Abstract
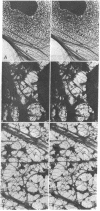
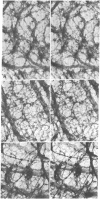
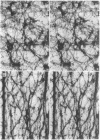
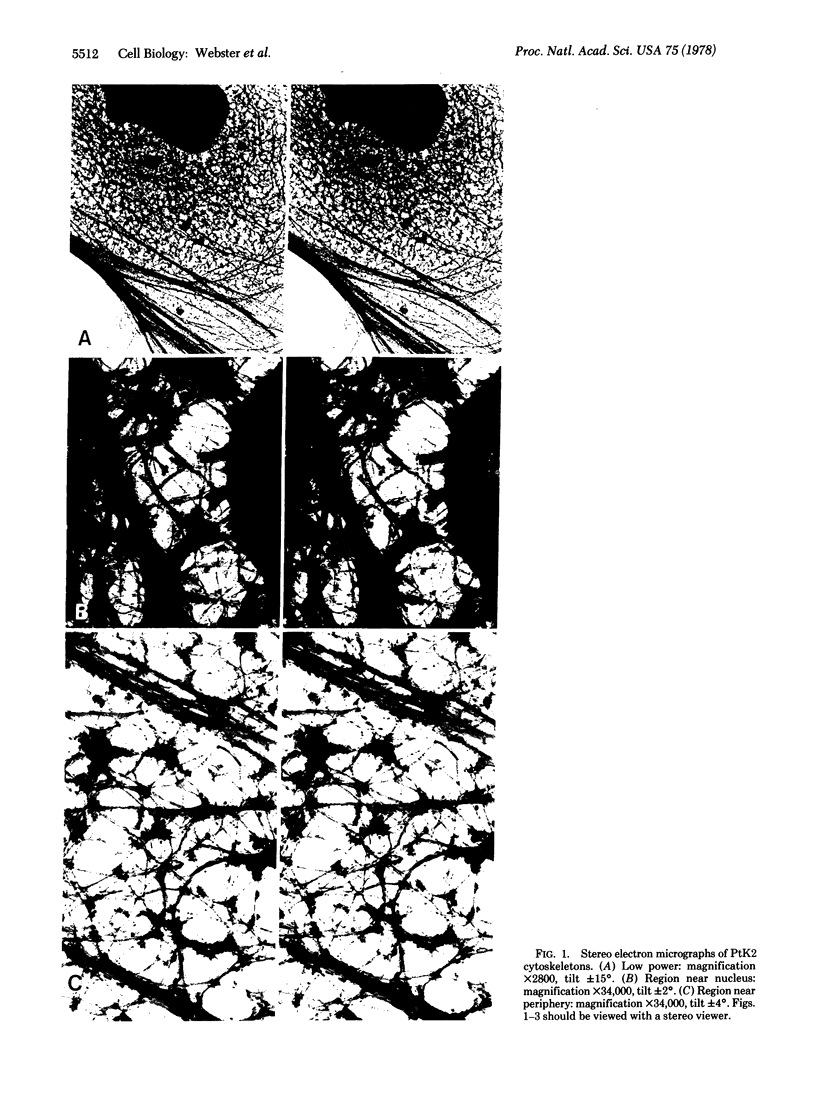
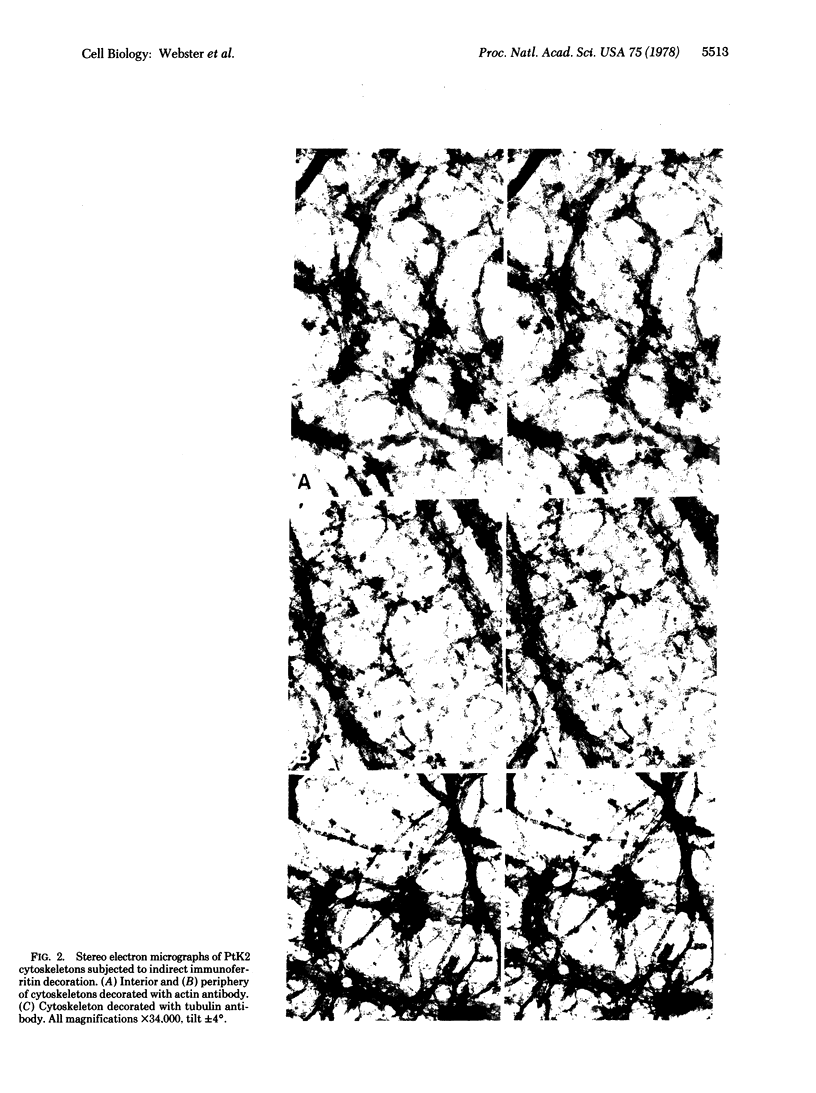
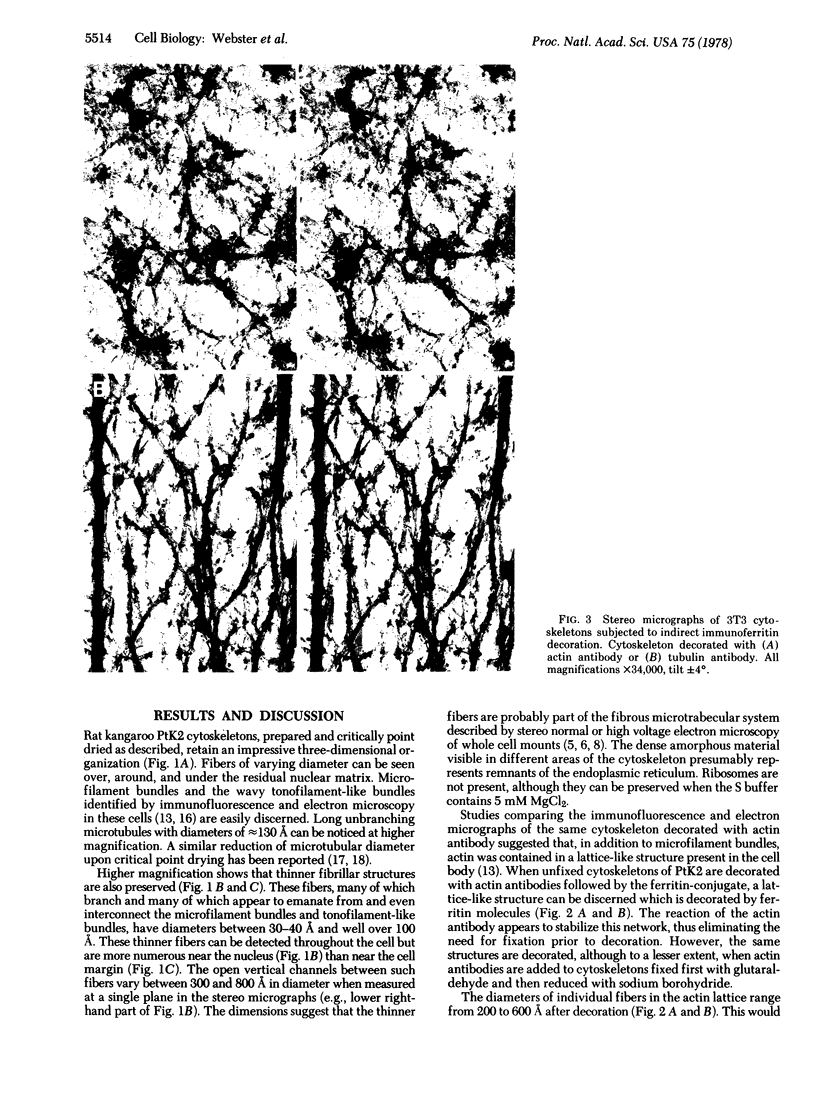
Cytoskeletons prepared by Triton X-100 treatment of tissue culture cells appear in stereo electron microscopy as a highly organized and interconnected three-dimensional matrix of different fibrous elements. Microfilament bundles and also tonofilament-like bundles are readily discerned when present in the cell type. In addition thinner fibers, some of which branch (smallest diameter 30--40 A), as well as fibers of larger diameter, some of which correspond to microtubules, can be seen. Since such cytoskeletons are an open, membrane-free system, individual fibrous organizations can be identified by specific antibodies. An indirect immunoferritin procedure using antibodies to tubulin or actin visualizes microtubules or actin-containing structures. Stereo electron microscopy of cytoskeletons decorated with actin antibody reveals, in addition to the F-actin-containing microfilament bundles, an extended fine actin lattice. This actin net is displayed throughout the cytoplasm not only between the microfilament bundles but also in those regions of the cytoskeleton that in the intact cell correspond to the submembraneous regions. Thus all actin-containing fibrous cytoplasmic structures may be interconnected in the living cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash J. F., Singer S. J. Concanavalin-A-induced transmembrane linkage of concanavalin A surface receptors to intracellular myosin-containing filaments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4575–4579. doi: 10.1073/pnas.73.12.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Buckley I. K., Raju T. R. Form and distribution of actin and myosin in non-muscle cells: a study using cultured chick embryo fibroblasts. J Microsc. 1976 Jul;107(2):129–149. doi: 10.1111/j.1365-2818.1976.tb02431.x. [DOI] [PubMed] [Google Scholar]
- Buckley I. K. Three dimensional fine structure of cultured cells: possible implications for subcellular motility. Tissue Cell. 1975;7(1):51–72. doi: 10.1016/s0040-8166(75)80007-3. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Kishida Y., Olsen B. R., Berg R. A., Prockop D. J. Two improved methods for preparing ferritin-protein conjugates for electron microscopy. J Cell Biol. 1975 Feb;64(2):331–339. doi: 10.1083/jcb.64.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metuzals J. Configuration of a filamentous network in the axoplasm of the squid (Loligo pealii L.) giant nerve fiber. J Cell Biol. 1969 Dec;43(3):480–505. doi: 10.1083/jcb.43.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Born T., Koitsch H. J., Weber K. Stereo immunofluorescence microscopy: I. Three-dimensional arrangement of microfilaments, microtubules and tonofilaments. Cell. 1978 Jul;14(3):477–488. doi: 10.1016/0092-8674(78)90234-9. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Osborn M., Webster R. E., Weber K. Individual microtubules viewed by immunofluorescence and electron microscopy in the same PtK2 cell. J Cell Biol. 1978 Jun;77(3):R27–R34. doi: 10.1083/jcb.77.3.r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Celis J. E. Filament arrangements in negatively stained cultured cells: the organization of actin. Cytobiologie. 1978 Feb;16(2):308–325. [PubMed] [Google Scholar]
- Temmink J. H., Spiele H. Preservation of cytoskeletal elements for electron microscopy. Cell Biol Int Rep. 1978 Jan;2(1):51–59. doi: 10.1016/0309-1651(78)90084-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M. Cytoplasmic microtubular images in glutaraldehyde-fixed tissue culture cells by electron microscopy and by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1820–1824. doi: 10.1073/pnas.75.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Stereo high-voltage electron microscopy of whole cells of the human diploid line, WI-38. Am J Anat. 1976 Nov;147(3):303–323. doi: 10.1002/aja.1001470305. [DOI] [PubMed] [Google Scholar]