Abstract
Leptin-deficient patients report higher “liking” ratings for food, and leptin replacement therapy normalizes these ratings even before weight loss is achieved. Since animals cannot report their ratings, we studied the relationship between leptin and food reward in leptin-deficient ob/ob mice using a optogenetic assay that quantifies the reward value of sucrose. In this assay, mice chose between one sipper dispensing the artificial sweetener sucralose coupled to optogenetic activation of dopaminergic (DA) neurons, and another sipper dispensing sucrose. We found that the reward value of sucrose was high under a state of leptin deficiency, as well as at a dose of leptin that does not suppress food intake (12.5 ng/h). Treatment with higher doses of leptin decreased the reward value of sucrose before weight loss was achieved (100 ng/h), as seen in leptin-deficient patients. These results phenocopy in mice the behavior of leptin-deficient patients.
Keywords: Sucrose, Food preference, Reward value, Optogenetics, Leptin, Obesity
1. Introduction
Leptin is an adipose tissue hormone that functions as an afferent signal in a negative feedback loop that maintains relative constancy of adipose tissue mass [3]. Leptin functions as a homeostatic signal to control food intake, and recent studies have shown that leptin reduces food intake in part by diminishing the reward value of sucrose [1,2]. In humans, the reward value of food is assessed using a subjective rating scale for “liking”. Leptin-deficient patients report higher “liking” ratings for food, and leptin replacement therapy normalizes these ratings even before weight loss is achieved [1]. Assays of “liking” in rodents are limited by the fact that animals cannot verbally report their ratings. In order to assay “liking” in mice, we recently developed a two-bottle choice assay that measures preference for sucrose versus a reference stimulus in which ingestion of sucralose induces optogenetic stimulation of dopaminergic (DA) neurons [2,4,5]. In this assay, changes in preference relative to this optogenetic reference stimulus reflect changes in the reward value of the nutrient in the second bottle, in this case sucrose [2]. Using this assay, we previously accessed in wild-type mice the reward value of sucrose under different metabolic states – food restriction leading to weight loss increases the reward value of sucrose, and this increase is reversed by leptin. However, these results involved weight loss, and hence do not dissociate the hormonal effect from an effect of the body mass. In this report, we studied the effects of leptin treatment of ob mice on the reward value of sucrose before significant weight loss is achieved. Our results phenocopy the behavior of leptin-deficient humans, in which leptin treatment normalized the reward value of food even before weight loss.
2. Materials and methods
2.1. Animals
Dat-cre transgenic mice were obtained from the Laboratory of Nils-Goran Larsson, Karolinska Institutet [6]. Heterozygous ob/+ mice were obtained from Jackson Laboratories. Animals of either sex were kept in C57BL/7 background. Unless specified otherwise, mice were single housed and maintained under an inverted 12-h light/dark cycle, so that behavioral tests could be performed during the rodent active phase (dark). Standard chow and water were provided ad libitum, except during acclimatization to the behavioral chambers and laser training (see Section 2.3). Experimental protocols were approved by The Rockefeller University IACUCs and met the guidelines of the national Institutes of Health guide for the Care and Use of Laboratory Animals.
2.2. Virus preparation
To construct Cre-inducible recombinant AAV vectors, the DNA cassette carrying two pairs of incompatible lox sites (loxP and lox2722) was synthesized and the ChR2-mCherry transgene was inserted between the loxP and lox2722 sites in the reverse orientation [4]. The resulting double floxed reverse ChR2-mCherry cassette was cloned into a modified version of the pAAV2-MCS vector carrying the EF-1α promoter and the Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) to enhance expression [4]. The recombinant AAV vectors were serotyped with AAV5 coat proteins and packaged by the viral vector core facility at the University of North Carolina. The final viral concentration was 2×1012 genome copies/mL [4].
2.3. Surgeries
Surgical procedures were adapted from [2]. Virus or vehicle was injected via a pressure injector through pulled glass pipettes, which were positioned to the ventral tegmental area (VTA) on coordinates AP=−3.5 mm ML=±0.5 mm, DV=4.8 mm (Paxinos) with a stereotaxic frame. The fiber optic ferrule (Thorlabs) was chronically implanted with a stereotaxic frame, being secured with dental cement (Lang), and aimed at the aforementioned coordinates. Animals were allowed to recover from surgery for one week prior to any behavioral assays.
2.4. Behavioral and optogenetic setup
The lick-induced optogenetic stimulation has been described in our previous publication [2]. Briefly, MedAssociates chambers were equipped with two contact lickometers and a laser source (solid state Crystal laser, 473-nm wavelength) controlled by MedPC via a TTL impulse to be triggered upon lick detection. The laser turns on every five consecutive licks on the same sipper, being ON for one second and OFF for the following second. Animals were water deprived for 16–23 h, and acclimated to the chambers until side preference for either sipper was even, which required about one week. During the acclimation period mice were water deprived and were given water through the sippers inside the chamber for half an hour. Two-bottle preference was calculated as the ratio: preference for sipper1=number of licks on sipper1/(number of licks on sipper1+number of licks on sipper2) and expressed as percentage values, with 50% representing the indifference ratio. Behavioral data were analyzed with Excel, and expressed as mean±SEM. Significance tests comparing groups were t-tests, and, when appropriate, followed by Bonferroni corrections for multiple comparisons. After recovering from surgery animals were given water inside the chamber for half an hour, for four consecutive days. During day 1 mice were given water through one of the sippers, with the laser source being triggered upon licking as mentioned above (Laser Sipper=LS). Laser/sipper coupling was balanced. On day 2 mice were given water only through the other sipper, in the absence of laser activation (Control Sipper=CS). Day 3 and day 4 were, respectively, repetitions of days 1 and day 2. On the fifth day animals were given LS and CS simultaneously for 10 min. Once a clear bias towards LS was established (above 55%), animals were subjected to experiments testing the effects of different doses of leptin on the reward value of sucrose (Figures 3–5). Concentrations of sucrose and sucralose were set as published elsewhere and were, respectively, 140 mM and 0.5 mM [2].
Figure 3.
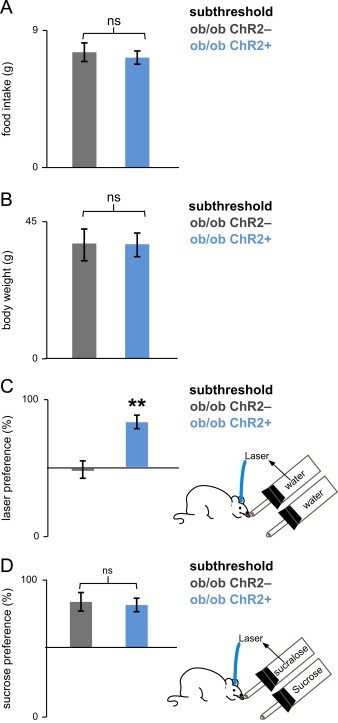
Subthreshold leptin treatment did not suppress the reward value of sucrose in ob/ob mice. (a) A subthreshold dose had no significant effect either on both eating behavior or on (b) body weight (statistics imbedded in text). (c) ob/ob;Chr2+, but not ob/ob;Chr2− mice, were sensitive to the rewarding effect of optogenetic activation of DA neurons during a regimen of leptin treatment of 12.5 ng per gram of body weight. ob/ob;Chr2+, but not ob/ob;Chr2− mice, preferred to lick the sipper coupled to laser stimulation of DA neurons. (d) Both ob/ob;Chr2+ and control ob/ob;Chr2− mice preferred sucrose to sucralose coupled to laser stimulation of DA neurons (statistics imbedded in text). Bars are mean±SEM, ⁎⁎p<0.0014.
Figure 4.
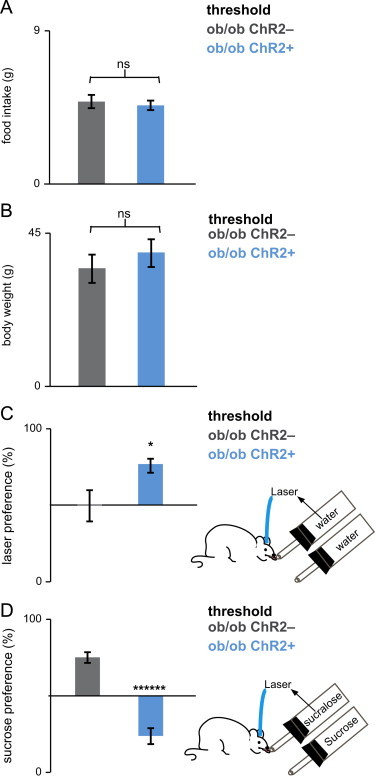
Threshold leptin treatment decreased the reward value of sucrose in ob/ob mice before weight loss. (a and b) A threshold dose of 100 ng per gram of body weight had an acute and significant effect on daily food intake, even before body weight decreases (statistics imbedded in text). (c) ob/ob;Chr2+ mice, but not ob/ob;Chr2− mice, were sensitive to the rewarding effect of optogenetic activation of DA neurons during a regimen of leptin treatment of 100 ng per gram of body weight. ob/ob;Chr2+, but not ob/ob;Chr2− mice, prefer to lick the sipper coupled to laser stimulation of DA neurons. (d) Control ob/ob;Chr2− mice preferred sucrose to sucralose coupled to laser stimulation of DA neurons, but ob/ob;Chr2+ mice preferred the reverse (statistics imbedded in text). Bars are mean±SEM, ⁎p<0.031, ⁎⁎⁎⁎p<0.00000023.
Figure 5.
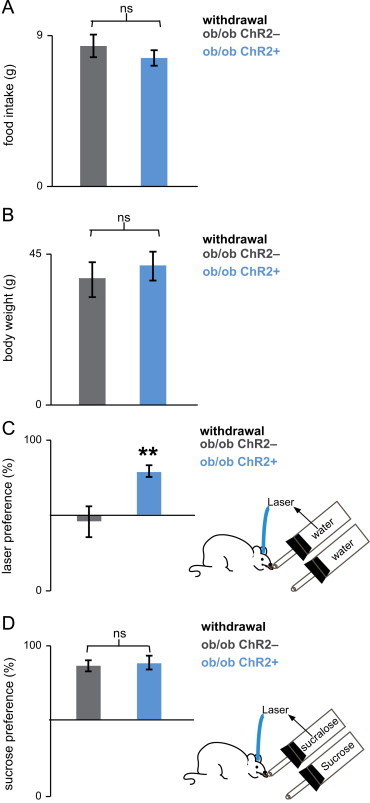
Leptin deficiency upregulates the reward value of sucrose. (a and b) Leptin deficiency by treatment withdrawal rapidly reverses changes in daily food intake (statistics imbedded in text). (c) ob/ob;Chr2+ mice, but not ob/ob;Chr2− mice, were sensitive to the rewarding effect of optogenetic activation of DA neurons after leptin treatment withdrawal. ob/ob;Chr2+, but not ob/ob;Chr2− mice, preferred to lick the sipper coupled to laser stimulation of DA neurons. (d) Both ob/ob;Chr2+ and control ob/ob;Chr2− mice preferred sucrose to sucralose coupled to laser stimulation of DA neurons 48 h after leptin treatment withdrawal (statistics imbedded in text). Bars are mean±SEM, ⁎⁎p<0.0012.
2.5. Immunohistochemistry
Immunohistochemistry was performed following the protocols as published elsewhere [2], using a rabbit anti-TH (Pel-Freez, 1:1000) antibody and appropriate host secondary antibodies.
2.6. Leptin treatment
Recombinant mouse leptin was obtained from Amylin Pharmaceuticals (San Diego, CA) and administered through subcutaneous osmotic pumps (Alzet; Palo Alto, CA) filled with the indicated concentrations of leptin and incubated overnight at 37 °C in sterile 0.9% NaCl, and implanted subcutaneously in 10- to 14-week-old mice. Posology is described in Figure 2.
Figure 2.
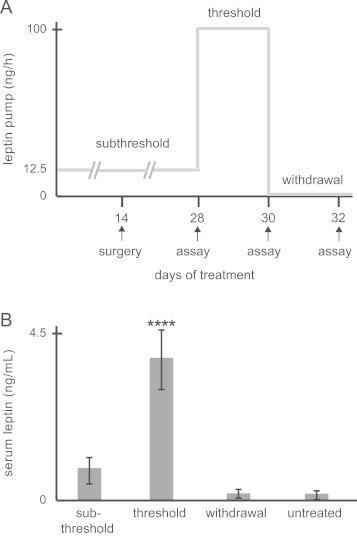
Leptin withdrawal protocol in implanted ob/ob Dat-cre mice. (a) This experimental design, in which the low dose was given before cranial surgery, was intended to improve implant stability, postoperative recovery and survival of ob/ob Dat-cre mice, minimizing the number of animals to be genotyped, implanted and euthanized. Mice had 14 days of leptin treatment before and after surgery and were behaviorally assayed before changing osmotic pumps. (b) Serum leptin levels were consistent with the dose regimen. Only the threshold dose induced serum leptin levels that were different from background serum leptin levels of untreated ob/ob mice (statistics imbedded in text). Bars are mean±SEM, ⁎⁎⁎⁎p<0.000022.
3. Results
3.1. Optogenetic targeting of dopaminergic neurons in ob/ob mice
We directed the expression of Channelrhodopsin-2 (ChR2) to DA neurons of the midbrain of genetically obese ob/ob mice as follows (Figure 1): Dat-cre mice were bred to heterozygous ob/+ mice, to generate ob/ob;Dat-cre mice. We stereotaxically injected a Cre-inducible adeno-associated virus carrying the gene encoding ChR2 fused to mCherry (AAV-DIO–ChR2-mCherry) into the ventral tegmental area (VTA) of ob/ob mice (Figure 1d). The nonrecombined construct is in the antisense orientation and is not expressed. Cre-mediated recombination activates ChR2-mCherry expression. The specificity of ChR2-expression in DA neurons was confirmed using immunohistochemistry, which revealed colocalization of mCherry and tyrosine hydroxylase (TH) (Figure 1a–c). Transduction efficiency averaged 75±8% (n=8) of TH-positive neurons, a value that is similar to that we have previously seen in viral transduction of wild type animals [2]. We next implanted an optical fiber into the VTA region of ob/ob;Dat-cre;AAV-DIo–ChR2-mCherry mice (Figure 1e) [2].
Figure 1.
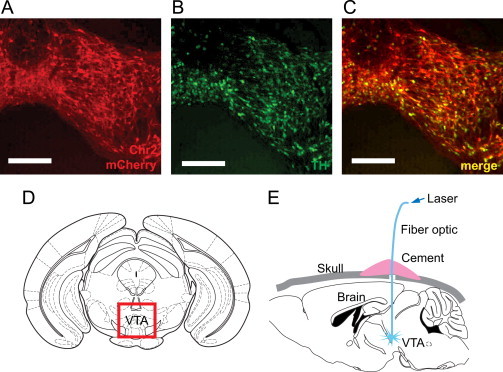
Tissue-specific expression of channelrhodopsin in DA neurons of ob/ob mice and cranial fiber optic implant. (a–c) AAV-DIO–ChR2-mCherry injection into the VTA of ob/ob;Dat-cre mice led to ChR2-mCherry expression in neurons colocalizing with tyrosine hydroxylase (TH), a marker for DA neurons (statistics imbedded in text, scale bars represent 100 µm). (d and e) Optical fibers were implanted above the VTA (red inset box in (d)) of ob/ob;Dat-cre mice.
3.2. Leptin treatment and withdrawal of implanted ob/ob Dat-cre mice
In initial experiments we encountered significant postoperative complications among ob animals that were not treated with leptin. Specifically, we noted that after implantation of the optical fiber, there was often excessive bleeding at the craniotomy site, which compromised the stability of the implant, and had a negative effect on postoperative recovery. This limited our ability to assay the preference of naïve ob mice. Therefore, we employed a leptin withdrawal protocol in which animals were first treated with a low dose of leptin (12.5 ng/h) before and after implantation of the optical fiber. Consistent with prior reports of the effect of leptin on immune and hematopoietic functions [7,8] leptin treatment dramatically reduced the frequency of post-operative complications in ob mice. After behavioral assays of animals treated with this dose of leptin, the osmotic pumps were removed and new pumps delivering a higher dose of leptin were implanted (100 ng/h), after which the animals were restudied at this higher dose. Finally, to study the behavior of animals in the absence of circulating leptin, the leptin osmotic pumps were removed after the assays of leptin treatment at the 100 ng/h dose were completed. Since the t1/2 of leptin in rodents is ~30 min, leptin levels decay to 0 within a day, and animals were studied 48 h post-leptin withdrawal. The use of this leptin withdrawal protocol allowed us to circumvent the aforementioned postoperative complications and perform behavioral assays in mice in the absence of circulating leptin.
In this leptin treatment/withdrawal protocol, animals are studied at three time points as indicated in Figure 2a. As described above, in this protocol, mice are first treated 14 days prior to surgery, and also 14 days postoperatively, with a dose of leptin (12.5 ng/h) which resulted in a leptin level of 0.91±0.12 ng/mL (Figure 2b). Throughout the text we refer to the 12.5 ng/h dose as a subthreshold dose. It is well established that leptin treatment with a higher dose (100 ng/h) has an acute effect on food intake [3]. We refer to this 100 ng/h dose as a threshold dose which led to a leptin level of 3.8±0.49 ng/mL (Figure 2b). The leptin level after withdrawal of the osmotic pumps was 0.24±0.09 ng/mL, which is not statistically different from background serum leptin levels of untreated ob/ob mice (Figure 2b) (p>0.18, t-test with Bonferroni correction for multiple comparisons). Indeed, only the threshold dose significantly increased serum leptin levels above background levels (⁎⁎⁎⁎p<0.000022, t-test with Bonferroni correction for multiple comparisons).
One week prior to implantation of the optical fiber, control ob/ob;Chr2− mice and ob/ob;Chr2+ mice received the subthreshold dose of leptin subcutaneously. This dose of leptin had no effect on body weight and food intake (Figure 3a and b) even after 28 days of treatment (ChR2−:p>0.2, ChR2+:p>0.17). Note, while the subthreshold dose of leptin did not alter food intake, it has been previously shown to be effective in correcting plasma glucose, which may also contribute to the diminished bleeding during the surgical procedure in animals treated with this or higher doses of leptin [9].
3.3. The reward value of sucrose in ob/ob mice treated with a subthreshold dose of leptin
We analyzed an animals' preference for (i) water versus optogenetic activation of DA neurons (Figure 3c) and (ii) sucrose versus sucralose plus optogenetic activation of DA neurons (Figure 3d), in ob mice treated with the subthreshold dose of leptin. ob/ob;Chr2+ mice (n=8) preferred water coupled to laser activation of DA neurons to water alone, displaying 83±5% preference for the sipper that was coupled to laser activation. This preference ratio was significantly different from the preference ratio of control ob/ob;Chr2− mice (n=8), that showed a 48±7% (iso)preference for the laser side (⁎⁎p<0.0014, two-sample t-test) (Figure 3c).
We next measured the reward value of sucrose in ob/ob;Dat-cre mice treated with the subthreshold dose (12.5 ng/h) (Figure 3d). Both ob/ob;Chr2− and ob/ob;Chr2+ animals preferred sucrose to sucralose coupled to laser activation with preference ratios of, 83±7% (n=8) and 82±5% respectively (n=8) (p>0.47 two-sample t-test) (Figure 3d). Thus a low dose of leptin that does not alter food intake has no effect on the animals' preference for sucrose suggesting that this leptin dose is insufficient to alter the reward value of sucrose (Figure 3a and d).
3.4. Threshold leptin treatment in ob/ob mice decreases the reward value of sucrose before weight loss
After implanting new osmotic pumps dispensing 100 ng/h of leptin (the threshold dose), animals consumed significantly less food relative to untreated ob mice or mice receiving the subthreshold dose: 33.6% decrease in the ob/ob;Chr2− mice and a 31.2% reduction in the ob/ob;Chr2+ at 48 h post-treatment was noted (Figure 4a, p>0.4, two-sample t-test). There was no difference in body weight after 48 h in these two groups. (p>0.3, two-sample t-test) (Figure 4b).
Behavioral tests were performed 48 h after treatment with the higher leptin dose. As in Section 3.3 we first tested whether leptin treatment of 100 ng per gram of body weight altered the rewarding effect of optogenetic activation of DA neurons per se (Figure 4c). We found that similar to control mice, ob/ob;Chr2+ mice (n=8) still preferred water coupled to optogenetic activation of DA neurons to water alone (Figure 4c). ob/ob;Chr2+ mice had 77±4% preference for the sipper that was coupled to laser activation compared to a of 50±11% preference ratio of ob/ob;Chr2− mice (n=8, ⁎p<0.031, two-sample t-test).
We next tested the effect of this leptin dose on the reward value of sucrose. When given a choice between sucrose versus sucralose coupled to laser activation, control ob/ob;Chr2− mice preferred the port with the sucrose with a preference ratio for sucrose of 78±4% (n=8) (Figure 4d). In contrast, for ob/ob;Chr2+ mice, threshold leptin treatment (100 ng/h) resulted in a reversal of this preference with a preference ratio for sucrose of 20±4% (n=8, significantly different from the preference ratio of ob/ob;Chr2− mice, ⁎⁎⁎⁎⁎⁎p<0.00000023, two-sample t-test) (Figure 4d). Thus, a dose of leptin capable of reducing food intake profoundly also suppressed the reward value of sucrose, even before weight loss was achieved (Figure 4).
3.5. Leptin deficiency increases the reward value of sucrose
At the conclusion of 48 h of threshold leptin treatment, the pumps delivering 100 ng/h of leptin were removed and replaced with new osmotic pumps filled with vehicle (Figure 5). At 48 h post-leptin withdrawal food intake of ob/ob;Chr2− and ob/ob;Chr2+ mice, was significantly increased compared to the food intake at the threshold dose (ob/ob;Chr2−:p<0.00013 and ob/ob;Chr2+:p< 0.023, two-sample t-test) (Figures 4 and 5a). Body weight increased by 5.2% in ob/ob;Chr2− mice and by 4.9% in ob/ob;Chr2+, but this increase was not statistically significant. (ob/ob;Chr2−:p>0.33 and ob/ob;Chr2+:p>0.35, two-sample t-test) (Figures 4 and 5b).
Forty-eight hours after removal of the leptin pumps, ob/ob;Chr2+ mice (n=8) preferred water coupled to laser activation of DA neurons with a 79±5% preference for the sipper that was coupled to laser activation versus the sipper containing water alone (Figure 5). This preference ratio was significantly different from the preference ratio of ob/ob;Chr2− mice (n=8), which scored a 45±7% preference for the laser side (⁎⁎p<0.0012, two-sample t-test) (Figure 5c). Finally, we measured the reward value of sucrose in leptin deficient mice, (post-leptin withdrawal) and found that both ob/ob;Chr2− and ob/ob;Chr2+ animals preferred sucrose to sucralose coupled to laser activation with preference ratios, of 86±2% (n=8) and 88±4% (n=8) (p>0.39 two-sample t-test) respectively (Figure 5d). This preference ratio is also similar to that of ob/ob;Chr2+ animals treated with a subthreshold dose of leptin (p>0.34 two-sample t-test, compared with ob/ob;Chr2+ in Figure 3d). These data indicate that leptin deficiency strongly increases the reward value of sucrose (Figure 5d).
4. Discussion
Animals and humans generally prefer sweeteners with nutrient value compared to non-nutritive sweeteners such as sucralose [2,9–13]. This preference is a result of the post-ingestive rewarding effect of sucrose [2]. The post-ingestive effect of sucrose was first described by showing that inert liquids that are paired to glucose administration either in the GI tract or in plasma, are greatly preferred to liquids that are not paired with nutrient [14,15]. In addition, sweet blind TRPM5 knockout mice can still sense the nutrient value of sucrose [16,17]. These studies have indicated that the nutrient value of sucrose is sensed and that, in turn, establishes preference for nutritive sweeteners [2,14–18]. The preference for sucrose relative to artificial sweeteners is associated with striatal dopamine release, resulting in a rewarding effect of sugar [2,14–18]. The post-ingestive effect plays a major role in driving nutrient choice rather than sweet taste [14–18]. The combination of sweet taste and an increase of dopamine accounts for the preference for natural versus artificial sweeteners [2].
In humans, food hedonics (“liking”) is measured with a subjective rating scale, but this scale cannot be used in studies of animals that cannot self-report. Current assays of “liking” in animals use subjective measures of orofacial expressions, similar to those used in human infants [19]. Rodent assays based on orofacial expressions have not been reported to distinguish between artificial and natural sweeteners. However, both humans and mice prefer natural to artificial sweeteners [2,10–14]. In addition, assays of orofacial expression in rodents predict that dopamine does not play a role in the hedonic value of sucrose [19] although several reports are consistent with a central role of dopamine: sucrose intake rapidly increases blood glucose, which leads to dopaminergic activation independent of taste perception, eliciting the post-ingestive rewarding effect of sucrose [2,15–19]. Artificial sweeteners, such as sucralose, do not raise blood glucose and lack this post-ingestive rewarding effect, which explains the innate preference for sucrose relative to sucralose [2,15–19]. As an alternative assay of food hedonics, we recently developed a two-bottle forced choice assay measuring preference for sucrose versus a reference stimulus in which ingestion of sucralose induces optogenetic stimulation of DA neurons [2]. In this assay, changes in preference relative to this reference stimulus reflect changes in the reward value of the nutrient in the second port, which in these studies is sucrose [2]. A choice behavior denotes the relative value of each option, as this optogenetic assay builds on neuroeconomic analyses of behavior, which can be generalized from humans to any species capable of making behavioral decisions [20–23]. In addition, choice behaviors can be automatically quantified with digital sensors, unlike subjective evaluation of orofacial expressions [2,20].
Using this optogenetic assay we have previously shown that the natural preference for sucrose versus sucralose could be inverted if ingestion of sucralose is supplemented by a proxy post-ingestive effect, in the form of optogenetic activation of DA neurons [2]. The reward value of sucrose was increased by fasting, and was decreased by leptin infusion [2]. However, these studies carried out in non-obese mice with intact leptin genes, and hence the effect of body weight and the hormonal action of endogenous leptin's on behavior could not be dissociated.
Leptin-deficient patients express high ratings for how much they “like” food prior to leptin treatment with a striking decrease in the “liking” score after leptin treatment. Hormone replacement of leptin deficient patients normalizes these ratings even before weight loss is achieved [1].
In this report we extend previous findings by showing that the reward value of sucrose in leptin-deficient ob mice is higher, and that leptin treatment normalizes the reward value of sucrose even before significant weight loss is achieved. These studies also allowed us to analyze the dose response relationship between leptin and the reward value of sucrose. This controlled dosage could not have been achieved in wild-type mice, which still have significant circulating levels of leptin even after weight loss.
As we implanted optical fibers that are required for the optogenetic reference stimulus, we found that leptin-deficient mice were highly susceptible to complications during and after the surgery. Similar to this observation, low leptin levels have also been associated with complicated postoperative course of cardiac surgery in humans [24]. Treatment with the low dose of leptin that significantly reduced surgical complications had no effect on body weight, food intake, and did not change the reward value of sucrose, even after 28 days of treatment. Infusion of a higher dose of leptin, which increased plasma leptin levels to about 4 ng/mL, decreased food intake before significant weight loss is achieved. To test the effect of leptin deficiency, the infusion pumps were removed. After 48 h serum leptin levels had dropped to the background levels as observed in untreated ob/ob mice. Food intake and the reward value of sucrose were strongly increased to levels that were observed during treatment with the low dose of leptin. Thus we show that leptin deficiency augments the reward value of sucrose, even before weight loss is achieved. We find that the effect of leptin on reward is dose dependent and correlated with its effects on food intake. In humans, changes in “liking” are correlated with changes in brain activity in reward-related regions such as the striatum [1]. This effect of leptin is in contrast to caloric restriction (dieting), which increases the perceived reward of food – “liking food” [1,2,25,26]. In humans, leptin deficiency is linked to food hedonics, but the same has been difficult to show in rodents. Others have analyzed the effects of leptin using operant conditioning assays such as progressive ratio (PR) schedules of reinforcement, or conditioned place preference (CPP) [27–29]. In a PR task, the subject is required to trigger an increasing number of operant responses for each successive reward. The number of responses triggered to obtain the last reward (“break point”) serves as an index of the willingness to work for food [27–29]. This operant procedure can be used in humans and animals [14,15,20]. Animals will work (press lever) because they are hungry. Animals will not work if not hungry. It is well known that leptin curbs hunger ratings, being known as an anorexigenic hormone. Hence lower hunger, or lower motivation to work, might be the reason why leptin decreased output in a lever-press operand task. Operant tasks are contingent upon a subject's motivational state to work, which limits its utility for assessing the pleasurable value of food. In fact, these assays cannot dissociate hunger, reward, and motivation work, hence preventing any conclusion about “food hedonics”. Because operant assays such as PR od CPP are one-way forced tasks (providing animals with only one choice – to work if hungry), reward and hunger are obscured by the motivation to work. In contrast, our assay provides two symmetric choices for ingesting a solution such that the amount of “work” required for licking either spout is the same. Because of this symmetry, we can exclude any confounds generated by “motivation to work”.
In summary, using a novel optogenetic assay we show that leptin deficiency augments the reward value of sucrose and that leptin treatment of ob mice reduces the reward value of sucrose, even before weight loss is achieved. These results phenocopy in a mouse model of obesity the clinical effects of leptin on a complex behavior of leptin-deficient patients, further validating our novel optogenetic assay to experimentally probe the reward value of food. The cellular and neural mechanisms by which leptin regulates the reward value of sucrose remain to be elucidated. Since leptin's primary site of action is the brain, further studies will be necessary to establish whether leptin acts on a heretofore unidentified nutrient sensor in the brain that conveys the reward value of sucrose [30–33]. As our assay can be easily generalized to ingestible fats, future studies will assay whether fat and sugar utilize the same circuitry to convey reward.
Conflict of interest
None declared.
Acknowledgments
We thank the JPB Foundation, The Klarman Family Foundation for Eating Disorders, and The Rockefeller Foundation for supporting this research. We thank the Gulbenkian Foundation and FCT (Portugal) for partial support to AID. The funding agencies had no involvement in how the research was conducted.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Ana I. Domingos, Email: dominan@igc.gulbenkian.pt.
Jeffrey M. Friedman, Email: friedj@rockefeller.edu.
Appendix A.
See Figure A1.
Figure A1.
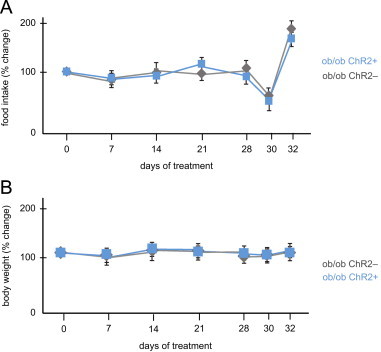
Food intake and body weight throughout leptin treatment: (a) percent change of food intake relative to the previous measurements, starting at pre-treatment on day 0; (b) percent change of body weight on the previous measurements, starting at pre-treatment on day 0.
References
- 1.Farooqi I.S., Bullmore E., Keogh J., Gillard J., O'Rahilly S., Fletcher P.C. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domingos A.I., Vaynshteyn J., Voss H.U., Ren X., Gradinaru V., Zhang F. Leptin regulates the reward value of nutrient. Nature Neuroscience. 2011;14:1–8. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman J.M. Modern science versus the stigma of obesity. Nature Medicine. 2004;10:563–569. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- 4.Tsai H., Zhang F., Adamantidis A., Stuber G., Bonci A., de Lecea L. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhury D., Walsh J.J., Friedman A.K., Juarez B., Ku S.M., Koo J.W., Fergusonet D. Rapid regulation of depression-related behaviors by control of midbrain dopamine neurons. Nature. 2012;493:1–7. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekstrand M.I., Terzioglu M., Galter D., Zhu S., Hofstetter C., Lindqvist E. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstantinides S., Schäfer K., Koschnick S., Loskutoff D.J. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. Journal of Clinical Investigation. 2001;108:1533–1540. doi: 10.1172/JCI13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lord G.M., Matarese G., Howard J.K., Baker R.J., Bloom S.R., Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 9.Hedbacker K., Birsoy K., Wysocki R.W., Asilmaz E., Ahima R.S., Farooqi I.S., Friedman J.M. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metabolism. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Walters, E.D., Orthoefer, F.E., DuBois, G., 1991. Sweeteners: discovery, molecular design, and chemoreception, p. 333.
- 11.Jacobson Michael F. Center for Science in the Public Interest; Washington, DC: 2005. Liquid candy; pp. pp. 1–46. [Google Scholar]
- 12.Sicher J. Soft drink market shares. Beverage Digest. 2011;59(5):1–2. 〈http://www.beverage-digest.com/pdf/top-10_2012.pdf〉 [Google Scholar]
- 13.Smiciklas-Wright H., Mitchell D., Mickle S., Cook A., Goldman J. 2002. Foods commonly eaten in the United States; pp. 1–264.〈http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/Portion.pdf〉 (US Department of Agriculture NFS report 965) [DOI] [PubMed] [Google Scholar]
- 14.Haley S. 2005. Sweetener consumption in the United States. (electronic outlook report from the Economic Research Service-USDA) [Google Scholar]
- 15.Sclafani A., Touzani K., Bodnar R.J. Dopamine and learned food preferences. Physiology and Behavior. 2011;104:64–68. doi: 10.1016/j.physbeh.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren X., Ferreira J.G., Zhou L., Shammah-Lagnado S.J., Yeckel C.W., De Araujo I.E. Nutrient selection in the absence of taste receptor signaling. Journal of Neuroscience. 2010;30(23):8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Araujo I.E., Oliveira-Maia A.J., Sotnikova T.D., Gainetdinov R.R., Caron M.G., Nicolelis M.A.L. Food reward in the absence of taste receptor signaling. Neuron. 2008;57(6):930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 18.de Araujo I.E., Ren X., Ferreira J.G. Metabolic sensing in brain dopamine systems. Results and Problems in Cell Differentiation. 2010;52:69–86. doi: 10.1007/978-3-642-14426-4_7. [DOI] [PubMed] [Google Scholar]
- 19.Ivan E., de Araujo I.E., Lin T., Veldhuizen M.G., Small D.M. Metabolic regulation of brain response to food cues. Current Biology. 2013;23(10):878–883. doi: 10.1016/j.cub.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berridge K. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neuroscience and Biobehavioral Reviews. 2000;24(2):173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 21.Glimcher P.W., Rustichini A. Neuroeconomics: the consilience of brain and decision. Science. 2004;306:447–452. doi: 10.1126/science.1102566. [DOI] [PubMed] [Google Scholar]
- 22.Sugrue L.P., Corrado G.S., Newsome W.T. Choosing the greater of two goods: neural currencies for valuation and decision making. Nature Reviews Neuroscience. 2005;6:363–375. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- 23.Padoa-Schioppa C., Assad J. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nature Neuroscience. 2008;11(1):9. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modan-Moses D., Kanety H., Dagan O., Ehrlich S., Lotan D., Pariente C., Novikovet I. Leptin and the post-operative inflammatory response. More insights into the correlation with the clinical course and glucocorticoid administration. Journal of Endocrinological Investigation. 2010;33(10):701–706. doi: 10.1007/BF03346673. [DOI] [PubMed] [Google Scholar]
- 25.Cameron J.D., Goldfield G., Cyr M., Doucet E. The effects of prolonged caloric restriction leading to weight-loss on food hedonics and reinforcement. Physiology and Behavior. 2008;94:474–480. doi: 10.1016/j.physbeh.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Epstein L.H., Truesdale R., Wojcik A., Paluch R.A., Raynor H.A. Effects of deprivation on hedonics and reinforcing value of food. Physiology and Behavior. 2003;78:221–227. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- 27.Figlewicz D.P., Higgins M.S., Ng-Evans S.B., Havel P.J. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiology and Behavior. 2001;73(1–2):229–234. doi: 10.1016/s0031-9384(01)00486-3. [DOI] [PubMed] [Google Scholar]
- 28.Figlewicz D.P., Bennett J.L., Naleid A.M., Davis C., Grimm J.M. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiology and Behavior. 2006;89(4):611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Davis J.F., Choi D.L., Schurdak J.D., Fitzgerald M.F., Clegg D.J., Figlewicz D.P. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biological Psychiatry. 2011;69(7):668–674. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong K., Vong L., Parton L.E., Ye C., Tong Q., Hu X., Choi B., Bruning J.C., Lowell B.B. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metabolism. 2010;12(5):545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanley S, Domingos AI, Kelly L, Garfield A, Damanpour S, Heisler L. Profiling of glucose-sensing neurons reveals that GHRH neurons are activated by hypoglycemia. Cell Metabolism. 2013;18(4):596–607. doi: 10.1016/j.cmet.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Dus M., Min S., Keene A.C., Lee G.Y., Suh G.S. Taste-independent detection of the caloric content of sugar in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dus M., Ai M., Suh G.S. Taste-independent nutrient selection is mediated by a brain-specific Na(+)/solute co-transporter in Drosophila. Nature Neuroscience. 2013;16(5):526–528. doi: 10.1038/nn.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]


