Abstract
Kawasaki disease (KD) is associated with generalized vasculitis with a predilection for coronary artery leading to ectasia and aneurysm in some cases. The aim of this study was to noninvasively assess the cutaneous microcirculation and correlate it with the coronary artery diameter in these patients. Laser Doppler flowmetry and dynamic capillaroscopy were performed at the nailbeds to assess total cutaneous blood flow and microcirculation in children with KD, both in the afebrile phase (after the resolution of fever) and convalescent phases, in comparison to controls. The 100 subjects analyzed in this study included 64 patients with KD (33 in afebrile phase and 31 in convalescent phase) and 36 normal controls. In KD, the capillary morphology was abnormal when compared to controls, with a larger diameter of the arterial and venous limbs, a higher intercapillary distance and a decrease in the loop numbers. Significantly decreased capillary blood cell velocity was noted in afebrile phase but not in convalescent phase. In the afebrile phase, a decreased capillary blood cell velocity significantly correlated with an increased coronary artery diameter. In conclusion, KD patients, both in the afebrile and convalescent phases, exhibited morphologic alterations in the microcirculation when compared to the controls. The results indicate the potential role of dynamic capillaroscopy for the noninvasive survey of microcirculation abnormalities in patients with KD.
Keywords: Capillary blood cell velocity, Dynamic capillaroscopy, Kawasaki disease, Microcirculation
Introduction
Kawasaki disease (KD), an acute febrile illness of unknown etiology, is characterized by diffuse vasculitis with predilection for coronary arteries sometimes leading to ectasia and aneurysmal changes by the subacute phase of the disease [1]. Although the incidence of coronary artery ectasia and aneurysm has decreased in the adequately treated children with the disease, some children continue to develop these abnormalities despite therapy [1, 2].
Whereas several studies have examined medium-sized muscular arteries, few have evaluated the microvasculature dysfunction in KD, even though edema of the extremity and mucus membrane with erythema is a prominent feature of KD indicating significant microvasculitis [3]. Furthermore, significantly increased incidences of myocardial microvascular lesions have been demonstrated in KD patients with coronary artery lesions [4]. These myocardial microvascular abnormalities have been reported to persist in late stage of the KD several years later [5–7]. In 1998, Hamaoka et al. used a small size of Doppler guide wire to measure coronary blood flow velocity in KD patients and reported that abnormal coronary microcirculation contributes to pathophysiologic response in KD [8].
Laser Doppler flowmetry (LDF) is a noninvasive tool to assess total cutaneous blood flow and microcirculation and has been used to study adults with conditions such as diabetes that are known to affect microcirculation [9]. In 2008, Kurio et al. investigated the microcirculation in KD patients using LDF and reported a transient abnormal endothelial function in the acute phase that does not persist in the convalescent phase [10]. Capillary microscopy, the preferred method of assessing capillary blood flow [11], has been coupled with videophotometric systems and software to analyze capillary blood cell velocity [12]. This unique technique has been applied to evaluate dynamic microcirculatory status in peripheral vascular disorders, including arterial occlusive diseases [11], collagen vascular diseases [13], and congenital heart disease [14].
The purpose of this study was to evaluate cutaneous hemodynamic alterations using LDF and capillary microscopy after the resolution of the febrile phase of KD and to evaluate the relationship between cutaneous microcirculation and the coronary artery diameter.
Materials and methods
Study Population and inclusion criteria
The study was approved by the institutional review board of Kaohsiung Medical University Hospital (KMUH) and Antai Tian-Sheng Memorial Hospital. Children diagnosed with KD and undergoing treatment in the KMUH pediatric ward were enrolled in this study and were evaluated in their afebrile phase from January 1, 2001 to December 31, 2009. At the time of enrollment, patients had been febrile for at least 3 days prior to enrollment, and all met at least four of five diagnostic criteria for KD or exhibited three criteria plus echocardiographic evidence of dilated coronary arteries (z-score>2.5, which was primarily useful for patients with Kawasaki Disease, this calculator return a z-score for the left main coronary artery (LMCA), left anterior descending (LAD), and right main coronary artery (RCA)) and were treated in the standard manner [2]. The subacute phase of KD begins when fever and other acute signs have abated, but irritability, anorexia, and conjunctiva injection may persist and it was at this point (afebrile phase) that the children were investigated for this study. Convalescent phase KD patients were recruited from the KMUH Pediatric Cardiology clinic. The convalescent phase begins when all clinical signs of illness have disappeared and continued until the erythrocyte sedimentation rate and C-reactive protein return to normal. Siblings of KD patients with no history of KD were enrolled as healthy controls. Additional healthy control subjects included infants and children undergoing outpatient echocardiograms either with or without chloral hydrate sedation. At the time of the evaluation by LDF, all the children were in the afebrile phase for at least 24 h. Analyses were performed only if patients had rectal or oral temperature lower than 37°C so that elevated body temperature would not complicate the interpretation of the test results. They underwent LDF and dynamic capillaroscopy study after their transthoracic echocardiograms. Patients with history of the cardiovascular abnormalities, hyperlipidemia, hypertension, or congenital heart disease were excluded. Subjects were also excluded if they had been febrile within 24 h prior to the study or were currently taking any medication. Participants were asked to refrain from consuming foods and beverages containing caffeine on the day of test. Patients older than 3 years and able to cooperate were evaluated without sedation. The remaining patients were studied by LDF and dynamic capillaroscopy after receiving chloral hydrate sedation for echocardiograms.
Microcirculatory assessments
Cutaneous microcirculatory evaluations including LDF [15] and dynamic capillary microscopy (dynamic capillaroscopy) [12] were performed after 30 min of equilibrium at a constant room temperature of 23–25°C. An LDF (PeriFlux PF3, Sweden) was used for cutaneous blood flow measurement. The standard probe (PF308) was fixed on the pulp of the left ring finger. All recordings were at least 10 min long, and the measurements were repeated six times. The system would be manually recalibrated each time for every single case. The results were analyzed by Perimed analysis program for PeriFlux. Cutaneous blood flow was expressed in perfusion units (pu), i.e., the product of the number of moving red blood cells and their velocity. Dynamic capillaroscopy was performed on the nail folds with a Leitz Laborlux 12 MEX capillary microscope connected to a TV camera (Ikegami CTC-2110, Japan). A drop of immersion oil was applied to make the nail fold transparent. With the finger resting on a plate, the distal end of the nail was lightly touched with a small bracket. The image was observed on external monitor with a final magnification of ×800. All recordings were videotaped and analyzed by the capiFlow computerized analysis system (capiFlow AB, Kista, Sweden). The incident laser angles to red blood cell (RBC) motion as well as RBC velocity were averaged, which thus obviates the need to consider the structure and angulations of the microvascular tree. Data analysis included number and morphology, intercapillary distance, caliber, and visibility of the capillary loops as well as capillary blood cell velocity measurement.
Coronary artery diameter assessments
The measurement of the proximal coronary artery diameter was performed in the parasternal short-axis view in every subject using high-resolution, two-dimensional ultrasound images obtained with Sonos 5500 (Philips Medical Systems, Andover, Massachusetts) with a 5 MHz transducer. The subject rested in the supine position for 10 min prior to the first scan and remained in the same position throughout the study. These images were analyzed by two trained, certified pediatric cardiologists, and interobserver discrepancies were resolved by the consensus of one other cardiologist.
Statistical analysis
Data were expressed as the means ± SD. All data were analyzed using the statistical package for the Social Sciences version 12.0 (SPSS, Inc., Chicago, IL, USA). The ANOVA test was used to compare KD subgroups and normal controls in terms of cutaneous blood flow, capillary blood cell velocity, capillary diameter, intercapillary distance, loop number, and capillary type. Pearson coefficient was applied to measure the correlation between capillary blood cell velocity and the coronary artery diameter. The correlation of capillary type and coronary artery diameter was evaluated by Spearman coefficient. The values were expressed as mean ± one standard deviation. A P value <0.05 was considered statistically significant.
Results
Clinical characteristics of the study population
The study analyzed 100 children with 64 KD patients and 36 normal children (Table 1) of similar ancestry (Asian). In Table 1, days after fever onset significantly differed (P< 0.001) among three groups such as afebrile KD, convalescent KD, and the control group with similar ages (4.09± 1.24, 3.73±1.30, and 4.03±1.04). None of the children with KD developed aneurysms in the proximal coronary arteries as evaluated by a transthoracic echocardiogram.
Table 1.
Characteristics of study population
| Afebrile KD | Convalescent KD |
Normal Control |
P value | |
|---|---|---|---|---|
| Case numbers | 33 | 31 | 36 | |
| Age (years) | 4.09±1.24 | 3.73±1.30 | 4.03±1.04 | 0.542 |
| Gender | 0.965 | |||
| Male | 17 | 17 | 19 | |
| Female | 16 | 14 | 17 | |
| Time of study (days after fever onset) | 12.69±8.87 | 352.05±112.80 | N/A | <0.001 |
Data are expressed as mean ± SD. P value is tested by Chi-square test
N/A not applicable
Comparison of laser Doppler flowmetry between KD and normal controls
Mean cutaneous blood flow measured by LDF was 129.88± 1.15 pu in afebrile KD group, 120.05±1.83 pu in convalescent KD group and 110.86±0.75 pu in normal controls. The LDF results showed that cutaneous blood flow in convalescent KD patients was significantly lower than normal controls (P<0.001).
Comparison of capillary morphology and capillary blood cell velocity between KD and normal controls
In normal controls, the capillary loops presented as regularly distributed hairpin-like structures with uniform diameter. The diameters of venous limbs and the intercapillary distance in KD groups were significantly larger than those in normal controls (Table 2). The arterial limb diameter was significantly larger in convalescent phase than in afebrile KD patients and controls (Table 2). The capillary blood cell velocity was significantly lower in afebrile phase KD patients than in convalescent KD patients and controls (Table 2).
Table 2.
Nail fold capillary morphology analysis and velocity measurements in normal controls and KD patients in afebrile and convalescent phases
| Afebrile KD | Convalescent KD | Normal control | P value | |
|---|---|---|---|---|
| Case numbers | 33 | 31 | 36 | |
| Diameter (um) | ||||
| Arterial limb | 10.55±0.59 | 11.26±0.52 | 10.78±0.43 | <0.001 |
| Venous limb | 15.99±0.33 | 15.93±0.26 | 13.44±1.26 | <0.001 |
| Intercapillary distance (um) | 129.88±1.15 | 120.05±1.83 | 110.86±0.75 | <0.001 |
| Loop number / mm | 5.16±0.13 | 6.12±0.12 | 7.56±0.19 | <0.001 |
| Abnormal loop (%) | 61.84 | 55.89 | 27.31 | <0.001 |
| CBV (mm/s) | 0.34±0.02 | 0.59±0.02 | 0.70±0.02 | <0.001 |
Data are expressed as mean ± SD. P<0.05; P value is compared between KD subgroups and the controls by ANOVA test
CBV capillary blood cell velocity
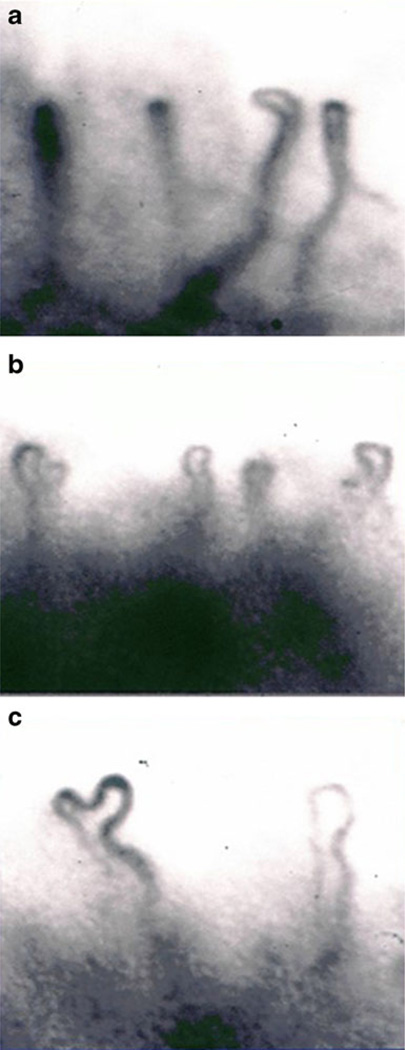
Capillary types of nail fold in KD patients
The Maricq capillary classification [16] was modified to assess afebrile and convalescent KD patients in this study. Three capillary types were classified under capillary microscopy: type I, normal loops; type II, definite enlargement of capillaries or subpapillary plexus; and type III, extremely enlarged capillaries or subpapillary plexus (Fig. 1a–c). Arterial limb diameter was significantly larger in the type III group in comparison to the normal controls (Table 3). Venous limb diameter in types I, II, and III groups was significantly larger than that in control groups (Table 3). Loop number per millimeter in the type III group was significantly higher than that in the type I and type II, even though it was lower than control groups (Table 3). The capillary blood cell velocity in types I, II, and III groups was significantly lower than that in control groups (Table 3). The intercapillary distance was significantly higher in capillary types I, II, and III than in control groups (Table 3).
Fig. 1.
Morphological changes in nail fold capillaries of KD patients. Three capillary types were classified under capillary microscopy. a shows Maricq type I capillaries, in which capillary loops appeared normal. b shows the type II capillaries, in which the venous limbs were dilated. c shows the type III capillaries, in which capillary loops were extremely enlarged and dilated
Table 3.
Nail fold capillary morphology analysis and velocity measurement in normal controls and KD patients categorized by capillary types
| Kawasaki disease | Normal control | P value* | |||
|---|---|---|---|---|---|
| Type I | Type II | Type III | |||
| Case numbers | 25 | 24 | 15 | 36 | |
| Afebrile phase | 16 | 12 | 5 | ||
| Convalescent phase | 9 | 12 | 10 | ||
| Diameter (um) | |||||
| Arterial limb | 10.54±0.54 | 10.84±0.46 | 11.59±0.58 | 10.78±0.43 | <0.001 |
| Venous limb | 16.10±0.22 | 15.90±0.31 | 15.83±0.32 | 13.44±1.27 | <0.001 |
| Intercapillary distance (um) | 126.30±5.18 | 124.89±5.19 | 123.53±4.99 | 110.86±0.75 | <0.001 |
| Loop number/mm | 5.50±0.51 | 5.64±0.48 | 5.81±0.49 | 7.56±0.19 | <0.001 |
| Abnormal loop (%) | 59.77 | 58.93 | 57.66 | 27.31 | <0.001 |
| CBV (mm/s) | 0.43±0.13 | 0.46±0.13 | 0.51±0.12 | 0.70±0.02 | <0.001 |
Data are expressed as mean ± SD
CBV capillary blood cell velocity
P<0.05, significant difference in comparison to the controls by ANOVA test
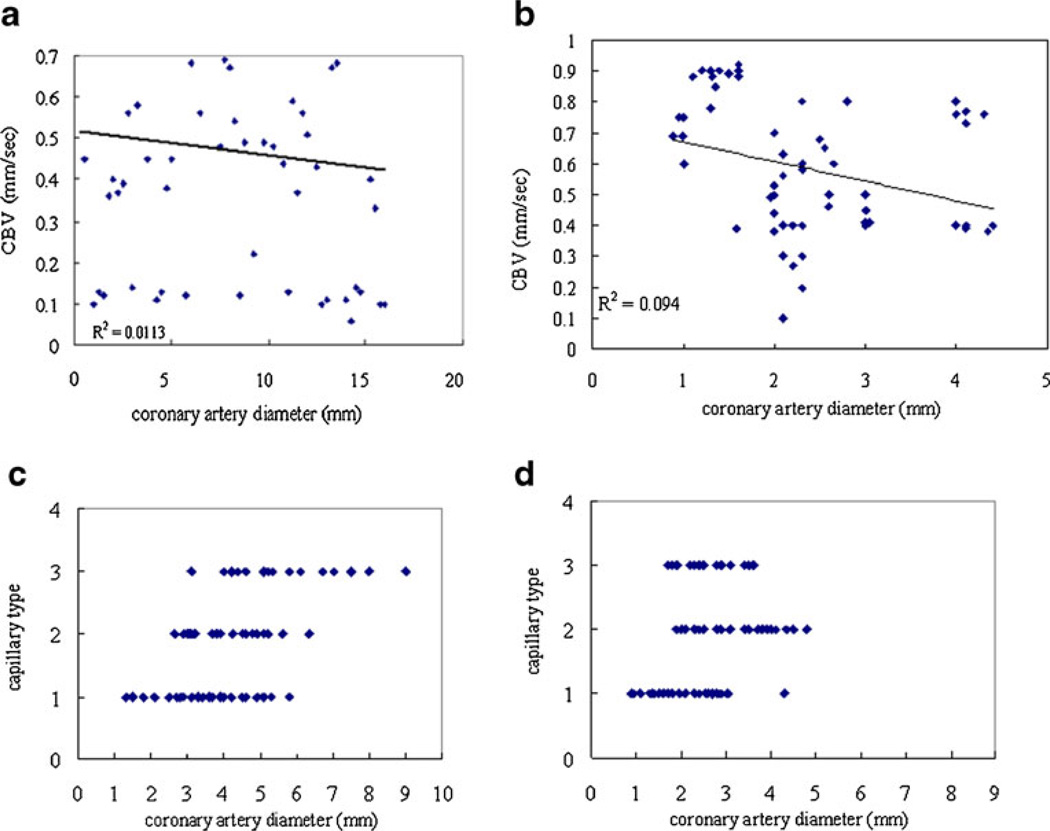
Correlation of coronary artery diameter and capillary blood cell velocity and capillary types in afebrile and convalescent KD patients
The mean value of coronary artery diameter was 5.16± 1.79 mm in the afebrile KD group and 2.43±1.11 mm in the convalescent KD group. Mean capillary blood cell velocity was 0.35±0.13 mm/s in afebrile phase KD patients and 0.59±0.21 mm/s in convalescent phase KD patients. In the afebrile phase, capillary blood cell velocity correlated with coronary artery diameter (Pearson correlation=−0.597, p=0.031; R2=0.6471, Fig. 2a). Diminished capillary blood cell velocity was significantly correlated with increased coronary artery diameter in afebrile phase KD patients. However, coronary artery diameter and capillary blood cell velocity revealed no correlation in convalescent phase (Pearson correlation=−0.326; P=0.328; Fig. 2b; R2=0.1052). Spearman coefficient analysis of capillary type and coronary artery diameter revealed no correlation in afebrile and convalescent phase KD patients (afebrile phase: Spearman correlation=0.473, P=0.103, Fig. 2c; convalescent phase: Spearman correlation=0.267, P=0.428, Fig. 2d).
Fig. 2.
Correlation of coronary artery diameter to capillary blood cell velocity and capillary type in KD patients. a shows the correlation between the mean coronary artery diameters and mean capillary blood cell velocity in afebrile phase KD patients. b shows the correlation between the mean coronary artery diameters and mean capillary blood cell velocity in the convalescent KD patients. c shows no correlation between the capillary type and coronary artery diameter in afebrile phase KD patients. d shows no correlation between the capillary type and coronary artery diameter in the convalescent KD patients
Discussion
Dynamic capillaroscopy revealed morphologic alterations in nail fold area capillaries, in both afebrile and convalescent KD patients, in comparison to controls. Morphological changes in nail fold capillaries were classified to three capillary types under capillary microscopy. Maricq type I capillaries were more common in KD patients at afebrile phase than convalescent phase. The patients with type III capillaries were more common at convalescent phase than afebrile phase. In KD, the capillary morphology was abnormal when compared to controls, with the findings of a larger diameter of the arterial and venous limbs, a higher intercapillary distance, and a decrease in the loop numbers. Compared to afebrile phase, abnormal loops and intercapillary distance were substantially improved in convalescent phase. Like coronary artery abnormality, transient microcirculatory alteration appears to be transient as well if adequately treated.
The evaluation by LDF in this study revealed that cutaneous blood flow in KD patients was significantly lower in the afebrile phase than that in normal controls. The capillary blood cell velocity was significantly lower in the afebrile KD group than in the convalescent and control groups. Comparisons of different categories of capillary loops in this study indicated that highly dilated and tortuous loops presented with a low capillary blood cell velocity. The comparisons of capillary blood cell velocity and coronary artery diameter revealed that capillary blood cell velocity inversely correlated with the severity of coronary arterial dilatation, especially in afebrile phase. The likely mechanism of a correlation between coronary artery diameter and capillary blood cell velocity could be a general process of vasodilatation or ectasia in the afebrile phase of KD. Endothelial cells were activated by increased flow in the arteries and capillaries, and endothelial activation is characterized as lumen protrusions, increase of cytoplasmic organelles, abluminal protrusions, basement membrane degradation, internal elastic lamina degradation in the arteries, and sprouting in the capillaries [17]. In 2008, Dobbe et al. reported RBC velocity was reduced by 70% during the first 10 s of cardiac luxation by analyzing microcirculatory videos [18]. The relationship between peripheral cutaneous vascular change and coronary arterial status observed in this study suggests that dynamic capillaroscopy is a noninvasive method of identifying small vessel changes and, indirectly, identifying coronary arterial abnormalities in KD patients. Further research will use this noninvasive dynamic capillaroscopy method to identify those KD patients who would be alerted for the high probability of coronary arterial aneurysm and for a more intensive medical intervention.
There were some limitations of this study including the small number of patients studied as well as the lack of a prospective follow-up. We did not have patients with aneurysms to determine the relationship of the cutaneous microcirculatory changes to the aneurysms.
In conclusion, KD patients, both in the afebrile and convalescent phases, exhibited morphologic alterations in the microcirculation when compared to the controls. The results indicate the potential role of dynamic capillaroscopy for the noninvasive survey of coronary arterial abnormalities in patients with KD.
Acknowledgments
The authors would like to thank Dr Kung-Kai Lin and Dr Hsin-Su Yu for their technological assistance.
Footnotes
Disclosures None.
Contributor Information
Ming-Yii Huang, Department of Radiation Oncology, Kaohsiung Medical University Hospital, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, No. 100 Tzyou 1st Road, Kaohsiung 807, Taiwan.
Joh-Jong Huang, Email: jjhua@seed.net.tw, Department of Family Medicine, Yuan’s General Hospital, No. 162 Cheng Kung 1st Road, Kaohsiung 80249, Taiwan.
Teh-Yang Huang, Email: huang190814@gmail.com, Department of Pediatrics, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Tzyou 1st Road, Kaohsiung 807, Taiwan.
Monesha Gupta-Malhotra, Department of Pediatrics, Division of Pediatric Cardiology, Children’s Memorial Hermann Hospital, University of Texas-Houston Medical School, 6411 Fannin Houston, Texas 77030, USA.
Fei-Kai Syu, The Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, 615 N Wolfe Street, Baltimore, MD 21205, USA.
References
- 1.Kato H, Ichinose E, Yoshioka F, et al. Fate of coronary aneurysms in Kawasaki disease: serial coronary angiography and long-term follow-up study. Am J Cardiol. 1982;49:1758–1766. doi: 10.1016/0002-9149(82)90256-9. [DOI] [PubMed] [Google Scholar]
- 2.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 3.Sohn S, Kwon K. Accelerated thrombotic occlusion of a medium-sized coronary aneurysm in Kawasaki disease by the inhibitory effect of ibuprofen on aspirin. Pediatr Cardiol. 2008;29:153–156. doi: 10.1007/s00246-007-9123-x. [DOI] [PubMed] [Google Scholar]
- 4.Liu AM, Ghazizadeh M, Onouchi Z, et al. Ultrastructural characteristics of myocardial and coronary microvascular lesions in Kawasaki disease. Microvasc Res. 1999;58:10–27. doi: 10.1006/mvre.1999.2155. [DOI] [PubMed] [Google Scholar]
- 5.Cicala S, Galderisi M, Grieco M, et al. Transthoracic echo-Doppler assessment of coronary microvascular function late after Kawasaki disease. Pediatr Cardiol. 2008;29:321–327. doi: 10.1007/s00246-007-9030-1. [DOI] [PubMed] [Google Scholar]
- 6.Furuyama H, Odagawa Y, Katoh C, et al. Altered myocardial flow reserve and endothelial function late after Kawasaki disease. J Pediatr. 2003;142:149–154. doi: 10.1067/mpd.2003.46. [DOI] [PubMed] [Google Scholar]
- 7.Muzik O, Paridon SM, Singh TP, et al. Quantification of myocardial blood flow and flow reserve in children with a history of Kawasaki disease and normal coronary arteries using positron emission tomography. J Am Coll Cardiol. 1996;28:757–762. doi: 10.1016/0735-1097(96)00199-4. [DOI] [PubMed] [Google Scholar]
- 8.Hamaoka K, Onouchi Z, Kamiya Y, et al. Evaluation of coronary flow velocity dynamics and flow reserve in patients with Kawasaki disease by means of a Doppler guide wire. J Am Coll Cardiol. 1998;31:833–840. doi: 10.1016/s0735-1097(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 9.Colberg SR, Parson HK, Nunnold T, et al. Effect of a single bout of prior moderate exercise on cutaneous perfusion in type 2 diabetes. Diabetes Care. 2006;29:2316–2318. doi: 10.2337/dc-06-1440. [DOI] [PubMed] [Google Scholar]
- 10.Kurio GH, Zhiroff KA, Jih LJ, et al. Noninvasive determination of endothelial cell function in the microcirculation in Kawasaki syndrome. Pediatr Cardiol. 2008;29:121–125. doi: 10.1007/s00246-007-9077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz RW, Freedman AM, Richardson DR, et al. Capillary blood flow: videodensitometry in the atherosclerotic patient. J Vasc Surg. 1984;1:800–808. doi: 10.1067/mva.1984.avs0010800. [DOI] [PubMed] [Google Scholar]
- 12.Norman M, Herin P, Fagrell B, et al. Capillary blood cell velocity in full-term infants as determined in skin by videophotometric microscopy. Pediatr Res. 1988;23:585–588. doi: 10.1203/00006450-198806000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht HP, Hiller D, Hornstein OP, et al. Microcirculatory functions in systemic sclerosis: additional parameters for therapeutic concepts? J Invest Dermatol. 1993;101:211–215. doi: 10.1111/1523-1747.ep12363834. [DOI] [PubMed] [Google Scholar]
- 14.Chang CH, Yu HS, Chen GS, et al. Deterioration of cutaneous microcirculatory status and its clinical correlation in tetralogy of Fallot. Microvasc Res. 1996;51:59–68. doi: 10.1006/mvre.1996.0007. [DOI] [PubMed] [Google Scholar]
- 15.Winsor T, Haumschild DJ, Winsor DW, et al. Clinical application of laser Doppler flowmetry for measurement of cutaneous circulation in health and disease. Angiology. 1987;38:727–736. doi: 10.1177/000331978703801001. [DOI] [PubMed] [Google Scholar]
- 16.Maricq HR. Wide-field capillary microscopy. Arthritis Rheum. 1981;24:1159–1165. doi: 10.1002/art.1780240907. [DOI] [PubMed] [Google Scholar]
- 17.Masuda H, Kawamura K, Nanjo H, et al. Ultrastructure of endothelial cells under flow alteration. Microsc Res Tech. 2003;60:2–12. doi: 10.1002/jemt.10237. [DOI] [PubMed] [Google Scholar]
- 18.Dobbe JG, Streekstra GJ, Atasever B, et al. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med Biol Eng Comput. 2008;46:659–670. doi: 10.1007/s11517-008-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]