Abstract
Purpose
Rectal hyposensitivity commonly causes anorectal disorders but its underlying mechanism is unknown. We hypothesized that subjects with rectal hyposensitivity have altered recto anal-reflexes and/or sensori-motor response.
Methods
We performed stepwise graded balloon distensions of the rectum in 30 subjects with constipation and rectal hyposensitivity and 23 healthy controls. Thresholds for first sensation, desire and urgency to defecate were assessed. The lowest balloon volume that evoked recto-anal inhibitory reflex, recto-anal contractile reflex and sensori-motor response, as well as their manometric characteristics and rectal compliance were examined.
Results
Reflex responses were present in all subjects. The balloon volumes were higher in rectal hyposensitivity subjects for inducing recto-anal inhibitory reflex ( p=0.008) and contractile reflex (p=0.001) when compared to controls. All controls showed a sensori-motor response, but in 13 (43%) hyposensitive subjects the onset of sensori-motor response was associated with absent sensation, and in 17 (57%) with a transient rectal sensation. Thresholds for eliciting sensori-motor response were similar between patients and controls, but the amplitude, duration and magnitude of response were higher (p<0.05) in patients. Rectal compliance was similar between controls and hyposensitive subjects with transient sensation but higher (p=0.001) in subjects with absent sensation.
Conclusions
Constipated subjects with rectal hyposensitivity demonstrate higher thresholds for inducing rectoanal reflexes and abnormal characteristics of sensori-motor response. These findings suggest either disruption of afferent gut-brain pathways or rectal wall dysfunction. These altered features may play a role in the pathogenesis of bowel dysfunction in rectal hyposensitivity.
Keywords: rectal hyposensitivity, recto anal inhibitory reflex, recto anal contractile reflex, sensori-motor response
Introduction
An intact sensory perception is essential for maintaining normal continence and normal evacuation.1 In contrast, a diminished perception of the rectum, usually described as Rectal hyposensitivity (RH), has been reported in up to 60% of patients 2,3 with chronic constipation, and in 10-18% of patients with fecal incontinence.4,5 The underlying mechanism(s) for RH is not known. It is possible that alterations in the pelvic and anorectal afferent nerves or mechanoreceptors of the rectal wall or recto-anal reflexes or brain-gut interactions may each play a role.3,6
Distension of the rectal wall evokes reflex motor responses that are controlled by the intrinsic and extrinsic innervations.1,3 The rectoanal inhibitory reflex (RAIR) is impaired in patients with chronic constipation,7 fecal incontinence,8 scleroderma,9 diabetic neuropathy10 and following radical hysterectomy.11 Rectal distension also induces a brief reflex contraction of the external anal sphincter, the rectoanal contractile reflex (RACR) which is either a subconscious effort or a primordial reflex that prevents accidental release of rectal contents, and is mediated by the pelvic splanchnic and pudendal nerves.1,12,13 Although some studies have assessed the clinical significance of the rectoanal reflexes in anorectal disorders,7-11 whether these responses are altered in patients with rectal sensory abnormalities, specifically in subjects with RH is unknown.
Recently, we have described that during rectal balloon distension the onset of a desire to defecate is associated with a unique, consistent and reproducible anal contractile response, often overlying the RAIR, the sensori-motor response (SMR).14 This reflex activity could be an important and integral component of the defecation reflex in humans. Unlike the RAIR and RACR that are independent of rectal sensation, the SMR appears to be associated with rectal sensory perception .14
We hypothesized that the rectoanal reflexes and the sensori-motor responses are altered in subjects with RH. We tested this by investigating the manometric characteristics of the RAIR, RACR and the SMR in subjects with RH and chronic constipation, and in healthy controls.
MATERIALS AND METHODS
Subjects
Thirty consecutive subjects who fulfilled the Rome II diagnostic criteria for functional constipation15 and with a diagnosis of rectal hyposensitivity (see below, measurements and analysis) were invited to participate in this study. All subjects were referred to a tertiary care center for assessment of chronic constipation and had comprehensive clinical evaluation that included flexible sigmoidoscopy, barium enema or colonoscopy, and blood tests to exclude either mucosal or metabolic disorders that cause constipation. Patients with drug induced constipation (i.e. chronic use of opioids) were excluded.
In addition, we recruited 23 healthy subjects through a hospital advertisement. All subjects completed a questionnaire regarding their bowel function. None had any gastrointestinal dysfunction, disturbance of defecation, history of previous surgery (except appendectomy) or were taking any medication (except aspirin, oral contraceptive or multivitamins). They all had normal physical examination. All subjects gave written informed consent for a study protocol that was approved by the Institutional Review Board.
Methods
Anorectal manometry
A digital rectal exam preceded probe insertion and a bowel preparation was not routinely used. The manometric assessment was performed by using a 6-mm. diameter probe containing 6 radially arrayed strain gauge microtransducers (Gaeltec, Isle of Skye, UK) and a central lumen for balloon inflation. A 4-cm. balloon was fashioned from a latex condom (Trojan-enz, non-lubricated, Carter Wallace, New York, NY) and was tied to the probe for the assessment of rectal sensation. When correctly positioned, the balloon was located between 7 and 11 cm. from the anal verge. The probe was connected to an amplifier and recorder (Gaeltec-7MPR, Gaeltec, Dunvegan, Isle of Skye, UK), and pressure activity was displayed on a computer monitor. With the patient lying in the left lateral position, the lubricated probe was introduced into the rectum and taped in place such that the pressure sensors were located at 1, 2, 3, 5, 9, and 14 cm from the anal verge. The manometric recording commenced 7-10 minutes after probe placement.
The rectal sensation and rectoanal reflexes were assessed simultaneously by inflating the rectal balloon with a hand-held syringe using the following volumes: 10, 20, 30, 40, 60, 80, 110, 140, 170, 200, 240, 280 and 320 cc. of air. The intermittent rectal balloon distention protocol was used 16 and the balloon was inflated at a rate of 10 cc/second. Each inflation was maintained for 1 min. After deflation, a rest period of 2 minutes was allowed before reinflating the balloon to the next volume. During the test, subjects were provided with a chart and were asked to grade their sensory responses as follows: no sensation (absent sensation), a transient or first sensation, a desire to defecate (that persisted > 15 seconds), an urge to defecate and the maximum tolerable volume. After this test the manometry probe was removed.
Measurements and data analysis
Anorectal physiology
Subjects underwent comprehensive evaluation using the solid state probe that included sphincter length, maximum resting sphincter pressure, maximum squeeze pressure, maximum sustained sphincter pressure and squeeze duration, as described previously.16
Rectal Sensation
The lowest balloon volume that evoked a first sensation, a desire to defecate, and an urgent desire to defecate and the maximum tolerable volume were assessed as described previously.14,16 Patients were considered to have RH if at least two of the three rectal sensory thresholds were greater than the 95% CI for the normative data from our laboratory.16 The mean (95% CI) values for normal subjects were: first sensation 20 (17-23 cc), desire to defecate 106 (90-122 cc), and urge to defecate 178 (159-197 cc).
Recto Anal Reflexes and Sensorimotor Response
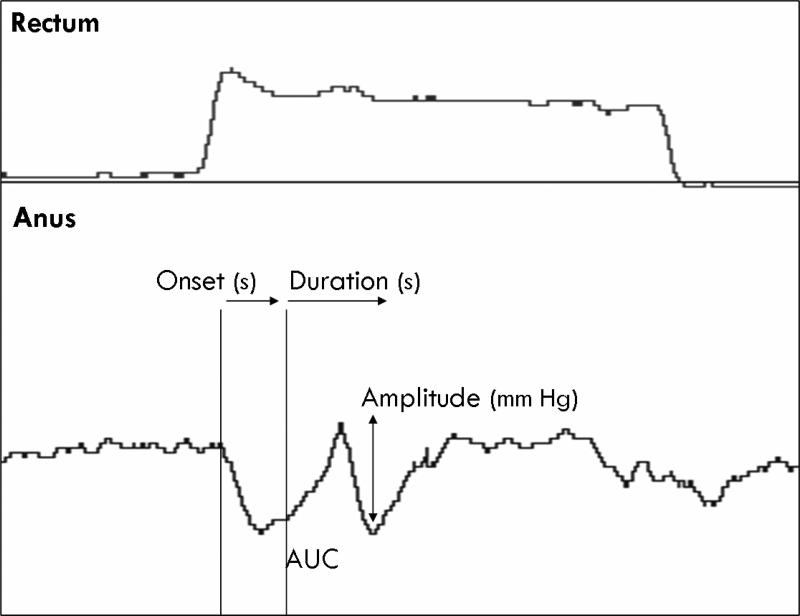
During the intermittent phasic rectal balloon distensions, the lowest balloon volumes required to elicit a brief reflex contraction of the external anal sphincter (RACR), and a sustained relaxation of the internal anal sphincter (RAIR) for one minute were also recorded. Also we assessed the onset, duration, amplitude and area under the curve (AUC) of the RACR. These reflexes were observed usually in the pressure sensor located at 1.5 cm from the anal verge (Figure 1).
Figure 1.
An example of the sensorimotor reflex (SMR). Three consecutive sets of phasic rectal balloon distensions in one subject are shown. In the first distension, there is an increase in rectal pressure (p1) with transient reflex relaxation of anal sphincter pressure (p3), demonstrating the RAIR. During the second distension a transient reflex contraction of the external anal sphincter – the recto-anal contractile reflex (RACR) is seen. In the third sequence, in addition to the RAIR and RACR, the subject reported a desire to defecate and simultaneously an anal contractile response is seen overlying the anal relaxation. Reproduced with permission from Dis Colon Rectum 2007; 50: 1639-1646.
Sensori-motor response (SMR) was defined as a transient, anal contractile response that occurred overlying the RAIR, and usually associated with the onset of a desire to defecate (Figure 1).14 When this response was seen at multiple sensors, the channel with the most optimal response was used for data analysis. We assessed the onset, duration, and amplitude of this response. The onset of SMR was defined as the time interval in seconds between the application of stimulus (inflation of the rectal balloon) and the onset of the anal pressure response, as described previously.14 The amplitude was defined as the maximum peak of the pressure wave, observed at any level in the anal canal, and was expressed in mm Hg. The duration was defined as the longest time interval in seconds, at any level in the anal canal, between the onset and the end of the pressure wave. Additionally, the rectal balloon volume (cc) and the sensation at which this reflex response occurred were noted.
Rectal Wall Compliance
Rectal distention usually induced an abrupt and initial increase in rectal pressure as air was introduced into the intrarectal balloon, followed by a slow decline in pressure to a steady state value as the rectum accommodated to the distention.16 The intralumenal rectal pressure for each distending volume was corrected by subtracting the pressure obtained during inflation of the balloon in ambient air. Rectal compliance was calculated from the slope describing the relationship between the intrarectal pressure (dP) and the balloon volume (dV) at steady state. A subject was designated as having an increased or more compliant rectum (or excessive laxity of the rectal wall) if the slope of the linear part of the curve significantly shifted to the right when compared to the curve in the healthy controls.16
Statistical analysis
The manometric data, the rectal balloon volume thresholds which induced the RACR, RAIR, SMR, and the onset, amplitude, duration and area under the curve (AUC) of the anal contractile response during the SMR are expressed as mean ± standard deviation. The gender differences in the onset, duration, amplitude and AUC of the SMR were assessed using either the student's t test or Mann Whitney test as appropriate. Statistical analyses were performed using a commercially available statistical software package (SPSS 10.0, SPSS, Inc, Chicago Illinois). A p value of < 0.05 was considered statistically significant.
RESULTS
Subjects
There were 30 patients with RH, 23 were female (76%), and their mean age was 45 ± 12 yrs). Among the 23 healthy controls, 15 were (65%) female and their mean age was 48 ± 28 yrs.
Anal Sphincter Pressures
The anal sphincter pressures were similar between the RH subjects and controls. The mean anal resting pressure (68 ± 22 vs. 70 ± 21 mm Hg, p= 0.78), mean maximum squeeze pressure (164 ± 53 vs. 173 ± 48 mm Hg, p= 0.52), and sustained squeeze pressure (144 ± 38 vs. 152 ± 52 mm Hg, p=0.53) were similar and showed no differences.
Rectal Sensation
The rectal sensory threshold data previously obtained from anorectal manometry were used to identify our cohort of subjects with RH. The mean threshold volumes for inducing a first sensation (40 ± 10 vs. 18 ± 8 cc, p=0.0001), desire to defecate (165 ± 38 vs. 111 ± 56 cc, p=0.003), and urge to defecate (233 ± 42 vs. 188 ± 53 cc, p=0.001) were significantly higher in rectal hyposensitivity group when compared to healthy controls.
Rectoanal Reflexes
Rectoanal inhibitory reflex
The RAIR was present in all subjects, both RH and healthy subjects. The lowest rectal balloon volume that induced an anal relaxation was significantly higher in RH subjects when compared to controls ( 20 ± 10 cc vs. 12 ± 6 cc, p=0.008) (Table 1).
Table 1.
| Controls (n=23) | Rectal Hyposensitivity (n=30) | p value | |
|---|---|---|---|
| RAIR | |||
| • Minimal volume to induce RAIR (cc) | 12 ± 6 | 20 ± 10 | 0.0008 |
| • Minimal volume to induce sustained RAIR(cc) | 44 ± 23 | 63 ± 29 | 0.04 |
| RACR | |||
| • Minimal volume to induce RACR(cc) | 32 ± 1.9 | 56 ± 19 | 0.001 |
| • Amplitude (mm Hg) | 19 ± 5 | 20 ± 8 | 0.8 |
| • Duration (seconds) | 2.8 ± 0.7 | 3.1 ± 0.1 | 0.2 |
| • Area under the curve (mm Hg/sec) | 51 ± 35 | 48 ± 33 | 0.75 |
Rectoanal contractile reflex
The RACR was seen in all subjects. Rectal hyposensitive patients had higher rectal balloon volumes for inducing the RACR when compared to controls ( 56 ± 19 vs. 32 ± 10 cc, p=0.001). The mean amplitude, duration and area under the curve of the contractile response in both groups were similar (Table 1).
Sensori-motor response (SMR)
A SMR was present in all healthy subjects and in all subjects with RH. However, the onset of SMR was either associated with a transient rectal sensation or no (absent) rectal sensation in subjects with RH (Fig 2). The lowest rectal balloon volume that induced a SMR was similar in RH subjects and in controls. The amplitude, duration and area under the curve for the SMR were higher in RH subjects (Table 2).
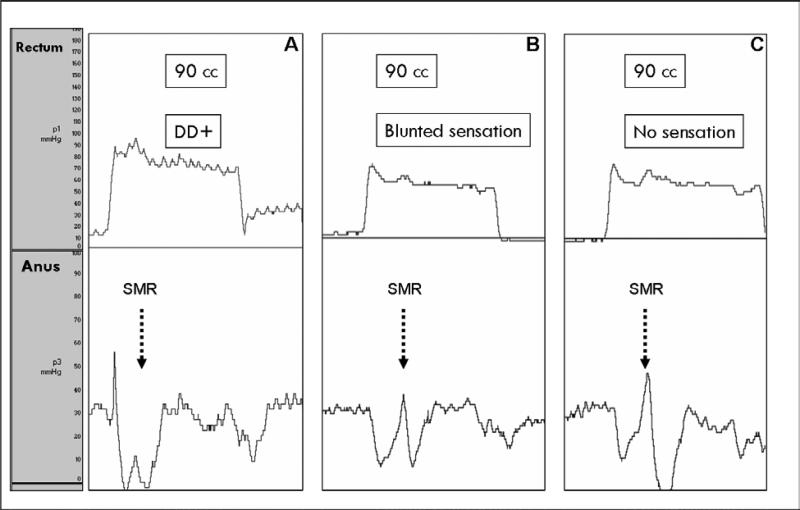
Figure 2.
Examples of the SMR. Figure A shows a SMR (black dotted arrow) evoked with 90 cc of rectal balloon distension and associated with a desire to defecate (DD +) in a healthy control. Figure B shows a SMR evoked with 90 cc and associated with a transient (blunted) rectal sensation in a patient with rectal hyposensitivity. Figure C shows a SMR evoked with 90 cc, but in this case the response is present but the subjects reported no rectal sensation. Note that the SMR is more prominent in subjects B and C when compared to healthy controls.
Table 2.
| Controls (n=23) | Rectal Hyposensitivity (n=30) | P value | |
|---|---|---|---|
| • Threshold balloon volume required for inducing SMR (cc) | 93 ± 27 | 93 ± 26 | 0.9 |
| • Onset of SMR (sec) | 9.2 ± 1.9 | 8.2 ± 3 | 0.1 |
| • Amplitude of SMR (mm Hg) | 19 ± 9 | 31.7 ± 3* | 0.01 |
| • Duration of SMR (sec) | 5.5 ± 1.5 | 7 ± 3* | 0.04 |
| • Area under the curve (mm Hg·sec) | 41 ± 33 | 177 ± 67* | 0.001 |
In controls, the onset of the SMR was always associated with a desire to defecate. In patients with rectal hyposensitivity, the onset of SMR was associated with a transient sensation (SMR-transient sensation group) in 17 (57%) and no rectal sensation (SMR-absent sensation group) in 13 (43%) subjects. The onset of SMR was prolonged in subjects with SMR-absent sensation group when compared to subjects with SMR-transient sensation group (13.9 ± 9 vs. 7.6 ± 3 seconds, p=0.04, Table 3). The amplitude, duration and area under the curve tended to be higher in the SMR-absent sensation group but the differences were not significant between the two RH groups (Table 3).
Table 3.
| SMR-Transient sensation (n=17) | SMR- Absent sensation (n=13) | P value | |
|---|---|---|---|
| • Threshold balloon volume for inducing SMR (cc) | 90 ± 22 | 96 ± 32 | 0.5 |
| • Onset of SMR (sec) | 7.6 ± 3 | 13 ± 9 | 0.04 |
| • Amplitude of SMR (mm Hg) | 29 ± 15 | 34 ± 12 | 0.5 |
| • Duration of SMR (sec) | 6.5 ± 3 | 7.3 ± 4 | 0.5 |
| • Area under the curve (mm Hg·sec) | 154 ± 76 | 188 ± 72 | 0.18 |
Rectal compliance
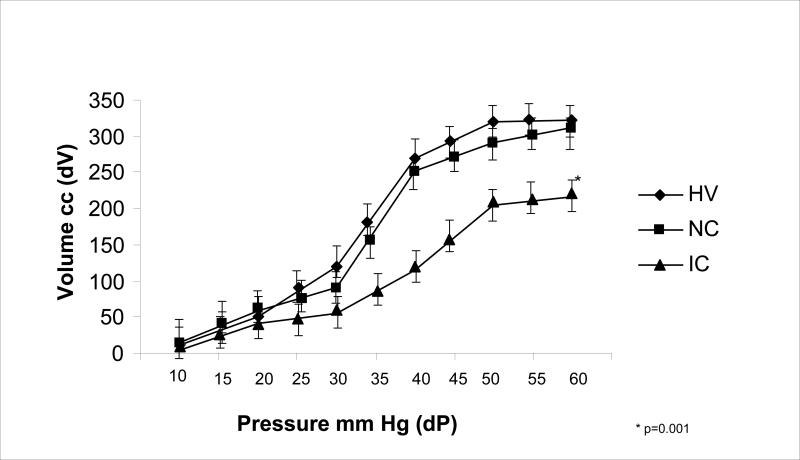
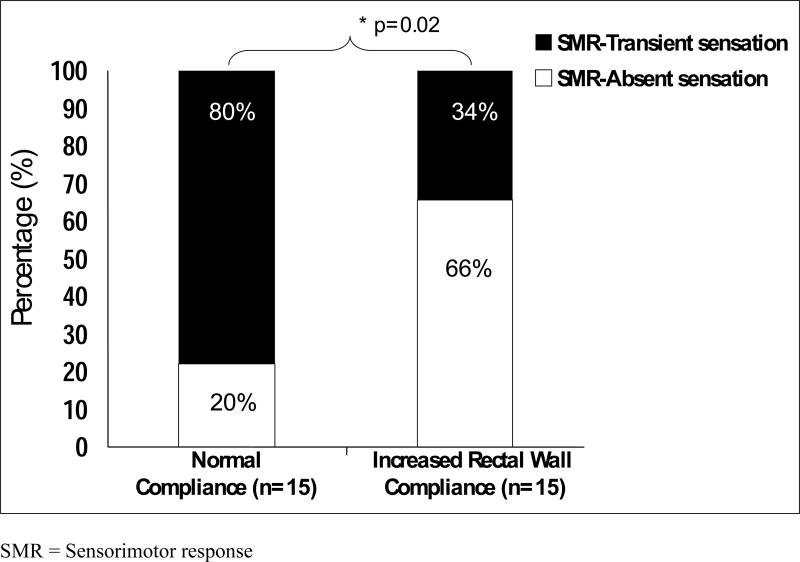
The data for rectal wall compliance in our healthy controls were similar to those previously published.16 In 15 subjects with RH, the rectal compliance values were similar to those of healthy controls (Figure 3). Twelve of the RH subjects (80%) with a normal rectal wall compliance exhibited an SMR with transient rectal sensation whereas 3 (20%) RH subjects exhibited an SMR with an absent rectal sensation. In 15 subjects with RH (50%), the slope of the linear part of the dP/dV curve significantly shifted to the right when compared to normal reference values suggesting an increased or a more compliant rectal wall. In subjects with an increased compliance of the rectal wall, 10 (66%) subjects had an absent rectal sensation (p=0.025, Figure 4) during the onset of an SMR and five (34%) subjects had a transient rectal sensation during the onset of the SMR.
Figure 3.
Figure showing the relationship between the changes in rectal balloon volume and the intrarectal pressure (compliance) in healthy volunteers (HV), patients with RH and normal rectal compliance (NC) and patients with RH and abnormal increased compliance (IC ) or excessive laxity of the rectal wall.
Figure 4.
Relationships between rectal hyposensitivity, sensorimotor response and abnormal increase rectal wall compliance. Most patients (80%) with a normal rectal wall compliance had transient sensation with SMR. In contrast, 66% of subjects with an increased compliance of the rectal wall, had absent rectal sensation during the onset of an SMR.
Discussion
Rectal hyposensitivity is associated with many common anorectal disorders such as constipation, fecal incontinence and irritable bowel syndrome, but the cause-effect relationship is poorly understood and its pathogenesis remains unclear.2,3,6
In this study, we found that all three recto-anal reflex responses were present, but were significantly altered in subjects with RH. The rectal balloon volumes required to evoke the RAIR and the RACR were significantly higher in RH subjects when compared to controls. These findings suggest that rectal hyposensitivity is associated with altered rectoanal reflex responses, which in turn may cause anorectal dysfunction.
The RAIR is mediated by the intramural myenteric neurons (myenteric plexus) and the noncholinergic and nonadrenergic nerves.8,12 Its physiological significance is not completely understood but this reflex may facilitate discrimination of the nature of rectal contents by permitting them to come into contact with the more sensitive anal mucosa.1,17 However, whether the receptors that mediate the RAIR response are located in the mucosa, submucosa or in the muscle plane is unknown, since this reflex is present in patients with spinal cord injury and in those with sacral root transection, whereas it is abolished after topical anesthesia.8,18 Furthermore, the preservation of RAIR after full rectal mobilization suggests that the receptors for the reflex are possibly located intramurally in the pelvic floor.19 This hypothesis is supported by the observation that bilateral hypogastric nerve block or total rectal extrinsic denervation does not affect the nature of the RAIR .20
Few studies have assessed the RAIR in constipated patients.7,21-23 Among these, some have reported that the volume of distention required to achieve an anal relaxation of 50% is significantly higher in constipated subjects when compared to controls.7 In contrast, others have reported either no changes in the RAIR 21,22 or a lower maximal amplitude of the reflex in patients with dyssynergic defecation.23 In this study, we found that the volume required to evoke this response was significantly higher in constipated subjects with RH when compared to healthy controls. These findings suggest that constipated subjects with RH may have an impaired mechanoreceptor transduction across the rectal wall in response to balloon distension. Another possibility is that prolonged retention of stool in the rectum, over time may lead to a blunting of rectal sensation (RH) and consequently the reflex responses. However, such a mechanism should lead to an attenuation of the reflex motor response and not an exaggeration of the response and hence this hypothesis seems unlikely.
We also found that the volume required to elicit the RACR was significantly higher in RH subjects when compared to controls. This primordial reflex that may prevent the premature release of rectal contents also appears to be dysfunctional in patients with RH.
Recently, we have described the SMR and have hypothesized that it may play an integral role in the regulation of anorectal sensation and function.14 This is based on the finding that an involuntary anal contraction occurs in response to rectal distension, and is associated with a desire to defecate, and can last for 5-6 seconds.14 Thus, the SMR may play an early warning role in preventing the inadvertent release of rectal contents in tandem with or independent of onset of voluntary squeeze. This response is partially under voluntary control and occurs along with awareness for rectal distension (i.e. desire to defecate). Furthermore, a more prominent SMR was observed when a stronger sensation such as an urge to defecate was elicited in healthy subjects.14 These findings suggest that the SMR may have a sensory component that involves the mechanoreceptors, rectal wall, afferent pathways, and the central nervous system, as well as a centrally mediated intramural motor component.
In subjects with RH, we found that although the SMR was present, 57% of subjects described a vague or transient rectal sensation (SMR-transient sensation group), unlike healthy subjects in whom SMR was associated with a desire to defecate. Furthermore, in the group of subjects with more severe RH (absent rectal sensation up to a volume of 320 cc air), although present, the SMR was altered and characteristics such as the onset, duration and amplitude were significantly higher when compared to the group of RH subjects with more blunted rectal sensation. These findings suggest that in subjects with RH, the motor component is intact, but the sensory component is defective. The higher amplitude, prolonged duration and larger AUC of the SMR in subjects with a more severe RH (SMR-absent sensation group) may represent a (exaggerated) compensatory response of the motor component in order to prevent the inadvertent release of rectal contents.
Our understanding of the mechanism(s) responsible for rectal sensory perception continues to evolve. Both thin myelinated Aδ and unmyelinated C fibers are present in the rectal mucosa, and the rectum can perceive thermal, chemical, and osmotic stimuli.1,12,18 Furthermore, rectal sensation is possibly evoked by stimulation of the mechanoreceptors that lie in the rectal wall 1,18 (1,30), and/or pelvic floor.12 These mechanoreceptors are stimulated by stretch-induced relaxation of the circular smooth muscle and depending on the degree of stretch relaxation, it has been proposed that a sensation of fullness, desire or urgency to defecate may be aroused.12,18 Recent evidence suggests that the rectal mechanoreceptors not only serve as tension receptors, but can also detect mechanical deformation of the myenteric ganglia, especially during stretching .24,25 Also, recent studies from rat models have confirmed the existence of intraganglionic laminar nerve endings (rIGLEs) in the myenteric plexus of the rectal wall that are sensitive to mechanical distension.25,26
Our study showed that 50% of subjects with RH had increased rectal wall compliance (i.e. excessive laxity of the rectum), confirming previous studies. If so, this suggests that the decreased rectal sensation may be due to the altered biomechanical properties such as abnormal collagen content or damage to smooth muscle fibers, rather than changes in the afferent pathways.3 Interestingly, we found an association between abnormal rectal sensation, SMR and rectal wall compliance. Patients who had normal compliance of the rectum demonstrated a SMR that was associated with transient sensation. This finding suggests that the altered SMR was due to a dysfunction of the afferent pathway and/or cortical processing of the response but the motor component was intact.27,28 In contrast, 66% of subjects who had a hyper-compliant rectum exhibited SMR, but had no rectal sensation (SRM-absent sensation group). These observations suggest that sensory perception from the rectum may have a component that is under conscious control and is perceived in the cerebral cortex and a reflex component. The conscious perception may be altered in some subjects with RH due to abnormalities within the rectal wall (i.e. alterations in both thin myelinated Aδ and unmyelinated C fiber, abnormal stimulation of mechanoreceptors), but the reflex component may be intact and may even be exaggerated.
Further studies are required to better characterize the relationship between the rectal sensory perception, rectoanal reflexes and SMR in patients with RH. These may include assessments of gut-brain-gut neuronal pathways, pudendal nerve terminal motor latencies, the effects of sleep, and pudendal nerve block and the effects of sensory training. Also, electromyographic recording in the EAS and IAS may identify the role of these muscles. Finally, he origin of the motor component of the response i.e whether it is due to the contraction of the puborectalis or the pelvic floor requires further delineation.
In conclusion, our study shows that patients with constipation and rectal hyposensitivity demonstrate significant abnormalities of the rectoanal reflexes and the sensori-motor responses. These altered manometric features may play an important role in the pathogenesis of constipation associated with RH.
Supplementary Material
Figure 5.
Relationship between Sensorimotor Response and Rectal Wall Compliance
Acknowledgments
Grant Support: This research was supported in part by grant R01DK 57100-03 National Institute of Health. Dr Remes-Troche was supported by the AGA, Jon I. Isenberg International Scholar Award. This work was presented at Digestive Disease Week, Los Angeles, CA, May 20-26, 2006 and published as an abstract in Gastroenterology 2006; 130 (Suppl 2) A 115. We thank Kimberly Klein for her superb secretarial support.
References
- 1.Rogers J. Anal and rectal sensation. Baillieres Clin Gastroenterol. 1992;6:179–91. doi: 10.1016/0950-3528(92)90026-b. [DOI] [PubMed] [Google Scholar]
- 2.Gladman MA, Lunniss PJ, Scott SM, Swash M. Rectal hyposensitivity. Am J Gastroenterol. 2006;101:1140–1151. doi: 10.1111/j.1572-0241.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 3.Gladman MA, Dvorkin LS, Lunniss PJ, Williams NS, Scott SM. Rectal hyposensitivity: a disorder of the rectal wall or the afferent pathway? An assessment using the barostat. Am J Gastroenterol. 2005;100:106–114. doi: 10.1111/j.1572-0241.2005.40021.x. [DOI] [PubMed] [Google Scholar]
- 4.Sun WM, Read NW, Miner PB. Relation between rectal sensation and anal function in normal subjects and patients with faecal incontinence. Gut. 1990;31:1056–1061. doi: 10.1136/gut.31.9.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun WM, Donnelly TC, Read NW. Utility of a combined test of anorectal manometry, electromyography, and sensation in determining the mechanism of ’idiopathic’ faecal incontinence. Gut. 1992;33:807–813. doi: 10.1136/gut.33.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gladman MA, Scott SM, Williams NS, Lunniss PJ. Clinical and physological findings, and possible aetiological factors of rectal hyposensitivity. Br J Surg. 2003;90:860–866. doi: 10.1002/bjs.4103. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Pasricha PJ, Sallam HS, Ma L, Chen JDZ. Clinical significance of quantitative assessment of rectoanal inhibitory réflex (RAIR) in patients with constipation. J Clin Gastroenterol. 2008;6:692–698. doi: 10.1097/MCG.0b013e31814927ba. [DOI] [PubMed] [Google Scholar]
- 8.Zbar AP, Jayne DGm Mathur D, Ambrose NS, Guillou PJ. The importante of the internal anal sphincter (IAS) in maintaining continence:anatomical, physiological and pharmacological considerations. Colorectal Disease. 2000;2:193–202. doi: 10.1046/j.1463-1318.2000.00159.x. [DOI] [PubMed] [Google Scholar]
- 9.Heyt GJ, Oh MK, Alemzadeh N, et al. Impaired rectoanal inhibitory response in scleroderma (systemic sclerosis): an association with fecal incontinente. Dig Dis Sci. 2004;49:1040–1045. doi: 10.1023/b:ddas.0000034569.85066.69. [DOI] [PubMed] [Google Scholar]
- 10.Deen KI, Premaratna R, Fonseka MM, DeSilva HJ. The recto-anal inhibitory reflex: abnormal response in diabetcis suggests an intrínsico neuropathy. J Gastroenterol Hepatol. 1998;13:1107–1110. doi: 10.1111/j.1440-1746.1998.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 11.Kelly JL, O'Riordain DS, Jones E, Alawi E, O'Riordain MG, Kirwan WO. The effect of hysterectomy on ano-rectal physiology. Int J Colorectal Dis. 1998;13:116–118. doi: 10.1007/s003840050147. [DOI] [PubMed] [Google Scholar]
- 12.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507–519. doi: 10.1111/j.1365-2982.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 13.Kumar D, Waldron D, Williams NS, Browning C, Hutton MR, Wingate DL. Prolonged anorectal manometry and external anal sphincter electromyography in ambulant human subjects. Dig Dis Sci. 1990;35:641–648. doi: 10.1007/BF01540414. [DOI] [PubMed] [Google Scholar]
- 14.De Ocampo S, Remes-Troche JM, Miller MJ, Rao SC. Rectoanal sensorimotor response in humans during rectal distensión. Dis Colon Rectum. 2007;50:1639–1646. doi: 10.1007/s10350-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 15.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773–83. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 17.Read MG, Read NW. Role of anorectal sensation in preserving continence. Gut. 1982;23:345–347. doi: 10.1136/gut.23.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sangwan YP, Solla JA. Internal anal sphincter: advances and insights. Dis Colon Rectum. 1998;41:1297–1311. doi: 10.1007/BF02258232. [DOI] [PubMed] [Google Scholar]
- 19.Gunterberg B, Kewenter J, Petersen I, Stener B. Anorectal function after major resections of the sacrum with bilateral or unilateral sacrifice of sacral nerves. Br J Surg. 1976;63:546–554. doi: 10.1002/bjs.1800630713. [DOI] [PubMed] [Google Scholar]
- 20.Sangwan YP, Coller JA, Barrett RC, Murray JJ, Roberts PL, Schoetz DJ., Jr Distal rectoanal excitatory reflex-a reliable index of pudendal neuropathy? Dis Colon Rectum. 1995;38:916–920. doi: 10.1007/BF02049725. [DOI] [PubMed] [Google Scholar]
- 21.Kaur G, Gardiner A, Duthie GS. Rectoanal reflex parameters in incontinence and constipation. Dis Colon Rectum. 2002;45:928–933. doi: 10.1007/s10350-004-6331-9. [DOI] [PubMed] [Google Scholar]
- 22.Zbar AP, Aslam M, Gold DM, Gatzen C, Gosling A, Kmiot WA. Parameters of the rectoanal inhibitory reflex in patients with idiopathic fecal incontinence and chronic constipation. Dis Colon Rectum. 1998;41:200–208. doi: 10.1007/BF02238249. [DOI] [PubMed] [Google Scholar]
- 23.Bartolo DC, Roe AM, Virjee J, Mortensen NJ, Locke-Edmunds JC. An analysis of rectal morphology in obstructed defecation. Int J Colorectal Dis. 1988;3:17–22. doi: 10.1007/BF01649677. [DOI] [PubMed] [Google Scholar]
- 24.Macefield VG. Physiological characteristics of low-threshold mechanoreceptors in joints, muscle and skin in human subjects. Clin Exp Pharmacol Physiol. 2005;32:135–144. doi: 10.1111/j.1440-1681.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- 25.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 26.Lynn PA, Olsson C, Zagorodnyuk V, Costa M, Brookes SJ. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology. 2003;125:786–794. doi: 10.1016/s0016-5085(03)01050-3. [DOI] [PubMed] [Google Scholar]
- 27.Turnbull G, Hamdy S, Aziz Q, Singh K. The cortical topography of human anorectal musculature. Gastroenterology. 1999;117:32–39. doi: 10.1016/s0016-5085(99)70547-0. [DOI] [PubMed] [Google Scholar]
- 28.Hobson AR, Aziz Q. Brain imaging and functional gastrointestinal disorders: has it helped our understanding? Gut. 2004;53:1198–1206. doi: 10.1136/gut.2003.035642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.