Abstract
BACKGROUND
We studied whether a melanoma survivor-centered intervention was more effective than materials available to the general public in increasing children’s sun protection.
METHODS
In a randomized controlled trial, melanoma survivors (n=340) who had a child ≤12 years received a targeted sun protection intervention (DVD and booklets) or standard education. Primary outcomes were children’s sunburns, children’s sun protection, and survivors’ psychosocial factors at baseline and postintervention (1 and 4 months).
RESULTS
The intervention increased children’s sunscreen reapplication at 1 month (P = 0.002) and use of wide-brimmed hats at 4 months (P = 0.045). There were no effects on other behaviors or sunburns. The intervention improved survivors’ hats/clothing self-efficacy at both follow-up assessments (P = 0.026, 0.009). At 4 months, the intervention improved survivors’ clothing intentions (P = 0.029), knowledge (P = 0.010), and outcome expectations for hats (P = 0.002) and clothing (P = 0.037). Children’s sun protection increased with survivors’ intervention use. The intervention was less effective in survivors who were female or who had a family history, older children, or children with higher baseline sun protection scores.
CONCLUSIONS
A melanoma survivor-centered sun protection intervention can improve some child and survivor outcomes. The intervention may be more effective in survivors who have younger children or less experience with sun protection. Intervention delivery must be enhanced to maximize use.
IMPACT
This is the first study to examine a sun protection intervention for children of melanoma survivors. Findings will guide interventions for this important population at increased melanoma risk.
Keywords: Melanoma, Prevention & Control, Survivors, Child, Health Behavior
INTRODUCTION
In 2013, about 76,690 new cases of invasive melanoma are expected in the United States, and incidence increases by about 3% per year (1, 2). Sun exposure during childhood increases melanoma risk (3), yet 43% to 76% of children experience sunburns (4–7). Recommended sun protection behaviors include using sunscreen, wearing protective clothing, seeking shade, and limiting time outdoors during midday (8).
Children of melanoma survivors are an important yet understudied population for sun protection intervention. Melanoma risk is approximately doubled in individuals who have a first-degree relative with melanoma, due to possible shared genotypic and/or phenotypic factors (9–11). A pilot study of melanoma survivors identified from the Los Angeles County cancer registry showed that 49% of survivors reported that their children experienced sunburns in the past year (12). To our knowledge, there are no studies of interventions to promote sun protection in the children of melanoma survivors.
Sun protection interventions involving melanoma survivors may have more relevance to and hence greater impact on survivors who are parents than standard sun protection education available to parents in the general public. We developed a melanoma survivor-centered sun protection intervention and hypothesized that it would be more effective than standard education in decreasing children’s sunburns, increasing children’s sun protection, and improving survivors’ psychosocial factors regarding children’s sun protection.
MATERIALS AND METHODS
Eligibility
Eligible survivors, identified from our hospital’s patient registry, were diagnosed between 1990 and 2008 with stage 0 to stage IIIB melanoma (13); were age ≥18 years; were able to speak, read, and write English; and had a child ≤12 years old. Survivors were mailed a study invitation letter and study information. Study personnel followed up by telephone to ascertain eligibility and obtain informed consent (ClinicalTrials.gov #NCT00394134). The study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center (Houston, TX).
Study design
We conducted a 2-armed randomized controlled trial. After baseline assessment, we used minimization, a covariate adaptive approach to randomization (14). Compared with techniques such as stratification, minimization results in better study arm balance with respect to participant characteristics (14). Participants were assigned to receive either a targeted sun protection intervention (n = 170) or standard education (n = 170), while balancing study arms on several characteristics: children’s and survivors’ baseline sun protection, children’s age, and survivors’ sex, melanoma stage, year of diagnosis, and melanoma family history. We randomly assigned the first participant to one of the study arms. Before assigning each subsequent participant, we totaled the numbers of participants in each study arm who had characteristics that were similar to the participant to be assigned. We then assigned the participant to the study arm that minimized characteristic imbalance between arms.
The baseline assessment was conducted in spring and summer. Participants received 3 mailings (targeted intervention or standard education) at their homes over a 5-month intervention period in fall and winter. Participants completed follow-up assessments in spring and summer at 1 month and 4 months postintervention.
Targeted sun protection intervention
The sun protection intervention included: (i) print booklet #1 and 10-minute DVD, (ii) print booklet #2 and magnet, and (iii) print booklet #3 and children’s activity booklet. We developed materials based on our previous experience in developing video and print interventions to increase children’s sun protection (15–17) and findings from qualitative research with melanoma survivors and their families. Materials development was further guided by social cognitive theory (SCT) (18) and the health belief model (HBM) (19), which have informed effective sun protection interventions for children and parents in the general population (15, 20–22).
SCT suggests mediators of behavior change, including self-efficacy (belief in one’s capability to perform a behavior), outcome expectations (beliefs regarding anticipated outcomes of behavior), and proximal goals (behavioral intentions) (23). Observational learning is a central tenet of SCT. Observing the actions, reinforcement, and positive self-evaluative reactions (e.g., feeling good about efforts to reduce children’s sun exposure) of role models depicted in the intervention was expected to improve survivors’ self-efficacy, outcome expectations, and intentions related to children’s sun protection. In HBM, health behavior performance is more likely if an individual perceives disease threat (risk and severity), believes that performance will reduce threat, perceives few performance barriers, and experiences cues to action (19). Knowledge also is an important determinant of health behavior (24). Parents’ self-efficacy, outcome expectations, intentions, risk perceptions, perceived benefits, perceived barriers, and knowledge have been associated with children’s sun protection (25–29).
Role models featured in the DVD and booklets were melanoma survivors who did not participate in the trial, their spouses, and their children of various ages. Families modeled the practice of sun protection in different settings (e.g., at home, in the park, and at the zoo). Through on-camera interviews in the DVD and personal stories and testimonials written by survivors for the booklets, survivors discussed sun protection expectations, why they protect their children (including risk perceptions and benefits), how they protect their children, and how they overcome sun protection barriers.
The children’s activity booklet contained educational puzzles, songs, and quizzes for children to educate them about sun protection, increase their cooperation with survivors’ sun protection efforts, and create opportunities for survivor-child interactions about sun protection. The magnet was designed to be a cue to action.
Standard education
Standard education comprised 3 health-related brochures available to the general public: (i) sun protection (30), (ii) physical activity (31), and (iii) nutrition (32). These brochures were mailed on the same schedule as the sun protection intervention. The standard education group received all intervention materials after the study.
Data collection
Survivors completed assessments by telephone at baseline, 1 month postintervention, and 4 months postintervention. If the survivor had 2 or more children ≤12 years old, one child was randomly selected and the survivor was asked to respond with this child in mind. Survivors were compensated $20 per assessment.
Measures
Child sunburn
We assessed the number of sunburns children experienced during the past 6 months (baseline and 1 month postintervention) and during the past 3 months (4 months postintervention).
Child sun protection
We measured individual behaviors and composite sun protection (a standard outcome that combines measures of each recommended behavior) (33). Questions were developed for this study and on the basis of our previous research (15, 34). Questions assessed behavior regarding sunscreen (7 questions), clothing (5 questions), shade (1 question), and limiting time outdoors between 10 a.m. and 4 p.m. (1 question) on 5-point response scales [1 (never) to 5 (always)]. Since we assessed multiple aspects of sunscreen (e.g., application, reapplication, and coverage) and clothing (e.g., hats, sunglasses, sleeved shirts, and pants) behavior, we averaged each survivor’s responses to these questions to create composite scores for children’s sunscreen behavior and for children’s clothing behavior. A composite sun protection score was computed by averaging the composite sunscreen score, the composite clothing score, the score for shade, and the score for limiting time outdoors.
Survivors’ psychosocial factors
We developed questions on the basis of our prior research (27, 34). Internal consistency reliability of multi-item scales was estimated by Cronbach’s alpha (α), which was averaged over the 3 assessments. Self-efficacy for children’s sun protection was assessed with behavior-specific measures: sunscreen (3 questions; α = .82), hats/clothing (2 questions; α = .60), shade (2 questions; α = .78), and limiting time outdoors (3 questions; α = .89). Questions (eg, “How confident are you that you can make sure that your child keeps a hat on each time he/she is outdoors, even on a windy day?”) had 5-point response scales [1 (not confident at all) to 5 (extremely confident)].
Outcome expectations were assessed for tanning (3 questions; α = .79) and children’s sunscreen (2 questions; α = .40), hats (3 questions; α = .72), clothing (3 questions; α = .76), shade (3 questions; α = .66), and limiting time outdoors (2 questions; α = .69). Questions (eg, “Wearing clothing that covers most of his/her body would make my child feel too hot”) had 5-point response scales [1 (strongly disagree) to 5 (strongly agree)]. Higher scores indicated more positive tanning outcome expectations and more negative sun protection outcome expectations.
Intentions were assessed for children’s sunscreen (2 questions; α = .79), hats/clothing (2 questions; α = .74), shade (1 question), and limiting time outdoors (1 question) behaviors. Survivors were asked about their intentions to protect their children each time they would be outdoors during the next 3 months with responses on 5-point scales [1 (strongly disagree) to 5 (strongly agree)].
Two perceived risk questions assessed survivors’ perceptions of the likelihood that their children would develop melanoma/skin cancer or sunburn in the future. Questions had 4-point response scales [1 (very unlikely) to 4 (very likely)].
Perceived benefits of children’s sun protection (5 questions; α = .73) was assessed by asking survivors whether sun protection would reduce their children’s risk of developing skin cancer [1 (strongly disagree) to 5 (strongly agree)].
Sun protection knowledge (6 questions; α = .39) was assessed. Questions (eg, “Does a sunscreen’s SPF number tell you the sunscreen’s level of protection against UVA and UVB rays from the sun?”) had yes/no/don’t know response options that were coded as correct/incorrect. Missing and don’t know responses were treated as incorrect.
Demographics and other variables
At baseline, demographics, sun sensitivity, and clinical characteristics were collected (Table 1).
Table 1.
Baseline demographics, sun sensitivity, and clinical characteristics of melanoma survivors and children
| Characteristic | Sun protection intervention (n = 170) | Standard education (n = 170) | P | ||
|---|---|---|---|---|---|
|
| |||||
| n (%) | Mean (SD) | n (%) | Mean (SD) | ||
| Survivor | |||||
| Age, y | 40.4 (6.4) | 40.5 (6.5) | 0.92a | ||
| Sex, male | 66 (38.8) | 64 (37.6) | 0.91b | ||
| Ethnicity | >0.99 | ||||
| Hispanic or Latino | 6 (3.5) | 6 (3.5) | |||
| Not Hispanic or Latino | 164 (96.5) | 164 (96.5) | |||
| Race, white | 169 (99.4) | 168 (100) | >0.99 | ||
| Education | 0.36 | ||||
| College graduate | 137 (80.6) | 129 (76.3) | |||
| Not a college graduate | 33 (19.4) | 40 (23.7) | |||
| Marital status, married | 150 (88.2) | 161 (95.3) | 0.03b | ||
| Sun sensitivity indexc | 2.16 (0.59) | 2.29 (0.60) | 0.06a | ||
| Smoking status | 0.82b | ||||
| Current smoker | 15 (8.8) | 12 (7.1) | |||
| Former smoker | 37 (21.8) | 39 (22.9) | |||
| Never smoker | 118 (69.4) | 119 (70.0) | |||
| Number of melanoma diagnoses | 0.18b | ||||
| 1 | 140 (82.8) | 149 (87.7) | |||
| 2 | 23 (13.6) | 13 (7.7) | |||
| ≥3 | 6 (3.6) | 8 (4.7) | |||
| Time since melanoma diagnosis, y | 4.56 (3.83) | 4.43 (3.45) | 0.73a | ||
| Stage of melanoma at diagnosis | 0.95b | ||||
| 0 | 30 (17.6) | 27 (15.9) | |||
| I | 116 (68.2) | 116 (68.2) | |||
| II | 10 (5.9) | 11 (6.5) | |||
| III | 14 (8.2) | 16 (9.4) | |||
| Number of first-degree relatives diagnosed with melanoma | >0.99 | ||||
| 0 | 136 (81.0) | 137 (81.6) | |||
| 1 | 26 (15.5) | 25 (14.9) | |||
| 2 | 6 (3.6) | 6 (3.6) | |||
| Child | |||||
| Age, y | 7.3 (3.9) | 7.3 (3.8) | 0.90a | ||
| Sex, male | 94 (55.3) | 79 (46.5) | 0.13b | ||
| Ethnicity | 0.39b | ||||
| Hispanic or Latino | 14 (8.3) | 9 (5.3) | |||
| Not Hispanic or Latino | 155 (91.7) | 160 (94.7) | |||
| Race, white | 166 (98.8) | 168 (99.4) | 0.62 | ||
| Sun sensitivity indexc | 2.29 (0.69) | 2.34 (0.66) | 0.48a | ||
Valid percentages are reported. Abbreviations: SD, standard deviation.
Two-sided t-test
Two-sided Fisher exact test
Computed from responses to questions about eye color, hair color, skin’s reaction to the sun and skin sensitivity.
Range 1–4; lower scores indicate higher sun sensitivity.
Process variables
We asked survivors in the intervention group if they viewed/read none, some, most or all of the DVD and each print booklet, and whether their families viewed/read (yes/no) these materials. We asked survivors whether they used the magnet (yes/no), their children liked the activity booklet (yes/no), and the activity booklet helped them teach their children about sun protection (yes/no). On a scale of 1 (strongly disagree) to 5 (strongly agree), survivors rated the attractiveness of materials and whether the information presented was credible, useful, new or interesting. Survivors in the standard education group were asked if they read none, some, most or all of each brochure. All survivors were asked if they received sun protection information from other sources during the study.
Statistical analyses
Linear mixed models (PROC MIXED of SAS 9.1.3 (35)) were used to examine main effects on continuous sun protection and psychosocial outcomes. Generalized linear mixed models (PROC GLIMMIX of SAS 9.1.3 (35)) were constructed using a logit link function to examine effects on binomial sunburn outcomes. Because we assessed sunburns for different recall periods, we divided the number of sunburns by the number of months in the recall period to calculate a sunburn rate for each assessment. Binary scores were defined as a success if either the sunburn rate decreased from baseline to postintervention assessments or no sunburns were reported at baseline and postintervention assessments. Covariates (demographics, sun sensitivity, and clinical characteristics) that were statistically significant (P < 0.05) in univariate models were retained in final models that included terms for intervention effect and assessment. The probability of a type I error was 5%. No adjustments were made for multiple testing. For measures with low estimates of internal consistency reliability (sun protection knowledge and sunscreen outcome expectations), we also examined the individual items as intervention outcomes. Findings were consistent with analyses based on the complete measures so we do not report item-level outcomes.
We explored whether the intervention was more effective in survivor subgroups by examining potential moderators (e.g., children’s age and baseline level of composite sun protection and survivors’ sex, number of melanomas, time since diagnosis, and melanoma family history). Mixed models included covariates and a 3-way interaction term (study arm by time by moderator). Mediation analyses will be reported in a separate paper.
We used linear mixed models to compare children’s composite sun protection outcomes for different levels of intervention use (none, some/most, and all) with outcomes for standard education. We also examined effects within the intervention group by assuming a linear dose response. To understand whether survivor subgroups were more likely to use the intervention, we used Wilcoxon rank-sum tests, Kruskal-Wallis tests, or Spearman correlation coefficients to examine the association between intervention use and survivor/child demographics, survivor/child sun sensitivity, survivor clinical characteristics, survivor baseline sun protection behaviors and psychosocial variables, and child baseline sun protection behaviors.
RESULTS
Participants
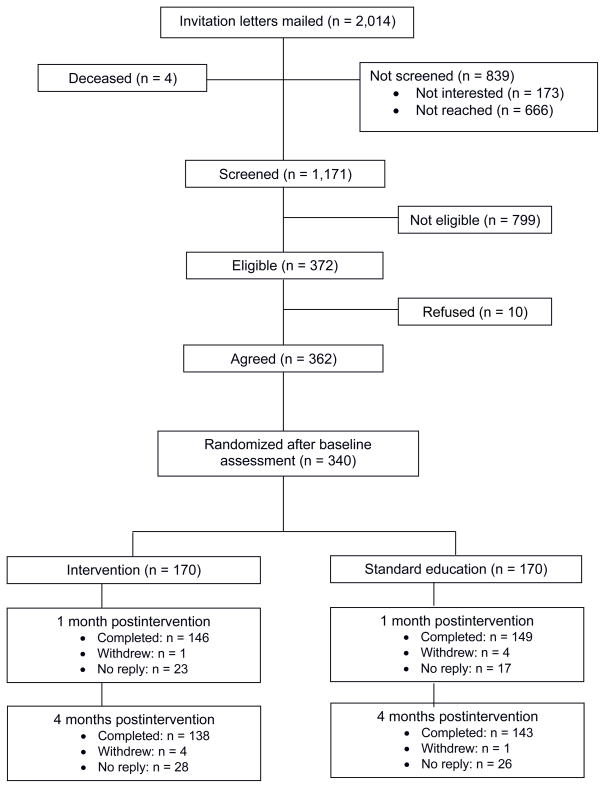
Of 2,014 screened melanoma survivors, 372 were eligible, 362 provided informed consent, and 340 completed the baseline assessment (Fig. 1). Most resided in Texas (82%) or other southern states (10%). Survivors randomized to standard education were more likely to be married (P = 0.03; Table 1); otherwise, study arms did not differ in baseline demographics, sun sensitivity, or clinical characteristics. Postintervention response rates were 87% (1 month, n = 295) and 83% (4 months, n = 281). Survivors who completed both follow-up assessments and those who did not complete one or both did not differ in baseline variables.
Figure 1.
CONSORT recruitment and study flow diagram for melanoma survivors
Intervention effects
Children’s sunburn and sun protection
We evaluated intervention effects on individual sun protection behaviors and a composite sun protection behavior score. We observed positive effects on children’s sunscreen reapplication after each hour outdoors at 1 month postintervention (Cohen’s effect size, d = 0.37) and on children’s wearing of wide-brimmed hats at 4 months postintervention (d = 0.24; Table 2). However, we did not observe intervention effects on other sun protection outcomes, including children’s sunscreen composite score, clothing composite score, shade behavior, limiting time outdoors behavior, or composite sun protection score. Children’s sunburn rate also did not decrease following the intervention (1 month, OR = 0.95, P = 0.90; 4 months, OR = 1.01, P = 0.98).
Table 2.
Main effects of sun protection intervention on children’s sun protection
| Outcome | Baselinea | 1 mo postintervention | 4 mo postintervention | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Scaleb | Meanc (SE) | Meanc (SE) | Bd | Pe | Meanc (SE) | Bd | Pe | |
| Sun protection (composite) | 1–5 | |||||||
| Intervention | 3.41 (0.13) | 3.62 (0.13) | 0.03 | 0.50 | 3.65 (0.13) | 0.00 | 0.94 | |
| Standard education | 3.41 (0.13) | 3.59 (0.13) | 3.65 (0.13) | |||||
| Sunscreen (composite) | 1–5 | |||||||
| Intervention | 2.64 (0.06) | 2.83 (0.07) | 0.06 | 0.35 | 2.89 (0.07) | 0.02 | 0.79 | |
| Standard education | 2.64 (0.06) | 2.78 (0.07) | 2.87 (0.07) | |||||
| Sunscreen 30 minutes before going outdoors | 1–5 | |||||||
| Intervention | 2.87 (0.09) | 3.08 (0.10) | 0.02 | 0.88 | 3.14 (0.10) | −0.11 | 0.30 | |
| Standard education | 2.87 (0.09) | 3.06 (0.10) | 3.24 (0.10) | |||||
| Sunscreen reapplied within 1 hour | 1–5 | |||||||
| Intervention | 3.05 (0.24) | 3.34 (0.25) | 0.11 | 0.23 | 3.42 (0.25) | 0.02 | 0.82 | |
| Standard education | 3.05 (0.24) | 3.22 (0.24) | 3.40 (0.24) | |||||
| Sunscreen reapplied after each hour outdoors | 1–5 | |||||||
| Intervention | 3.18 (0.24) | 3.43 (0.25) | 0.28 | 0.002 | 3.41 (0.25) | 0.10 | 0.27 | |
| Standard education | 3.18 (0.24) | 3.15 (0.25) | 3.31 (0.25) | |||||
| Clothing (composite) | 1–5 | |||||||
| Intervention | 3.03 (0.14) | 3.21 (0.15) | 0.00 | 0.99 | 3.18 (0.15) | 0.04 | 0.46 | |
| Standard education | 3.03 (0.14) | 3.21 (0.15) | 3.14 (0.15) | |||||
| Wide-brimmed hat | 1–5 | |||||||
| Intervention | 2.23 (0.29) | 2.37 (0.29) | 0.06 | 0.56 | 2.51 (0.29) | 0.20 | 0.045 | |
| Standard education | 2.23 (0.29) | 2.32 (0.29) | 2.31 (0.29) | |||||
| Sleeved shirt/longer pants | 1–5 | |||||||
| Intervention | 3.33 (0.05) | 3.44 (0.06) | −0.05 | 0.50 | 3.27 (0.06) | 0.10 | 0.88 | |
| Standard education | 3.33 (0.05) | 3.49 (0.06) | 3.49 (0.06) | |||||
| Shade | 1–5 | |||||||
| Intervention | 3.60 (0.14) | 3.76 (0.15) | 0.01 | 0.92 | 3.79 (0.15) | −0.04 | 0.53 | |
| Standard education | 3.60 (0.14) | 3.75 (0.15) | 3.83 (0.15) | |||||
| Limit time outdoors | 1–5 | |||||||
| Intervention | 3.90 (0.27) | 4.25 (0.28) | 0.07 | 0.52 | 4.29 (0.28) | 0.02 | 0.85 | |
| Standard education | 3.90 (0.27) | 4.18 (0.28) | 4.27 (0.28) | |||||
Abbreviations: SE, standard error.
No significant differences at baseline.
Higher scores indicate higher frequency of behavior.
Means are adjusted for survivors’ and children’s demographics and sun sensitivity covariates and for survivors’ clinical covariates.
B indicates the difference in the change from baseline between the intervention and standard education groups.
P indicates the significance of the test of the intervention by time interaction. Significant P values (<0.05) are in bold.
Child age significantly moderated effects on children’s sunscreen (1 month, B = −0.03, P = 0.005; 4 months, B = −0.04, P = 0.001) and clothing (1 month, B = −0.02, P = 0.043) behaviors: as child age increased, the intervention was less effective. For example, greatest effects on sunscreen behavior were in survivors who had children younger than 8 years old (1 month B = 0.22, P = 0.013; 4 months B = 0.31, P = 0.001). Survivors’ sex moderated intervention effects on children’s clothing (1 month, B = −0.17, P = 0.026; 4 months, B = −0.16, P = 0.037) and limiting time outdoors (4 months, B = −0.31, P = 0.049) behaviors: the intervention was less effective in female survivors. Family history of melanoma moderated effects on children’s limiting time outdoors behaviors (1 month, B = −0.42, P = 0.028): the intervention was less effective in survivors who had a family history of melanoma. Moderators of the intervention effect on children’s sunburns or shade behavior were not identified.
Baseline composite sun protection scores in children moderated intervention effects on children’s clothing (1 month, B = −0.16, P = 0.029; 4 months, B = −0.17, P = 0.026) and limiting time outdoors (1 month, B = −0.77, P < 0.0001; 4 months, B = −0.47, P = 0.001) behaviors: as baseline scores increased, the intervention was less effective. The strongest intervention effect on children’s clothing behavior was in survivors whose children had baseline sun protection scores lower than 3.4 (1 month, B = 0.32, P = 0.001; 4 months, B = 0.35, P < 0.0001). The strongest effect on limiting time behavior was in survivors whose children had scores lower than 3.0 (1 month, B = 0.45, P = 0.002; 4 months, B = 0.42, P = 0.004).
Survivors’ psychosocial outcomes
At 1 month postintervention, the sun protection intervention increased survivors’ hats/clothing self-efficacy (d = 0.26; Table 3). At 4 months postintervention, the intervention improved survivors’ hats/clothing self-efficacy (d = 0.33), clothing intentions (d = 0.27), sun protection knowledge (d = 0.32), and outcome expectations for children’s hat (d = −0.40) and clothing (d = −0.24) behaviors.
Table 3.
Main effects of sun protection intervention on survivors’ psychosocial outcomes
| Outcome | Scaleb | Baselinea | 1 mo postintervention | 4 mo postintervention | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean (SE) | Mean (SE) | B | P | Mean (SE) | B | P | ||
| Self-efficacy | ||||||||
| Sunscreen | 1–5 | |||||||
| Intervention | 4.04 (0.25) | 3.99 (0.26) | 0.01 | 0.89 | 3.97 (0.27) | 0.04 | 0.65 | |
| Standard education | 4.04 (0.25) | 3.98 (0.25) | 3.93 (0.26) | |||||
| Hats/Clothing | 1–5 | |||||||
| Intervention | 3.99 (0.26) | 4.26 (0.27) | 0.18 | 0.026 | 4.27 (0.27) | 0.22 | 0.009 | |
| Standard education | 3.99 (0.26) | 4.08 (0.27) | 4.06 (0.27) | |||||
| Shade | 1–5 | |||||||
| Intervention | 3.96 (0.23) | 4.09 (0.24) | 0.02 | 0.83 | 4.19 (0.24) | 0.04 | 0.64 | |
| Standard education | 3.96 (0.23) | 4.07 (0.24) | 4.15 (0.24) | |||||
| Limit time outdoors | 1–5 | |||||||
| Intervention | 4.65 (0.25) | 4.70 (0.25) | −0.03 | 0.71 | 4.74 (0.25) | 0.00 | 0.98 | |
| Standard education | 4.65 (0.25) | 4.73 (0.25) | 4.74 (0.25) | |||||
| Outcome expectations | ||||||||
| Sunscreen | 1–5 | |||||||
| Intervention | 1.89 (0.25) | 2.00 (0.26) | 0.05 | 0.65 | 1.89 (0.26) | −0.16 | 0.12 | |
| Standard education | 1.89 (0.25) | 1.95 (0.26) | 2.04 (0.26) | |||||
| Hats | 1–5 | |||||||
| Intervention | 2.58 (0.25) | 2.40 (0.26) | −0.15 | 0.10 | 2.40 (0.26) | −0.30 | 0.002 | |
| Standard education | 2.58 (0.25) | 2.56 (0.26) | 2.70 (0.26) | |||||
| Clothing | 1–5 | |||||||
| Intervention | 2.67 (0.26) | 2.51 (0.27) | −0.03 | 0.75 | 2.45 (0.27) | −0.20 | 0.037 | |
| Standard education | 2.67 (0.26) | 2.54 (0.27) | 2.65 (0.27) | |||||
| Shade | 1–5 | |||||||
| Intervention | 2.96 (0.27) | 2.81 (0.27) | −0.07 | 0.43 | 2.70 (0.27) | −0.12 | 0.19 | |
| Standard education | 2.96 (0.27) | 2.88 (0.27) | 2.82 (0.27) | |||||
| Limit time outdoors | 1–5 | |||||||
| Intervention | 1.83 (0.26) | 1.82 (0.27) | 0.00 | 0.97 | 1.65 (0.27) | −0.11 | 0.28 | |
| Standard education | 1.83 (0.26) | 1.81 (0.27) | 1.76 (0.27) | |||||
| Tanning | 1–5 | |||||||
| Intervention | 1.60 (0.23) | 1.52 (0.23) | −0.02 | 0.80 | 1.54 (0.23) | −0.06 | 0.46 | |
| Standard education | 1.60 (0.23) | 1.54 (0.23) | 1.59 (0.23) | |||||
| Intentions | ||||||||
| Sunscreen | 1–5 | |||||||
| Intervention | 3.85 (0.26) | 4.09 (0.27) | 0.13 | 0.15 | 3.79 (0.27) | 0.03 | 0.74 | |
| Standard education | 3.85 (0.26) | 3.96 (0.27) | 3.76 (0.27) | |||||
| Clothing | 1–5 | |||||||
| Intervention | 3.59 (0.12) | 3.79 (0.13) | 0.06 | 0.51 | 3.79 (0.13) | 0.20 | 0.029 | |
| Standard education | 3.59 (0.12) | 3.73 (0.13) | 3.59 (0.13) | |||||
| Shade | 1–5 | |||||||
| Intervention | 4.58 (0.25) | 4.71 (0.26) | −0.01 | 0.91 | 4.73 (0.26) | 0.07 | 0.46 | |
| Standard education | 4.58 (0.25) | 4.72 (0.26) | 4.65 (0.26) | |||||
| Limit time outdoors | 1–5 | |||||||
| Intervention | 4.46 (0.31) | 4.59 (0.31) | −0.02 | 0.86 | 4.53 (0.31) | 0.03 | 0.80 | |
| Standard education | 4.46 (0.31) | 4.61 (0.31) | 4.50 (0.31) | |||||
| Perceived risk – child | ||||||||
| Melanoma/skin cancer | 1–4 | |||||||
| Intervention | 3.53 (0.13) | 3.51 (0.14) | −0.03 | 0.68 | 3.57 (0.14) | 0.12 | 0.10 | |
| Standard education | 3.53 (0.13) | 3.54 (0.14) | 3.44 (0.14) | |||||
| Sunburn | 1–4 | |||||||
| Intervention | 4.09 (0.16) | 4.13 (0.17) | −0.13 | 0.09 | 4.10 (0.17) | −0.12 | 0.12 | |
| Standard education | 4.09 (0.16) | 4.26 (0.17) | 4.22 (0.17) | |||||
| Perceived benefits of sun protection | 1–5 | |||||||
| Intervention | 4.52 (0.03) | 4.52 (0.04) | −0.06 | 0.30 | 4.51 (0.04) | −0.02 | 0.68 | |
| Standard education | 4.52 (0.03) | 4.58 (0.04) | 4.53 (0.04) | |||||
| Knowledge | 0–6 | |||||||
| Intervention | 1.93 (0.32) | 2.07 (0.33) | 0.10 | 0.39 | 2.07 (0.33) | 0.32 | 0.010 | |
| Standard education | 1.93 (0.32) | 1.97 (0.32) | 1.75 (0.32) | |||||
Abbreviations: SE, standard error. Analyses are described in footnotes (c to e) to Table 2.
No significant differences at baseline.
Higher scores indicate more negative sun protection outcome expectations and higher levels of other variables.
Process evaluation
Of survivors in the intervention group, 71% used at least some of the DVD and booklets (Table 4). Relatively few reported that their family members used the materials (Table 5). A greater proportion of survivors in the standard education group than in the intervention group reported receiving sun protection information from sources other than the study materials (36% vs. 22% at 1 month, P < 0.05).
Table 4.
Melanoma survivors’ use of sun protection intervention DVD and print booklets and standard education
| Level of use | Sun protection intervention (%)a | Standard education (%)a | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| DVD | Booklet #1 | Booklet #2 | Booklet #3 | DVD and bookletsb | Sun protection brochure | Physical activity brochure | Nutrition brochure | |
| Did not receive | 16 | 14 | 30 | 28 | -- | 11 | 24 | 20 |
| Viewed/read none | 44 | 14 | 21 | 24 | 29 | 11 | 18 | 25 |
| Viewed/read some | 16 | 24 | 12 | 17 | 32 | 22 | 13 | 15 |
| Viewed/read most | 7 | 21 | 10 | 12 | 22 | 24 | 20 | 14 |
| Viewed/read all | 18 | 28 | 26 | 19 | 17 | 33 | 26 | 26 |
Percentages may not total 100 because of rounding. Data collected at 1 month postintervention.
Responses to questions about use of DVD and three booklets were averaged, resulting in a composite score that indicated overall use.
Table 5.
Use of and opinions about sun protection intervention, reported by melanoma survivors
| Process variable | % | Mean (SD)a |
|---|---|---|
| Family viewed DVD | 26 | |
| Family read booklet #1 | 27 | |
| Family read booklet #2 | 20 | |
| Family read booklet #3 | 22 | |
| Survivor used magnet | 54 | |
| Child liked activity booklet | 49 | |
| Activity booklet helped survivor teach child about sun protection | 50 | |
| Intervention materials… | ||
| Were attractive | 4.60 (0.65) | |
| Presented credible information | 4.69 (0.59) | |
| Presented useful information | 4.63 (0.58) | |
| Presented interesting information | 3.98 (0.91) | |
| Presented new information | 3.27 (1.06) | |
Response scale: 1 (strongly disagree) to 5 (strongly agree).
Survivors who used all of the intervention DVD and booklets reported higher children’s composite sun protection scores than did survivors in the standard education group (B = 0.27, P = 0.002). There were no effects for survivors who used none or some/most of the intervention materials. Dose-response analyses showed that while controlling for survivors’ baseline sun protection intentions, children’s composite sun protection increased as survivors’ use of the DVD and booklets increased (B = 0.11, P < 0.0001).
Intervention use was higher in survivors who had higher baseline levels of sunscreen (P < 0.05), clothing (P < 0.05), limiting time outdoors (P < 0.05), and composite sun protection (P < 0.05) behaviors. Intervention use also was higher in survivors who reported more positive outcome expectations about limiting their children’s time outdoors (P < 0.01) and more frequent children’s limiting time outdoors behavior (P < 0.05) at baseline. Intervention use was not associated with other survivor or child characteristics.
DISCUSSION
In general, the intervention increased sunscreen reapplication and use of wide-brimmed hats in children. Other effects on children’s sun protection or sunburns were not observed. The sunscreen reapplication findings were encouraging, especially considering that reapplication rates are low in children (36), children use less sunscreen per application than is recommended (37), and reapplication enhances protection (38). The effects on children’s hat behavior and survivors’ psychosocial factors that facilitate hat and clothing behaviors also were encouraging, as few interventions directed to parents have increased children’s hat outcomes (15, 39, 40) and, in general, 8% or fewer of children wear wide-brimmed hats (7, 36).
At baseline, children were protected by sunscreen and clothing only “sometimes” or less frequently. Thus, this sample of melanoma survivors provided an important opportunity to test the effect of this new intervention on these sun protection behavioral and related psychosocial outcomes. The limited intervention effect on other outcomes may be partly due to the relatively high (ie, desirable) baseline scores on measures and the relatively low baseline prevalence of children’s sunburn (28%), which may have limited our ability to evaluate intervention effects. Shade use and limiting time outdoors may be influenced by external factors not addressed by this intervention, such as shade availability in the environment and scheduling barriers. We cannot exclude the possibility that survivors in the standard education group engaged in compensatory behaviors, which would likely result in an underestimation of the intervention effect.
Effects may be strengthened by tailoring the intervention. Tailored interventions have increased sun protection (41, 42) and skin examination (41–43) in adult first-degree relatives of melanoma survivors and other adults at higher melanoma risk, suggesting that tailoring has the potential to be effective in melanoma survivors and their children. Personalized interventions may also draw from research on communication and melanoma risk attributions in families with melanoma (44–46).
Incomplete use of the intervention by survivors also may have contributed to the modest intervention effects. The DVD, in particular, had low rates of use, possibly because of inconvenience; substantially more survivors reported viewing none of the DVD compared with reading none of the booklets which could be easily skimmed upon receipt. We do not know to what extent perceived need influenced intervention use; however, survivors reported that they found the intervention to be useful and interesting.
We developed the intervention to be low cost and easily disseminated through regular mail. Future studies should identify strategies that increase intervention use by survivors and their families. For example, very little is known about how tailored materials may enhance intervention use by melanoma survivors. There is some evidence that tailored, compared with generic, print materials are read for a longer period of time by adult first-degree relatives of melanoma survivors (41). Mobile health and other e-health approaches [e.g., texts (47), mobile phone applications (48), web-based videos] are promising vehicles for sun protection interventions and need to be studied to determine whether they enhance the use of interventions designed to increase children’s sun protection. Communication technology is rapidly evolving and parents may consider it more convenient to access video content through mobile devices than a DVD, as was used in this study.
Results from moderation analyses suggest that the intervention may be more effective in survivors who have less experience with sun protection, including males, survivors without a melanoma family history, and survivors whose children had lower levels of sun protection before the intervention. We speculate that the intervention may be more effective in this group of survivors because the booklets and DVD could be revisited as sun protection skills were developed, the information addressed frequently asked questions about sun protection, and the role modeling stories, video scenes, and booklet photographs facilitated engagement with, and absorption of, the content. Our intervention may be an important first step in increasing children’s sun protection in this group of survivors. Although the intervention was developed for widespread dissemination to survivors regardless of child age, survivors with younger children benefited most from this intervention.
In community-based research, interventions guided by SCT and HBM have increased sun protection in children and parents (15, 20–22). Mediation analyses, currently underway, will help us determine whether a similar conceptual framework is relevant for interventions for melanoma survivors and their children. Interventions should be guided by theory to increase effectiveness in target audiences (33), and may need to combine multiple theories (24). Longitudinal studies are lacking in the literature on children’s skin cancer prevention (49), and are needed to better understand the theoretical predictors of sun protection in melanoma survivors and their children. Our findings suggest that our theoretical approach may have been more useful in subgroups of survivors. Further study is needed to understand whether and how to adapt and apply theory to different subgroups in this population. The timing of intervention relative to the survivor’s melanoma diagnosis also may suggest the use of theories such as Protection Motivation Theory (50) which address threat appraisals and coping processes.
Social desirability and recall biases are potential measurement limitations of this study. Validated psychosocial measures regarding children’s sun protection are lacking (49). We developed measures for the current study to assess psychosocial constructs specific to individual sun protection behaviors. A few of the measures, namely sunscreen outcome expectations and sun protection knowledge, had low Cronbach’s alpha estimates of internal consistency reliability, and this may limit the interpretation of findings based on these measures. Continual testing and refinement of measures will enhance their validity and reliability for future research.
We were unable to compare respondents with nonrespondents because the registry had no records on the age of survivors’ children. A large proportion of survivors screened for the study did not have a child in the eligible age range. The mean age of melanoma survivors in the patient registry (51 years) was older compared to population-based estimates for parents who have children younger than 12 years (37 years) (51). Most of the sample resided in Texas or another southern state, but we acknowledge that the potential for seasonal or geographic variation in intervention effect is a challenge in sun protection research and a possible limitation of this study. Our sample had a relatively high education level, which has been associated with increased melanoma incidence (52). The sample’s distributions of education, race, and ethnicity were similar to those reported in other studies of melanoma survivors recruited from population-based cancer registries and comprehensive cancer centers (53–55), although distributions may vary nationally (12).
This is the first study to examine the effects of an intervention to promote sun protection in children of melanoma survivors. Our findings provide a valuable starting point for developing effective sun protection interventions in this group of children at higher risk of melanoma. Our melanoma survivor-centered sun protection intervention can improve some outcomes related to children’s sun protection and the intervention may be more effective in survivors who have younger children or less experience with sun protection. Furthermore, intervention delivery must be enhanced to maximize use. Future research also should focus on evaluating this intervention in different samples of melanoma survivors to test the external validity of our findings and better describe the populations that may receive the most benefit.
Acknowledgments
The authors thank Valen Johnson, PhD, and Ying Yuan, PhD, for their statistical contributions during this study.
Grant support: This research was funded by a grant from the American Cancer Society (RSGPB 04-010-01-CPPB; to E.R. Gritz, PhD), and supported by the NIH/NCI under award number P30CA016672 and used the Patient-Reported Outcomes, Survey & Population Research Shared Resource and the Biostatistics Resource Group.
Footnotes
Disclosure of Potential Conflicts of Interest:
No potential conflicts of interest were disclosed.
References
- 1.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–74. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Trends Progress Report – 2009/2010 Update. National Cancer Institute, NIH, DHHS; Bethesda, MD: Apr, 2010. [cited 2013 March 1]. Available from: http://progressreport.cancer.gov. [Google Scholar]
- 3.Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69–82. doi: 10.1023/a:1008980919928. [DOI] [PubMed] [Google Scholar]
- 4.Hall HI, McDavid K, Jorgensen CM, Kraft JM. Factors associated with sunburn in white children aged 6 months to 11 years. Am J Prev Med. 2001;20:9–14. doi: 10.1016/s0749-3797(00)00265-8. [DOI] [PubMed] [Google Scholar]
- 5.Dusza SW, Halpern AC, Satagopan JM, Oliveria SA, Weinstock MA, Scope A, et al. Prospective study of sunburn and sun behavior patterns during adolescence. Pediatrics. 2012;129:309–17. doi: 10.1542/peds.2011-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis KJ, Cokkinides VE, Weinstock MA, O’Connell MC, Wingo PA. Summer sunburn and sun exposure among US youths ages 11 to 18: national prevalence and associated factors. Pediatrics. 2002;110:27–35. doi: 10.1542/peds.110.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Cokkinides V, Weinstock M, Glanz K, Albano J, Ward E, Thun M. Trends in sunburns, sun protection practices, and attitudes toward sun exposure protection and tanning among US adolescents, 1998–2004. Pediatrics. 2006;118:853–64. doi: 10.1542/peds.2005-3109. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 9.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, Masini C, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040–59. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Ford D, Bliss JM, Swerdlow AJ, Armstrong BK, Franceschi S, Green A, et al. Risk of cutaneous melanoma associated with a family history of the disease. The International Melanoma Analysis Group (IMAGE) Int J Cancer. 1995;62:377–81. doi: 10.1002/ijc.2910620403. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama S, Woods SL, Boyle GM, Aoude LG, MacGregor S, Zismann V, et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;480:99–103. doi: 10.1038/nature10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glenn BA, Bastani R, Chang LC, Khanna R, Chen K. Sun protection practices among children with a family history of melanoma: a pilot study. J Cancer Educ. 2012;27:731–37. doi: 10.1007/s13187-012-0377-5. [DOI] [PubMed] [Google Scholar]
- 13.Balch CM, Soong SJ, Atkins MB, Buzaid AC, Cascinelli N, Coit DG, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–49. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 14.Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials. a review. Control Clin Trials. 2002;23:662–74. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 15.Gritz ER, Tripp MK, James AS, Carvajal SC, Harrist RB, Mueller NH, et al. An intervention for parents to promote preschool children’s sun protection: Effects of Sun Protection is Fun! Prev Med. 2005;41:357–66. doi: 10.1016/j.ypmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Gritz ER, Tripp MK, James AS, Harrist RB, Mueller NH, Chamberlain RM, et al. Effects of a preschool staff intervention on children’s sun protection: Outcomes of Sun Protection Is Fun! Health Educ Behav. 2007;34:562–77. doi: 10.1177/1090198105277850. [DOI] [PubMed] [Google Scholar]
- 17.Tripp MK, Herrmann NB, Parcel GS, Chamberlain RM, Gritz ER. Sun Protection is Fun! A skin cancer prevention program for preschools. J Sch Health. 2000;70:395–401. doi: 10.1111/j.1746-1561.2000.tb07226.x. [DOI] [PubMed] [Google Scholar]
- 18.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice-Hall, Inc; 1986. [Google Scholar]
- 19.Becker MH. The health belief model and personal health behavior. Thorofare: Slack; 1974. [Google Scholar]
- 20.Glanz K, Geller AC, Shigaki D, Maddock JE, Isnec MR. A randomized trial of skin cancer prevention in aquatics settings: The Pool Cool program. Health Psychol. 2002;21:579–87. [PubMed] [Google Scholar]
- 21.Rodrigue JR. Promoting healthier behaviors, attitudes, and beliefs toward sun exposure in parents of young children. J Consult Clin Psychol. 1996;64:1431–36. doi: 10.1037//0022-006x.64.6.1431. [DOI] [PubMed] [Google Scholar]
- 22.Crane LA, Deas A, Mokrohisky ST, Ehrsam G, Jones RH, Dellavalle R, et al. A randomized intervention study of sun protection promotion in well-child care. Prev Med. 2006;42:162–70. doi: 10.1016/j.ypmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–64. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 24.Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31:399–418. doi: 10.1146/annurev.publhealth.012809.103604. [DOI] [PubMed] [Google Scholar]
- 25.Turner LR, Mermelstein RJ. Psychosocial characteristics associated with sun protection practices among parents of young children. J Behav Med. 2005;28:77–90. doi: 10.1007/s10865-005-2565-9. [DOI] [PubMed] [Google Scholar]
- 26.Lescano CM, Rodrigue JR. Skin cancer prevention behaviors among parents of young children. Child Health Care. 1997;26:107–14. [Google Scholar]
- 27.Tripp MK, Carvajal SC, McCormick LK, Mueller NH, Hu SH, Parcel GS, et al. Validity and reliability of the Parental Sun Protection Scales. Health Educ Res. 2003;18:58–73. doi: 10.1093/her/18.1.58. [DOI] [PubMed] [Google Scholar]
- 28.van Osch L, Reubsaet A, Lechner L, Candel M, Mercken L, de Vries H. Predicting parental sunscreen use: Disentangling the role of action planning in the intention-behavior relationship. Psychol Health. 2008;23:829–47. doi: 10.1080/08870440701596577. [DOI] [PubMed] [Google Scholar]
- 29.Glanz K, Lew RA, Song V, Cook VA. Factors associated with skin cancer prevention practices in a multiethnic population. Health Educ Behav. 1999;26:344–59. doi: 10.1177/109019819902600305. [DOI] [PubMed] [Google Scholar]
- 30.American Cancer Society. A parent’s guide to skin protection. Atlanta, GA: American Cancer Society; 2004. [Google Scholar]
- 31.American Academy of Pediatrics. Encourage your child to be physically active. Chicago, IL: American Academy of Pediatrics; 2003. [Google Scholar]
- 32.American Academy of Pediatrics. Right from the start: ABC’s of good nutrition for young children. Chicago, IL: American Academy of Pediatrics; 2005. [Google Scholar]
- 33.Saraiya M, Glanz K, Briss PA, Nichols P, White C, Das D, et al. Interventions to prevent skin cancer by reducing exposure to ultraviolet radiation: a systematic review. Am J Prev Med. 2004;27:422–66. doi: 10.1016/j.amepre.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Tripp MK, Diamond PM, Vernon SW, Swank PR, Dolan Mullen P, Gritz ER. Measures of parents’ self-efficacy and perceived barriers to children’s sun protection: construct validity and reliability in melanoma survivors. Health Educ Res. 2012 Nov 30; doi: 10.1093/her/cys114. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SAS Institute Inc. Base SAS® 9.1.3 Procedures Guide. 2. 1, 2, 3, and 4. Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- 36.Hall HI, Jorgensen CM, McDavid K, Kraft JM, Breslow R. Protection from sun exposure in US white children ages 6 months to 11 years. Public Health Rep. 2001;116:353–61. doi: 10.1093/phr/116.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz A, Neale RE, Kimlin MG, Jones L, Janda M. The children and sunscreen study: a crossover trial investigating children’s sunscreen application thickness and the influence of age and dispenser type. Arch Dermatol. 2012;148:606–12. doi: 10.1001/archdermatol.2011.2586. [DOI] [PubMed] [Google Scholar]
- 38.Diffey BL. When should sunscreen be reapplied? J Am Acad Dermatol. 2001;45:882–5. doi: 10.1067/mjd.2001.117385. [DOI] [PubMed] [Google Scholar]
- 39.Mayer JA, Slymen DJ, Eckhardt L, Johnston MR, Elder JP, Sallis JF, et al. Reducing ultraviolet radiation exposure in children. Prev Med. 1997;26:516–22. doi: 10.1006/pmed.1997.0166. [DOI] [PubMed] [Google Scholar]
- 40.Crane LA, Asdigian NL, Baron AE, Aalborg J, Marcus AC, Mokrohisky ST, et al. Mailed intervention to promote sun protection of children: a randomized controlled trial. Am J Prev Med. 2012;43:399–410. doi: 10.1016/j.amepre.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manne S, Jacobsen PB, Ming ME, Winkel G, Dessureault S, Lessin SR. Tailored versus generic interventions for skin cancer risk reduction for family members of melanoma patients. Health Psychol. 2010;29:583–93. doi: 10.1037/a0021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glanz K, Schoenfeld ER, Steffen A. A randomized trial of tailored skin cancer prevention messages for adults: Project SCAPE. Am J Public Health. 2010;100:735–41. doi: 10.2105/AJPH.2008.155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geller AC, Emmons KM, Brooks DR, Powers C, Zhang Z, Koh HK, et al. A randomized trial to improve early detection and prevention practices among siblings of melanoma patients. Cancer. 2006;107:806–14. doi: 10.1002/cncr.22050. [DOI] [PubMed] [Google Scholar]
- 44.Harris JN, Hay J, Kuniyuki A, Asgari MM, Press N, Bowen DJ. Using a family systems approach to investigate cancer risk communication within melanoma families. Psychooncology. 2010;19:1102–11. doi: 10.1002/pon.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hay J, Shuk E, Brady MS, Berwick M, Ostroff J, Halpern A. Family communication after melanoma diagnosis. Arch Dermatol. 2008;144:553–4. doi: 10.1001/archderm.144.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hay J, DiBonaventura M, Baser R, Press N, Shoveller J, Bowen D. Personal attributions for melanoma risk in melanoma-affected patients and family members. J Behav Med. 2011;34:53–63. doi: 10.1007/s10865-010-9286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong AW, Watson AJ, Makredes M, Frangos JE, Kimball AB, Kvedar JC. Text-message reminders to improve sunscreen use: a randomized, controlled trial using electronic monitoring. Arch Dermatol. 2009;145:1230–6. doi: 10.1001/archdermatol.2009.269. [DOI] [PubMed] [Google Scholar]
- 48.Gold J, Aitken CK, Dixon HG, Lim MS, Gouillou M, Spelman T, et al. A randomised controlled trial using mobile advertising to promote safer sex and sun safety to young people. Health Educ Res. 2011;26:782–94. doi: 10.1093/her/cyr020. [DOI] [PubMed] [Google Scholar]
- 49.Tripp MK, Vernon SW, Gritz ER, Diamond PM, Mullen PD. Children’s skin cancer prevention: a systematic review of parents’ psychosocial measures. Am J Prev Med. 2013;44:265–73. doi: 10.1016/j.amepre.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 50.Rogers RW. Cognitive and physiological processes in fear appeals and attitude change: a revised theory of protection motivation. In: Cacioppo JT, Petty RE, editors. Social psychophysiology: a sourcebook. New York: Guilford; 1983. pp. 153–76. [Google Scholar]
- 51.U.S. Census Bureau. 2012 Annual Social and Economic Supplement. Current Population Survey. Internet Release Date: November 2012. [Google Scholar]
- 52.Harrison RA, Haque AU, Roseman JM, Soong SJ. Socioeconomic characteristics and melanoma incidence. Ann Epidemiol. 1998;8:327–33. doi: 10.1016/s1047-2797(97)00231-7. [DOI] [PubMed] [Google Scholar]
- 53.Manne S, Lessin S. Prevalence and correlates of sun protection and skin self-examination practices among cutaneous malignant melanoma survivors. J Behav Med. 2006;29:419–34. doi: 10.1007/s10865-006-9064-5. [DOI] [PubMed] [Google Scholar]
- 54.Bowen D, Jabson J, Haddock N, Hay J, Edwards K. Skin care behaviors among melanoma survivors. Psychooncology. 2012;21:1285–91. doi: 10.1002/pon.2017. [DOI] [PubMed] [Google Scholar]
- 55.Mujumdar UJ, Hay JL, Monroe-Hinds YC, Hummer AJ, Begg CB, Wilcox HB, et al. Sun protection and skin self-examination in melanoma survivors. Psychooncology. 2009;18:1106–15. doi: 10.1002/pon.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]