Abstract
A new tripodal tris(hydroxypyridinone) bifunctional chelator for gallium allows easy production of 68Ga-labelled proteins rapidly under mild conditions in high yields at exceptionally high specific activity and low concentration.
The germanium-68/gallium-68 generator promises to make molecular imaging with positron emission tomography (PET) more widely available clinically, much as the molybdenum-99/technetium-99m generator did in the last century for single photon imaging, by providing a convenient, economic and reliable source of positron-emitting radionuclide without the need for a local cyclotron.1 Gallium-68 has a half life of 68 min and a high positron yield (90%, 1.9 MeV).2 To achieve its full potential, incorporation of this isotope into biomolecules for molecular imaging requires bifunctional chelators able to bind the Ga3+ ion rapidly under mild aqueous conditions, to give a complex with sufficient kinetic stability in vivo to withstand challenge from plasma transferrin, allowing imaging over several hours.
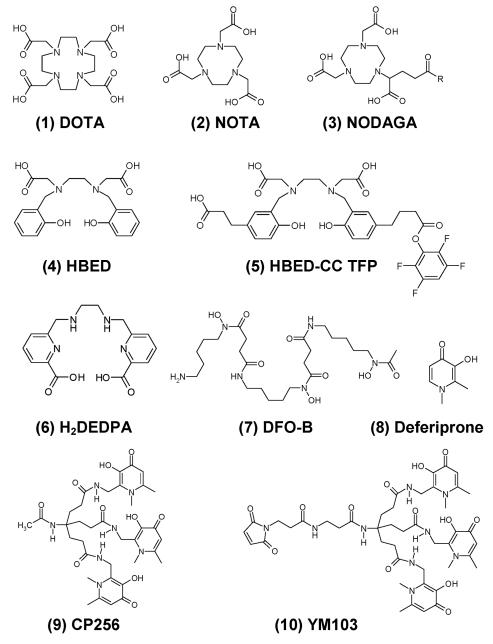
The current “gold standard” bifunctional 68Ga chelator is the aminocarboxylate macrocycle DOTA (1, Fig. 1) and 67Ga-DOTA bioconjugates have been reported to be stable in serum in vitro for at least 1250 h.3 The most well known 68Ga-DOTA bioconjugate is 68Ga-DOTATOC, a somatostatin analogue used clinically for somatostatin receptor imaging.4 Despite the high kinetic stability of the 68Ga-DOTA complex, DOTA has several drawbacks as a 68Ga chelator. Typical radiolabelling conditions entail heating (e.g. 95 °C) for up to 30 min at pH 4.6. The long radiolabelling time allows extensive decay during labelling and the high temperature and low pH are unsuitable for many proteins of interest for molecular imaging. Other chelators are being evaluated to circumvent some of these problems. The macrocycle NOTA (2) can be radiolabelled with 68Ga at room temperature within 10 min at pH 3–5.5 and the complex has excellent stability in plasma.5 Its derivative NODAGA (3) has been conjugated to proteins and radiolabelled with 68Ga at pH 3.5-4 in 7 min.6 Another promising chelator for 68Ga is HBED (4), which forms a Ga complex with a very high logK of 38.5.7 The derivative HBED-CC TFP (5) has been used for protein labeling in 80% yield by incubating at pH 4.1 for 5 min.8 The acyclic ligand DTPA complexes gallium with high affinity but poor kinetic stability.9 Derivatising the carbon backbone of DTPA can increase the stability of the 68Ga complexes. Nevertheless these 68Ga complexes undergo significant dissociation in serum.10 Recently the acyclic H2DEDPA (6) and a bifunctional derivative have shown promise as 67/68Ga chelators, radio-labelling at pH 4.5 in 10 min.11
Fig. 1. Structures of some 68Ga chelators.
The similarity in coordination chemistry between Fe3+ and Ga3+ suggests the use of siderophore-like chelators for gallium. Indeed the siderophore Desferrioxamine-B (DFO-B, 7) was used previously as a bifunctional chelator for 67/68Ga 12 but blood clearance of 68Ga-DFO-Octreotide in patients is slow.13 Deferiprone (8) is a bidentate 3-hydroxy-4-pyridinone (HPO) that is used clinically for the treatment of iron overload. Deferiprone and its analogues are effective scavengers of 67Ga with the ability to remove 67Ga bound to transferrin.14 Hexadentate tris-HPO derivatives have been developed for Fe3+ and Al3+ sequestration15 and have recently been shown to bind gallium with high affinity.16 However, these ligands cannot be derivatised for bioconjugation, and they are conformationally ill-suited to the formation of mononuclear six-coordinate complexes. Here we evaluate the use of CP256 (9), a powerful hexadentate HPO Fe3+ chelator which overcomes both these limitations,17 for radiolabelling with gallium radioisotopes. We report its radiolabelling properties with 68Ga (≤ 5 min at room temperature and at mild pH, ~6.5) in comparison with those of other chelators widely used for this purpose, and the stability of its 67Ga complex in biological media (see ESI for further information). We also report the synthesis of its bifunctional maleimide derivative YM-103 (10) and its use to label a thiol-containing protein C2Ac with 68Ga as a potential PET imaging agent for cell death.
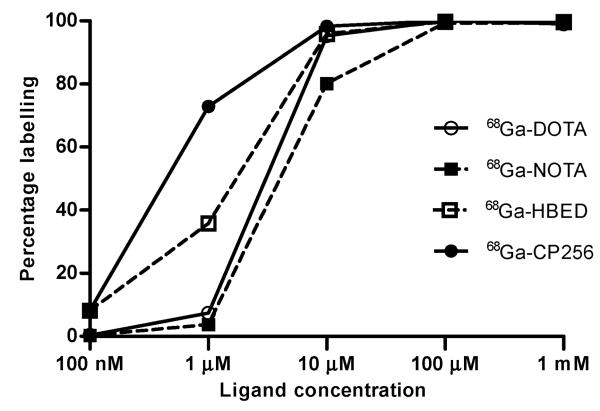
CP256 was found to complex natural-abundance gallium rapidly at neutral pH and room temperature. Mass spectrometry showed the presence of only a 1 : 1 complex at m/z 740.3631 for singly protonated Ga-CP256 and 370.6865 for the doubly protonated species. CP256 is designed to form neutral, six-co-ordinate complexes with 3+ metals such as Fe3+ and Ga3+ and these results indicate such a species. No multinuclear complexes were detected, and only a single species was detected by HPLC. Radio-HPLC analysis after 1 min incubation of CP256 with 67Ga-citrate showed a single radioactive peak with a retention time matching that of the cold Ga-complex. A side-by-side comparison was made of the 68Ga-chelating efficiency of CP256 with that of other established Ga chelators (DOTA, NOTA and HBED), using instant thin layer chromatography (ITLC) at progressively lower chelator concentration, using the same 68Ga generator (IGG100, Eckert & Ziegler, Germany) eluate for each ligand (Fig. 2). A radiochemical yield (RCY) of 68Ga-CP256 of 98–100% was achieved at a ligand concentration of 10 μM after 5 min, pH 6.5 at room temperature. At 1 μM, the RCY was 73%. In contrast, the RCY of 68Ga-NOTA (at room temp) and 68Ga-DOTA (with heating at 100 °C for 30 min at pH 4.4) fell to approximately 80% and 95% respectively at 10 μM and 4% and 7% at 1 μM (Fig. 2). The RCY of 68Ga-HBED dropped from 96–100% at 10 μM and above to 36% at 1 μM. Further data supporting the comparative labelling efficiency of CP256 are provided in the ESI.
Fig. 2. Radiolabelling yield versus ligand concentration for 68Ga-DOTA (pH 4.4, 30 min, 100 °C), 68Ga-NOTA (pH 3.6, 10 min, room temp), 68Ga-HBED (pH 4.6, 10 min, room temp) and 68Ga-CP256, (pH 6.5, 5 min, room temp).
All experiments were conducted with the same batch of 68Ga eluate. All radiolabelling buffers were 0.2 M acetic acid/sodium acetate.
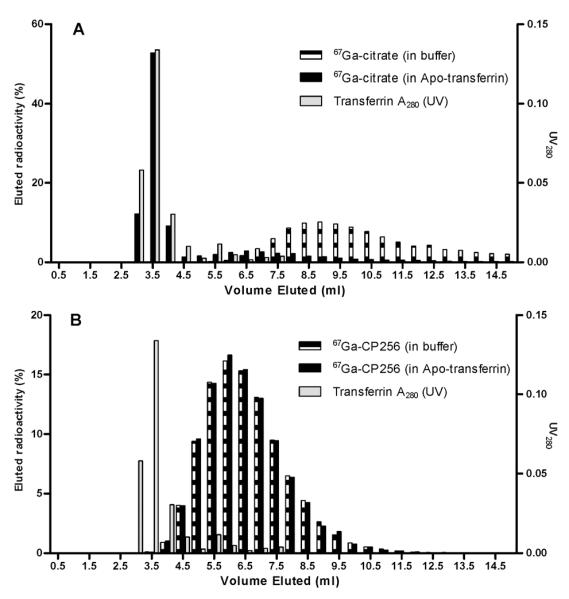
Incubation of 67Ga-CP256 in human serum for 4 h at 37 °C showed no evidence of protein binding or release of 67Ga when analysed by size exclusion chromatography. To provide a more stringent test of resistance to transchelation with transferrin, the complex was incubated with a 130-fold excess of apotransferrin (a 260 fold excess in terms of Ga-binding capacity since there are two metal binding sites per transferrin molecule) in the presence of bicarbonate at 37 °C. Again no transchelation was observed (Fig. 3), whereas significant transchelation by transferrin was observed with 67Ga-citrate as the control.
Fig. 3. Size exclusion elution profiles of (A) 67Ga-citrate and (B) 67Ga-CP256 in buffer and apo-transferrin after 4 h incubation.
The outstanding radiolabelling kinetics of 68Ga-CP256 and in vitro stability observed for 67Ga-CP256 encouraged us to synthesise a bifunctional derivative for protein labelling. The maleimide derivative 10 was chosen to confer site-specificity on conjugation to proteins containing an engineered free cysteine residue. To evaluate the potential of 10 for 68Ga labelling of biomolecules, the protein C2Ac was selected. C2Ac is an analogue of C2A (the phosphatidylserine (PS)-binding domain of synaptotagmin I, an amphipathic protein which binds to PS in a Ca2+-dependent manner and is being investigated as an imaging agent for cell death) into which a cysteine residue has been engineered18 for purposes of bioconjugate synthesis. Incubation of 10 (or its HCl addition product, which is also formed as an intermediate during the synthesis of 10, see ESI) with C2Ac produced the bioconjugate YM-103-C2Ac whose electrospray mass spectrum showed the incorporation of a single molecule of 10. The conjugate (20 μg, 1 mg ml−1, 63 μM) was incubated with freshly-eluted 68Ga in 0.5 M ammonium acetate buffer, pH ~5.5, giving quantitative labelling after 5 min as determined by TLC.
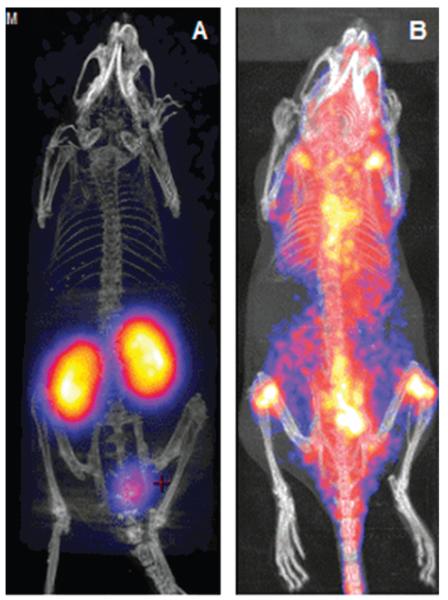
The radiolabelled conjugate showed retention of calcium-dependent binding to PS in a red blood cell binding assay in vitro. A PET imaging study was therefore carried out in a normal mouse (Fig. 4A).‡ After 90 min post-injection the 68Ga was located almost exclusively in the kidney, with some excretion to the bladder, in contrast to the distribution throughout the whole mouse observed (Fig. 4B) when uncomplexed 68Ga was injected. This demonstrates that 68Ga is not released from the conjugate in vivo during the imaging period. We conclude that the tris(hydroxypyridinone) ligand CP256 is an excellent chelator for gallium and can be radiolabelled with gallium isotopes more quickly, with higher yield, under milder conditions and to significantly higher specific activity than the currently established chelators DOTA, NOTA and HBED. The complex is extremely stable in serum and when challenged with excess apotransferrin. The maleimide derivative 10 can be conjugated site-specifically to cysteine residues and the resulting bioconjugate is efficiently labelled with 68Ga to high specific activity. It is likely to become the bifunctional chelator of choice for labelling of sensitive proteins with 68Ga.
Fig. 4. PET-CT images of mice 90 min after intravenous injection of (A) 68Ga-YM-103-C2Ac and (B) unchelated 68Ga.
Supplementary Material
Acknowledgments
This work was conducted within the King’s College London-UCL Comprehensive Cancer Imaging Centre supported by Cancer Research UK & EPSRC, in association with MRC and DoH (UK). The work was supported by an MRC studentship to DJB and by the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC under grants (WT 088641/Z/09/Z). JRB was supported in part by a National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. YM was supported in part by a grant from the British Heart Foundation Centre of Excellence at King’s College London. We thank Wellcome Trust for grant support enabling PET-CT imaging. Selected samples were sent for mass spectral analysis at the EPSRC Mass Spectrometry Service Centre (Swansea) and at the Mass Spectrometry Facility, King’s College, London (Franklin Wilkins Building).
Footnotes
Animal studies were carried out in accordance with UK Research Councils’ and Medical Research Charities’ guidelines on Responsibility in the Use of Animals in Bioscience Research, under a UK Home Office licence and were approved by the King’s College London Local Ethics Committee.
Notes and references
- 1.Breeman WAP, Verbruggen AM. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:97. doi: 10.1007/s00259-007-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reichert D, Lewis J, Anderson C. Coord. Chem. Rev. 1999;184:3. [Google Scholar]
- 3.Heppeler A, Froidevaux S, Mäcke H, Jermann E, Behe M, Powell P, Hennig M. Chem. Eur. J. 1999;5:1974. [Google Scholar]
- 4.Kowalski J, Henze M, Schuhmacher J, Mäcke HR, Hofmann M, Haberkorn U. Mol. Imaging Biol. 2003;5:42. doi: 10.1016/s1536-1632(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 5.Velikyan I, Maecke H, Langstrom B. Bioconjug. Chem. 2008;19:569. doi: 10.1021/bc700341x. [DOI] [PubMed] [Google Scholar]
- 6.Wangler C, Wangler B, Lehner S, Elsner A, Todica A, Bartenstein P, Hacker M, Schirrmacher R. J. Nucl. Med. 2011;52:586. doi: 10.2967/jnumed.110.082198. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Anderson C, Pajeau T, Reichert D, Hancock R, Motekaitis R, Martell A, Welch M. J. Med. Chem. 1996;39:458. doi: 10.1021/jm9505977. [DOI] [PubMed] [Google Scholar]
- 8.Eder M, Wangler B, Knackmuss S, LeGall F, Little M, Haberkorn U, Mier W, Eisenhut M. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:1878. doi: 10.1007/s00259-008-0816-z. [DOI] [PubMed] [Google Scholar]
- 9.Wagner S, Welch M. J. Nucl. Med. 1979;20:428. [PubMed] [Google Scholar]
- 10.Koop B, Reske SN, Neumaier B. Radiochim. Acta. 2007;95:39. [Google Scholar]
- 11.Boros E, Ferreira CL, Cawthray JF, Price EW, Patrick BO, Wester DW, Adam MJ, Orvig C. J. Am. Chem. Soc. 2010;132:15726. doi: 10.1021/ja106399h. [DOI] [PubMed] [Google Scholar]
- 12.Smith-Jones P, Stolz B, Bruns C, Albert R, Reist H, Fridrich R, Mäcke H. J. Nucl. Med. 1994;35:317. [PubMed] [Google Scholar]
- 13.Eisenwiener K, Prata M, Buschmann I, Zhang H, Santos A, Wenger S, Reubi J, Mäcke H. Bioconjug. Chem. 2002;13:530. doi: 10.1021/bc010074f. [DOI] [PubMed] [Google Scholar]
- 14.Santos M, Gil M, Marques S, Gano L, Cantinho G, Chaves S. J. Inorg. Biochem. 2002;92:43. doi: 10.1016/s0162-0134(02)00483-x. [DOI] [PubMed] [Google Scholar]
- 15.Chaves S, Marques SM, Matos AMF, Nunes A, Gano L, Tuccinardi T, Martinelli A, Santos MA. Chem.–Eur. J. 2010;16:10535. doi: 10.1002/chem.201001335. [DOI] [PubMed] [Google Scholar]
- 16.Chaves S, Mendonça AC, Marques SM, Prata MI, Santos AC, Martins AF, Geraldes CFGC, Santos MA. J. Inorg. Biochem. 2011;105:31. doi: 10.1016/j.jinorgbio.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Zhou T, Neubert H, Liu DY, Liu ZD, Ma YM, Kong XL, Luo W, Mark S, Hider RC. J. Med. Chem. 2006;49:4171. doi: 10.1021/jm0600949. [DOI] [PubMed] [Google Scholar]
- 18.Tavaré R, Torres Martin De Rosales R, Blower PJ, Mullen GED. Bioconjug. Chem. 2009;20:2071. doi: 10.1021/bc900160j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.