Abstract
Background
In animal models of ischaemic stroke, 25% albumin reduced brain infarction and improved neurobehavioral outcome. In a pilot clinical trial, albumin doses as high as 2 g per kg were safely tolerated.
Trial Design and Methods
This was a randomised, parallel-group, double-blind trial to test the superiority of 25% albumin (dose 2 g [8 ml] per kg; maximum, 750 ml) over an equivalent volume of isotonic saline in improving the outcome of acute ischaemic stroke. Eligibility criteria were an ischaemic (i.e., non-haemorrhagic) stroke with baseline National Institutes of Health Stroke Scale (NIHSS) score of 6 or above, ability to treat within 5 hours of onset, age 18 through 83 years, and written informed consent. The major exclusion criteria were cardiovascular. The objective was to test the hypothesis that the primary outcome (defined as either a modified Rankin Scale score of 0 or 1, or a NIHSS score of 0 or 1, or both, at 90 days) with albumin treatment was superior to saline by an absolute margin of 10 percentage points. Centralised web-based randomisation was by a minimisation-plus-biased-coin algorithm. Thrombolytic therapies were permitted. The trial is registered with ClinicalTrials.gov, Identifier: NCT00235495.
Findings
The trial was stopped prematurely for futility after 841 participants were randomised (422 patients to albumin and 419 to saline). The primary outcome did not differ by treatment assignment (albumin, 44.1%; saline, 44.2%; relative benefit, 0.96; 95% confidence interval [CI] 0.84 – 1.10 adjusted for baseline NIHSS score and thrombolysis stratum). Secondary outcomes were also neutral. The chief adverse event was mild-to-moderate pulmonary edema, which was more common with albumin than saline (13.1% and 1.2%, respectively), as was symptomatic intracranial haemorrhage within 24 hours (albumin, 4.1%; saline, 1.7%). While the favourable outcome rate in albumin-treated subjects remained consistent at 44–45% over the course of the trial, the cumulative rate in the saline arm rose steadily from 31% early in the trial to 44% at its conclusion.
Interpretation
This trial failed to demonstrate a clinical benefit of albumin in ischaemic stroke.
Introduction
At present, stroke is the fourth-leading cause of death in North America, the second-leading cause of death globally, and a major cause of disability 1. By 2030, stroke prevalence in the U.S. is projected to rise to 4%, and stroke-related disability is estimated become the fourth most important cause of disability-adjusted life-years in western countries 2. In the developing world, stroke incidence has increased in recent decades, and the rates of disability and mortality in medically underserved regions are at least 10 times greater than in developed countries 3. Approximately 80% of all strokes are ischaemic 4. The only specific therapy proven to improve outcome in acute ischaemic stroke is intravenous tissue-type plasminogen activator (tPA), whose therapeutic benefit declines sharply over the first few hours after stroke onset 5.
Preclinical studies have implicated the contribution of multiple injurious biochemical, molecular and vascular events in ischaemic brain injury and have shown in principle that the ischaemic brain can be protected by strategies designed to counter these injury-mechanisms 6. To date, however, the translation of these approaches to treat patients with ischaemic stroke has been disappointing 7,8.
In experimental studies of acute ischaemic stroke, high doses of 25% human albumin have proven to be consistently neuroprotective, reducing the volume of brain infarction, diminishing cerebral edema, and improving behavioral function, with a therapeutic window of efficacy of at least 4 hours after stroke onset 9,10. Albumin improved perfusion to the ischaemic penumbra 11, normalised the apparent diffusion coefficient within the residual infarct 9, reversed stagnation in postischaemic cortical venules 12, and improved microvascular haemodynamics distal to an arterial thrombosis 13–15. In a pilot safety trial in 82 subjects with acute ischaemic stroke, 25% albumin administered in doses encompassing the neuroprotective range shown in experimental studies 10 was tolerated without major complications, and an exploratory analysis suggested possible efficacy 16,17.
A randomised multicenter efficacy trial – the Albumin in Acute Stroke (ALIAS) Trial – began in July, 2006, to assess whether 25% albumin treatment within 5 hours of the onset of an acute ischaemic stroke would confer neurological and functional benefit. After 434 subjects had been enrolled, the Data and Safety Monitoring Board (DSMB) suspended further enrollment for safety reasons 18. An unblinded safety analysis of this trial (termed “ALIAS Part 1”) disclosed excess deaths within 30 days in the albumin group relative to the saline group, chiefly affecting subjects older than 83 years. Deaths were also higher in the albumin-treated subjects who had received excessive intravenous fluids. We modified the study design on the basis of these safety findings; and with frequent DSMB oversight, the trial recommenced as a separate “ALIAS Part 2” trial 18, whose results we report here.
METHODS
Study design and participants
The Albumin in Acute Stroke (ALIAS) Part 2 Trial was a placebo-controlled, double-blind, phase 3 multicenter clinical trial with the primary objective to ascertain whether a weight-adjusted intravenous infusion of 25% albumin solution (2 g per kg estimated body weight) begun within 5 hours of stroke onset would increase the proportion of subjects with a favourable outcome at 90 days from randomisation, compared to subjects treated with a similar volume of isotonic saline. A favourable primary outcome was defined as either a modified Rankin Scale (mRS) score of 0 or 1 or a National Institutes of Health Stroke Scale (NIHSS) score of 0 or 1, or both. The mRS ranges from 0 to 6, with higher scores denoting greater disability; scores of 0 or 1 denote lack of any functional disability. The NIHSS score ranges from 0 to 42; a score of 0 suggests a normal neurological examination, and a score of 1, a minimal abnormality. The study was conducted in accordance with the protocol, which is available online at https://dcu.musc.edu/Projects/MajorProjects.asp.
The ALIAS Part 2 Trial Executive Committee had sole responsibility for the design of the trial, data collection and analysis, and writing of the manuscript, and it vouches for its accuracy and fidelity to the protocol. Approval to conduct the study was obtained from an Institutional Review Board/Research Ethics Board at each study site. A DSMB appointed by the National Institute of Neurological Disorders and Stroke (NINDS) oversaw the safety and performance of the trial. Safety events were adjudicated by two independent medical monitors. The study was performed under an Investigational New Drug application with the U.S. Food and Drug Administration and a Clinical Trial Application at Health Canada.
Patients aged 18 through 83 years were eligible for the study if they had an acute ischaemic stroke (with a non-contrast CT or an MRI scan required to exclude intracranial haemorrhage), had a baseline NIHSS score ≥ 6, and could be started on study-drug treatment within 5 hours of the onset of stroke. Written informed consent was obtained from the patient or a legal representative before enrollment. Major exclusion criteria included: an episode of congestive heart failure, cardiac surgery with thoracotomy, or acute myocardial infarction within the preceding 6 months; signs of myocardial infarction or congestive heart failure on admission; an elevated baseline serum troponin level > 0.1 mcg/L; significant chronic lung disease; an historical mRS score >2; and stroke occurring in-hospital (Panel 1). Standard-of-care thrombolytic therapy was permitted and consisted of intravenous tissue plasminogen activator (tPA), and/or intra-arterial tPA or endovascular thrombolysis with approved devices and catheters, based upon local clinical judgment. Endovascular procedures, if used, had to begin within 5 hours and be completed within 7 hours after the onset of stroke.
Panel 1. Inclusion and Exclusion Criteria.
Inclusion Criteria
Acute ischaemic stroke
Age 18 years through 83 years (subjects must not have had their 84th birthday)
NIHSS score of 6 or greater as assessed immediately prior to thrombolysis treatment if the patient is eligible for thrombolysis or immediately prior to randomisation for patients not eligible for thrombolysis
Initiation of albumin/placebo within 5 hours of stroke onset, and within 90 minutes of the start of thrombolysis with intravenous (IV) tPA if that therapy is used
Signed and dated informed consent has been obtained
Exclusion Criteria
Episode/exacerbation of congestive heart failure (CHF) from any cause in the last 6 months. (An episode of congestive heart failure is any heart failure that required a change in medication, change in diet or hospitalisation.)
Known valvular heart disease with CHF in the last 6 months
Known (or in the Investigator’s clinical judgment) existence of severe aortic stenosis or mitral stenosis
Cardiac surgery involving thoracotomy (e.g., coronary artery bypass graft (CABG), valve replacement surgery) in the last 6 months
Acute myocardial infarction in the last 6 months
Signs or symptoms of acute myocardial infarction, including EKG findings, on admission
Elevated serum troponin level on admission (> 0.1 mcg/L)
Suspicion of aortic dissection on admission
Acute arrhythmia (including any tachy-or bradycardia) with haemodynamic instability on admission (systolic blood pressure < 100 mmHg)
Findings on physical examination of any of the following: (1) jugular venous distention (JVP > 4 cm above the sternal angle); (2) 3rd heart sound; (3) resting tachycardia (heart rate > 100/min) attributable to congestive heart failure; (4) lower extremity pitting edema attributable to congestive heart failure; (5) bilateral rales; and/or (6) if a chest x-ray is performed, definite evidence of pulmonary edema, bilateral pleural effusion, or pulmonary vascular redistribution
Current acute or chronic lung disease requiring supplemental chronic or intermittent oxygen therapy
Historical modified Rankin Scale (mRS) >2. Patients who live in a nursing home or who are not fully independent for activities of daily living (toileting, dressing, eating, cooking and preparing
meals, etc.), immediately prior to the stroke are not eligible for the trial.
In-patient stroke
Profound dehydration
Fever, defined as core body temperature > 38.0°C (100.4°F)
Serum creatinine > 2.0 mg/dL or 180 μmol/L
Severe chronic anemia (haemoglobin < 7.5 g/dL or 75g/L)
Evidence of intracranial haemorrhage (intracerebral haematoma, intraventricular haemorrhage, subarachnoid haemorrhage (SAH), epidural haemorrhage, acute or chronic subdural haematoma (SDH)) on the baseline CT or MRI scan
History of or known allergy to albumin
History of or known allergy to natural rubber latex
Pregnancy, breastfeeding or positive pregnancy test. (Women of childbearing age must have a negative pregnancy test prior to study drug administration.)
Concurrent participation in any other therapeutic clinical trial
Evidence of any other major life-threatening or serious medical condition that would prevent completion of the study protocol, impair the assessment of outcome, or in which albumin therapy would be contraindicated or might cause harm to the subject
Human albumin, 25% solution, was manufactured for the trial and prepared in glass bottles by Baxter Healthcare Corp., Westlake Village, California, which had no role in the study’s design, execution or analysis. Isotonic saline in glass bottles was purchased commercially. Study-drug kits were assembled and distributed to the sites by the U.S. Department of Health and Human Services’ Program Support Center – Supply Service Center in Perry Point, Maryland.
Randomisation and masking
A centralised web-based randomisation process assigned subjects 1:1 to either albumin or saline via a minimisation-plus-biased-coin algorithm that accounted for the current status of treatment group balance within and across sites 19. The time of randomisation was defined as the time at which the study-drug kit was unsealed. In subjects who received IV tPA, study-drug infusion needed to begin within 90 minutes after starting IV tPA. All study personnel and subjects were blinded to the identity of the study drug. Each sealed study-drug kit contained two bottles (initially, 500 ml and 250 ml; later, for manufacturing reasons, both 500 ml) of the same substance (either albumin or saline) encased in cardboard blinding-boxes similar to those used in the Saline versus Albumin Fluid Evaluation (SAFE) Trial 20, along with filters and opaque sheathing to mask the intravenous tubing.
Procedures, clinical management, and follow-up
A bedside nurse or other personnel not involved with the trial administered the study drug (8 ml per kg estimated body weight) by constant intravenous infusion over 2 hours (± 15 minutes). Subjects weighing 94 kg or more received 750 ml. Vital signs were monitored frequently. Serum chemistries were collected at 24 and 48 hours. Intravenous fluid intake was recorded at 24 and 48 hours. A follow-up brain CT or MRI scan was obtained at 24 hours. An electrocardiogram was repeated at 24 to 48 hours. Neurological and cardiac status, including the NIHSS score, was assessed at 24 and 48 hours and at 7 days or discharge, whichever came first.
Intravenous fluid management and diuretic administration were mandated. Subjects were not to receive total IV fluids in excess of 4,200 ml during the first 48 hours, including the volumes of study drug and thrombolytic agent (if used). For subjects exceeding this amount, investigators were required to provide justifications, which were centrally adjudicated as to clinical reasonableness. Subjects were also required to receive at least a single dose of furosemide, 20 mg IV (or an equivalent loop diuretic), between 12 and 24 hours after study-drug administration. Physicians withholding diuretics were asked to provide a written justification. Antiplatelet therapy was recommended in all subjects within 48 hours of their stroke. Blood pressure was managed according to the local standard of care.
Subjects were followed for 1 year. At 90 days (±30 days) after randomisation, subjects were assessed in person on the NIHSS, the mRS, the Barthel Index, the Stroke-Specific Quality of Life instrument 21, and Trailmaking A and B tests 22, administered by a certified and blinded site investigator. Subjects were also followed by telephone contact at 1 month (±7 days), 6 months (±14 days), 9 months (±14 days), and 1 year (±14 days) after randomisation to assess the mRS, record serious adverse events (SAEs), and complete the EuroQol 23,24 at 3 months and 1 year, and the Questionnaire to Validate a Stroke-Free Status 25 at 3, 6, 9, and 12 months.
Statistical analysis
The ALIAS Part 2 Trial was conducted in a single cohort that included both non-thrombolysed subjects and subjects receiving thrombolysis (defined as any intravenous and/or endovascular thrombolytic/thrombectomy procedures). Based on simulation, the total required sample size was 1,100, to yield 84% power to detect a 20% interaction effect between treatment and thrombolysis status, i.e., a 20% absolute difference in the treatment effect between thrombolysis and non-thrombolysis strata with a two-sided alpha of 0.10 and assuming a 4:1 ratio of thrombolysis to non-thrombolysis subjects. Concurrently, we calculated that a total of 980 subjects would be required to test the primary hypothesis, i.e., to detect a minimum effect size of 10 absolute percentage points in the primary outcome. We assumed that the control group’s favourable primary outcome would be 40%, based on the NINDS rt-PA Stroke Study 26 and the ALIAS Part 1 Trial 27. Sample size was estimated based on 85% power, a one-sided type 1 error probability of 0.025, and allowing for a group-sequential design for three interim analyses 28. For overwhelming efficacy, we adopted the alpha spending function approach 29, and for futility, the beta spending function approach 30. For both overwhelming efficacy and futility, O’Brien and Fleming-type stopping guidelines were used 28, as well as the binding boundaries and corrected pooled variance estimates 31. The intention-to-treat principle was to be applied to the primary analysis, and the final maximum sample size of 1,092 was derived to account for approximately 5% lost-to-follow-up using the approach of Friedman and colleagues 32. Hence, we concluded that the 1,100 subjects required to detect the interaction effect should provide sufficient power for the primary analysis of the main treatment effect. Statistical analyses were performed with SAS software, version 9.3 (SAS Institute).
Role of funding source
The trial was funded by cooperative agreements from the U.S. National Institutes of Health/National Institute of Neurological Disorders and Stroke. A representative of NINDS (CSM) participated in the study leadership committee and contributed to design decisions as well as the interpretation and writing of the manuscript. Baxter Healthcare Corporation provided funds to extend the trial to Finland but played no role in the design, execution, or interpretation of the study.
RESULTS
Enrollment began in February, 2009. Sixty-nine U.S. sites, 13 sites in Canada, 2 sites in Finland, and 5 sites in Israel randomised 841 subjects to the trial. Half (47%) of the subjects were from hub- or spoke-sites of the NINDS-funded Neurological Emergencies Treatment Trial (NETT) Network [http://net.umich.edu/net/welcome]. Two prespecified interim analyses for overwhelming efficacy or futility were conducted at N=275 and N=550 subjects, respectively. On September 10, 2012, after requesting and reviewing a third, unscheduled interim analysis (at N=732 subjects), the DSMB recommended to the NINDS that enrollment be halted for futility and that all subjects then in the trial (N=841) be followed only through 90 days.
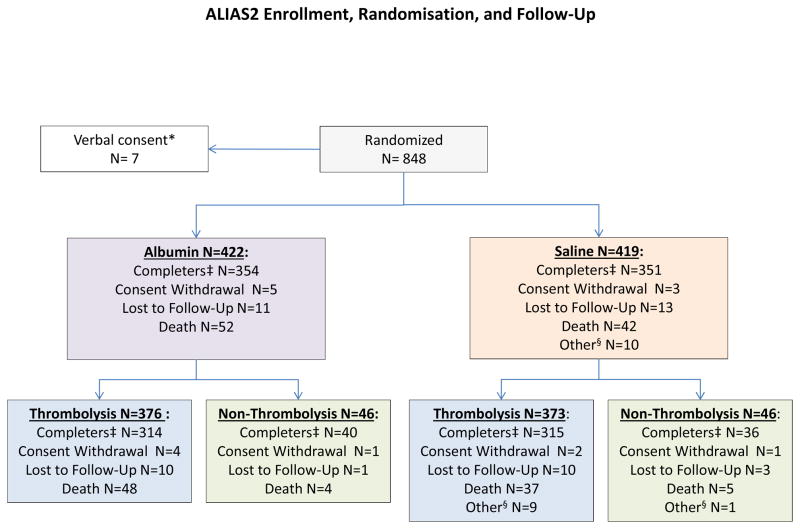
Figure 1 shows a CONSORT diagram of the trial. Table 1 presents baseline and treatment characteristics. Overall, the mean (±SD) age of the subjects was 64.1 years (12.9), and the median baseline NIHSS score was 11 (range, 6–40). Demographic and baseline clinical characteristics were similar between groups. For all subjects, mean (±SD) time from stroke onset to study-drug initiation was 206 (52) minutes. A total of 749 subjects (89.1%) received some form of thrombolytic treatment. Intravenous tPA alone was given to 575 subjects. Another 137 subjects received both IV tPA as well as endovascular thrombolytic therapy. Thirty-seven subjects received only endovascular therapy.
Figure 1.
CONSORT diagram showing enrollment, randomisation, and follow-up in the ALIAS Part 2 Trial.
* Verbally consented subjects were deleted from the database since written consent was required by the FDA.
‡ Those who completed the 3-month follow-up. Not all completed the 12-month follow-up due to early termination of the Trial.
§ Those whose Day 90 visit was outside the ±30 day window, Day 90 visit was missing, or Day 90 assessments were missing.
Table 1.
Baseline and Treatment Characteristics of the Subjects*
| Characteristic | Albumin (N = 422) | Saline (N = 419) | |
|---|---|---|---|
| Age [mean (SD)] | 63.4 (13.0) | 64.8 (12.9) | |
| Male sex [n/N (%)] | 220/422 (52.1%) | 235/419 (56.1%) | |
| Race [n/N (%)]† | White | 314/422 (74.4%) | 305/419 (72.8%) |
| Black | 69/422 (16.4%) | 86/419 (20.5%) | |
| Asian | 25/422 (5.9%) | 20/419 (4.8%) | |
| American Indian/Alaska Native/First Nations People | 2/422 (0.5%) | 0/419 (0.0%) | |
| Native Hawaiian or Pacific islander | 2/422 (0.5%) | 1/419 (0.2%) | |
| Multiple, Other, or Unknown | 10/422 (2.4%) | 7/419 (1.7%) | |
| Ethnic group [n/N (%)]† | Non-Hispanic/Latino | 379/422 (89.8%) | 372/419 (88.8%) |
| Hispanic/Latino | 24/422 (5.7%) | 26/419 (6.2%) | |
| Unknown | 19/422 (4.5%) | 21/419 (5.0%) | |
| Medical history [n/N (%)] | Hypertension | 304/422 (72.0%) | 295/419 (70.4%) |
| Atrial fibrillation | 79/422 (18.7%) | 78/419 (18.6%) | |
| Past congestive heart failure | 18/422 (4.3%) | 27/419 (6.4%) | |
| Past myocardial infarction | 45/422 (10.7%) | 51/419 (12.2%) | |
| Past stroke | 89/422 (21.1%) | 79/419 (18.9%) | |
| Past transient ischaemic attack | 52/422 (12.3%) | 50/419 (11.9%) | |
| Diabetes mellitus | 78/422 (18.5%) | 94/419 (22.4%) | |
| Hyperlipidemia | 196/422 (46.4%) | 213/419 (50.8%) | |
| Peripheral vascular disease | 21/422 (5.0%) | 25/419 (6.0%) | |
| Baseline NIHSS score [median, (inter-quartile range)]‡ | 11 (8 – 17) | 11 (8 – 17) | |
| Baseline ASPECTS score§ | Baseline ASPECTS > 7 [n/N, (%)] | 325/418 (77.8%) | 327/415 (78.8%) |
| Clinical findings [mean (SD)] | Systolic blood pressure, mm Hg | 155.9 (28.0) | 156.6 (29.9) |
| Plasma glucose, mmol/L | 7.1 (2.5) | 7.7 (3.8) | |
| Haemoglobin, g/L | 13.9 (1.7) | 14.0 (1.8) | |
| Creatinine, μmol/L | 86.3 (23.3) | 90.5 (26.9) | |
| Oxfordshire Community Stroke Project classification [N (%)] | |||
| Total anterior circulation syndrome | 102/422 (24.2 %) | 98/419 (23.4 %) | |
| Partial anterior circulation syndrome | 232/422 (55.0 %) | 235/419 (56.1 %) | |
| Posterior circulation syndrome | 35/422 (8.3 %) | 39/419 (9.3 %) | |
| Lacunar stroke | 53/422 (12.6 %) | 47/419 (11.2 %) | |
| Stroke onset to initiation of study drug infusion (min) [median, (inter-quartile range)] | 200 (167 – 249) | 198 (167 – 243) | |
| Subjects receiving intravenous thrombolysis [N (%)] | 351 (83.2%) | 361 (86.2%) | |
| Characteristics of subjects receiving intravenous thrombolysis | |||
| Stroke onset to initiation of intravenous tPA (min) [median, (inter-quartile range)] | 126 (98 – 170) | 130 (100 – 166) | |
| Initiation of IV tPA to initiation of study drug infusion (min) [median, (inter-quartile range)] | 60 (49 – 80) | 60 (48 – 80) | |
| Distribution of thrombolytic procedures by type | |||
| Intravenous tPA only | 282/422 (66.8%) | 293/419 (69.9%) | |
| Intravenous tPA plus any endovascular procedure | 69/422 (16.4%) | 68/419 (16.2%) | |
| Any endovascular procedure only | 25/422 (5.9%) | 12/419 (2.9%) | |
| No thrombolysis | 46/422 (10.9%) | 46/419 (11.0%) | |
| Intravenous fluids | |||
| IV fluids administered within 24 hours of randomization (ml) (mean (SD); [min, max]) | 2101 (958); [173, 7503] | 2077 (986);[408, 7040] | |
| IV fluids administered 24–48 hours from randomization (ml) (mean (SD); [min, max]) | 1178 (1000); [0, 7503] | 1178 (926); [0, 5353] | |
| Total IV fluids administered within 48 hours of randomization (ml) (mean (SD); [min, max]) | 3279 (1671); [173,15006] | 3255 (1624); [432, 11235] | |
The abbreviation tPA denotes tissue plasminogen activator.
There were no significant differences between groups.
Race and ethnic group were self-reported.
The National Institutes of Health Stroke Scale (NIHSS) is a 42-point scale that quantifies neurological deficits in 11 categories, with 0 indicating normal function without deficits, and higher scores indicating greater severities of deficit.
The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) uses computed tomography to assess 10 regions of the brain; a score of 1 indicates a normal region and 0 indicates a region showing signs of ischaemia. Total scores range from 10 (no evidence of early ischaemia) to 0 (all 10 regions of the affected hemisphere show early ischaemic changes).
Primary outcome
There was no significant difference between subjects assigned to albumin and saline in the proportion achieving a favourable primary outcome at 90 ± 30 days (albumin, 44.1%; saline, 44.2%; 95% confidence interval (CI) 0.84 – 1.10 with adjustment for baseline NIHSS score and thrombolysis stratum (defined as any intravenous or endovascular treatment)) (Table 2).
Table 2.
Efficacy Outcomes Adjusted for Baseline NIHSS and Thrombolysis Stratum
| Outcome | Albumin (N=422) | Saline (N=419) | Risk Ratio | 95% CI | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Primary outcome: NIHSS and/or mRS 0-1 at 90 (± 30) days | 186 | 44.1 | 185 | 44.2 | 0.96 | 0.84–1.10 |
| 99% CI for rest | ||||||
| Composite outcome at 90 (±30) days: mRS 0-1 and/or NIHSS 0-1 and/or decrease in NIHSS from baseline by 10 or more points | 227 | 53.8 | 241 | 57.5 | 0.93 | 0.80–1.09 |
| NIHSS 0-1 at 24 hours | 65 | 15.4 | 62 | 14.8 | 0.99 | 0.66–1.49 |
| NIHSS 0-1 at 90 days | 150 | 35.6 | 164 | 39.1 | 0.88 | 0.71–1.10 |
| mRS 0-1 at 90 days | 155 | 36.7 | 145 | 34.6 | 1.03 | 0.82–1.28 |
| mRS 0-2 at 90 days | 227 | 53.8 | 221 | 52.7 | 0.99 | 0.85–1.13 |
| mRS at 90 days by sliding-dichotomy analysis 53,54 | 142 | 33.6 | 141 | 33.7 | 0.98 | 0.87– 1.11 |
| Barthel Index (BI) 95–100 at 90 days | 227 | 53.8 | 231 | 55.1 | 0.95 | 0.83–1.10 |
| EuroQol EQ-5D 24 favourable score (<0.78 vs. otherwise) at 90 days | 155 | 36.7 | 152 | 36.3 | 0.99 | 0.84–1.17 |
| SSQOL21 favourable score (>=3 vs. otherwise) at 90 days | 258 | 61.1 | 268 | 63.9 | 0.93 | 0.84–1.02 |
| Global scale (NIHSS,mRS,BI, EuroQoL,SSQOL) at 90 days | 0.93 | 0.82–1.05 | ||||
| Trailmaking A and B at 90 days | Median | IQR | Median | IQR | p-value * | |
| Trails A | 55 | 39–105 | 56 | 39–90 | 0.913 | |
| Trials B | 111 | 78–173 | 110 | 82–180 | 0.923 | |
Abbreviation: IQR, inter-quartile range.
Two-sided probability, Wilcoxon rank sum test with normal approximation
There was also no significant difference in the primary outcome by treatment assignment in any of the pre-defined subgroups (thrombolysis; sex; race; ethnicity; stroke type; baseline ASPECTS; baseline NIHSS; stroke onset to randomisation or to treatment; clinical site subject-volume; baseline glucose; and history of diabetes, atrial fibrillation, or hypertension) (Table 3).
Table 3.
Subgroup Efficacy Analysis Adjusted for Baseline NIHSS and Thrombolysis Stratum
| Subgroup | RR | 99% Confidence Limits | ||
|---|---|---|---|---|
| Thrombolysis treatment* | Thrombolysis | 0.95 | 0.79 | 1.15 |
| Non-Thrombolysis | 1.09 | 0.53 | 2.22 | |
| Gender | Male | 1.01 | 0.80 | 1.28 |
| Female | 0.89 | 0.67 | 1.19 | |
| Race | Caucasian | 0.96 | 0.79 | 1.18 |
| Black/African American | 1.09 | 0.69 | 1.73 | |
| Ethnicity | Hispanic/Latino | 0.64 | 0.26 | 1.60 |
| Not Hispanic/Latino | 1.00 | 0.83 | 1.20 | |
| Stroke type | TACS | 0.98 | 0.52 | 1.85 |
| Others | 0.96 | 0.80 | 1.17 | |
| Baseline ASPECTS | >7 | 0.95 | 0.79 | 1.16 |
| <=7 | 1.04 | 0.60 | 1.81 | |
| Baseline NIHSS** | >=18 | 1.05 | 0.53 | 2.08 |
| <18 | 0.95 | 0.79 | 1.15 | |
| Baseline NIHSS Quartiles | 0 – 7 | 0.99 | 0.72 | 1.35 |
| 8 – 10 | 0.93 | 0.69 | 1.25 | |
| 11 – 16 | 0.88 | 0.59 | 1.32 | |
| 17 and above | 1.08 | 0.56 | 2.06 | |
| Stroke onset to Randomisation | <= 2 hours | 0.98 | 0.49 | 1.95 |
| > 2 hours | 0.96 | 0.80 | 1.16 | |
| Stroke onset to Treatment | <= 3 hours | 0.93 | 0.71 | 1.22 |
| > 3 hours | 0.99 | 0.78 | 1.27 | |
| Site volume | 1–2 subjects [21 sites] | 1.10 | 0.37 | 3.30 |
| 3–4 subjects [20 sites] | 1.18 | 0.62 | 2.23 | |
| 5–11 subjects [23 sites] | 1.05 | 0.67 | 1.65 | |
| 12+ subjects [25 sites] | 0.92 | 0.75 | 1.14 | |
| Baseline Glucose (mmol/L) | < 8 | 0.96 | 0.79 | 1.17 |
| >= 8 | 0.93 | 0.61 | 1.42 | |
| History of Diabetes | No | 0.95 | 0.78 | 1.16 |
| Yes | 0.97 | 0.61 | 1.54 | |
| History of Atrial Fibrillation | No | 0.98 | 0.80 | 1.20 |
| Yes | 0.87 | 0.56 | 1.35 | |
| History of Hypertension | No | 0.91 | 0.66 | 1.24 |
| Yes | 0.99 | 0.79 | 1.23 | |
Adjusted for Baseline NIHSS only
Adjusted for thrombolysis stratum only
TACS = total anterior circulation syndrome
Secondary outcomes
Other predefined outcomes assessed at 90 days -- a composite outcome, mRS assessed by sliding-dichotomy, Barthel Index, EuroQol, Stroke-Specific Quality of Life, and the Trailmaking tests -- also showed no significant differences by treatment assignment (Table 2). Figure 2 shows the distribution of 90-day mRS scores in albumin- and saline-treated subjects.
Figure 2.
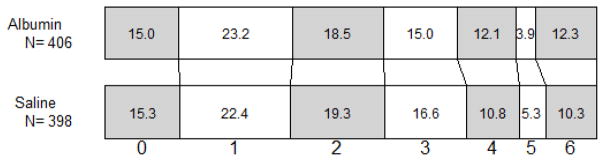
Distribution of modified Rankin Scale scores at 90 days post-randomisation in subjects treated with albumin or saline.
Safety outcomes
The major safety outcome was pulmonary edema/congestive heart failure within 48 hours, which was observed in 13.1% of subjects treated with albumin – a rate consistent with that of albumin-treated subjects in the ALIAS Pilot Trial 16 and the Part 1 Trial 18. By contrast, pulmonary edema/CHF arose in only 1.2% of patients treated with saline (relative risk (RR), 10.8; 95% CI, 4.4 – 26.7) (Table 4). This adverse event was typically of mild-to-moderate severity and was readily managed. Shortness of breath within 48 hours, and atrial fibrillation within 48 hours were also more common in albumin- than in saline-treated subjects (RR, 2.6; 95% CI, 1.1 – 6.1; and RR, 1.7; CI, 0.98 – 2.9, respectively). Although the rate of symptomatic or large intracranial haemorrhage (ICH) within 24 hours in both treatment groups was as low or lower than rates reported in other major intravenous thrombolytic trials 26,33–35, albumin-treated subjects nonetheless had a 2.4-fold greater risk than subjects receiving saline (CI 1.02 – 5.8). Correspondingly, 24-hour centrally read CT scans showed a 2.8-fold greater risk of larger (type PH2 36) parenchymal haemorrhages with albumin compared to saline (CI 1.01 – 7.7).
Table 4.
Prespecified Safety Events in the Safety Sample*
| ---------Albumin--------- | ---------Saline ---------- | |||||||
|---|---|---|---|---|---|---|---|---|
| Event | N at risk | N events | Event proportion (%) | N at risk | N events | Event proportion (%) | Relative Risk | 95% CI |
| Neurological deterioration within 48 hours | 411 | 47 | 11.4 | 412 | 40 | 9.7 | 1.18 | 0.79, 1.76 |
| Neurological death within 7 days | 404 | 13 | 3.2 | 407 | 13 | 3.2 | 1.01 | 0.47, 2.15 |
| Recurrent stroke within 30 days | 373 | 7 | 1.9 | 370 | 6 | 1.6 | 1.16 | 0.39, 3.41 |
| Recurrent stroke within 90 days | 337 | 10 | 3.0 | 331 | 12 | 3.6 | 0.82 | 0.36, 1.87 |
| Atrial fibrillation within 48 hours | 410 | 32 | 7.8 | 412 | 19 | 4.6 | 1.69 | 0.98, 2.94 |
| Pulmonary edema/CHF within 48 hours | 412 | 54 | 13.1 | 412 | 5 | 1.2 | 10.8 | 4.37, 26.72 |
| Shortness of breath within 48 hours | 410 | 18 | 4.4 | 412 | 7 | 1.7 | 2.58 | 1.09, 6.12 |
| Symptomatic ICH within 24 hours | 415 | 17 | 4.1 | 414 | 7 | 1.7 | 2.42 | 1.02, 5.78 |
| Asymptomatic ICH within 24 hours | 415 | 27 | 6.5 | 414 | 23 | 5.6 | 1.17 | 0.68, 2.01 |
| Parenchymal haemorrhage 1 (PH1) on 24h-CT scan (central reader)¶ | 407 | 8 | 2.0 | 405 | 5 | 1.2 | 1.59 | 0.53, 4.83 |
| Parenchymal haemorrhage 2 (PH2) on 24h-CT scan (central reader)¶ | 407 | 14 | 3.4 | 405 | 5 | 1.2 | 2.79 | 1.01, 7.66 |
| Death within 30 days | 409 | 39 | 9.5 | 406 | 37 | 9.1 | 1.05 | 0.68, 1.61 |
| Death within 90 days | 378 | 46 | 12.2 | 369 | 41 | 11.1 | 1.10 | 0.74, 1.63 |
The safety sample consisted of the 823 subjects who received at least 20% of the intended dose of study-drug.
Abbreviations: CI, confidence interval; CHF, congestive heart failure; ICH, intracranial haemorrhage; CT, computed tomography.
Parenchymal haemorrhage PH1 denotes a blood clot not exceeding 30% of the infarcted area with some mild space-occupying effect. Parenchymal haemorrhage PH2 refers to a dense blood clot or clots exceeding 30% of the infarct volume with significant space-occupying effect 36.
Other prespecified major safety outcomes showed no significant differences by treatment (Table 4). These included neurological deterioration within 48 hours (overall rate, 10.6%); neurological death within 7 days (3.2%); recurrent stroke within 30 days (1.8%); asymptomatic intracranial haemorrhage within 24 hours (6.0%); mild parenchymal haemorrhage (PH1) on the 24-hour centrally read CT scan (1.6%); deaths within 30 days (9.3%); and deaths within 90 days (11.6%).
Outcome trends over the course of the trial
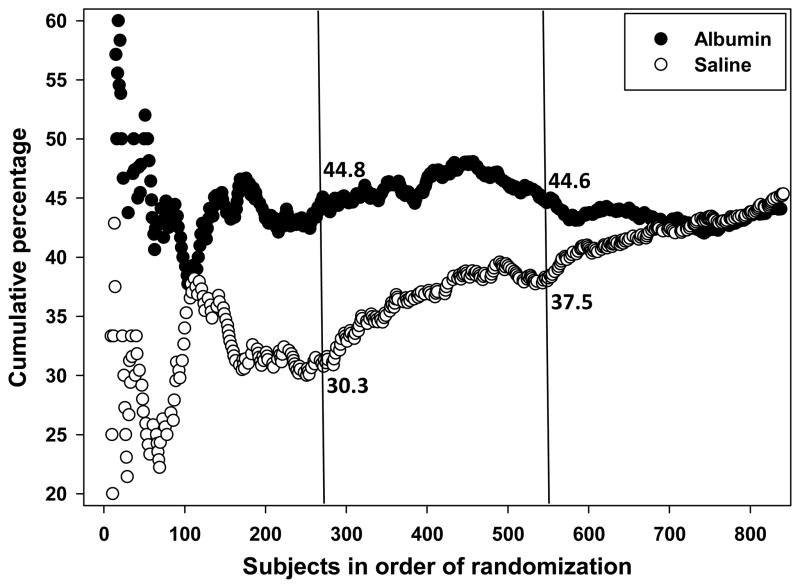
The favourable primary outcome rate with albumin remained steady at 44–45% throughout the trial. However, our assumption that the saline-placebo group would show a favourable rate of 40% proved initially to be incorrect (Figure 3); rather, the rate in saline-treated subjects averaged 32% over the first quarter of the trial but rose steadily thereafter, so that an interaction of treatment-by-randomisation order was evident. At the first prespecified interim analysis (of N=275 subjects) presented to the DSMB (Figure 3), albumin- and saline-treated subjects showed respective favourable outcome rates of 44.8% and 30.3%, with an effect size of 14.5% and a relative benefit of 1.48 favouring albumin therapy (95% CI 1.12–1.97 adjusted for baseline NIHSS and thrombolysis status; one-sided p = 0.0028), suggesting that the likelihood of favourable outcome was approximately 50% higher for those in the albumin group. At the second prespecified interim analysis (N=550 subjects) (Figure 3), the saline-placebo rate had risen to 37.5% while the albumin rate remained steady at 44.6%, for a relative benefit of 1.21 favouring albumin (adjusted 95% CI 1.01–1.44; one-sided p = 0.0176). At neither of these prespecified analyses, however, did the differences cross the interim-analysis boundary for overwhelming efficacy 28 By the time the trial was halted, there was no difference by treatment assignment (Figure 3). Table 5 presents the stopping boundaries and the statistics observed in these interim analyses. A detailed analysis of outcome trends-over-time will be the subject of a subsequent report.
Figure 3.
Cumulative primary outcome rates in subjects treated with albumin and with saline-placebo. The two vertical lines denote the points at which two prespecified interim analyses were conducted, at N=275 and N=550 subjects, respectively. The cumulatively computed primary-outcome rate in albumin-treated subjects was consistent at 44–45% throughout the trial, while the cumulative rate in the saline-placebo group was initially much lower but subsequently improved steadily.
Table 5.
Stopping Boundaries and Observed Statistics for Overwhelming Efficacy and Futility Based on One-sided α=0.02528–31
| Analysis | N | Nominal Critical Point to Reject H0 for Overwhelming Efficacy | Nominal Critical Point to Reject H1 for Futility | Actual Test Statistic |
|---|---|---|---|---|
| 1 | 275 | 4.3326 | −1.3491 | 2.76 |
| 2 | 550 | 2.9631 | 0.3293 | 2.11 |
| 3 | 732 | 2.5410 | 0.9539 | 0.14 |
DISCUSSION
Despite convincing experimental evidence supporting ischaemic neuroprotection by high-dose albumin 9–15, and despite an exploratory efficacy analysis of a “target population” of ALIAS-Part 1 subjects who would have satisfied the ALIAS Part 2 Trial’s revised eligibility criteria that showed a trend toward a favourable outcome with albumin treatment 27, neither the primary nor secondary outcomes of the present definitive trial differed by treatment assignment. (See Research in Context panel.)
Panel 2. Research in Context.
Systematic review
We searched the PubMed database for articles published prior to July 12, 2013, describing the therapeutic use of albumin in stroke. We used the search string, “albumin [ti] AND brain AND (stroke OR ischemia OR hemorrhage)”. The resulting reports were manually reviewed. Prior to our own clinical trials, custom-tailored haemodilution with albumin plus crystalloids was studied in a prospective single-center study, and reduced 3-month mortality and increased functional independence were reported 51. Another study was a retrospective review of the safety of high-dose albumin treatment in 30 subjects treated within 24 hours of stroke onset and 60 case-controls; albumin treatment was associated with a non-significant trend toward cardiopulmonary adverse events, which, however, did not influence mortality 52. The only other studies identified were our own trials that formed the antecedents to the multicenter trial reported here: the ALIAS Pilot Trial 16,17, and the ALIAS Part 1 Multicenter Trial 18,27, which are reviewed in the present article. As of July 12, 2013, the U.S. National Institutes of Health website, ClinicalTrials.gov, listed the following trials of albumin in cerebrovascular disorders: a) A phase 2B study (NCT01684462) investigating the safety and efficacy of 20% albumin, 1.25 g/kg, administered within 12 hours of stroke onset, currently recruiting subjects in Korea; b) an inactive phase 2 trial of albumin therapy for intracerebral haemorrhage, utilizing MRI imaging outcome markers (NCT00990509); and c) a phase 1 dose-escalation trial, now terminated, of albumin therapy for subarachnoid haemorrhage (NCT01747408).
Interpretation
The ALIAS Part 2 Multicenter Trial represents the translation of the concept of high-dose albumin neuroprotection from therapeutically successful pre-clinical studies in rodent models of focal cerebral ischaemia, through a pilot clinical trial and an antecedent multicenter clinical trial, in which both the albumin-dose and the time-window of treatment were selected to be similar to those shown to be therapeutically efficacious in the animal studies. The predominant safety event throughout the clinical trials has been the consistent induction of mild-to-moderate pulmonary edema in around 13% of albumin-treated subjects, which we have shown can be successfully managed without serious consequences by fluid restriction and diuretic therapy. The neutral primary and secondary efficacy outcomes in the present trial were unexpected in view of suggestive signals of possible efficacy emerging from the pilot trial 17 and in a target population of the antecedent Part 1 trial 27, and interpretation of the present results is markedly complicated by the fact that the outcome of placebo- treated subjects improved steadily over the course of the current trial, such that strong signals of albumin’s therapeutic efficacy were present during the first half of this trial but had disappeared by its end. These results challenge interpretation and will be the focus of extensive subsequent analysis.
This trial was limited by its early termination. In addition, we were unable to obtain complete 12-month assessments due to the sponsor’s decision not to provide funding for follow-up beyond the 3-month primary outcome assessment. Biologically, we restricted our study design to the estimated time-window of treatment effect based upon preclinical findings 10, but we recognize that a 5-hour window-to- treatment might still be too long. Furthermore, we did not control for reperfusion, either in application or timing, and since the only proven neuroprotectant strategy in humans is the induction of prompt reperfusion, this might have affected our results.
The neutral outcomes of this and other major multicenter trials of neuroprotective strategies for acute ischaemic stroke (e.g., 37–42) raise the concern as to whether positive results in animal stroke models can legitimately be translated to humans. This question has elicited extensive discussion in the recent literature 7,8,43–46. The preclinical studies supporting albumin-neuroprotection were conducted in rodents but not in primates; focal cerebral ischemia was typically induced mechanically (by an intraluminal filament to occlude the middle cerebral artery) rather than by a thrombus or embolus; and reperfusion was induced by withdrawing the filament rather than by thrombolysis. The animals used were young and free of medical co-morbidities, contrasting with human ischaemic stroke, which typically afflicts middle-aged and elderly patients who often suffer from other medical conditions. Any or all of these differences may be relevant here.
The interpretation of this trial is challenged by the fact that subjects treated with saline-placebo had a steadily improving favourable-outcome rate over the course of the trial, while this did not occur in albumin-treated participants (Figure 3). Thus, the first prespecified interim analysis at N=275 subjects revealed an apparent relative benefit of 48% favouring albumin treatment (Figure 3) while by the trial’s end the effect had completely disappeared. Early fluctuation in cumulative effect size over time has been reported for large cardiac trials, with a conclusion that continuing to the prespecified fixed sample size is critically important 47.
We do not have a satisfactory explanation at this time for the steadily improving favourable outcome rate observed in the placebo but not the albumin arm over the course of the trial. We cannot exclude that ongoing improvements in general stroke care over the 3.5-year enrollment period may have affected the placebo group preferentially. Under this hypothetical scenario, the stable favourable-outcome rate of 44–45% in the albumin group would be viewed as a “ceiling effect”, that is, not sensitive to further improvements in stroke care; while the saline-placebo group, whose favourable outcome was initially quite low (31%), was susceptible to increases over time afforded by steadily improving standards of care and possibly by the increasing rate of IV tPA use observed over the course of the trial.
Supporting the notion of an albumin-related “ceiling effect” is a recent experimental study in which albumin treatment was shown to exert a significant therapeutic effect after ischaemic stroke by augmenting the brain’s collateral perfusion when studied in a mouse strain with sparse brain collaterals, while this effect was not seen in a mouse strain having more abundant brain collateral circulation 13.
As in our earlier trials, mild-to-moderate pulmonary edema was the major drug-related adverse event but was usually readily managed, and serious adverse events related to CHF were rare. The findings of significantly greater symptomatic ICH and of type-PH2 parenchymal haemorrhages by CT-scan with albumin treatment were unanticipated and may relate to albumin’s reported platelet anti-aggregatory effect 48–50. This adverse event, however, did not appear to influence the rates of neurological deterioration or death, which were similar by treatment assignment.
In summary, this randomised multicenter trial failed to demonstrate that high doses of albumin improve the neurological or functional outcome of patients with acute ischaemic stroke. Nonetheless, we do not believe that the neutral results of this, or of any well-designed, clinical trial should discourage future efforts to identify efficacious strategies to protect the ischaemic brain as an abundant preclinical literature continues to provide cogent proof-of-principle that this may be possible.
Supplementary Material
Acknowledgments
The study was supported by cooperative agreements from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (University of Miami, U01NS040406; Medical University of South Carolina, U01NS054630; University of Michigan, 2U01NS056975) and by grant BT10-000272 from Baxter Healthcare Corporation to support extension of the trial to Finland.
Appendix. Study Committees and Investigators
ALIAS Trial Executive Committee
Myron D. Ginsberg, University of Miami, Miami, FL -- Study Chair and Principal Investigator, Clinical Coordinating Center;
Yuko Y. Palesch, Medical University of South Carolina, Charleston, SC -- Principal Investigator, Statistics and Data Coordinating Center;
Michael D. Hill, University of Calgary, Canada -- Director, Canadian Coordinating Center;
Bonnie D. Waldman, Medical University of South Carolina, Charleston, SC -- Project Manager;
Kristen M. Clasen (ex officio), Medical University of South Carolina, Charleston, SC -- Project Manager Assistant;
Richard Leinster, Medical University of South Carolina, Charleston, SC -- Data Manager;
Isabel Mendez, University of Miami, Miami, FL -- Financial Manager
Diego Tamariz, University of Miami, Miami, FL -- Clinical Project Coordinator;
Karla J. Ryckborst, University of Calgary, Calgary, Canada -- Study Coordinator;
Claudia S. Moy, NINDS, National Institutes of Health, Rockville, MD -- NINDS Project Scientist.
Unblinded Study Statisticians
Renee H. Martin, Medical University of South Carolina, Charleston, SC;
Sharon D. Yeatts, Medical University of South Carolina, Charleston, SC.
External Safety Monitors
Andrew M. Naidech, Northwestern University, Chicago;
Alejandro A. Rabinstein, Mayo Clinic, Rochester, MN.
Data and Safety Monitoring Board
Patrick D. Lyden, Cedars–Sinai Medical Center, Los Angeles, CA (Chair);
Christopher S. Coffey, University of Iowa, Iowa City, Iowa;
Marco DiTullio, Columbia University, New York, NY;
Christine Wijman, Stanford University, Palo Alto, CA;
and Janice Cordell (ex officio), NINDS, National Institutes of Health, Rockville, MD.
PARTICIPATING CLINICAL SITES
[Site Principal Investigator, Primary Study Coordinator (number of subjects enrolled)] [* denotes Neurological Emergency Treatment Trials (NETT) Network hub or spoke sites]
UNITED STATES
Abington Memorial Hospital, Abington, PA*: David Weisman, Jennifer McGowan Trainer (39);
Memorial Hermann - Texas Medical Center, Houston, TX*: Elizabeth Jones, Misty Ottman (36);
University of Kentucky Medical Center, Lexington, KY*: Roger Humphries, Linda Dechtenberg (33);
Oregon Health and Science University, Portland, OR: Wayne Clark, Darren Larsen (26);
Mayo Clinic Hospital, Phoenix, AZ: Maria Aguilar, Erica Boyd (24);
New York Presbyterian/Columbia University Medical Center, New York City, NY*: Stephan Mayer, Cristina Falo (22);
Oregon Health and Science University - Providence Portland Medical Center, Portland, OR: Wayne Clark, Darren Larsen (18);
Stanford University Medical Center, Stanford, CA*: James Quinn, Stephanie Casal (18);
Temple University Hospital, Philadelphia, PA*: Nina Gentile, Brent Freeman (18);
University of Florida - Shands Jacksonville Medical Center, Jacksonville, FL: Scott Silliman, Mary Stegmaier (18);
Buffalo General Medical Center, Buffalo, NY: Robert Sawyer, Annemarie Crumlish (16);
Detroit Receiving Hospital, Detroit, MI*: Robert Welch, Valerie Mika (16);
Grady Memorial Hospital, Atlanta, GA*: David Wright, Andrea McDougal (16);
Jackson Memorial Hospital, University of Miami, Miami, FL: Sebastian Koch, Diego Tamariz (16);
Froedtert Memorial Lutheran Hospital, Milwaukee, WI*: Michel Torbey, Melissa Boettcher (15);
San Francisco General Hospital, San Francisco, CA*: Claude Hemphill, Michele Meeker (14);
Emory University Hospital, Atlanta, GA*: David Wright, Harriet Nevarez (12);
Hennipin County Medical Center, Minneapolis, MN*: Mustapha Ezzeddine, Kathleen Miller (12);
Henry Ford Hospital, Detroit, MI*: Christopher Lewandowski, Paula Crouse (12);
Oregon Health and Science University - Providence St. Vincent Medical Center, Portland, OR: Wayne Clark, Barbara Dugan (11);
University Hospital, Cincinnati, OH*: Pooja Khatri, Irene Ewing (11);
Virginia Commonwealth University/Medical College of Virginia, Richmond, VA*: Warren Felton, Margot Lee (11);
Sinai-Grace Hospital, Detroit, MI*: Robert Welch, Valerie Mika (10);
University of Maryland Medical Center, Baltimore, MD*: Marcella Wozniak, Virginia Ganley (10);
Hahnemann University Hospital, Philadelphia, PA*: Mark Saks, Romy Nocera (9);
University of North Carolina at Chapel Hill, Chapel Hill, NC: Souvik Sen & David Huang; Sierra Marino (9);
Baylor College of Medicine, Houston, TX: Jose Suarez, Eusebia Calvillo (7);
Beaumont Hospital, Royal Oak, Royal Oak, MI*: Robert Swor, LynnMarie Mango (7);
El Camino Hospital Mountain View, Mountain View, CA*: Peter Fung, Rosen Mann (7);
Intercoastal Neurology - Intercoastal Medical Research Center, Sarasota, FL: Mauricio Concha, Jeanette Wilson (7);
New York Methodist Hospital, Brooklyn, NY*: Robert Birkhahn, Paris Datillo (7);
Bethesda North Hospital, Cincinnati, OH*: Pooja Khatri, Irene Ewing (6);
Fairview Southdale Hospital, Edina, MN*: Mustapha Ezzeddine, Kathleen Miller (6);
University of Pennsylvania Perelman School of Medicine, Philadelphia, PA: Steven Messe, Maryliz Desanto (6);
Loyola University Medical Center, Maywood, IL: Rima Dafer & Michael Schneck, Linda Chadwick (5);
University of California San Francisco Medical Center, San Francisco, CA*: Claude Hemphill, Michele Meeker (5);
Upper Chesapeake Medical Center, Bel Air, MD*: Michael Abraham, Barbara Cysyk (5);
Villages Research Group, Ocala, FL: Gregory Howell, Sharon Howell (5);
Yale University School of Medicine, New Haven, CT: Joseph Schindler, Janet Halliday (5);
California Pacific Medical Center - Pacific Campus, San Francisco, CA*: Nobi Barazangi, Jessica Redford (4);
Good Samaritan Hospital, Cincinnati, OH*: Pooja Khatri, Irene Ewing (4);
HealthEast Care System, St. Joseph’s Hospital, St. Paul, MN: Sandra Hanson, Amy Castle (4);
Mercy Hospital of Buffalo, Buffalo, NY: Catalina Ionata, Kathleen Parkes (4);
Sacred Heart Medical Center, Springfield, OR: Raymond Englander, Becky Hammerschmith (4);
Saint Elizabeth South Hospital, Cincinnati, OH*: Pooja Khatri, Irene Ewing (4);
University of Arizona Medical Center, Tucson, AZ*: Kurt Denninghoff, Ginny Stasinski (4);
University of Minnesota Medical Center, Fairview, Minneapolis, MN*: Mustapha Ezzeddine, Kathleen Miller (4);
Christiana Care - Christiana Hospital, Newark, DE*: Jason Nomura, Christy Poole (3);
Metro Health Medical Center, Cleveland, OH: Michael Bahntge, Scott Bailey (3);
Neuroscience Institute - Florida Hospital Orlando, Orlando, FL: Carlos Villar, Cherlynn Basignani (3);
Oregon Health and Science University - Legacy Emanuel Medical Center, Portland, OR: Wayne Clark, Darren Larsen (3);
Saint Louis University, St. Louis, MO: Salvador Cruz-Flores, Eve Holzemer (3);
Winthrop-University Hospital, Mineola, NY*: Jay Yasen, Kim Byrnes (3);
California Pacific Medical Center - Davies Campus, San Francisco, CA*: Nobi Barazangi, Jessica Redford (2);
Duke University School of Medicine, Durham, NC: Daniel Laskowitz, Ellen Bennett (2);
John Muir Medical Center – Concord, Concord, CA: Raymond Stephens, Peggy Newsom (2);
Penn State Hershey Medical Center, Hershey, PA*: Thomas Terndrup, Deborah Hoffman (2);
St. Elizabeth Healthcare – Florence, Florence, KY*: Pooja Khatri, Irene Ewing (2);
The Christ Hospital, Cincinnati, OH*: Pooja Khatri, Irene Ewing (2);
University of Pittsburgh Medical Center, Pittsburgh, PA: Lawrence Wechsler, Sharon DeCesare (2);
WakeMed Health and Hospitals, Raleigh, NC: Keith Hull, Keisha Fuller (2);
Atlantic Neuroscience Institute, Overlook Medical Center, Summit, NJ: Shalini Bansil, Angela Brown (1);
Mercy Fairfield Hospital, Cincinnati, OH*: Pooja Khatri, Irene Ewing (1);
John Muir Medical Center - Walnut Creek, Walnut Creek, CA: Raymond Stephens, Peggy Newsom (1);
O’Connor Hospital, San Jose, CA*: Raul Guisado, Karen De La Cuesta (1);
Ohio State University Wexner Medical Center, Columbus, OH: Michel Torbey, April Spangler (1);
Seton Medical Center Austin, Austin, TX: Darryl Camp, Lisa Houy (1);
UCLA Medical Center - Santa Monica, Los Angeles, CA: Sidney Starkman, Judy Guzy (1);
University Physicians Healthcare Hospital at Kino, Tucson, AZ*: Kurt Denninghoff, Ginny Stasinski (1).
CANADA
Foothills Hospital, University of Calgary, Calgary, AB: Michael Hill, Karla Ryckborst (96);
University of Alberta, Edmonton, AB: Ashfaq Shuaib, Brenda Schwindt (25);
Vancouver General Hospital, Vancouver, BC: Phillip Teal, Nathalie Esplana (17);
Centre de recherche de l’Hôpital Charles LeMoyne, Greenfield Park, QC: Jean-Martin Boulanger, Lise Blais (16);
The Ottawa Hospital, Ottawa, ON: Grant Stotts, Melodie Mortensen (13);
University of Toronto, St. Michael’s Hospital, Toronto, ON: Neville Bayer & Daniel Selchen, Pawel Kostyrko (13);
Royal Inland Hospital, Kamloops, BC: Todd Collier, Lauren Kembel (10);
Centre de Santé et de Services Sociaux de Chicoutimi, Saguenay (Chicoutimi), QC: Michel Beaudry, Marie-Josee Chartrand (9);
Trillium Health Centre, Mississauga, ON: Daniel Selchen, Heather Hink (7);
Grey Nuns Community Hospital, Edmonton, AB: Brian Buck, Theresa Griffin-Stead (4);
Queen Elizabeth II Health Sciences Centre, Halifax, NS: Gord Gubitz, Judith Jarrett (4);
London Health Sciences Centre - University Hospital, London, ON: Vladimir Hachinski, Kim Hesser (2);
Thunder Bay Regional Health Sciences Centre, Thunder Bay, ON: David Howse, Sandra Stoger (2).
FINLAND
Tampere University Hospital, Tampere: Heikki Numminen, Anne Simi (4);
Helsinki University Central Hospital, Helsinki: Turgut Tatlisumak, Saija Eirola (1).
ISRAEL
Hadassah Medical Center - Hadassah University Hospital, Ein Kerem, Jerusalem: Ronen Robert Leker, Ruth Paniri (4);
Rambam Health Care Campus, Haifa: Gregory Telman, Svetlana Afanasiev (4);
Tel Aviv Sourasky Medical Center, Tel Aviv: Hen Hallevi, Rotem Elimelech (4);
Chaim Sheba Medical Center at Tel Hashomer, Tel Hashomer, Ramat Gan: David Tanne, Meirav Izchak (1);
Soroka Medical Center, Beersheba: Gal Ifergane, Ruthie Bekore (1).
Footnotes
Contributors
MDG was the principal investigator and wrote the manuscript. MDG, YYP, MDH, and CSM designed the study. MDG, MDH, WGB, DT, and KJR supervised clinical aspects of the study. RHM and YYP performed biostatistical analyses. MDG, YYP, RHM, and MDH analyzed and interpreted data. BDW directed project management and supervised site monitoring. MDH performed central readings of CT scans and electrocardiograms. DT and KJR collected clinical data and guided the study coordinators. All authors contributed substantively to the final paper.
Conflicts of Interest
Dr. Ginsberg reports receiving consulting fees from Aldagen, Inc. Dr. Palesch reports being a paid DSMB member of one of the trials of BrainsGate Ltd. Dr. Hill reports receiving funding from the Heart & Stroke Foundation of Alberta. MDG, YYP, MDH, RHM, CSM, WGB, BDW, DT, and KJR declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 3.Norrving B, Kissela B. The global burden of stroke and need for a continuum of care. Neurology. 2013;80:S5–12. doi: 10.1212/WNL.0b013e3182762397. [DOI] [PubMed] [Google Scholar]
- 4.Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Incidence of the major stroke subtypes: initial findings from the North East Melbourne stroke incidence study (NEMESIS) Stroke. 2001;32:1732–1738. doi: 10.1161/01.str.32.8.1732. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke. 2012;7:407–418. doi: 10.1111/j.1747-4949.2012.00770.x. [DOI] [PubMed] [Google Scholar]
- 8.Cook DJ, Tymianski M. Translating promising preclinical neuroprotective therapies to human stroke trials. Expert Rev Cardiovasc Ther. 2011;9:433–449. doi: 10.1586/erc.11.34. [DOI] [PubMed] [Google Scholar]
- 9.Belayev L, Zhao W, Pattany PM, et al. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke. 1998;29:2587–2599. doi: 10.1161/01.str.29.12.2587. [DOI] [PubMed] [Google Scholar]
- 10.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- 11.Huh PW, Belayev L, Zhao W, Busto R, Saul I, Ginsberg MD. The effect of high-dose albumin therapy on local cerebral perfusion after transient focal cerebral ischemia in rats. Brain Res. 1998;804:105–113. doi: 10.1016/s0006-8993(98)00674-x. [DOI] [PubMed] [Google Scholar]
- 12.Belayev L, Pinard E, Nallet H, et al. Albumin therapy of transient focal cerebral ischemia: in vivo analysis of dynamic microvascular responses. Stroke. 2002;33:1077–1084. doi: 10.1161/hs0402.105555. [DOI] [PubMed] [Google Scholar]
- 13.DeFazio RA, Zhao W, Deng X, Obenaus A, Ginsberg MD. Albumin therapy enhances collateral perfusion after laser-induced middle cerebral artery branch occlusion: a laser speckle contrast flow study. J Cereb Blood Flow Metab. 2012;32:2012–2022. doi: 10.1038/jcbfm.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimmagadda A, Park H-P, Prado R, Ginsberg MD. Albumin therapy improves local vascular dynamics in a rat model of primary microvascular thrombosis: a two-photon laser-scanning microscopy study. Stroke. 2008;39:198–204. doi: 10.1161/STROKEAHA.107.495598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park HP, Nimmagadda A, DeFazio RA, Busto R, Prado R, Ginsberg MD. Albumin therapy augments the effect of thrombolysis on local vascular dynamics in a rat model of arteriolar thrombosis: a two-photon laser-scanning microscopy study. Stroke. 2008;39:1556–1562. doi: 10.1161/STROKEAHA.107.502195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg MD, Hill MD, Palesch YY, Ryckborst KJ, Tamariz D. The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke. I. Physiological responses and safety results. Stroke. 2006;37:2100–2106. doi: 10.1161/01.STR.0000231388.72646.05. [DOI] [PubMed] [Google Scholar]
- 17.Palesch YY, Hill MD, Ryckborst KJ, Tamariz D, Ginsberg MD. The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke. II. Neurological outcome and efficacy-analysis. Stroke. 2006;37:2107–2114. doi: 10.1161/01.STR.0000231389.34701.b5. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg MD, Palesch YY, Martin RH, et al. The albumin in Acute Stroke (ALIAS) multicenter clinical trial: safety analysis of part 1 and rationale and design of part 2. Stroke. 2011;42:119–127. doi: 10.1161/STROKEAHA.110.596072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pocock S. Clinical Trials --a Practical Approach. Wiley; New York: 1983. [Google Scholar]
- 20.Myburgh J, Cooper J, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- 21.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell WE, Reynolds DM, De Soto CB. Neuropsychological impairment scale (NIS): initial validation study using trailmaking test (A & B) and WAIS digit symbol (scaled score) in a mixed grouping of psychiatric, neurological, and normal patients. J Clin Psychol. 1983;39:746–748. doi: 10.1002/1097-4679(198309)39:5<746::aid-jclp2270390517>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.The EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 24.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–2236. doi: 10.1161/hs1001.096191. [DOI] [PubMed] [Google Scholar]
- 26.NINDS rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 27.Hill MD, Martin RH, Palesch YY, et al. The Albumin in Acute Stroke Part 1 Trial: an exploratory efficacy analysis. Stroke. 2011;42:1621–1625. doi: 10.1161/STROKEAHA.110.610980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 29.Lan KKG, DeMets DI. Design and analysis of group sequential tests based on type-I error spending rate function. Biometrika. 1987;74:149–154. [Google Scholar]
- 30.Pampallona S, Tsiatis AA, Kim KM. Interim monitoring of group sequential trials using spending functions for the Type I and Type II error probabilities. Drug Information Journal. 2001;35:1113–1121. [Google Scholar]
- 31.Fleiss JL, Tytun A, Ury HK. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics. 1980;36:343–346. [PubMed] [Google Scholar]
- 32.Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. Springer-Verlag; New York: 1998. [Google Scholar]
- 33.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4. 5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 34.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 35.Shobha N, Buchan AM, Hill MD. Thrombolysis at 3–4. 5 hours after acute ischemic stroke onset--evidence from the Canadian Alteplase for Stroke Effectiveness Study (CASES) registry. Cerebrovasc Dis. 2011;31:223–228. doi: 10.1159/000321893. [DOI] [PubMed] [Google Scholar]
- 36.Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30:2280–2284. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 37.Davalos Alvarez-Sabin J, Castillo J, et al. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380:349–357. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg G, Bornstein N, Diener HC, Gorelick PB, Shuaib A, Lees K. The Membrane-Activated Chelator Stroke Intervention (MACSI) Trial of DP-b99 in acute ischemic stroke: a randomized, double-blind, placebo-controlled, multinational pivotal phase III study. Int J Stroke. 2011;6:362–367. doi: 10.1111/j.1747-4949.2011.00608.x. [DOI] [PubMed] [Google Scholar]
- 39.Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 40.Shuaib A, Lees KR, Lyden P, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 41.Sacco RL, DeRosa JT, Haley EC, Jr, et al. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: a randomized controlled trial. JAMA. 2001;285:1719–1728. doi: 10.1001/jama.285.13.1719. [DOI] [PubMed] [Google Scholar]
- 42.Lees KR, Asplund K, Carolei A, et al. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. GAIN International Investigators. Lancet. 2000;355:1949–1954. doi: 10.1016/s0140-6736(00)02326-6. [DOI] [PubMed] [Google Scholar]
- 43.Dirnagl U. Bench to bedside: the quest for quality in experimental stroke research. J Cereb Blood Flow Metab. 2006;26:1465–1478. doi: 10.1038/sj.jcbfm.9600298. [DOI] [PubMed] [Google Scholar]
- 44.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 47.Yusuf S. Challenges in the conduct and interpretation of phase II (pilot) randomized trials. Am Heart J. 2000;139:S136–S142. doi: 10.1016/s0002-8703(00)90061-9. [DOI] [PubMed] [Google Scholar]
- 48.Ammit AJ, O’Neill C. Studies of the nature of the binding by albumin of platelet-activating factor released from cells. J Biol Chem. 1997;272:18772–18778. doi: 10.1074/jbc.272.30.18772. [DOI] [PubMed] [Google Scholar]
- 49.Gresele P, Deckmyn H, Huybrechts E, Vermylen J. Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem Pharmacol. 1984;33:2083–2088. doi: 10.1016/0006-2952(84)90577-x. [DOI] [PubMed] [Google Scholar]
- 50.Jorgensen KA, Stoffersen E. On the inhibitory effect of albumin on platelet aggregation. Thromb Res. 1980;17:13–18. doi: 10.1016/0049-3848(80)90289-3. [DOI] [PubMed] [Google Scholar]
- 51.Goslinga H, Eijzenbach V, Heuvelmans JH, et al. Custom-tailored hemodilution with albumin and crystalloids in acute ischemic stroke. Stroke. 1992;23:181–188. doi: 10.1161/01.str.23.2.181. [DOI] [PubMed] [Google Scholar]
- 52.Koch S, Concha M, Wazzan T, Romano JG, Forteza A. High dose human serum albumin for the treatment of acute ischemic stroke: a safety study. Neurocrit Care. 2004;1:335–341. doi: 10.1385/NCC:1:3:335. [DOI] [PubMed] [Google Scholar]
- 53.Adams HP, Jr, Effron MB, Torner J, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II) Stroke. 2008;39:87–99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]
- 54.Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of a randomized phase 2 trial. Stroke. 2005;36:880–890. doi: 10.1161/01.STR.0000157668.39374.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.