Abstract
Background:
β-Catenin is a potent oncogenic protein in colorectal cancer (CRC), but the targets and regulation of this important signalling molecule are not completely understood. Hypoxia is a prominent feature of solid tumours that contributes to cancer progression.
Methods:
Here, we analysed the regulation between Nur77 and β-catenin under hypoxic conditions. Cell proliferation, migration, and invasion assays were performed to assess functional consequences.
Results:
We showed that hypoxia stimulated co-upregulation of β-catenin and Nur77 in a number of human CRC cell lines. Interestingly, expression of β-catenin and Nur77 by hypoxia formed a mutual feedback regulation circuits that conferred aggressive growth of CRC. Overexpression of β-catenin increased Nur77 transcription through hypoxia-inducible factor-1α rather than T-cell factor. Nur77-mediated activation of β-catenin by hypoxia was independent of both DNA binding and transactivation. Further, we showed that hypoxic activation of β-catenin was independent of the classical adenomatous polyposis coli and p53 pathways, but stimulated by phosphatidylinositol 3-kinase/Akt in a Nur77-dependent manner. Under hypoxic conditions, enhanced β-catenin and Nur77 expression synergistically stimulated CRC cell migration, invasion, and epithelial–mesenchymal transition.
Conclusion:
These findings provide a novel molecular mechanism for hypoxic CRCs that may contribute to tumour progression, and its targeting may represent an effective therapeutic avenue.
Keywords: β-catenin, Nur77, hypoxia, Akt, colorectal cancer
β-Catenin is a key mediator that regulates cell fate decisions during development. Accordingly, aberrant β-catenin signalling is observed at high frequency in many cancers, including colorectal cancer (CRC), where it is the most common genetic cause of oncogenesis (Morin et al, 1997; Bienz and Clevers, 2000; Giles et al, 2003). Thus, the mechanisms that regulate the stabilisation of β-catenin in the cytosol, which regulates its target genes, have been an area of intense interest. The adenomatous polyposis coli (APC)-dependent proteasomal degradation pathways through glycogen synthase kinase 3β (GSK-3β) and p53/Siah-1 are well known to regulate the levels of β-catenin (Munemitsu et al, 1995; Korinek et al, 1997; Liu et al, 2001; Matsuzawa and Reed, 2001). However, both Axin/GSK-3β and p53/Siah-1 are often mutated in CRC. Moreover, given the clinical challenges and limited success of APC/Axin and p53 gene replacement, identifying new mechanisms that regulate β-catenin signalling will have a great therapeutic significance.
Nur77 (also known as NR4A1, TR3, or NGFIB), encoded by an immediate early gene, belongs to the NR4A subgroup of the nuclear receptor superfamily, which also includes Nurr1 (NR4A2) and Nor-1 (NR4A3). Without a physiological ligand yet identified, Nur77 is classified as an orphan receptor. Nur77 is overexpressed in CRC cells, and its expression levels are often elevated in human CRC tumours as compared with the corresponding non-tumour tissues (Cho et al, 2007; Wu et al, 2011; Chen et al, 2012). Since Nur77 gene amplification is a rare event, the mechanisms underlying Nur77 overexpression in CRC remain obscure. Moreover, Nur77 exhibits a wide range of functions depending on the cell type and the nature of stimulus. The precise roles of Nur77 in specific cellular and physiological contexts are yet to be fully elucidated.
The importance of tumour microenvironment in cancer is increasingly recognised (Peddareddigari et al, 2010; Swarta et al, 2012). Note that high hypoxia-inducible factor-1α (HIF-1α) expression is associated with advanced stage colon cancer and has been associated with poor patient survival (Cao et al, 2009; Baba et al, 2010). A correlation between Nur77 and β-catenin has been suggested (Rajalin and Aarnisalo, 2011; Smith et al, 2011; Wu et al, 2011; Sun et al, 2012). However, the conflicting reports provide an unclear picture of the relationship. Moreover, these studies mainly employ cells maintained at atmospheric oxygen (∼20%) that far exceeds levels found within tumours in situ (Bertout et al, 2008; Ivanovic, 2009). Hypoxia is known to cause alterations in gene expression and changes in cellular function (Lal et al, 2001; Liu et al, 2007; Sermeus and Michiels, 2011). Whether and by what mechanism(s) oxygen may regulate Nur77 and β-catenin interaction remains to be explored. In this study, we show for the first time a positive feedback loop between Nur77 and β-catenin that drives the invasive growth properties of CRC cells under hypoxic condition. This provides an attractive mechanism which may explain the highly aggressive behaviour of CRC expressing these two molecules and highlights the importance of the hypoxic microenvironment in Nur77–β-catenin crosstalk.
Materials and methods
Cell culture and hypoxic treatment
Human colon cancer cells HT29, HT29-APC, HT29-β-gal, SW480, and HEK293 were grown in DMEM, and SW620, HCT116, and HCT116-p53−/− were grown in RPMI-1640 medium. The two paired cell lines (HT29-APC and HT29-β-gal; HCT116 and HCT116-p53−/−) were kind gifts from Dr B Volgelstein (Johns Hopkins Oncology Center, Baltimore, MD), and the other lines were obtained from ATCC. Media were supplemented with 100 units ml−1 penicillin, 100 mg ml−1 streptomycin and 5% FBS. Cultures were maintained in a humidified incubator at 37 °C with 5% CO2. For hypoxic conditions, the cells were serum starved for 24 h and then grown at 1% O2 in a modulator. Alternatively, hypoxia was chemically induced by 100 μM cobalt chloride (CoCl2) or desferrioxamine (DFX) (Sigma, St Louis, MO, USA).
Constructs and transfections
To express cDNA constructs, cells were transfected with 0.25 μg of plasmid DNA per well in 24-well plates for 24 h using Lipofectamine (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Transfected cells were serum starved for 24 h before incubation at hypoxic conditions. Constructs encoding wild-type β-catenin, β-catenin S37A, Nur77, and Nur77ΔDBD were previously described (Pon and Wong, 2006; Wu et al, 2011). DN-Akt was purchased from Upstate Biotechnology. DN-TCF and VP16-TCF were provided by Dr Barry Gumbiner (University of Virginia, Charlottesville, VA). GSK-3β-S9A was a kind gift of Dr James Woodgett (Ontario Cancer Institute, Toronto, ON, Canada).
Small interfering RNA transfection
Small interfering RNA (siRNA) targeting Nur77 (SMARTpool 5′-UCGAGGACUUCCAGGUGUA-3′, 5′-GGACAGAGCAGCUGCCCAA-3′, 5′-GAAGGCCGCUGUGCUGUGU-3′, 5′-CGGCUACACAGGAGAGUUU-3′), β-catenin (5′-AAGUCCUGUAUGAGUGGGAAC-3′), HIF-1α (5′-GGACACAGAUUUAGACUUG-3′), and a non-specific duplex oligo (5′-GGCTACGTCCAGGAGCGCA-3′) were purchased from Dharmacon (Lafayette, CO, USA). Cells were transfected with 20 nmol l−1 siRNA using siLectFect (Bio-Rad, Hercules, CA, USA) following the manufacturer's instructions. Transfected cells were serum starved for 24 h before incubation under hypoxic conditions.
Western blot analysis
Cells were lysed with sodium dodecyl sulphate (SDS) lysis buffer. Total proteins were separated by a 7.5% polyacrylamide gel and transferred onto a nitrocellulose membrane. After blocking with non-fat dry milk for 1 h, the membrane was incubated with antibodies specific for β-catenin (1 : 5000), HIF-1α (1 : 1000) (BD Transduction Laboratories, BD Biosciences, Palo Alto, CA, USA), Nur77 (1 : 1000), phospho-Akt (Ser473) (1 : 1000), total Akt (1 : 1000), phospho-ERK1/2 (Thr202/Tyr204) (1 : 1000), total ERK1/2 (1 : 1000), phospho-JNK (Thr183/Tyr185) (1 : 1000), or total JNK (1 : 1000) (Cell Signaling Technology, Beverley, MA, USA) overnight at 4 °C. β-Actin (1 : 5000) (Sigma) was used as a loading control. After incubation with appropriate secondary antibodies conjugated with peroxidase, proteins were detected by Western-Lightning Plus Enhanced Chemiluminescence (Amersham, Little Chalfont, UK). Band intensities were determined by densitometry using the ImageJ software (Bethesda, MD, USA), and analysis of Nur77 proteins included the intensities of all bands.
Reverse transcription-PCR
Total RNA was extracted with Trizol Reagent (Invitrogen) and reverse transcribed into cDNAs using the SuperScript first-strand synthesis system (Invitrogen). The following specific oligonucleotide primers were used: β-catenin: 5′-GGCTGCAGTTATGGTCCATCA-3′ (sense) and 5′-GCTGCACAAACAATGGAATGG-3′ (antisense); Nur77: 5'-TCTGCTCAGGCCTGGTGCTAC-3′ (sense) and 5′-GGCACCAGTCCTCCAGCTTG-3′ (anti-sense); HIF-1α: 5′-TCCAGCAGACTCAAATACAAGAAC-3′ (sense) and 5′-GTATGTGGGTAGGAGATGGAGATG-3′ (antisense); E-cadherin: 5′-GGGTGACTACAAAATCAATC-3′ (sense) and 5′-GGGGGCAGTAAGGGCTCTTT-3′ (antisense); N-cadherin: 5′-CACTGCTCAGGACCCAGAT-3′ (sense) and 5′-TAAGCCGAGTGATGGTCC-3′ (antisense); MMP-2: 5′-TCTGTGTTGTCCAGAGGCAAT-3′ (sense) and 5′-AACAGGTTGCAGCTCTCCT-3′ (antisense); and 18s rRNA: 5′-GGTGAAATTCTTGGACCGGC-3′ (sense); and 5′-GACTTTGGTTTCCCGGAAGC-3′ (antisense). Cycle parameters were determined in pilot experiments and PCR products were resolved in 1.5% agarose gel.
MTT assays
MTT (3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyltetrazolium bromide; Sigma) assays were performed following the manufacturer's instructions. Briefly, MTT solution was added to each well and the plates were incubated for 3 h at 37 °C. Medium was then aspirated from each well, and 250 μl DMSO was added. Colorimetric analysis was performed at a wavelength of 570 nm using a microplate reader (Bio-Rad).
Boyden chamber migration and Matrigel invasion assays
Cell migration was quantified using a Boyden chamber (8-μm pore size) (BD Biosciences). Approximately 5 × 104 cells were seeded onto each upper chamber in serum-free medium. In the lower chambers, medium was supplemented with 10% fetal bovine serum. The cells were then incubated under hypoxia for 24 h. Non-migrating cells were removed from the upper chamber with a moistened cotton swab. Cells that had migrated through the membrane to the lower surface were fixed with ice-cold methanol, stained with 0.5% crystal violet, and counted in five different fields under a light microscope. To assess cellular invasion potential, the same protocol was used, except that inserts were precoated with Matrigel (1 : 10 dilution, 100 μl per well; BD Biosciences).
Statistical analysis
Experiments were repeated at least thrice and the statistical significance of differences was analysed by Student's t-test (GraphPad Software, San Diego, CA, USA). P-value<0.05 was considered as significant.
Results
Hypoxia induces expression of both Nur77 and β-catenin
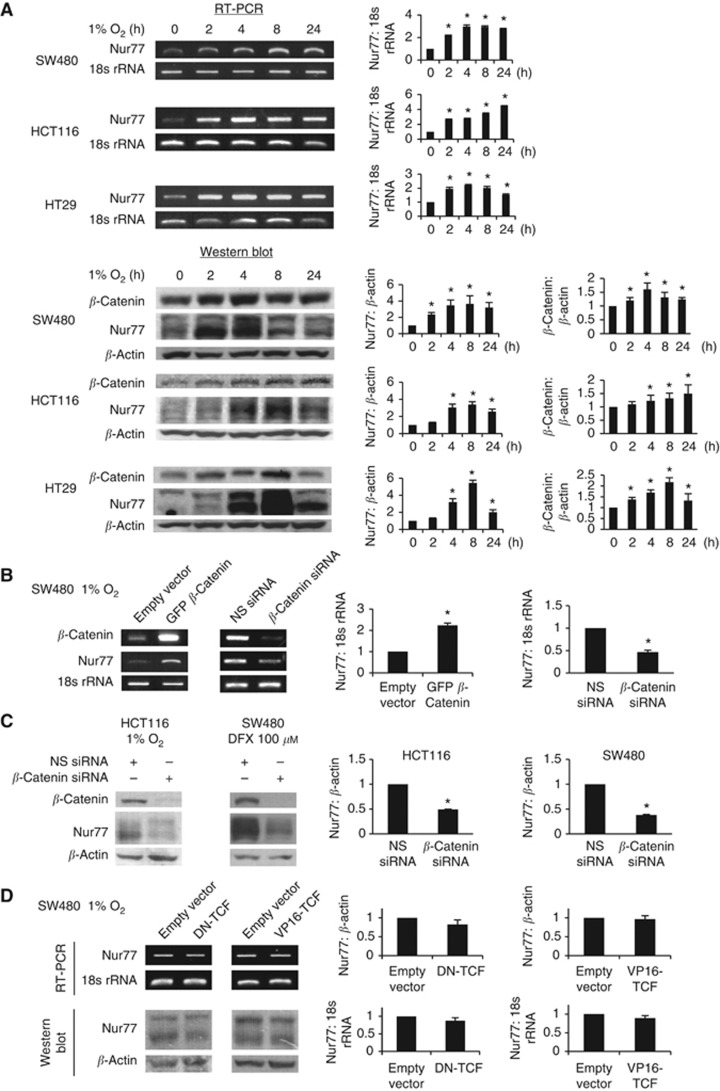
To investigate a possible role of Nur77 in the cellular response to hypoxia, we analysed the expression of the Nur77 mRNA and protein in CRC cells cultured in 1% O2. As shown in Figure 1A, hypoxia markedly increased both the mRNA and protein levels of Nur77 in all cells analysed. Nur77 protein is recognised by western blot analysis as multiple bands as previously reported in different studies, and the slow-migrating bands may represent post-translational modifications (Kolluri et al, 2003; Han et al, 2006). Similarly, the use of CoCl2 or deferoxamine mesylate (DFX) to chemically induce hypoxia also activated the expression of Nur77 (Supplementary Figure 1B and C). By contrast, normoxic conditions did not change the expression of Nur77 (Supplementary Figure 1D). To study the association of Nur77 with β-catenin, the levels of β-catenin were also examined in the same blot. As shown in Figure 1A, hypoxia-induced Nur77 expression was closely accompanied by increased β-catenin, demonstrating that hypoxia could potently stimulate co-upregulation of Nur77 and β-catenin in CRC.
Figure 1.
Hypoxia induces Nur77 expression by β-catenin in a TCF-independent manner. (A) Colon cancer cells were serum starved and then incubated at 1% O2 in a modulator for different time intervals as indicated. Total RNA or proteins were extracted from cell lysates for subsequent RT-PCR and western blotting to determine the mRNA and protein expression, respectively. (B) SW480 cells were transfected with empty vector or GFP-β-catenin, or non-specific (NS) siRNA or β-catenin siRNA for 24 h. Transfected cells were incubated at 1% O2 for 4 h and harvested for RT-PCR. (C) HCT116 and SW480 cells were transfected with NS siRNA or β-catenin siRNA. Cells were incubated at 1% O2 or with 100 μM desferrioxamine (DFX) for 4 h and harvested for western blotting. (D) SW480 cells were transfected with dominant-negative TCF (DN-TCF) or constitutively active TCF (VP16-TCF). Cells were then incubated at 1% O2 for 4 h and harvested for RT-PCR or western blotting. 18s rRNA was used as an internal control for RT-PCR, whereas β-actin was used as a loading control for western blotting. All blots shown are representative of three independent experiments. Bar, ±s.d. *P<0.05.
Hypoxic induction of Nur77 requires β-catenin and HIF-1α
The concurrent increase in Nur77 and β-catenin suggests a possible crosstalk between these two pathways under hypoxia. We therefore asked whether β-catenin has any role in regulating Nur77 activity. We showed that hypoxia-induced expression of Nur77 mRNA could be substantially enhanced by overexpression of β-catenin (Figure 1B). Conversely, depletion of β-catenin with a specific siRNA abrogated the effect of hypoxic treatment on Nur77 expression (Figure 1B and C). Since the transcriptional activity of β-catenin is largely dependent on its association with T-cell factor (TCF)/lymphoid enhancing factor (Korinek et al, 1997), we decided to explore whether TCF is involved in regulation of β-catenin-induced Nur77 expression in response to hypoxia. However, expression of a dominant-negative mutant of TCF (DN-TCF) shown previously to inhibit β-catenin/TCF transcription (Wong and Gumbiner, 2003) did not alter hypoxia-increased Nur77 expression (Figure 1D). Nor did the expression of constitutively active TCF (VP16-TCF) (Wong and Gumbiner, 2003) affect Nur77 expression induced by hypoxia (Figure 1D). Thus, the hypoxic induction of Nur77 by β-catenin does not seem to be attributable to changes in β-catenin/TCF-dependent nuclear signalling.
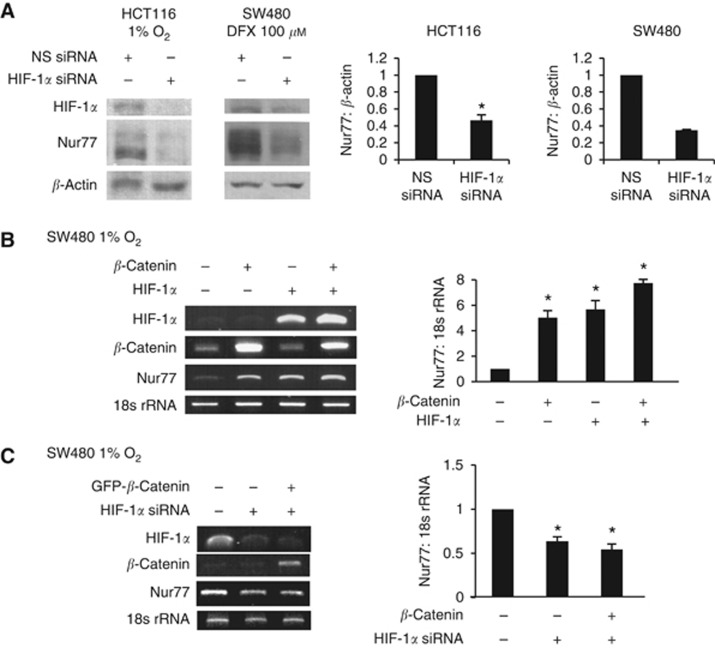
The critical role of HIF-1α as an important mediator of the hypoxic response prompted us to ask whether HIF-1α had a direct role in β-catenin-mediated Nur77 expression. As shown in Figure 2A, there was a clear reduction in Nur77 expression by inhibition of HIF-1α with a specific siRNA shown to deplete HIF-1α, but not the non-specific siRNA. In contrast, the overexpression of β-catenin or HIF-1α caused a marked increase in Nur77 mRNA expression, and that this effect was enhanced when β-catenin and HIF-1α were simultaneously co-expressed (Figure 2B). Furthermore, knocking down of HIF-1α greatly impaired β-catenin in enhancing Nur77 expression (Figure 2C). These results strongly suggest a critical role of HIF-1α in the regulation between β-catenin and Nur77.
Figure 2.
Hypoxia induces Nur77 expression through the coordination between β-catenin and HIF-1α. (A) HCT116 and SW480 cells were transfected with non-specific (NS) siRNA or HIF-1α siRNA. Cells were then incubated at 1% O2 or with 100 μM DFX for 4 h and harvested for western blotting. (B) SW480 cells were transfected with expression vector encoding β-catenin and/or HIF-1α. Cells were then incubated at 1% O2 for 4 h and harvested for RT-PCR. (C) SW480 cells were transfected with NS siRNA or HIF-1α siRNA, empty vector or GFP-β-catenin as indicated for 24 h, followed by 4 h hypoxic incubation. 18s rRNA was used as an internal control for RT-PCR, whereas β-actin was used as a loading control for western blotting. The blots were quantified by densitometry and the values were normalised with 18r RNA or β-actin. All blots shown are representative of three independent experiments. Bar, ±s.d. *P<0.05.
Nur77 is required for the activation of β-catenin expression
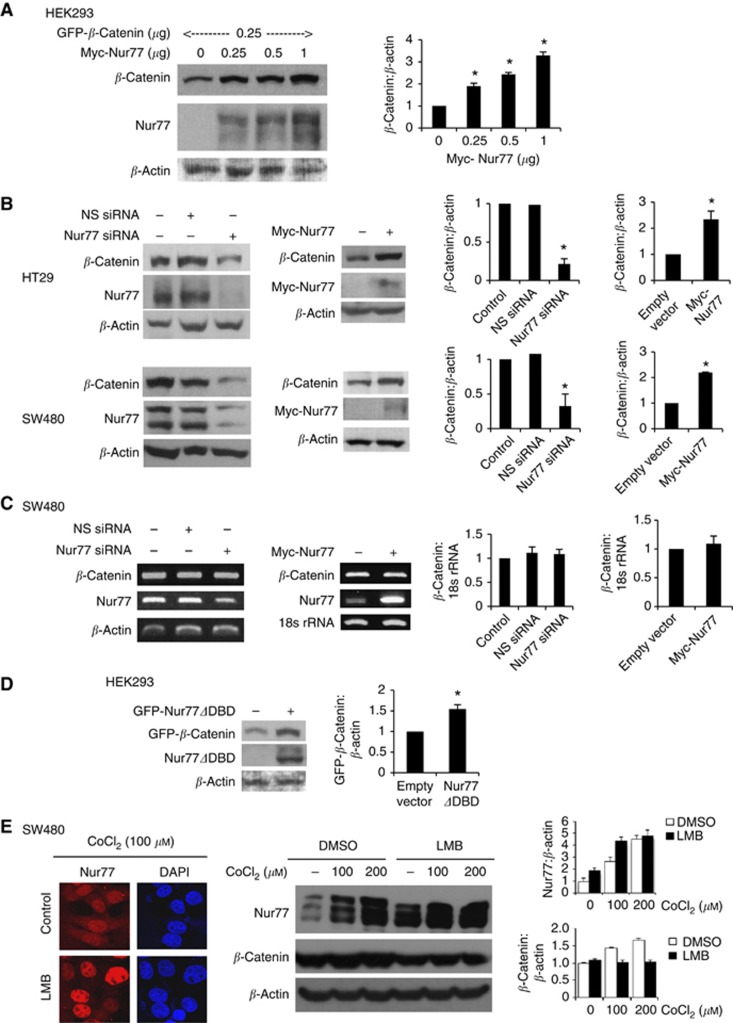
We have previously shown that Nur77 can downregulate β-catenin under normoxic conditions (Sun et al, 2012). Therefore, we asked whether β-catenin-induced Nur77 activation might modulate β-catenin expression in the presence of hypoxia. Interestingly, as shown in Figure 3A, overexpression of Nur77 increased β-catenin expression in a dose-dependent manner in HEK293 cells, which have relatively low levels of cytoplasmic β-catenin (Gan et al, 2008; Li and Wang, 2008). On the other hand, when we introduced specific siRNA to inhibit Nur77 expression in CRC cells known to contain high endogenous β-catenin expression, β-catenin expression decreased with decreasing level of Nur77 (Figure 3B), suggesting the presence of an endogenous Nur77–β-catenin positive feedback loop under hypoxia.
Figure 3.
Hypoxia increases β-catenin protein expression by Nur77 independent of DNA binding. (A) HEK293 cells were transfected with β-catenin expression vector (0.25 μg) and the indicated amount of Nur77 expression vector for 24 h. Transfected cells were then incubated at 1% O2 for 4 h. The total amount of plasmid DNA in all transfections was kept constant using appropriate parental empty expression vectors. Levels of overexpressed Nur77 and β-catenin were analysed by western blotting, with β-actin as a loading control. (B) HT29 and SW480 cells were transfected with non-specific (NS) siRNA or Nur77 siRNA or empty vector and Myc-Nur77. After transfection, cells were exposed to 1% O2 for 4 h, followed by western blotting to detect endogenous β-catenin and Nur77, with β-actin as a loading control. (C) SW480 cells were transfected with NS siRNA or Nur77 siRNA or empty vector or Myc-Nur77. After 24 h of transfection, cells were exposed to 1% O2 for 4 h, followed by RT-PCR to detect mRNA levels of β-catenin and Nur77. 18s rRNA was included as an internal control. (D) HEK293 cells were transfected with Nur77 lacking DNA-binding domain (Nur77ΔDBD) as indicated for 24 h. Cells were then incubated at 1% O2 for 4 h after transfection for 24 h, followed by western blotting. (E) SW480 cells were pretreated with leptomycin B (LMB; 2.5 nM) for 1 h and then exposed to different concentrations of CoCl2 for 4 h. The effect of CoCl2 on Nur77 expression and translocation was analysed by immunostaining using anti-Nur77 antibody (1 : 200). Cells were co-stained with DAPI to visualise the nucleus. β-catenin and Nur77 expression was detected by western blotting. β-Actin expression was served as a loading control. All the immunoblots were quantified by densitometry and the values were normalised with β-actin. The blots shown are representative of three independent experiments. Bar, ±s.d. *P<0.05.
Nur77 positively regulates β-catenin independent of its transcriptional function
It has been known that Nur77, through its transactivation activity, can regulate gene expression and mediate diverse signalling (Wansa et al, 2002). To examine the molecular mechanism underlying the regulation of Nur77 on β-catenin, we asked whether Nur77 affects β-catenin expression at the transcriptional level. Our data showed a similar level of β-catenin transcripts in the presence or absence of Nur77, suggesting that Nur77-mediated enhancement of β-catenin expression by hypoxia was possibly through modulating the protein stability (Figure 3C). Interestingly, while transfection of wild-type Nur77 resulted in an increase in β-catenin expression, expression of a Nur77 mutant known to lack DNA binding (Nur77ΔDBD) showed a similar effect (Figure 3D), suggesting that the induction of β-catenin may be mediated through a non-genomic mechanism of Nur77. Since expression of Nur77ΔDBD is cytoplasmic (Sun et al, 2012), we further study whether leptomycin B (LMB), a specific inhibitor of the nuclear export factor CRM1, could affect hypoxia on inducing β-catenin expression. Our data showed that a significant amount of Nur77 by hypoxia (CoCl2 treatment) was translocated to cytoplasm, which was almost completely inhibited by LMB (Figure 3E). Interestingly, LMB treatment could strongly enhance the basal and hypoxia-induced Nur77 expression. However, hypoxia-increased β-catenin expression was abrogated by LMB (Figure 3E). Taken together, our results suggest that hypoxia-induced interaction between β-catenin and Nur77 is possibly occurred outside the nucleus.
Regulation of β-catenin by Nur77 is independent of APC and p53
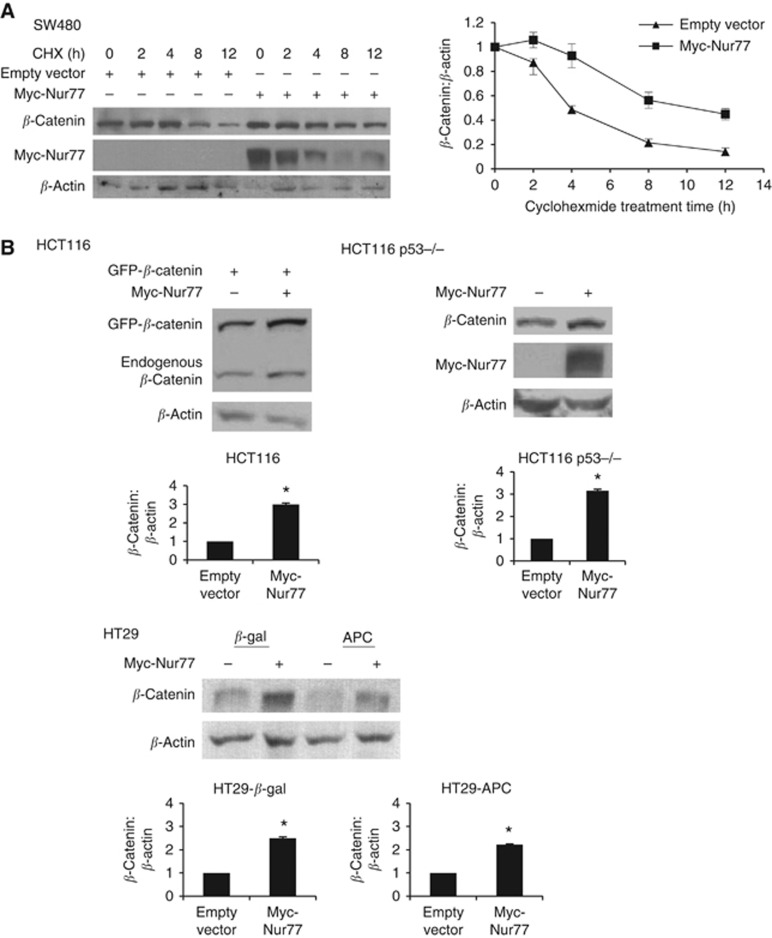
To gain insight into the non-genomic mechanism by which Nur77 increased the protein levels of β-catenin, the protein synthesis inhibitor cycloheximide (CHX) was used to determine whether Nur77 decreased the turnover of β-catenin. Figure 4A showed that the half-life of β-catenin was estimated to be ∼4 h as deduced from the kinetics after treatment of cells with CHX, while overexpression of Nur77 could significantly prolong the stability of β-catenin (∼8 h). It is known that APC/GSK-3β and APC/p53/Siah have important roles in regulation of β-catenin degradation. We therefore asked whether Nur77 might regulate β-catenin through APC and p53. We showed that under 1% O2 conditions, overexpression of Nur77 could potently enhance β-catenin in HCT116 cells normally expressing both APC and p53 (Figure 4B), as well as SW480 and HT29 cells with mutations in both APC and p53 (Figure 3B), suggesting that Nur77 could modulate β-catenin expression independent of APC and p53. These observations were further confirmed by using two matched cell lines, HT29-β-gal/HT29-APC and HCT116-wt/HCT116-p53−/−. Figure 4B showed that overexpression of Nur77 significantly increased β-catenin to a similar extent in HT29-β-gal and HT29-APC, as well as in HCT116-wt and HCT116-p53−/− cells.
Figure 4.
Nur77 enhances β-catenin stability independent of APC and p53. (A) SW480 cells transfected with empty vector or Myc-Nur77 for 24 h were treated with 100 μM cycloheximide (CHX) under 1% O2 for the time indicated. Changes in β-catenin and Nur77 levels were detected by western blotting. β-Actin was included as a loading control. (B) Two paired cell lines were used: HCT116 and HCT116-p53−/− (both with mutant β-catenin), HT29-β-gal (with mutations in both p53 and APC) and H292-APC (with mutant p53 but expressing full-length APC). Cells were transfected with expression constructs as indicated for 24 h. Transfected cells were incubated with 100 μM desferrioxamine (DFX) or 1% O2 for 4 h. For HT29 paired cells, 100 μM zinc chloride was added to induce the expression of APC during starvation. β-Actin served as the loading control. Immunoblots were quantified by densitometry and the values were normalised with β-actin. All blots shown are representative of three independent experiments. Bar, ±s.d. *P<0.05.
Nur77 induces β-catenin expression through PI3K/Akt
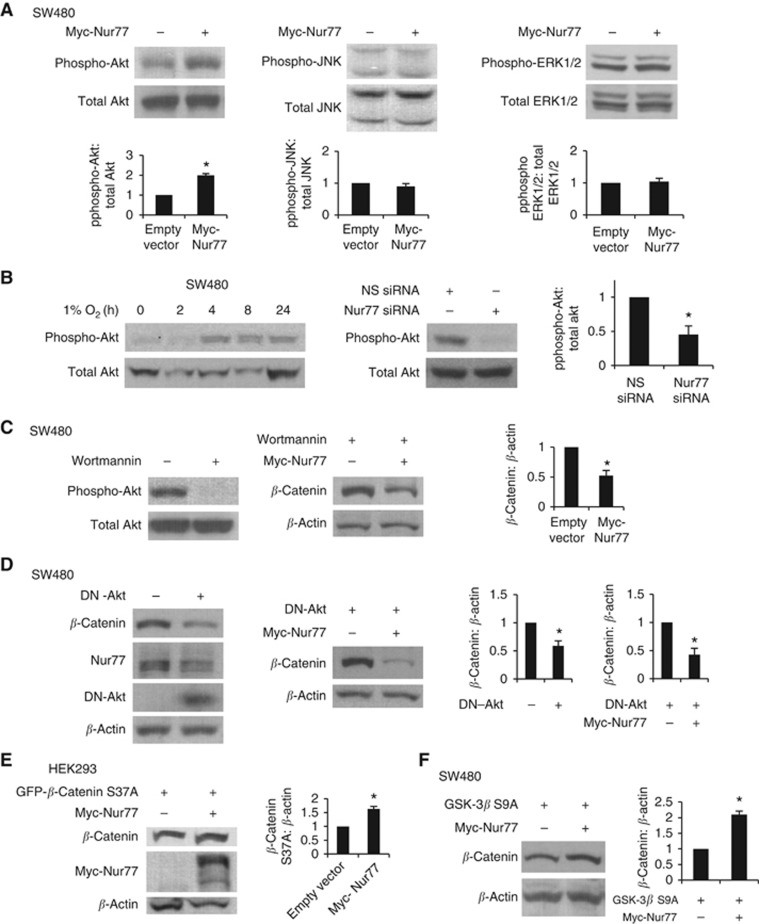
To determine the mechanism by which Nur77 induced β-catenin expression, we first examined the phosphorylation (activation) states of several signal pathways. Interestingly, we showed that overexpression of Nur77 significantly increased the phosphorylation of Akt, but not that of ERK1/2 and JNK (Figure 5A). We then asked whether hypoxia could induce Akt activation and whether such effect was mediated by Nur77. Indeed, we showed that hypoxia increased Akt phosphorylation in a time-dependent manner (Figure 5B), while normoxia did not (Supplementary Figure 1D). Such effect could be greatly inhibited by siRNA-mediated knocking down of Nur77 (Figure 5B), demonstrating that endogenous Nur77 was a mediator of hypoxic activation of Akt. Further, we explored whether hypoxia induced Nur77-mediated activation of Akt was essential for its elevation of β-catenin. Our results showed that while the PI3K inhibitor Wortmannin strongly suppressed Akt activation, it largely impaired the ability of exogenous Nur77 to induce β-catenin (Figure 5C). Moreover, a dominant-negative mutant of Akt (DN-Akt) could also strongly inhibit exogenous Nur77 to induce β-catenin expression (Figure 5D). These observations demonstrated that Nur77-mediated Akt activation by hypoxia has a critical role in hypoxia-induced activation of β-catenin.
Figure 5.
Nur77 stabilises β-catenin by activating PI3K/Akt. (A) SW480 cells transfected with empty vector or Myc-Nur77 for 24 h were exposed to 1% O2, followed by western blotting to detect the levels of phosphorylated forms of (phospho)-Akt, phospho-JNK, and phospho-ERK1/2. Total Akt, JNK, and ERK1/2 expression was included as loading controls. (B) SW480 cells were exposed to 1% O2 for different time intervals as indicated. Cells were transfected with non-specific (NS) siRNA or Nur77 siRNA for 24 h, and then incubated at 1% O2 for 4 h. (C) SW480 cells transfected with empty vector or Myc-Nur77 for 24 h were then treated with 50 nM Wortmannin for 1 h, followed by exposure to 1% O2 for 4 h. (D) SW480 cells were cotransfected with dominant-negative Akt (DN-Akt) and Myc-Nur77. After 24 h transfection, cells were incubated at 1% O2 for 4 h. (E) HEK293 cells were cotransfected with β-catenin S37A mutant and Myc-Nur77 or empty vector for 24 h, followed by incubation at 1% O2 for 4 h. (F) SW480 cells were cotransfected with GSK-3β S9A and Myc-Nur77. After 24 h transfection, cells were incubated at 1% O2 for 4 h, followed by western blotting. β-Actin was included as a loading control. Immunoblots were quantified by densitometry and the values were normalised with β-actin. All blots shown are representative of three independent experiments. Bar, ± s.d. *P<0.05.
A well-studied mechanism by which Akt regulates β-catenin is through GSK-3β (Cross et al, 1995; Delcommenne et al, 1998). Therefore, we next examined the involvement of GSK-3β by Nur77 in its induction of β-catenin. We found that Nur77 was able to increase the expression of a mutant form of β-catenin (S37A), which is insensitive to GSK-3β-mediated phosphorylation and subsequent proteasomal degradation (Figure 5E). Consistently, a constitutively active form of GSK-3β (S9A), which prevents phosphorylation and activates the kinase, did not inhibit the effect of Nur77 on inducing β-catenin expression (Figure 5F), suggesting a mechanism that relied upon PI3K/Akt but not GSK-3β.
Positive feedback between Nur77 and β-catenin promotes the invasive growth of CRC
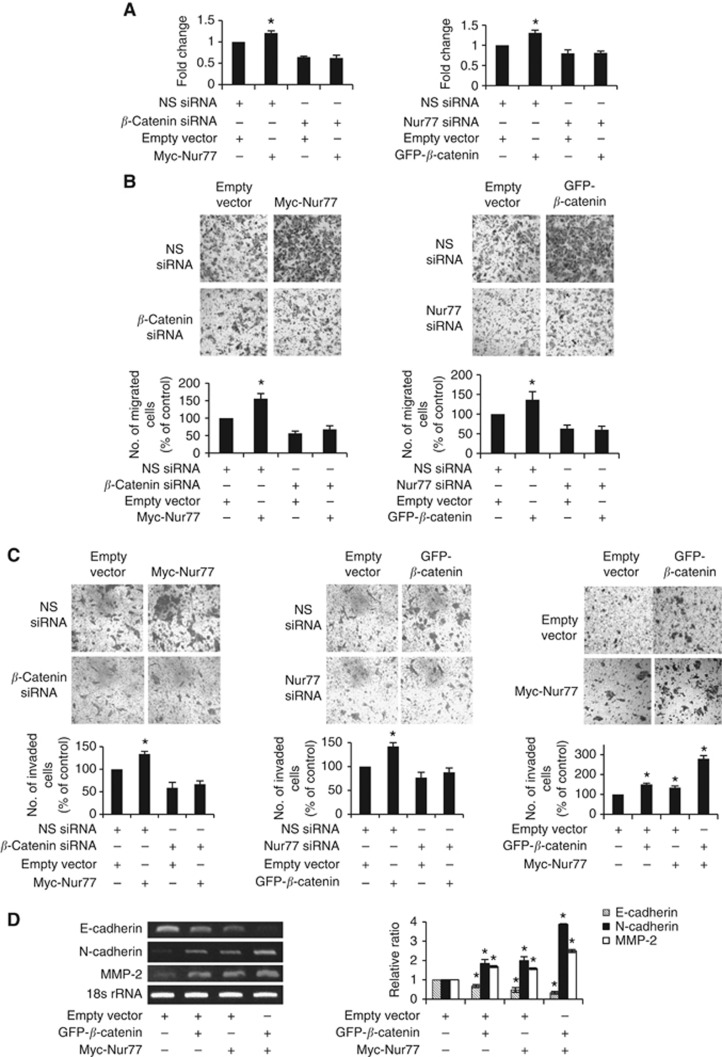
Hypoxia unleashes the invasive and metastatic potential of tumour cells. We investigated whether a positive feedback loop between Nur77 and β-catenin could have a role in growth, migration, and invasion of CRCs. We found that overexpression of Nur77 or β-catenin alone increased the growth of hypoxic CRC cells (Figure 6A). Notably, Nur77-induced survival was abolished by knocking down of β-catenin, whereas β-catenin-dependent survival was reduced in cells with Nur77 knockdown by specific siRNA (Figure 6A). We also found that either Nur77 or β-catenin siRNA decreased the number of migrated cells (Figure 6B). Nur77-induced migration was abolished in β-catenin siRNA-treated cells, and on the other hand, β-catenin-induced migration was decreased in Nur77 siRNA-treated cells (Figure 6B). This Nur77–β-catenin loop also induced cellular invasion across the Matrigel-coated membrane barrier (Figure 6C). To determine whether the molecular alterations of an epithelial-to-mesenchymal transition (EMT) occurred in these cells, we examined the expression of epithelial and mesenchymal markers by reverse transcription-PCR. As shown in Figure 6D, overexpression of Nur77 or β-catenin led to decreased expression of epithelial marker E-cadherin and increased expression of mesenchymal markers N-cadherin and matrix metalloproteinase-2, which is a well-documented extracellular matrix degrading enzyme and whose activity is closely associated with colorectal tumour invasiveness (Langers et al, 2008). Such effect on EMT markers could be synergistically enhanced by cotransfection of β-catenin and Nur77 (Figure 6D), suggesting a functional connection between Nur77 and β-catenin in the invasive growth of CRC cells under hypoxia.
Figure 6.
Hypoxia-induced Nur77 enhances the survival and migration of colon cancer cells. (A) HCT116 cells transfected with empty vector or Myc-Nur77 were treated with non-specific (NS) siRNA or β-catenin siRNA. Similarly, HCT116 cells transfected with empty vector or GFP-β-catenin were treated with NS siRNA or Nur77siRNA. After 24 h of transfection, cells were grown under hypoxia (1% O2) for an additional 48 h. Cell viability was then determined by MTT assays as described in Materials and Methods. Results were represented as a fold change compared with the control. (B) Cell migration was performed using Boyden chambers and (C) invasion through Matrigel-coated filters. After 24 h of incubation under hypoxia, cells remained in the upper chambers were removed and cells migrated to the lower chambers were fixed, stained, and counted. (D) Expression of epithelial cellular adhesion molecule E-cadherin, mesenchymal markers N-cadherin and matrix metalloproteinase (MMP)-2 was assessed by RT-PCR. 18s rRNA was used as an internal control. All gels shown are representative of three independent experiments. Bar, ±s.d. *P<0.05.
Discussion
Since hypoxia readily occurs in CRC and other solid tumours, deciphering hypoxia-regulated signalling pathways involved in tumorigenesis is of paramount importance for the understanding of tumour progression. In this study, an important positive reciprocal regulation of Nur77–β-catenin signalling that results in the enhancement of invasive growth in response to hypoxia is revealed, adding a new dimension to the understanding of the dysregulation of these two important signalling molecules that are characteristics of CRC.
The critical role of β-catenin in transcription is underscored by its ability to bind and activate the TCF transcription factors (Korinek et al, 1997; Morin et al, 1997). However, accumulating evidence shows that β-catenin can regulate transcription in a TCF-independent manner by interacting with a panel of different partners (Le et al, 2008). Indeed, our data suggest that β-catenin activates Nur77 transcription through HIF-1α, which is independent of TCF. Consistently, two functional hypoxia-responsive element (HRE) binding sites were found within the Nur77 promoter (Choi et al, 2004). It has recently been reported that HIF-1α competes with TCF-4 for direct binding to β-catenin under hypoxia, suggesting a functional switch is instigated to promote cell survival and adaption to hypoxia (Kaidi et al, 2007). The HIF-1α/β-catenin interaction has been demonstrated in various cancer cell types and in clinical samples, including colon tumours (Kaidi et al, 2007; Wincewicz et al, 2010). It is intriguing to speculate that hypoxia-induced interaction between β-catenin and Nur77 is possibly orchestrated by HIF-1α, a novel mechanism for survival and aggressive behaviour of CRC under hypoxic microenvironment. Although we did not further explore this interaction, the fragment encompassing amino acids 530–722 of β-catenin has been shown to interact with HIF-1α (Kaidi et al, 2007), and the ligand binding domain of Nur77 is required for β-catenin binding (Chen et al, 2012).
In the previous years, most molecular studies on tumorigenesis have focused on single pathways. However, it is becoming increasingly clear that these signals occur in a complex network of independent and interacting actions to cause malignant transformation. We have discovered here a positive feedback loop in the Nur77/β-catenin crosstalk by hypoxia. Consistently, we previously reported that both β-catenin and Nur77 were overexpressed in human colon tumours and deoxycholic acid, a tumour promoter in human colorectal carcinogenesis, could significantly enhance β-catenin-mediated Nur77 expression (Wu et al, 2011). This positive feedback may have a regulatory role in the Nur77/β-catenin pathway that was not explicitly realised by previous studies. In fact, the positive feedback in Nur77/β-catenin may casually participate in the pathogenesis of CRC, in which Nur77 reinforces the neoplastic potential of CRC via β-catenin largely independent of external cues. This self-enforced reciprocal activation may be of particular importance in tumour cells, which need to be constantly replenished to sustain unrestricted cell proliferation and migration observed.
Our findings that Nur77ΔDBD could induce β-catenin activation, suggesting the involvement of a non-genomic rather than genomic action of Nur77. Although the rapid non-genomic effects of Nur77 on activation of signalling cascades have attracted increasing attention in recent years (To et al, 2012), its underlying mechanisms are not yet clear. It is noteworthy that unlike many other members of the nuclear receptors that are predominantly localised in the nucleus, Nur77 can translocate from nucleus to the cytoplasm (Han et al, 2006). It is largely recognised that cytoplasmic Nur77 is pro-apoptotic. It is demonstrated that many anticancer drugs or apoptosis-inducing agents can target Nur77 to mitochondria where it interacts with Bcl-2 resulting in cell killing (Liu et al, 2008). Further, our recent report shows that two digitalis-like compounds H-9 and ATE-i2-b4 induce JNK-mediated Nur77 nuclear export leading to β-catenin degradation and tumour growth inhibition (Sun et al, 2012). Our present results demonstrate that cytoplasmic Nur77 by hypoxia can be also pro-survival due to its activation of Akt and β-catenin. The complex and paradoxical relationship between β-catenin and Nur77 may be due to different stimuli with distinct pathways. In our search of how Nur77 might regulate β-catenin, our results suggest that this may act independently of APC and p53, which are classical pathways in the regulation of β-catenin (Munemitsu et al, 1995; Korinek et al, 1997; Liu et al, 2001; Matsuzawa and Reed, 2001), suggesting the importance in developing novel therapeutic strategy to combat CRC.
Although it has been shown that PI3K/Akt acts through GSK-3β to regulate β-catenin, our data indicate that GSK-3β is not involved in regulation of β-catenin. The most likely explanation is that PI3K/Akt may act directly on β-catenin to facilitate binding to an interacting partner other than GSK-3β. For example, β-catenin has been shown to be a direct phosphorylation target of Akt, and that phosphorylation can prime β-catenin for 14-3-3ζ binding and subsequent stabilisation (Tian et al, 2004). Another formal possibility is that PI3K/Akt could recruit other signalling molecules to regulate β-catenin. For example, PI3K/Akt has also been found to mediate β-catenin through activation of IκB kinase (Agarwal et al, 2005). Therefore, the exact mechanism by which PI3K/Akt upregulates β-catenin remains to be elucidated. Increased PI3K/Akt levels or altered PI3K/Akt signalling is commonly observed in CRCs and that aberrant PI3K/Akt activation correlates with tumour grade and poor survival (Kang et al, 2008; Hsu et al, 2011; Liao et al, 2012). Although the mechanism of β-catenin activation could also be mediated through a mitogen-activated protein kinase pathway (Ishitani et al, 1999), we ruled out this possibility in CRCs, since treatment of cells with a specific inhibitor of ERK1/2 (PD98059) or JNK (SP600125) had no effect on Nur77-inducd β-catenin (data not shown). Nor did Nur77 alter the level of ERK1/2 or JNK (Figure 5A). Recently, cross-talks between Nur77 and Wnt/β-catenin signalling have been reported in several tumour types. However, the findings regarding this interplay are inconsistent. Whereas Nur77 represses Wnt/β-catenin signalling in some studies (Smith et al, 2011; Chen et al, 2012), it activates Wnt/β-catenin in other cases (Rajalin and Aarnisalo, 2011). These discrepancies are indeed not surprising due to the fact that Nur77 can act differently with various partners, which are highly dependent on the specific cellular context and the nature of stimulus. Our findings showing that Nur77 drives a positive feedback loop to enhance β-catenin signalling through HIF-1α under hypoxia are highly suggestive of this view. The different or frequently opposite functions under hypoxia as reported for other proteins (Koumenis et al, 2001; Liu et al, 2007) highlight the importance of the microenvironment in signal transduction. There is accumulating evidence for the contribution of hypoxic microenvironment to CRC progression. For instance, hypoxia has been found to promote invasive behaviour in the colon cancer cells through enhancing EMT (Cannito et al, 2008; Hongo et al, 2013), and it also protects colon cancer stem cells from chemotherapy (Mao et al, 2013). Given the important role of hypoxia in cancer, it has become an attractive target for therapeutic intervention and thus the identification of molecular targets contributing to the survival of hypoxic cells could be a promising approach. The Nur77/β-catenin cross-talk reported here is of particular interest as it may represent a novel therapeutic opportunity against CRC and other hypoxic tumours.
In summary, we reveal a new mechanism by which functions in a positive feedback loop of β-catenin signalling may promote the aggressive behaviour of CRC, with implications for development of novel target therapies. Further studies will undoubtedly enhance the understanding of specific mechanisms by which Nur77/β-catenin crosstalk contributes to the development and progression of cancer.
Acknowledgments
We thank Dr B Vogelstein for providing HT29-β-gal, HT29-APC, HCT116, and HCT116-p53−/− cells. This work was supported by the Hong Kong Research Grant Council grants NSFC/RGC (30931160431/N_HKU 735/09) and NSFC (No. 31340029). Alice ST Wong is a recipient of the Croucher Senior Research Fellowship.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G, Sizemore N. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene. 2005;24:1021–1031. doi: 10.1038/sj.onc.1208296. [DOI] [PubMed] [Google Scholar]
- Baba Y, Nosho K, Shima K, Irahara N, Chan AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS, Ogino S. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176:2292–2301. doi: 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Cannito S, Novo E, Compagnone A, Valfre di Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A, Bozzo F, Cravanzola C, Bravoco V, Colombatto S, Parola M. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29:2267–2278. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- Cao D, Hou M, Guan YS, Jiang M, Yang Y, Gou HF. Expression of HIF-1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Cancer. 2009;9:432. doi: 10.1186/1471-2407-9-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HZ, Liu QF, Li L, Wang WJ, Yao LM, Yang M, Liu B, Chen W, Zhan YY, Zhang MQ, Cai JC, Zheng ZH, Lin SC, Li BA, Wu Q. The orphan receptor TR3 suppresses intestinal tumorigenesis in mice by downregulating Wnt signalling. Gut. 2012;61:714–724. doi: 10.1136/gutjnl-2011-300783. [DOI] [PubMed] [Google Scholar]
- Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Lei P, Hamilton S, Khan S, Ramaiah SK, Safe S. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Res. 2007;67:674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- Choi JW, Park SC, Kang GH, Liu JO, Youn HD. Nur77 activated by hypoxia-inducible factor-1alpha overproduces proopiomelanocortin in von Hippel-Lindau-mutated renal cell carcinoma. Cancer Res. 2004;64:35–39. doi: 10.1158/0008-5472.can-03-0145. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J Cell Biol. 2008;180:1087–1100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Han YH, Cao X, Lin B, Lin F, Kolluri SK, Stebbins J, Reed JC, Dawson MI, Zhang XK. Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene. 2006;25:2974–2986. doi: 10.1038/sj.onc.1209358. [DOI] [PubMed] [Google Scholar]
- Hongo K, Tsuno NH, Kawai K, Sasaki K, Kaneko M, Hiyoshi M, Murono K, Tada N, Nirei T, Sunami E, Takahashi K, Nagawa H, Kitayama J, Watanabe T. Hypoxia enhances colon cancer migration and invasion through promotion of epithelial-mesenchymal transition. J SurgRes. 2013;182:75–84. doi: 10.1016/j.jss.2012.08.034. [DOI] [PubMed] [Google Scholar]
- Hsu CP, Kao TY, Chang WL, Nieh S, Wang HL, Chung YC. Clinical significance of tumor suppressor PTEN in colorectal carcinoma. Eur J Surg Oncol. 2011;37:140–147. doi: 10.1016/j.ejso.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta29 catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol. 2009;219:271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- Kang B, Hao C, Wang H, Zhang J, Xing R, Shao J, Li W, Xu N, Lu Y, Liu S. Evaluation of hepatic-metastasis risk of colorectal cancer upon the protein signature of PI3K/AKT pathway. J Proteome Res. 2008;7:3507–3515. doi: 10.1021/pr800238p. [DOI] [PubMed] [Google Scholar]
- Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han YH, Dawson MI, Zhang XK. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol. 2003;23:8651–8667. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Koumenis C, Alarcon R, Hammond E, Sutphin P, Hoffman W, Murphy M, Derr J, Taya Y, Lowe SW, Kastan M, Giaccia A. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol Cell Biol. 2001;21:1297–1310. doi: 10.1128/MCB.21.4.1297-1310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Peters H, St Croix B, Haroon ZA, Dewhirst MW, Strausberg RL, Kaanders JH, van der Kogel AJ, Riggins GJ. Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst. 2001;93:1337–1343. doi: 10.1093/jnci/93.17.1337. [DOI] [PubMed] [Google Scholar]
- Langers AM, Sier CF, Hawinkels LJ, Kubben FJ, van Duijin W, van der Reijden JJ, Lamers CB, Hommes DW, Verspaget HW. MMP-2 geno-phenotype is prognostic for colorectal cancer survival, whereas MMP-9 is not. Br J Cancer. 2008;98:1820–1823. doi: 10.1038/sj.bjc.6604380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le NH, Franken P, Fodde R. Tumour-stroma interactions in colorectal cancer: converging on beta-catenin activation and cancer stemness. Br J Cancer. 2008;98:1886–1893. doi: 10.1038/sj.bjc.6604401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang CY. TBL1-TBLR1 and beta-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat Cell Biol. 2008;10:160–169. doi: 10.1038/ncb1684. [DOI] [PubMed] [Google Scholar]
- Liao X, Morikawa T, Lochhead P, Imamura Y, Kuchiba A, Yamauchi M, Nosho K, Qian ZR, Nishihara R, Meyerhardt JA, Fuchs CS, Ogino S. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18:2257–2268. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Stevens J, Rote CA, Yost HJ, Hu Y, Neufeld KL, White RL, Matsunami N. Siah-1 mediates a novel beta-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Liu M, Li D, Aneja R, Joshi HC, Xie S, Zhang C, Zhou J. PO(2)-dependent differential regulation of multidrug resistance 1 gene expression by the c-Jun NH2-terminal kinase pathway. J Biol Chem. 2007;282:17581–17586. doi: 10.1074/jbc.M702206200. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou W, Li SS, Sun Z, Lin B, Lang YY, He JY, Cao X, Yan T, Wang L, Lu J, Han YH, Cao Y, Zhang XK, Zeng JZ. Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogs. Cancer Res. 2008;68:8871–8880. doi: 10.1158/0008-5472.CAN-08-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q, Zhang Y, Fu X, Xue J, Guo W, Meng M, Zhou Z, Mo X, Lu Y. A tumor hypoxic niche protects human colon cancer stem cells from chemotherapy. J Cancer Res Clin Oncol. 2013;139:211–222. doi: 10.1007/s00432-012-1310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010;3:149–166. doi: 10.1007/s12307-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon YL, Wong AS. Gonadotropin-induced apoptosis in human ovarian surface epithelial cells is associated with cyclooxygenase-2 up-regulation via the beta-catenin/T-cell factor signaling pathway. Mol Endocrinol. 2006;20:3336–3350. doi: 10.1210/me.2006-0125. [DOI] [PubMed] [Google Scholar]
- Rajalin AM, Aarnisalo P. Cross-talk between NR4A orphan nuclear receptors and beta-catenin signaling pathway in osteoblasts. Arch Biochem Biophys. 2011;509:44–51. doi: 10.1016/j.abb.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Sermeus A, Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2011;2:e164. doi: 10.1038/cddis.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Lim W, Pearen M, Muscat GE, Sturm RA. Regulation of NR4A nuclear receptor expression by oncogenic BRAF in melanoma cells. Pigment Cell Melanoma Res. 2011;24:551–563. doi: 10.1111/j.1755-148X.2011.00843.x. [DOI] [PubMed] [Google Scholar]
- Sun Z, Cao X, Jiang MM, Qiu Y, Zhou H, Chen L, Qin B, Wu H, Jiang F, Chen J, Liu J, Dai Y, Chen HF, Hu QY, Wu Z, Zeng JZ, Yao XS, Zhang XK. Inhibition of beta-catenin signaling by nongenomic action of orphan nuclear receptor Nur77. Oncogene. 2012;31:2653–2667. doi: 10.1038/onc.2011.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72:2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Feetham MC, Tao WA, He XC, Li L, Aebersold R, Hood L. Proteomic analysis identifies that 14-3-3zeta interacts with beta-catenin and facilitates its activation by Akt. Proc Natl Acad Sci USA. 2004;101:15370–15375. doi: 10.1073/pnas.0406499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To SK, Zeng JZ, Wong AS. Nur77: a potential therapeutic target in cancer. Expert Opin Ther Targets. 2012;16:573–585. doi: 10.1517/14728222.2012.680958. [DOI] [PubMed] [Google Scholar]
- Wansa KD, Harris JM, Muscat GE. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J Biol Chem. 2002;277:33001–33011. doi: 10.1074/jbc.M203572200. [DOI] [PubMed] [Google Scholar]
- Wincewicz A, Koda M, Sulkowski S, Kanczuga-Koda L, Sulkowska M. Comparison of beta-catenin with TGF-beta1, HIF-1alpha and patients' disease-free survival in human colorectal cancer. Pathol Oncol Res. 2010;16:311–318. doi: 10.1007/s12253-009-9217-2. [DOI] [PubMed] [Google Scholar]
- Wong AS, Gumbiner BM. Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol. 2003;161:1191–1203. doi: 10.1083/jcb.200212033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Lin Y, Li W, Sun Z, Gao W, Zhang H, Xie L, Jiang F, Qin B, Yan T, Chen L, Zhao Y, Cao X, Wu Y, Lin B, Zhou H, Wong AS, Zhang XK, Zeng JZ. Regulation of Nur77 expression by beta-catenin and its mitogenic effect in colon cancer cells. FASEB J. 2011;25:192–205. doi: 10.1096/fj.10-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.