy or to undergo a delayed CPM, without starting radiotherapy). Also, some women may have chosen to undergo surgery as soon as possible to reduce their level of psychological distress (Drageset et al, 2011).
The logistics of scheduling and rescheduling a surgical procedure is also a part of the clinical situation that may have had a role in some women's decision not to await the results of the rapid DNA testing. Another relevant factor is that the Dutch quality of care guidelines for treatment of breast cancer recommends that surgery be performed within 3 weeks of diagnosis (The National Breast cancer Organisation the Netherlands (NABON) and Comprehensive Cancer Center the Netherlands (VIKC), 2008). However, because of laboratory capacity, scheduling and budgets in The Netherlands, DNA test results could not be provided in less than 4 weeks after blood sampling. In planning the trial, we did not anticipate that a slight delay in scheduling surgery (e.g., of 1 additional week beyond the 3-week guideline) would be an issue. In practice, however, it was. In some cases, patients were simply unwilling to accept any delay in their treatment. In other cases, participating surgeons were reluctant to postpone surgery because of concerns about the possible effect of doing so on their hospital's quality of care audit.
As expected, when women received their DNA test results before surgery, being a carrier often influenced their treatment decisions. Of the carriers in this subgroup, 71% had a direct BLM as compared with 15% of those with an inconclusive test result. This latter percentage is in line with other studies that have reported that between 5 and 24% of those without a pathogenic mutation opt for a BLM (Weitzel et al, 2003; Schwartz et al, 2004; Stolier and Corsetti, 2005; Tilanus-Linthorst et al, 2006). Apart from carrier status, other as yet unidentified factors probably have a role in the decisional process, as not all carriers opted for a direct BLM and some women without a pathogenic mutation did. The importance given to the various benefits and risks of a direct BLM will vary across patients, and such individual variations in the choice of primary surgery merit further study.
We would also note that we included both patients with unilateral and bilateral breast cancer in our study. This may seem counterintuitive, as one may assume that a BLM is the surgery of choice for bilateral breast cancer. However, BLM is not always necessary in the case of bilateral breast cancer. Indeed, four women with bilateral breast cancer underwent bilateral breast-conserving surgery and one had breast-conserving surgery of one breast and a mastectomy of the other breast.
As participating hospitals did not maintain a screening log, we do not know how many eligible patients were not referred to the trial. On the basis of information from 2010, ∼11% (range 3–24%) of breast cancer patients across the participating hospitals were invited to participate, which is slightly lower than was expected (Schlich-Bakker et al, 2008). Also, some participating surgeons indicated that they did not invite women to join the study who they believed were psychologically vulnerable. Furthermore, the women who participated were younger than those who declined participation (44.9 vs 53.0 years, P<0.001) and more highly educated than their general population peers (Statistics Netherlands, 2011). This suggests that the results cannot necessarily be generalised to older women or women with a lower education.
Clinical implications
As described in the draft of the new NICE guideline (January 2013) on familial breast cancer, research is needed on the benefits and harms of RGCT (National Institute for Health and Clinical Excellence, 2013). Our results suggest that knowledge of one's carrier status before surgery influences a woman's treatment choice. In fact, as the genetic status of women with familiar breast cancer is prognostic in determining contralateral breast cancer risk, this information is important in deciding on the benefits of CPM (Rhiem et al, 2012). A sine qua non for effective RGCT is that there is sufficient time available to provide genetic test results in the relatively small window between diagnosis and surgery. Although in our trial this was not always possible, given budgetary (and thus laboratory capacity) constraints, hopefully this limitation will be resolved as the technology of DNA testing improves. In the meantime, it is important for surgeons to accept that it may be better clinical practice to delay primary surgery slightly in a small subset of patients to ensure that high-risk women have the opportunity to avail themselves of this extra information selecting their treatment than to adhere rigidly to general quality of care guidelines that dictate the maximum allowable interval between diagnosis and surgery.
Finally, before recommending that RGCT be offered to all breast cancer patients with a high risk of carrying a BRCA1/2 mutation, it is important to assess its psychosocial effects, satisfaction with treatment decisions, and possible regret about having undergone a direct BLM. Previous research has shown that GCT during adjuvant radiotherapy does not result in heightened levels of psychological distress (Schlich-Bakker et al, 2008). Whether this also holds for the RGCT setting, where the patients have not yet received any breast cancer treatment, will be addressed in a subsequent paper. If that proves to be the case, and if patients are satisfied with their decisions, then it can be argued that RGCT is both safe and potentially useful in helping high-risk breast cancer patients in selecting the surgical treatment that is right for them. In addition, we hope to be able to conduct a follow-up of the women in our trial to determine longer-term satisfaction with treatment choice.
Acknowledgments
We thank the clinical geneticists and genetic counsellors who counselled the participants, and Jacobien Kieffer for her advice on the statistical analyses. Funding for this study was provided by the NutsOHRA Foundation, Grant number SNO-T-0701-95.
Disclaimer
The trial is registered at http://www.clinicaltrials.gov, number NCT00783822. Full details of the trial protocol can be found there.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Ardern-Jones A, Kenen R, Eeles R. Too much, too soon? Patients and health professionals' views concerning the impact of genetic testing at the time of breast cancer diagnosis in women under the age of 40. Eur J Cancer Care (Engl) 2005;14 (3:272–281. doi: 10.1111/j.1365-2354.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- Comprehensive Cancer Center the Netherlands (IKNL) 2008. Breast cancer guideline 1.1 www.oncoline.nl/mammacarcinoom .
- Drageset S, Lindstrom TC, Giske T, Underlid K. Being in suspense: women's experiences awaiting breast cancer surgery. J Adv Nurs. 2011;67 (9:1941–1951. doi: 10.1111/j.1365-2648.2011.05638.x. [DOI] [PubMed] [Google Scholar]
- Evans DG, Lalloo F, Ashcroft L, Shenton A, Clancy T, Baildam AD, Brain A, Hopwood P, Howell A. Uptake of risk-reducing surgery in unaffected women at high risk of breast and ovarian cancer is risk, age, and time dependent. Cancer Epidemiol Biomarkers Prev. 2009;18 (8:2318–2324. doi: 10.1158/1055-9965.EPI-09-0171. [DOI] [PubMed] [Google Scholar]
- Evans DG, Lalloo F, Hopwood P, Maurice A, Baildam A, Brain A, Barr L, Howell A. Surgical decisions made by 158 women with hereditary breast cancer aged <50 years. Eur J Surg Oncol. 2005;31 (10:1112–1118. doi: 10.1016/j.ejso.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Fackenthal JD, Olopade OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer. 2007;7 (12:937–948. doi: 10.1038/nrc2054. [DOI] [PubMed] [Google Scholar]
- Graeser MK, Engel C, Rhiem K, Gadzicki D, Bick U, Kast K, Froster UG, Schlehe B, Bechtold A, Arnold N, Preisler-Adams S, Nestle-Kraemling C, Zaino M, Loeffler M, Kiechle M, Meindl A, Varga D, Schmutzler RK. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27 (35:5887–5892. doi: 10.1200/JCO.2008.19.9430. [DOI] [PubMed] [Google Scholar]
- Herrinton LJ, Barlow WE, Yu O, Geiger AM, Elmore JG, Barton MB, Harris EL, Rolnick S, Pardee R, Husson G, Macedo A, Fletcher SW. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol. 2005;23 (19:4275–4286. doi: 10.1200/JCO.2005.10.080. [DOI] [PubMed] [Google Scholar]
- Katz SJ, Lantz PM, Janz NK, Fagerlin A, Schwartz K, Liu L, Deapen D, Salem B, Lakhani I, Morrow M. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23 (24:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- Koumpis C, Dimitrakakis C, Antsaklis A, Royer R, Zhang S, Narod SA, Kotsopoulos J. Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Greece. Hered Cancer Clin Pract. 2011;9:10. doi: 10.1186/1897-4287-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010;11:CD002748. doi: 10.1002/14651858.CD002748.pub2. [DOI] [PubMed] [Google Scholar]
- Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, Xue S, Teraoka S, Bernstein L, Capanu M, Reiner AS, Riedel ER, Thomas DC, Mellemkjaer L, Lynch CF, Boice JD, Jr., Anton-Culver H, Bernstein JL. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 2010;28 (14:2404–2410. doi: 10.1200/JCO.2009.24.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser B, Tucker K, Friedlander M, Barlow-Stewart K, Lobb E, Saunders C, Mitchell G. Genetic counselling and testing for inherited gene mutations in newly diagnosed patients with breast cancer: a review of the existing literature and a proposed research agenda. Breast Cancer Res. 2008;10 (6:216. doi: 10.1186/bcr2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe KA, Lubinski J, Ghadirian P, Lynch H, Kim-Sing C, Friedman E, Foulkes WD, Domchek S, Ainsworth P, Isaacs C, Tung N, Gronwald J, Cummings S, Wagner T, Manoukian S, Moller P, Weitzel J, Sun P, Narod SA. Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: the Hereditary Breast Cancer Clinical Study Group. J Clin Oncol. 2008;26 (7:1093–1097. doi: 10.1200/JCO.2007.12.6078. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence 2013. Familial breast cancer: full guideline DRAFT (January http://www.nice.org.uk/guidance/index.jsp?action=folder&o=62324 .
- Piantadosi S. Clinical Trials: A Methodologic Perspective. John Wiley & Sons: New York, NY, USA; 1997. [Google Scholar]
- Reavey P, McCarthy CM. Update on breast reconstruction in breast cancer. Curr Opin Obstet Gynecol. 2008;20 (1:61–67. doi: 10.1097/GCO.0b013e3282f2329b. [DOI] [PubMed] [Google Scholar]
- Rhiem K, Engel C, Graeser M, Zachariae S, Kast K, Kiechle M, Ditsch N, Janni W, Mundhenke C, Golatta M, Varga D, Preisler-Adams S, Heinrich T, Bick U, Gadzicki D, Briest S, Meindl A, Schmutzler RK. The risk of contralateral breast cancer in patients from BRCA1/2 negative high risk families as compared to patients from BRCA1 or BRCA2 positive families: a retrospective cohort study. Breast Cancer Res. 2012;14 (6:R156. doi: 10.1186/bcr3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlich-Bakker KJ, Ausems MG, Schipper M, ten Kroode HF, Warlam-Rodenhuis CC, van den Bout J. BRCA1/2 mutation testing in breast cancer patients: a prospective study of the long-term psychological impact of approach during adjuvant radiotherapy. Breast Cancer Res Treat. 2008;109 (3:507–514. doi: 10.1007/s10549-007-9680-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Lerman C, Brogan B, Peshkin BN, Halbert CH, DeMarco T, Lawrence W, Main D, Finch C, Magnant C, Pennanen M, Tsangaris T, Willey S, Isaacs C. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol. 2004;22 (10:1823–1829. doi: 10.1200/JCO.2004.04.086. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Lerman C, Brogan B, Peshkin BN, Isaacs C, DeMarco T, Halbert CH, Pennanen M, Finch C. Utilization of BRCA1/BRCA2 mutation testing in newly diagnosed breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2005;14 (4:1003–1007. doi: 10.1158/1055-9965.EPI-03-0545. [DOI] [PubMed] [Google Scholar]
- Statistics Netherlands 2011 ) http://www.cbs.nl/nl-NL/menu/methoden/classificaties/overzicht/soi/2006/default.htm .
- Stolier AJ, Corsetti RL. Newly diagnosed breast cancer patients choose bilateral mastectomy over breast-conserving surgery when testing positive for a BRCA1/2 mutation. Am Surg. 2005;71 (12:1031–1033. [PubMed] [Google Scholar]
- Stolier AJ, Fuhrman GM, Mauterer L, Bolton JS, Superneau DW. Initial experience with surgical treatment planning in the newly diagnosed breast cancer patient at high risk for BRCA-1 or BRCA-2 mutation. Breast J. 2004;10 (6:475–480. doi: 10.1111/j.1075-122X.2004.21543.x. [DOI] [PubMed] [Google Scholar]
- The National Breast cancer Organisation the Netherlands (NABON) and Comprehensive Cancer Center the Netherlands (VIKC) 2008. The National Breast cancer Organisation the Netherlands (NABON) - Breast Cancer Guideline 2.0. Available at http://www.oncoline.nl/index.php?pagina=/richtlijn/item/pagina.php&richtlijn_id=608 (Accessed on 7 February 2013).
- The Netherlands Foundation for the Detection of Hereditary Tumours (STOET) and the Dutch Society of Clinical Genetics (VKGN) 2005Erfelijke tumoren: richtijnen voor diagnostiek en preventie. 3ed edn, pp 10–13.
- Tilanus-Linthorst MM, Bartels KC, Alves C, Bakri B, Crepin E, van den Ouweland A, Klijn JG, Meijers-Heijboer H, Brekelmans CT. Selection bias influences reported contralateral breast cancer incidence and survival in high risk non-BRCA1/2 patients. Breast Cancer Res Treat. 2006;95 (2:117–123. doi: 10.1007/s10549-005-9054-2. [DOI] [PubMed] [Google Scholar]
- Trainer AH, Lewis CR, Tucker K, Meiser B, Friedlander M, Ward RL. The role of BRCA mutation testing in determining breast cancer therapy. Nat Rev Clin Oncol. 2010;7 (12:708–717. doi: 10.1038/nrclinonc.2010.175. [DOI] [PubMed] [Google Scholar]
- Turnbull C, Hodgson S. Genetic predisposition to cancer. Clin Med. 2005;5 (5:491–498. doi: 10.7861/clinmedicine.5-5-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk DM, de Bock GH, Leegte BK, Schaapveld M, Mourits MJ, de Vries J, van der Hout AH, Oosterwijk JC. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treat. 2010;124 (3:643–651. doi: 10.1007/s10549-010-0805-3. [DOI] [PubMed] [Google Scholar]
- Van Riel E, Warlam-Rodenhuis CC, Verhoef S, Rutgers EJ, Ausems MG. BRCA testing of breast cancer patients: medical specialists' referral patterns, knowledge and attitudes to genetic testing. Eur J Cancer Care (Engl) 2010;19 (3:369–376. doi: 10.1111/j.1365-2354.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- van Sprundel TC, Schmidt MK, Rookus MA, Brohet R, van Asperen CJ, Rutgers EJ, Van't Veer LJ, Tollenaar RA. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer. 2005;93 (3:287–292. doi: 10.1038/sj.bjc.6602703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel JN, McCaffrey SM, Nedelcu R, MacDonald DJ, Blazer KR, Cullinane CA. Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis. Arch Surg. 2003;138 (12:1323–1328. doi: 10.1001/archsurg.138.12.1323. [DOI] [PubMed] [Google Scholar]
- Wevers MR, Ausems MG, Verhoef S, Bleiker EM, Hahn DE, Hogervorst FB, van der Luijt RB, Valdimarsdottir HB, van Hillegersberg R, Rutgers EJ, Aaronson NK. Behavioral and psychosocial effects of rapid genetic counseling and testing in newly diagnosed breast cancer patients: design of a multicenter randomized clinical trial. BMC Cancer. 2011;11:6. doi: 10.1186/1471-2407-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Hunt KK, Arun BK, Bedrosian I, Barrera AG, Do KA, Kuerer HM, Babiera GV, Mittendorf EA, Ready K, Litton J, Meric-Bernstam F. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res (Phila) 2010;3 (8:1026–1034. doi: 10.1158/1940-6207.CAPR-09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.