Abstract
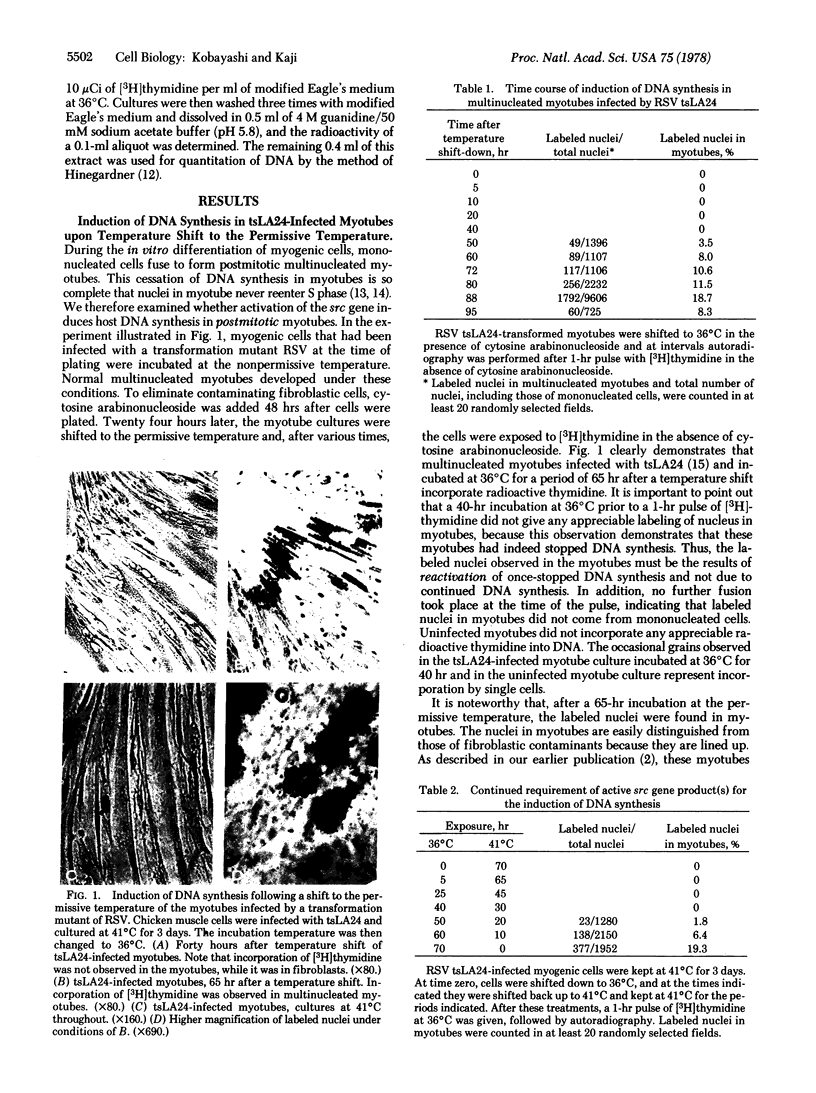
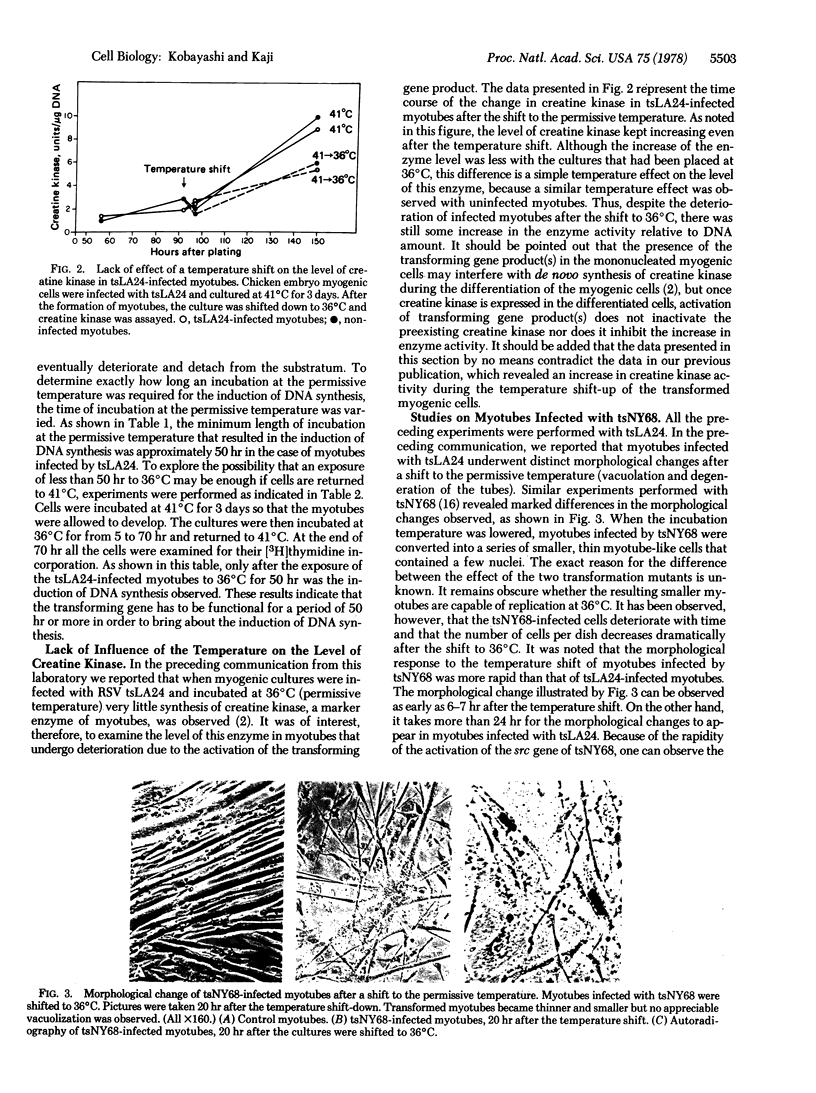
Mononucleated myogenic cells from 11-day-old chicken embryos were infected with tsLA24 or tsNY68, temperature-sensitive transformation mutants of Rous sarcoma virus. The infected mononucleated myogenic cells were incubated at the nonpermissive temperature (41 degrees C) and allowed to develop into multinucleated myotubes. These myotubes have withdrawn from the cell cycle, and no DNA synthesis was observed as long as the cultures were maintained at the nonpermissive temperature. However, when the incubation temperature of these cultures was lowered to the permissive temperature (36 degrees C) for expression of the src gene, DNA synthesis was induced in multinucleated myotubes. For this induction of DNA synthesis, cells infected with tsLA24 had to be incubated at the permissive temperature for at least 50 hr, while the induction of DNA synthesis in cells infected with tsNY68 required less than 20 hr. Induction of DNA synthesis was observed by autoradiography as well as by measuring incorporation of [3H]thymidine into the macromolecule fraction in these myotube cultures. For the maintenance of capacity to induce DNA synthesis, constant presence of the src gene product is necessary, because when the temperature of these cultures was returned to 41 decrees C, the myotubes lost the capacity to induce DNA synthesis. During the process of DNA induction one biochemical marker of muscle (creatine kinase) remained unchanged.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Johnson G. S., Pastan I. Transformation of chick-embryo fibroblasts by wild-type and temperature-sensitive Rous sarcoma virus alters adenylate cyclase activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1055–1059. doi: 10.1073/pnas.70.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. B., Lovelace E., Pastan I. Adenylate cyclase activity is decreased in chick embryo fibroblasts transformed by wild type and temperature sensitive Schmidt-Ruppin Rous sarcoma virus. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1293–1299. doi: 10.1016/0006-291x(73)90641-4. [DOI] [PubMed] [Google Scholar]
- Arbogast B. W., Yoshimura M., Kefalides N. A., Holtzer H., Kaji A. Failure of cultured chick embryo fibroblasts to incorporate collagen into their extracellular matrix when transformed by Rous sarcoma virus. An effect of transformation but not of virus production. J Biol Chem. 1977 Dec 25;252(24):8863–8868. [PubMed] [Google Scholar]
- Bader J. P. Temperature-dependent transformation of cells infected with a mutant of Bryan Rous sarcoma virus. J Virol. 1972 Aug;10(2):267–276. doi: 10.1128/jvi.10.2.267-276.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. G., Wyke J. A., Macpherson I. A. Transformation by a temperature sensitive mutant of Rous sarcoma virus in the absence of serum. J Gen Virol. 1975 May;27(2):127–134. doi: 10.1099/0022-1317-27-2-127. [DOI] [PubMed] [Google Scholar]
- Calothy G., Pessac B. Growth stimulation of chicl embryo neuroretinal cells infected with Rous sarcoma virus: relationship to viral replication and morphological transformation. Virology. 1976 May;71(1):336–345. doi: 10.1016/0042-6822(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Claycomb W. C. DNA synthetic activity of nuclei isolated from differentiating cardiac muscle and association of DNA polymerase alpha with the outer nuclear membrane. Dev Biol. 1977 Dec;61(2):245–251. doi: 10.1016/0012-1606(77)90295-0. [DOI] [PubMed] [Google Scholar]
- EPHRUSSI B., TEMIN H. M. Infection of chick iris epithelium with the Rous sarcoma virus in vitro. Virology. 1960 Jul;11:547–552. doi: 10.1016/0042-6822(60)90099-4. [DOI] [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Fogel M., Defendi V. Infection of muscle cultures from various species with oncogenic DNA viruses (SV40 and polyoma). Proc Natl Acad Sci U S A. 1967 Sep;58(3):967–973. doi: 10.1073/pnas.58.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Fogel M. The relationship of polyoma virus-induced tumor (T) antigen to activation of DNA synthesis in rat myotubes. Dev Biol. 1973 Nov;35(1):180–186. doi: 10.1016/0012-1606(73)90016-x. [DOI] [PubMed] [Google Scholar]
- Grippo P., Cossu G., Molinaro M. DNA replication during muscle cell differentiation: identification of multiple DNA-dependent DNA polymerases. Cell Differ. 1977 Jun;6(1):9–16. doi: 10.1016/0045-6039(77)90040-9. [DOI] [PubMed] [Google Scholar]
- Gruen R., Graessmann M., Graessmann A., Fogel M. Infection of human cells with polyoma virus. Virology. 1974 Mar;58(1):290–293. doi: 10.1016/0042-6822(74)90162-7. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovi T., Keski-Oja J., Vaheri A. Growth control in chick embryo fibroblasts; no evidence for a specific role for cyclic purine nucleotides. Cell. 1974 Aug;2(4):235–240. doi: 10.1016/0092-8674(74)90016-6. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Martin G. S., Shearer M., Critchley D. R., Epstein C. J. Viral transformation of rat myoblasts: effects on fusion and surface properties. Dev Biol. 1976 Jan;48(1):35–46. doi: 10.1016/0012-1606(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Wyke J. A. Alterations in surface proteins in chicken cells transformed by temperature-sensitive mutants of Rous sarcoma virus. Virology. 1975 Apr;64(2):492–504. doi: 10.1016/0042-6822(75)90126-9. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG I. R., MCELVAIN N., TOOTLE M., HERRMANN H. The dissociability of deoxyribonucleic acid synthesis from the development of multinuclearity of muscle cells in culture. J Biophys Biochem Cytol. 1960 Oct;8:333–343. doi: 10.1083/jcb.8.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. II. Alterations in transport following transformation by a temperature-sensitive mutant of the Rous sarcoma virus. J Biol Chem. 1974 Jun 10;249(11):3375–3382. [PubMed] [Google Scholar]
- Lee H. H., Kaighn M. E., Ebert J. D. Induction of thymidine-3H incorporation in multinucleated myotubes by Rous sarcoma virus. Int J Cancer. 1968 Jan 15;3(1):126–136. doi: 10.1002/ijc.2910030115. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto M., Yoshimura M., Okayama M., Kaji A. Cellular transformation and differentiation. Effect of Rous sarcoma virus transformation on sulfated proteoglycan synthesis by chicken chondrocytes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4173–4177. doi: 10.1073/pnas.74.10.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M., Strohman R. C. Changes in DNA polymerase activity associated with cell fusion in cultures of embryonic muscle. J Cell Physiol. 1969 Feb;73(1):61–68. doi: 10.1002/jcp.1040730109. [DOI] [PubMed] [Google Scholar]
- OLIVER I. T. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955 Sep;61(1):116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama M., Yoshimura M., Muto M., Chi J., Roth S., Kaji A. Transformation of chicken chondrocytes by Rous sarcoma virus. Cancer Res. 1977 Mar;37(3):712–717. [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Wickus G. G., Branton P. E., Gaffney B. J., Hirschberg C. B., Fuchs P., Blumberg P. The chick fibroblast cell surface after transformation by Rous sarcoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1173–1180. doi: 10.1101/sqb.1974.039.01.135. [DOI] [PubMed] [Google Scholar]
- Roth S. L., Delotto R., Kaji A. Changes in leucine aminotransferase isozymes by viral transformation and its correlation with the isozyme changes occurring during differentiation. Cancer Res. 1977 Apr;37(4):1147–1153. [PubMed] [Google Scholar]
- Stockdale F. E., O'Neill M. C. Repair DNA synthesis in differentiated embryonic muscle cells. J Cell Biol. 1972 Mar;52(3):589–597. doi: 10.1083/jcb.52.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P., Okayama M., Orkin R. W., Yoshimura M., Muto M., Kaji A. Developmental roles of hyaluronate and chondroitin sulfate proteoglycans. Soc Gen Physiol Ser. 1977;32:139–154. [PubMed] [Google Scholar]
- Wyke J. A., Linial M. Temperature-sensitive avian sarcoma viruses: a physiological comparison of twenty mutants. Virology. 1973 May;53(1):152–161. doi: 10.1016/0042-6822(73)90474-1. [DOI] [PubMed] [Google Scholar]
- YAFFE D., FELDMAN M. THE FORMATION OF HYBRID MULTINUCLEATED MUSCLE FIBERS FROM MYOBLASTS OF DIFFERENT GENETIC ORIGIN. Dev Biol. 1965 Apr;11:300–317. doi: 10.1016/0012-1606(65)90062-x. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Gershon D. Multinucleated muscle fibres: induction of DNA synthesis and mitosis by polyoma virus infection. Nature. 1967 Jul 22;215(5099):421–424. doi: 10.1038/215421a0. [DOI] [PubMed] [Google Scholar]