Abstract
Objective
To ascertain prevalence of peripheral sensory and motor neuropathy; to evaluate impairments in relation to function.
Design
St. Jude Lifetime Cohort Study, a clinical follow-up study designed to evaluate adverse late effects in adult survivors of childhood cancer.
Setting
St. Jude Children’s Research Hospital (SJCRH).
Participants
Eligibility required treatment for an extracranial solid malignancy between 1962 and 2002, age ≥18 years, ≥10 years post-diagnosis, no history of cranial radiation. 531 survivors were included in the evaluation: median age 32 years, median time from diagnosis 25 years.
Interventions
Not applicable.
Main Outcome Measures
Primary exposure measures were cumulative doses of vinca-alkaloid and platinum-based chemotherapies. Survivors with scores ≥ 1 on the sensory subscale of the modified Total Neuropathy Score were classified with prevalent sensory impairment. Those with sex-specific Z-scores of ≤−1.3 for dorsiflexion strength were classified with prevalent motor impairment. Participants completed the 6-minute walk test (endurance), the timed up and go test (mobility), and the sensory organization test (balance).
Results
The prevalence of sensory and motor impairment was 20% and 17.5%, respectively. Vinca-alkaloid exposure was associated with an increased risk of motor impairment (adjusted odds ratio (OR)=1.66, 95% Confidence Interval (CI): 1.04–2.64) without evidence for a dose response. Platinum exposure was associated with increased risk of sensory impairment (adjusted OR= 1.62, 95% CI: 0.97–2.72) without evidence of a dose response. Sensory impairment was associated with poor endurance (OR=1.99, 95% CI: 0.99–4.00) and mobility (OR=1.65, 95% CI: 0.96–2.83).
Conclusion
Vincristine and cisplatin exposure may increase risk for long-term motor and sensory impairment, respectively. Survivors with sensory impairment are at increased risk for functional performance limitations.
Keywords: Peripheral nervous system diseases, vincristine, cisplatin, neoplasm
Chemotherapy-induced peripheral neuropathies, both motor and sensory, are well-described acute toxicities of vincristine and platinum drugs.1–3 Often dose-limiting in treatment settings2, 4–6, acute neuropathy can manifest as painful dysesthesia; numbness and sensory loss to vibration, temperature, and proprioception; suppression or loss of deep tendon reflexes; impaired balance and coordination; distal muscle weakness; ataxia and, in extreme cases, paralysis.1, 2, 7 Most children with newly diagnosed solid tumor are treated with platinum drugs (cisplatin or carboplatin or both), and/or vinca-alkaloid drugs (usually vincristine, occasionally vinblastine). These solid tumor diagnoses include sarcomas (osteosarcoma, Ewing sarcoma family of tumors, rhabdomyosarcoma, and other soft tissue sarcomas), embryonal tumors (Wilms tumor, neuroblastoma, retinoblastoma, germ cell tumors, hepatoblastoma), and several types of carcinomas of childhood (nasopharyngeal carcinoma, adrenocortical carcinoma). Cisplatin-induced acute neuropathy is known to persist and progress many weeks after treatment completion (so called “coasting”).2, 6 Despite the common use of platinum-based chemotherapy in pediatric solid tumor treatment protocols, very little is known about long term (chronic) peripheral nervous system sequelae from these exposures. It is generally believed that acute vincristine-induced neurotoxicity in children, unless severe, completely resolves within months after treatment completion.2, 8 However, we9, 10 and others11, 12 have provided evidence suggesting that mild peripheral motor neuropathy may be a long-term effect in survivors of childhood acute lymphoblastic leukemia, in whom relatively high cumulative doses of vincristine are received over a 2 to 3 year treatment period. There is very little long-term follow-up data on patients treated for solid tumors during childhood in whom doses of vincristine may be lower and exposure duration is considerably shorter than that of leukemia patients. The aims of this study were to evaluate: 1) whether exposure to vinca-alkaloid and platinum drugs are associated with symptoms of motor and/or sensory impairment in adult survivors of childhood solid tumors; and 2) whether adult survivors with symptoms of motor and/or sensory impairment demonstrate impaired function in mobility, endurance, or balance.
METHODS
St. Jude Lifetime Cohort
Study participants were enrolled in the IRB-approved St. Jude Lifetime Cohort Study (SJLIFE) as described in detail by Hudson et al.13 Eligibility for SJLIFE is restricted to those aged 18 years or older who were treated for cancer at St. Jude Children’s Research Hospital and were at least 10 years post diagnosis at the time of enrollment. Medical record abstraction is conducted for chemotherapeutic agents, radiation treatment fields and doses, surgical interventions, life-threatening organ toxicities, and subsequent malignancies. Participants complete an extensive health questionnaire. In addition, an on-campus clinical evaluation is performed consistent with the risk-based screening and surveillance recommendations of the Children’s Oncology Group Long Term Follow-Up Guidelines for Treatment of Children, Adolescent and Young Adult Cancers.14, 15 Transportation to the St. Jude campus in Memphis, TN, and other travel and research-related lodging expenses are paid for by SJLIFE. The risk-based medical assessment includes testing of physical functioning and performance in the SJLIFE Human Performance Laboratory. Current eligible SJLIFE participants were diagnosed and treated between 1962 and 2002.
Subjects
SJLIFE is an ongoing study with continuous enrollment. The recruitment cutoff date for this analysis was April 30th, 2012. Eligibility was restricted to participating SJLIFE survivors with an extracranial solid malignancy, no cranial radiation exposure, and no diagnosis of a second primary neoplasm. Of 1002 potential subjects, 396 (39.5%) were either active refusals, passive non-participants, or were lost to follow-up. Of the 606 who agreed to participate, 75 did not complete the physical functioning and performance testing to qualify for this analysis. Thus, 531 survivors with extracranial solid tumors were included for evaluation of the first aim (assessment of neuropathic symptoms). For the second aim (assessment of impaired function), because lower extremity amputation precludes fair comparisons with normative data for the functional measures, 48 of 531 survivors with lower extremity amputations, all performed as part of local control for their bone sarcomas, were not included in the analysis, leaving 475 participants for evaluation of impaired function.
Exposures
Cumulative doses of vincristine, vinblastine, cisplatin and carboplatin were abstracted from medical records. Only 6 participants were treated with vinblastine (4 of whom also received vincristine) and they were included with the 287 participants with vincristine exposure in the vinca-alkaloid group (N=289). No vincristine dose equivalence adjustment was made for vinblastine exposure. For platinum exposure, 88 participants were treated with cisplatin and 32 with carboplatin (including 7 who received both drugs). Thus 113 participants were included in the platinum exposure group. Because the pharmacokinetics of carboplatin differs substantially from that of cisplatin, a 4:1 carboplatin to cisplatin dose equivalence adjustment was applied.16, 17 There were 418 participants with no platinum exposure and 242 with no vinca-alkaloid exposure.
Outcomes
For aim 1, sensory impairment was assessed from the sensory subscale of the Modified Total Neuropathy Score (mTNS).18 The mTNS includes all components of the validated Total Neuropathy Score (TNS) measure19–21 except nerve conduction studies, and was shown to be highly correlated with TNS (r=0.99) in a study of breast cancer survivors treated with platinum drugs.18 A sensory subscale score of ≥ 1 was coded as “impaired” and 0 as “not impaired”. Motor impairment was assessed by categorizing peak dorsiflexion ankle strength measured at 60 degrees/second on an isokinetic dynamometer (Biodex System 3 Proa).22 A sex-specific standard deviation score (Z-score) of ≤ −1.3 (lower 10th percentile) was defined as “impaired” and a Z-score of >−1.3 as “not impaired”. The normative values for calculating the dorsiflexion Z-scores were taken from 343 healthy adults (median age28.4, range 18.0–50.8 years, 50.7% male) who were recruited as a control sample for a study of motor proficiency and physical activity. For aim 2, mobility was evaluated with the timed up and go test23 and endurance with the 6-minute walk test.24 The timed up and go test measures the time in seconds required to rise from sitting in a standard arm chair, walk 3 meters, turn, walk back to the chair, and sit down; > 6 seconds is one standard deviation above the mean in young adults and was considered impaired.17 The 6-minute walk test measures the distance in meters that an individual can quickly walk on a flat, hard surface in a period of 6 minutes; < 500 meters was considered impaired.16 Functional balance was assessed using dynamic posturography. The composite score of the Sensory Organization Test (SOT) was used for the balance outcome, measuring the percentage of time spent inside a 12 degree sway envelope during 6 conditions referenced to somatosensory, visual, and vestibular inputs;25 <70% was considered impaired (SMART EquiTest®b). All testing was conducted by trained and experienced exercise specialists in the Human Performance Lab.
Statistical approach
Descriptive statistics and frequency distributions were generated to compare demographic and cancer-related characteristics between the evaluable patient population and non-participants known or presumed to be eligible for the study. Frequencies and percentages were calculated to enumerate the prevalence of sensory and motor impairments and of functional limitations in endurance, mobility, and balance. The strength of the association (odds ratios [OR] with corresponding 95% confidence limits [95%CL]) between platinum and vinca-alkaloid exposures and motor and sensory impairments were evaluated in logistic regression models26 with treatment exposure represented in four categories (none versus each tertile of exposure dose in mg/m2) and as a dichotomous exposure variable (any versus none). We also evaluated the association between platinum exposure at >200 mg/m2 versus ≤200 mg/m2 and sensory impairment because a previous investigation among adults treated with cisplatin for testicular cancer indicated that this value was a potential threshold for sensory loss.27 All models were adjusted for age at the time of testing. Associations between sensory and motor impairments and functional limitations were evaluated in both linear and logistic regression models. These models were adjusted for age, sex, weight and height.
RESULTS
Characteristics of the study population
A thorough evaluation of potential selection bias from differential distributions in cancer-related, personal, and neighborhood-level characteristics between participants and presumed eligible non-participants in SJLIFE was previously analyzed and published.28 Table 1 shows demographic and cancer-related characteristics of the 513 evaluable participants compared with the 396 non-participants for this specific analysis. Non-participants were more likely to be male (61% vs. 49%) and less likely to be of white race (77% vs. 83%) than were participants. Median age and median time since diagnosis were similar between the groups. Among those with platinum and/or vinca-alkaloid exposure, no statistical differences in mean exposure dose were observed between participants and non-participants. Among participants, the most common diagnosis types were Wilms tumor (24%), rhabdomyosarcoma (15%), neuroblastoma (14%) and osteosarcoma (12%).
Table 1.
Characteristics of participants and non-participants
| Characteristic | Evaluable Participants (N=531) N (%) |
Non-participants (N=396) N (%) |
P-value | |
|---|---|---|---|---|
| Current age (years) | Median | 31.6 | 33.0 | <0.001 * |
| Range | 18.7–63.8 | 18.5–66.8 | ||
| Age at diagnosis (years) | Median | 4.9 | 5.8 | 0.278 * |
| Range | 0.0–24.8 | 0.0–28.6 | ||
| Time since diagnosis (years) | Median | 25.2 | 26.2 | 0.005 * |
| Range | 10.7–48.2 | 10.7–48.4 | ||
| Sex | Male | 261 (49.2) | 240 (60.6) | <.001† |
| Female | 270 (50.8) | 156 (39.4) | ||
| Race/ethnicity | White | 440 (82.9) | 306 (77.3) | 0.049† |
| Black | 87 (16.4) | 82 (20.7) | ||
| Other | 4 (0.8) | 8 (2.0) | ||
| Diagnosis | Carcinoma | 26 (4.9) | 27 (6.8) | 0.017† |
| Ewing sarcoma | 54 (10.2) | 35 (8.8) | ||
| Neuroblastoma | 73 (13.7) | 62 (15.7) | ||
| Osteosarcoma | 64 (12.1) | 37 (9.3) | ||
| Other malignancies | 54 (10.2) | 58 (14.6) | ||
| Retinoblastoma | 54 (10.2) | 25 (6.3) | ||
| Rhabdomyosarcoma | 79 (14.9) | 77 (19.4) | ||
| Wilms tumor | 127 (23.9) | 75 (18.9) | ||
| Vincristine (mg/m2) | N | 287 | 176 | |
| Mean | 30.8 | 29.4 | 0.419‡ | |
| Standard Deviation | 16.7 | 19.4 | ||
| Median | 29.1 | 23.8 | ||
| Range | 1.5–95.9 | 1.9–107.9 | ||
| Vinblastine (mg/m2) | N | 6 | 8 | |
| Mean | 35.2 | 32.4 | 0.865‡ | |
| Standard Deviation | 16.1 | 36.6 | ||
| Median | 36.8 | 20.3 | ||
| Range | 13.6–61.7 | 6.7–120.0 | ||
| Cisplatin (mg/m2) | N | 88 | 62 | |
| Mean | 429.4 | 472.4 | 0.228‡ | |
| 0.393T | 183.9 | 232.8 | ||
| Median | 400.0 | 403.9 | ||
| Range | 92.7–1045.0 | 90.2–1175.4 | ||
| Carboplatin (mg/m2) | N | 32 | 31 | |
| Mean | 2673.4 | 2460.6 | 0.393‡ | |
| Standard Deviation | 1027.4 | 933.1 | ||
| Median | 2766.7 | 2459.4 | ||
| Range | 895.4–4775.0 | 856.7–5131.7 |
T-test
Chi-square test
Wilcoxon rank sum test
Sensory and motor impairment
Inclusive of 21 participants who tested positive for both motor and sensory impairment, 107 (20%) had symptoms of sensory impairment and 93 (17.5%) had symptoms of motor impairment (Table 2).
Table 2.
Relative odds of sensory or motor impairment in relation to chemotherapy exposure
| (Total N=531) N | Sensory Impairment (N=107) | Motor Impairment (N=93) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| % | OR | 95% CL | % | OR | 95% CL | ||
| Vinca-alkaloid (mg/m2) | |||||||
| None | 242 | 19.8 | 1.00 | 13.6 | 1.00 | ||
| Any | 289 | 20.4 | 1.04 | 0.67–1.59 | 20.8 | 1.66 | 1.04–2.64 |
| None | 242 | 19.8 | 1.00 | 13.6 | 1.00 | ||
| 1.5–20.5 (mg/m2) | 96 | 20.8 | 1.05 | 0.58–1.89 | 17.7 | 1.36 | 0.72–2.58 |
| 20.6–39.5 (mg/m2) | 97 | 19.6 | 1.04 | 0.57–1.90 | 22.7 | 1.87 | 1.03–3.42 |
| 39.6–95.9 (mg/m2) | 96 | 20.8 | 1.02 | 0.57–1.84 | 21.9 | 1.76 | 0.96–3.24 |
| Platinum (mg/m2) | |||||||
| None | 418 | 19.1 | 1.00 | 17.0 | 1.00 | ||
| Any | 113 | 23.9 | 1.62 | 0.97–2.72 | 19.5 | 1.22 | 0.71–2.10 |
| None | 418 | 19.1 | 1.00 | 17.0 | 1.00 | ||
| 92.7–400 (mg/m2) | 35 | 28.6 | 2.04 | 0.92–4.49 | 20.0 | 1.25 | 0.52–3.01 |
| 400–560.8 (mg/m2) | 40 | 25.0 | 1.57 | 0.73–3.37 | 20.0 | 1.24 | 0.55–2.81 |
| 580–1,597.5 (mg/m2) | 38 | 18.4 | 1.30 | 0.54–3.15 | 18.4 | 1.15 | 0.48–2.78 |
| None | 418 | 19.1 | 1.00 | ||||
| 1–200 (mg/m2) | 10 | 30.0 | 2.49 | 0.61–10.1 | |||
| >200 (mg/m2) | 103 | 23.3 | 1.56 | 0.91–2.66 | |||
All models adjusted for age at evaluation.
Models adjusted for time from diagnosis showed similar results.
Vinca-Alkaloid Exposure
We found no evidence to support an association between vinca-alkaloid exposure and sensory impairment (age adjusted OR=1.04 for any vs. no exposure and no indication of a dose response). The age-adjusted OR for any vinca-alkaloid exposure vs. no exposure in relation to motor impairment was 1.66 (95% CL 1.04, 2.64). Some indication of increased risk for motor impairment with higher dose categories was observed, although the evidence was not strong (Table 2).
Platinum Exposure
The age-adjusted OR for sensory impairment comparing any platinum exposure vs. no exposure was 1.62 (95% CL 0.97, 2.72). However, we did not find a clear dose-response relation when comparing increasing dose categories of exposure or when categorizing participants by dose levels of 0, 1–200 mg/m2, and ≥200 mg/m2. There was little indication of an association between platinum exposure and motor impairment, either in dose response models or dichotomous exposure models (adjusted OR for any vs. no exposure = 1.22, 95% CL 0.71, 2.10).
Functional impairment
The distributions of scores on the performance measures are shown in Figure 1. Among the 475 study participants without a lower limb amputation, 14% were unable to walk 500 meters in 6 minutes, indicating poor endurance capabilities. A larger percentage, 25%, did not meet the normal threshold of < 6 seconds to complete the timed up and go test, suggesting poor mobility skills. A composite score of < 70% on the SOT, indicating problems with functional balance, was evident in 12% of the study population (Table 3). Forty participants (8.4%) had two of the three measures indicating poor function and 9 (1.9%) had poor scores on all three function measures (not shown). In a logistic regression model that together considered sensory impairment (yes vs. no) with ankle dorsiflexion strength in Newton-Meters as a continuous variable, the OR (adjusted for age, sex, height and weight) for poor performance on the 6-minute walk test among those with sensory impairment was 1.99 (95% CL 0.99, 4.00) when compared to those without sensory impairment (Table 3). A similar finding was observed for sensory impairment in relation to the risk of having poor performance on the timed up and go test, with an adjusted OR of 1.65 (95%CL 0.96, 2.83). No statistical association was observed between sensory impairment and poor balance in this model, and there was no indication of a linear association between ankle dorsiflexion strength and any functional outcome (Table 3).
Figure 1.
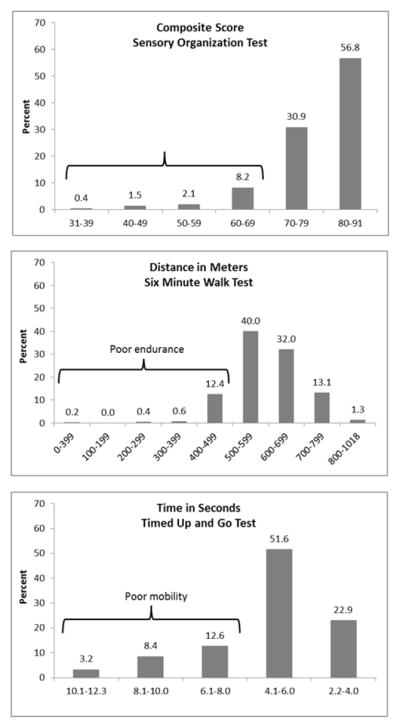
Distribution of scores on performance measures
Table 3.
Relative odds of poor endurance, mobility, and balance in relation to sensory impairment and dorsiflexion strength
| (Total N=475) N | Poor Endurance (N=65) | Poor Mobility (N=121) | Poor Balance (N=58) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| % | OR | 95% CI | % | OR | 95% CI | % | OR | 95% CI | ||
| Sensory Impairment | 90 | 18.9 | 1.99 | 0.99–4.00 | 32.2 | 1.65 | 0.96–2.83 | 12.2 | 1.17 | 0.55–2.50 |
| None | 385 | 12.5 | 1.00 | 23.9 | 1.00 | 12.2 | 1.00 | |||
| Ankle dorsiflexion strength (Newton-Meters)* | 475 | --- | 1.00 | 0.99–1.00 | --- | 1.00 | 0.99–1.00 | --- | 0.99 | 0.99–1.0 |
The regression models for each functional outcome analysis (endurance, mobility, and balance) provide estimates of the simultaneous effect of sensory impairment as a dichotomous variable controlling for ankle dorsiflexion strength, and the effect of ankle dorsiflexion strength as a continuous variable controlling for sensory impairment status. The models are also adjusted for age at evaluation, gender, height and weight, with the exception of the model for balance which is not adjusted for weight.
DISCUSSION
In this analysis of motor, sensory, and functional outcomes among long-term survivors of extracranial childhood solid malignancies (median time since diagnosis, 25 years), we found some evidence of an association between previous vinca-alkaloid exposure (primarily vincristine) and deficits in ankle dorsiflexion strength. Nearly 21% of those who were treated with vincristine, compared with fewer than 14% who were not so treated, demonstrated evidence of abnormally low ankle dorsiflexion strength (adjusted OR=1.66), which we interpret as a motor impairment. No evidence was apparent in our analysis that vincristine exposure was associated with long-term sensory impairment. We found that past platinum exposure was a potential risk factor for sensory impairment (adjusted OR=1.62 for any vs. no exposure) but we did not observe stronger effects with increasing dose levels of exposure. Platinum exposure was not found to be associated with increased risk for chronic motor impairment in our study. Thus, our clinical evidence for long-term neuropathic impairment due to exposure in childhood to vinca-alkaloid and/or platinum chemotherapy certainly cannot be dismissed, but it is not strong. Previous reports with smaller sample sizes have also suggested evidence for an association between vincristine and chronic motor problems. Studies by Harila-Saari et al29 and Ramchandren et al10 among survivors of childhood acute lymphoblastic leukemia demonstrated that conduction delays in peripheral motor nerves are prevalent after vincristine exposure. Harila-Saari29 evaluated motor evoked potentials among 32 children at the end of treatment for acute lymphoblastic leukemia and compared them to healthy controls. Treated patients demonstrated delayed peripheral motor responses with diminished amplitudes when compared to the control population and the deficits were associated with the time between the last vincristine administration and nerve conduction testing. Depressed deep tendon reflexes were also associated with total dose and time since last vincristine administration in that study. Ramchandren et al10 reported abnormally low motor amplitudes in nearly 30% of a group of 37 children and adolescents who were a mean of 7.4 years post-treatment for acute lymphoblastic leukemia. In that study there was no evidence of a cumulative vincristine dose gradient, nor evidence of an association between impaired motor nerve conduction and functional motor outcome. Their results are similar to those of Hartman et al,22 who reported impaired dorsiflexion strength among 92 survivors (ages 6 to 12 years) of acute lymphoblastic leukemia, Wilms tumor, non-Hodgkin lymphoma, and malignant mesenchymal tumors, but no association with vincristine dose.
The number of survivors in our cohort who did not receive vincristine or platinum, but who nevertheless had sensory loss or dorsiflexion motor weakness is higher than expected in healthy young adults. We did not find any specific cancer treatment related reasons for these impairments. However, it is possible that receiving cellular toxic agents of nearly any kind may damage peripheral nerves or the vasculature that supplies them. A recent review by Gilchrist identifies six different classes of agents used to treat pediatric cancers that damage peripheral nerves by disrupting axonal transport at microtubules, by inducing cellular damage or apoptosis in dorsal root ganglia, or by interfering with immune or angiogenic function.30 One study of vascular injury among survivors of childhood cancer indicates an effect of anthracycline on vascular compliance and stiffness;31 another reports evidence of vascular inflammation, dyslipidemia, and injury suggestive of early atherogenesis among survivors of Hodgkin Lymphoma.32
In our study of young adults, dorsiflexion muscle weakness was not associated with poor performance on the 6-minute walk test, the timed up and go test, or the SOT balance test after controlling for sensory impairment and several other variables. These results are consistent with a study of 102 survivors of childhood cancer in whom reduced strength and ankle range of motion were not associated with performance on a global test of movement and mobility (Movement ABC).22 However, our results are in contrast to a study of adult survivors of childhood acute lymphoblastic leukemia, in whom neuromuscular deficits were associated with balance limitation and limited walking inefficiency.9 In another study that compared motor function between 13 adults with peripheral neuropathy of varying etiologies to age- and sex-matched healthy controls, individuals with peripheral neuropathy walked, on average, 100 meters less during the 6-minute walk test, and performed less optimally (had increased sway velocity) when asked to stand in place and maintain their balance on a force plate.33
Among those in our study who demonstrated sensory impairment, we found an elevated risk for poor performance on the 6-minute walk test (adjusted OR=1.99) and decreased mobility on the timed up and go test (adjusted OR=1.65), even after controlling for ankle dorsiflexion strength, age, sex, weight, and height. This finding suggests that a subset of young adult survivors of extracranial solid tumors of childhood (median age in our study population was 32 years) may have diminished physical function and could potentially benefit from assessment and intervention. Our finding of a potential association between platinum exposure and peripheral sensory impairment in adult survivors of childhood solid tumors is consistent with previous reports documenting long term sensory loss among survivors of testicular cancer whose treatment included platinum compounds. Glendenning et al27 evaluated 384 platinum treated survivors of testicular cancer 23–33 years post treatment and reported that 21.7% had detectable peripheral neuropathy. As noted previously, they observed a cisplatin dose threshold for peripheral neuropathy of 200 mg/m2. Another study examined a group of 32 cisplatin-treated survivors of testicular cancer 15 years after treatment and found disabling neuropathy in 6%, symptomatic neuropathy in 38%, and asymptomatic neuropathy in 28% of their population. Platinum dose was not associated with symptom scores in that study.34
LIMITATIONS
Interpretation of our results must take into account potential study limitations. First, the measures of function that we selected are not the only ones available. All three required standing or walking, most likely to be impacted by neuropathy in the lower extremities. We did not include a measure of hand function, important for survivors with neuropathy that affects the upper extremities. Second, we did not conduct formal nerve conduction testing and our use of a formal measure of muscle strength to characterize the motor component of peripheral neuropathy may not be completely representative of motor nerve damage. Third, individuals can have muscle weakness for reasons other than peripheral nerve damage, which may have attenuated our estimates of the strength of the association between vincristine and motor loss. We did however, account for body size in our analyses, and we excluded survivors treated with cranial radiation because of potential central nervous system damage that could influence motor function and confuse the chemotherapy treatment effects we were evaluating. Additionally, because this was a cross sectional analysis, we did not have information on physical therapy services received by these survivors during the years between treatment and our assessment. Previous participation in rehabilitation services may have influenced overall functional outcome. Fifth, 48 participants with lower extremity amputation, were excluded from the evaluation of the association between neuropathy and function. This group of survivors is likely to be most affected. The compound effects of limb loss and neuropathy on function will requires research in larger cohorts of bone sarcoma survivors. Finally, not everyone who was eligible for the study participated. Male survivors and those who were of non-white race were less likely to participate. It is possible that this could bias our results. However, we did not find an association between sex or race and any of our outcomes.
CONCLUSION
In conclusion, we found that nearly 18% of survivors of childhood-onset extracranial solid tumors, a median of 25 years from diagnosis, had ankle dorsiflexion muscle weakness and 20% had peripheral sensory loss. We observed that motor impairment was associated with vincristine exposure and that sensory impairment was associated with platinum exposure during cancer treatment. We also found that sensory impairment was a risk factor for poor endurance and poor mobility. These findings are important because, although no established preventive or curative agents are available presently for motor and sensory loss associated with chemotherapy-induced peripheral neuropathy,1, 2, 4, 7, 35 rehabilitative services and adaptive equipment provided for children during or soon after treatment may improve symptoms and provide strategies for joint protection, pain reduction, and strengthening to minimize long-term functional limitations that may persist into adulthood. Adult survivors of childhood cancer who present to clinical practice with motor or sensory neuropathy that interferes with function may benefit from rehabilitative services that provide them strength and balance training, or with instruction in compensatory strategies that minimize their risk for falls or for the development of joint injuries.36–38 Future research needs to look at peripheral neuropathy among childhood cancer survivors longitudinally, beginning at diagnosis, and develop interventions specific to this patient population.
Acknowledgments
This work was supported by the Cancer Center Support (CORE) grant CA 21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
List of Abbreviations
- SJCRH
St. Jude Children’s Research Hospital
- OR
Odds ratio
- CI
Confidence interval
- SJLIFE
St. Jude Lifetime Cohort Study
- mTNS
Modified Total Neuropathy Scale
- TNS
Total Neuropathy Scale
- SOT
Sensory Organization Test
- CL
Confidence limits
- mg
milligrams
- m
meters
Footnotes
Biodex System 3 Pro, Biodex Medical Systems, Inc.,20 Ramsey Road, Shirley, NY 11967-4704
SMART EquiTest®, NeuroCom®, a division of Natus®, 9570 SE Lawnfield Road, Clackamas, OR 97015, USA
Conflict of Interests: None of the authors has any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2011;(2) doi: 10.1002/14651858.CD005228.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 3.Ross CJ, Visscher H, Rassekh SR, Castro-Pastrana LI, Shereck E, Carleton B, Hayden MR. Pharmacogenomics of serious adverse drug reactions in pediatric oncology. J Popul Ther Clin Pharmacol. 2011;18:e134–51. [PubMed] [Google Scholar]
- 4.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6(12):657–66. doi: 10.1038/nrneurol.2010.160. [DOI] [PubMed] [Google Scholar]
- 5.Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25(2):116–31. [PubMed] [Google Scholar]
- 6.Cavaletti G, Alberti P, Frigeni B, Piatti M, Susani E. Chemotherapy-Induced Neuropathy. Curr Treat Options Neurol. 2011;13(2):180–90. doi: 10.1007/s11940-010-0108-3. [DOI] [PubMed] [Google Scholar]
- 7.Argyriou AA, Zolota V, Kyriakopoulou O, Kalofonos HP. Toxic peripheral neuropathy associated with commonly used chemotherapeutic agents. J BUON. 2010;15(3):435–46. [PubMed] [Google Scholar]
- 8.Legha SS. Vincristine neurotoxicity. Pathophysiology and management. Med Toxicol. 1986;1(6):421–7. doi: 10.1007/BF03259853. [DOI] [PubMed] [Google Scholar]
- 9.Ness KK, Hudson MM, Pui CH, Green DM, Krull KR, Huang TT, Robison LL, Morris EB. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer. 2012;118(3):828–38. doi: 10.1002/cncr.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramchandren S, Leonard M, Mody RJ, Donohue JE, Moyer J, Hutchinson R, Gurney JG. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst. 2009;14(3):184–9. doi: 10.1111/j.1529-8027.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright MJ, Halton JM, Martin RF, Barr RD. Long-term gross motor performance following treatment for acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;31(2):86–90. doi: 10.1002/(sici)1096-911x(199808)31:2<86::aid-mpo7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Lehtinen SS, Huuskonen UE, Harila-Saari AH, Tolonen U, Vainionpaa LK, Lanning BM. Motor nervous system impairment persists in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2002;94(9):2466–73. doi: 10.1002/cncr.10503. [DOI] [PubMed] [Google Scholar]
- 13.Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, Spunt SL, Metzger ML, Krull KR, Klosky JL, Srivastava DK, Robison LL. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–36. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, Darling J, Armstrong FD, Blatt J, Constine LS, Freeman CR, Friedman DL, Green DM, Marina N, Meadows AT, Neglia JP, Oeffinger KC, Robison LL, Ruccione KS, Sklar CA, Hudson MM. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–90. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 15.COG. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. [cited 2008]. Available from: URL: http://www.survivorshipguidelines.org/
- 16.Pollentier B, Irons SL, Benedetto CM, Dibenedetto AM, Loton D, Seyler RD, Tych M, Newton RA. Examination of the six minute walk test to determine functional capacity in people with chronic heart failure: a systematic review. Cardiopulm Phys Ther J. 2010;21(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Brotherton SS, Williams HG, Gossard JL, Hussey JR, McClenaghan BA, Eleazer P. Are measures employed in the assessment of balance useful for detecting differences among groups that vary by age and disease state? J Geriatr Phys Ther. 2005;28(1):14–9. doi: 10.1519/00139143-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Wampler MA, Miaskowski C, Hamel K, Byl N, Rugo H, Topp KS. The Modified Total Neuropathy Score: A Clinically Feasible and Valid Measure of Taxane-Induced Peripheral Neuropathy in Women With Breast Cancer. J Support Oncol. 2006;4(8):W9–W16. [Google Scholar]
- 19.Cavaletti G, Frigeni B, Lanzani F, Piatti M, Rota S, Briani C, Zara G, Plasmati R, Pastorelli F, Caraceni A, Pace A, Manicone M, Lissoni A, Colombo N, Bianchi G, Zanna C. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12(3):210–5. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith EM, Beck SL, Cohen J. The total neuropathy score: a tool for measuring chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum. 2008;35(1):96–102. doi: 10.1188/08.ONF.96-102. [DOI] [PubMed] [Google Scholar]
- 21.Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, Joh T. Total neuropathy score: validation and reliability study. Neurology. 1999;53(8):1660–4. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- 22.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr Blood Cancer. 2008;50(4):833–7. doi: 10.1002/pbc.21325. [DOI] [PubMed] [Google Scholar]
- 23.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 24.Enright PL, Sherrill DL. Physiology/Epidemiology: Reference Equations for the Six-Minute Walk in Healthy Adults. Am J Respir Crit Care Med. 1998;158(5):1384. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 25.Ford-Smith CD, Wyman JF, Elswick RK, Jr, Fernandez T, Newton RA. Test-retest reliability of the sensory organization test in noninstitutionalized older adults. Arch Phys Med Rehabil. 1995;76(1):77–81. doi: 10.1016/s0003-9993(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 26.Allison PD. Logistic Regression Using SAS® System: Theory and Application. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- 27.Glendenning JL, Barbachano Y, Norman AR, Dearnaley DP, Horwich A, Huddart RA. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer. 2010;116(10):2322–31. doi: 10.1002/cncr.24981. [DOI] [PubMed] [Google Scholar]
- 28.Ojha RP, Oancea SC, Ness KK, Lanctot JQ, Srivastava DK, Robison LL, Hudson MM, Gurney JG. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: Results from the St. Jude lifetime cohort study. Pediatr Blood Cancer. 2012 doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harila-Saari AH, Huuskonen UE, Tolonen U, Vainionpaa LK, Lanning BM. Motor nervous pathway function is impaired after treatment of childhood acute lymphoblastic leukemia: a study with motor evoked potentials. Med Pediatr Oncol. 2001;36(3):345–51. doi: 10.1002/mpo.1084. [DOI] [PubMed] [Google Scholar]
- 30.Gilchrist L. Chemotherapy-induced peripheral neuropathy in pediatric cancer patients. Semin Pediatr Neurol. 2012;19(1):9–17. doi: 10.1016/j.spen.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Jenei Z, Bardi E, Magyar MT, Horvath A, Paragh G, Kiss C. Anthracycline Causes Impaired Vascular Endothelial Function and Aortic Stiffness in Long Term Survivors of Childhood Cancer. Pathol Oncol Res. 2012 doi: 10.1007/s12253-012-9589-6. [DOI] [PubMed] [Google Scholar]
- 32.Mulrooney DA, Ness KK, Solovey A, Hebbel RP, Neaton JD, Peterson BA, Lee CK, Kelly AS, Neglia JP. Pilot study of vascular health in survivors of Hodgkin lymphoma. Pediatr Blood Cancer. 2012;59(2):285–9. doi: 10.1002/pbc.24082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doherty A, Li L, Manor B. The effects of peripheral neuropathy on physical function. Journal of Biomechanics; XXI ISB Congress, Podium Sessions; 2007. p. 0449. [Google Scholar]
- 34.Strumberg D, Brugge S, Korn MW, Koeppen S, Ranft J, Scheiber G, Reiners C, Mockel C, Seeber S, Scheulen ME. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. 2002;13(2):229–36. doi: 10.1093/annonc/mdf058. [DOI] [PubMed] [Google Scholar]
- 35.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–15. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Dingwell JB, Cusumano JP, Sternad D, Cavanagh PR. Slower speeds in patients with diabetic neuropathy lead to improved local dynamic stability of continuous overground walking. J Biomech. 2000;33(10):1269–77. doi: 10.1016/s0021-9290(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 37.Allet L, Armand S, de Bie RA, Golay A, Monnin D, Aminian K, Staal JB, de Bruin ED. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53(3):458–66. doi: 10.1007/s00125-009-1592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568–79. doi: 10.2522/ptj.20090362. [DOI] [PubMed] [Google Scholar]


