Abstract
Background
Both abused and well cared for infants show attachment to their caregivers, although the quality of that attachment differs. Moreover, the infant’s attachment to the abusive caregiver is associated with compromised mental health, especially under stress. In an attempt to better understand how abuse by the caregiver can compromise mental health, we explore the neural basis of attachment in both typical and abusive environments using infant rats, which form attachments to the mother through learning her odor. Here, we hypothesize that the neural circuitry for infant attachment differs based on the quality of the attachment, which can be uncovered during stressful situations.
Methods
We used infant rats to compare infant attachment social behaviors and supporting neurobiology using natural maternal odor, as well as two odor-learning attachment paradigms: odor-stroke (mimics typical attachment) and odor-.5 mA shock conditioning (mimics abusive attachment). Next, to uncover differences in behavior and brain, these pups were injected with systemic corticosterone. Finally, pups were reared with an abusive mother to determine ecological relevance.
Results
Our results suggest that the natural and learned attachment odors indistinguishably control social behavior in infancy (approach to the odor and interactions with the mother). However, with corticosterone injection, pups with an abusive attachment show disrupted infant social behavior with the mother and engagement of the amygdala.
Conclusions
This animal model of attachment accommodates both abusive and typical attachment and suggests that pups’ social behavior and underlying neural circuitry may provide clues to understanding attachment in children with various conditions of care.
Keywords: Abuse, amygdala, attachment, fear conditioning, infant, stress, trauma
Clinical studies suggest that attachments are formed in both abusive and typical infant-caregiver relationships (1). However, abusive attachment is associated with compromised physical and mental health, including psychopathologies such as anxiety disorders and depression (2–5). This suggests divergence in neural processing of stimuli during infancy associated with abusive versus typical attachment, which may account for later abnormalities identified within the adult amygdala, prefrontal cortex, and hippocampus (2,5,6).
Altricial species have a unique attachment neural circuit that ensures infants attach to the caregiver, regardless of the quality of care received. We have chosen to develop this model in infant rats because of their well-documented attachment learning to maternal odor. As rat pups are born with nonfunctional visual and auditory systems, they depend upon their olfactory system to detect the maternal odor so they can approach the mother, but the odor also controls interactions with the mother, including nipple attachment. Maternal odor was initially thought to be a pheromone, because at birth, pups respond to the maternal odor with approach behaviors and nipple attachment (7). Now, however, it is understood that the maternal odor is learned at birth through tactile stimulation and relearned repeatedly throughout the postnatal period, presumably because changing maternal diets alter the maternal odor (8,9).
Maternal odor learning is heightened during the first 10 days of the pup’s life (9–12). This specialized sensitive period of learning can be mimicked with classical olfactory conditioning using either milk or stroking, which mimics maternal care. Similar to natural maternal odor, the learned attachment odor supports an odor preference, orientation to the odor, and nipple attachment (8,9,11,13,14). Nevertheless, the infant sensitive period is also characterized by learning constraints such as attenuated avoidance/fear learning (10,15–21). For example, a novel odor becomes preferred if it is paired with aversive stimuli such as tail pinch (11), .5 mA shock (10,16,19,21), or rough handling by the mother (22). The attenuated avoidance/fear learning is not due to an inability to detect pain; pups show similar pain thresholds to shock before and after the age when shock transitions from supporting odor preference learning to supporting odor aversion learning (21,23,24). Rather, the inability of odor-.5 mA shock conditioning to support avoidance/fear learning in young pups is because of the amygdala’s failure to become incorporated into the infant learning circuit (18,19,21).
Here, we develop an animal model of abusive attachment by comparing the naturally learned maternal odor with two other learned odors produced by conditioning with either odor-.5 mA shock (abusive attachment) or odor-stroke (typical attachment) and question whether these odors all support social attachment behaviors during the sensitive period (postnatal day [PN] 8). We used two prominent infant social behaviors: 1) Y-maze testing measures the approach response to odors that serve as the primary control of pup interactions with the mother; and 2) the Mother-Pup Interactions test directly measures social interactions with the mother. Since the clinical literature suggests that stressful situations seem to exacerbate differences in typical and abusive attachment in children (25–28), we also examine the impact of stress on abusive and typical attachment. We stressed pups through systemic corticosterone (CORT) injections or through a more naturalistic paradigm in which pups’ endogenous CORT was increased by rearing them with a stressed mother.
Methods and Materials
Subjects
Male and female Long-Evans rat pups were bred in the University’s animal care facilities and housed (polypropylene cages 34 × 29 × 17 cm, wood chips, ad libitum food and water) in a temperature (20°C) and light (06:00–18:00 hours) controlled room. The birthdate was PN0. Litters were culled to 12 pups on PN1 and only 1 male and/or female per litter was used in any conditioning/test condition. Procedures were approved by the Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines.
Conditioning
Odor-.5 mA Shock (Abusive Attachment) and Odor-Stroke (Typical Attachment)
Pups received 11 conditioning trials with a 4-minute intertrial interval. The paired group received 11 pairings of peppermint odor (2 L/min, 1:10 peppermint vapor; olfactometer controlled by ChronTrol, ChonTrol Corporation, San Diego, California), with either 1-second .5 mA shock (hind limb) or 20-second stroking with a sable-haired paintbrush, and terminated simultaneously with odor termination. The unpaired group received the shock or stroking 1.5 to 2 minutes after the odor presentation, while the odor-only group received only odor. Pups were removed from the nest immediately before conditioning, placed in the conditioning apparatus (600 mL plastic jars), given 10-minute acclimation, conditioned, and returned to the nest (18,19,21,29).
Y-Maze
The next day, pups’ were given a 5-trial Y-maze test to assess odor preference/avoidance using a video tracking system (Columbus Instruments, Columbus, Ohio) to record pups’ choice between two alleys (start box: 8.5 × 10 × 8 cm; arms: 8.5 × 24 × 8 cm). Odors were placed at the end of the alleys: maternal odor (air stream) or peppermint odor (20-µL peppermint odor on KimWipe, Kimthech Science, Dallas, Texas) versus a familiar odor (20-mL clean aspen shavings in petri dish). Pups were placed in the start box for 5 seconds and alley doors opened to initiate the 60-second choice period. A choice required the pup’s entire body to be beyond the alley entrance. The maternal odor air stream used a mother placed in an airtight glass enclosure (20 × 21 cm) connected to the olfactometer for odor delivery of 2 L/min, 1:10 maternal odor:air.
Mother-Pup Interactions
Before testing, the anesthetized (urethane to prevent milk letdown) mother’s maternal odor was removed (wash of acetone, then alcohol, and finally water), which disrupts nipple attachment and interactions with the mother (30,31). The washed mother was then placed in the testing chamber (25 × 40 × 20 cm) on her side to provide pups access to nipples. The odor (maternal odor or learned attachment odor) was presented in an air stream 2 cm from the center of the mother’s ventrum (2 L/min, 1:10 maternal odor:air). During the 10-minute test, the latency and duration of pups’ probing/nipple searching and nipple attachment were recorded.
Systemic CORT Injection
Pups were injected with either CORT (corticosterone HBC complex, Sigma, St. Louis, Missouri; 3.0 mg/kg, intraperitoneally) or .9% saline 30 minutes before testing (17,18).
Naturalistic Stress-Mother Paradigm
Mother and pups were housed with either limited (1000 mL, 1.2 cm layer) or abundant (4500 mL, 5 cm layer) nesting/bedding material from PN3 to PN8. This limited bedding environment decreased mothers’ ability to construct a nest, which resulted in frequent nest building, more time away from pups, rough handling and stepping on pups, and less licking/grooming. Pups nursed less but weight gain was normal (Table 1). This treatment was adapted from the Baram Laboratory (32,33), although we did use the wire mesh floor and mothers were provided with clean bedding every 2 days.
Table 1.
Frequency of Mother and Pups Behaviors Observed During Mother-Infant Interactions
| Percent of Observation Periods in Which Behaviors Occurred | ||
|---|---|---|
| Stressed Mother | Normal Mother | |
| Mother Abnormal Behaviors | ||
| Steps or jumps on | 24.78% | .00% |
| Rough handing | 5.56% | .00% |
| Nest building | 10.19% | .00% |
| Mother Normal Behaviors | ||
| Nursing | 19.44% | 80.56% |
| Mother′s time in the nest | 33.40% | 85.22% |
| Pups Body Weight at PN8 (g) | 17.75 ± .28 | 17.69 ± .22 |
| Pups Vocalization | 73.15% | 4.63% |
| Pups CORT Level (ng/mL) | 58.33 ± 9.41 | 38.57 ± 2.04a |
CORT, corticosterone; PN, postnatal day.
p < .05 between groups.
Maternal and pup behaviors were recorded three times a day (15-minute sessions): stepping on pups, rough handling (i.e., mother aggressively grooms pups, transporting pups by limb), pup vocalization, nest building, mother’s time in the nest, and nursing.
Radioimmunoassay
Plasma of PN8 normal- and stress-mother reared pups collected by trunk blood (centrifuged 14,000 rpm for 6 minutes and stored at −70°C) was used to and analyze CORT levels using the Rat Corticosterone Coat-a-Count kit (Diagnostic Products Corporation, Los Angeles, California; sensitivity 5 ng/mL; intra-assay coefficient 1%–9%).
Neural Assessment
The day following conditioning, pups were placed in beakers (600 mL), given 5-minute acclimation and 5-minute odor presentation (maternal or learned attachment odors), and decapitated 90 minutes later. The brains were removed, frozen, and stored in a −70°C freezer. Olfactory bulb and amygdala were sectioned (20 µm) and c-Fos immunohistochemistry was performed, as previously described (22). The c-Fos-positive cells were counted bilaterally using a microscope (Olympus Optical Co., Tokyo, Japan; 10× objective) and drawing tube. The c-Fos-positive cells were distinguished from background by density of staining, shape, and size of cells. Cells were counted without knowledge of experimental conditions and averaged over three sections per brain area. Amygdala nuclei were identified using corresponding cresyl violet sections and stereotaxic atlas (34). Olfactory bulb c-Fos cells were counted in the midlateral region bounded by a 30° angle centered between the two bulbs on the horizontal line positioned at half the dorsal-ventral extent of the entire section (35).
Statistical Analysis
Data were analyzed by analysis of variance followed by post hoc Fisher tests or Student t test case with two experimental groups. Differences were considered significant when p < .05.
Results
Experiment 1
Natural and Learned Attachment Odors (Typical and Abusive) Support Social Behaviors and Activate a Converging Neural Circuit
Here, we explore infant responses to natural maternal odor compared with two learned attachment odors produced through odor-stroke and odor-.5 mA shock conditioning during the sensitive period (PN8).
Pups’ Approach to Odor
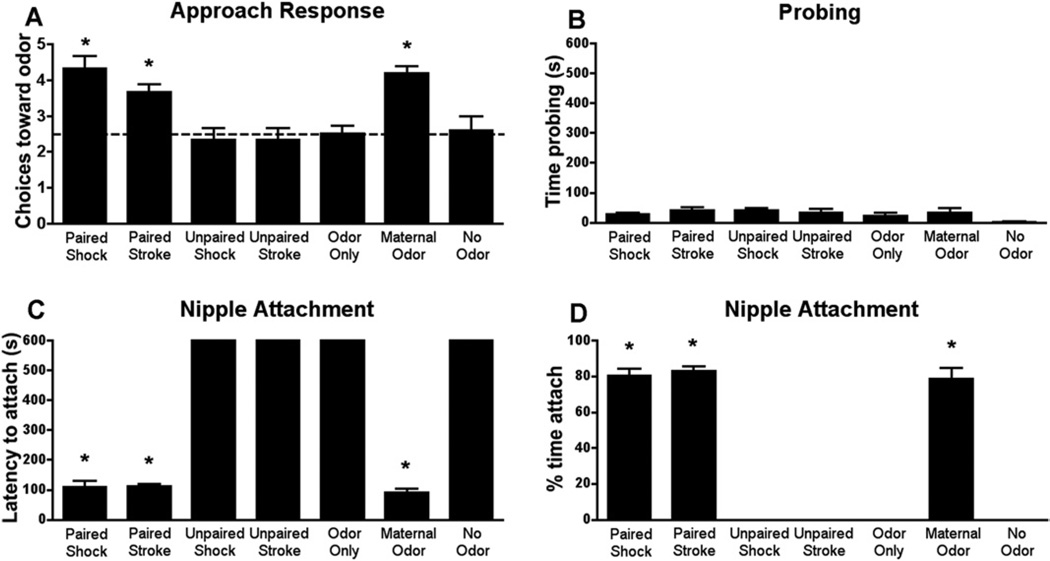
Our results show that pups approach both learned and natural maternal odors [Figure 1A; F(6,33) = 8.999, p < .0001; replicates: 8–11, 13–16, 19, 21]. Control odor (unpaired, odor-only groups) did not elicit approach.
Figure 1.
During the sensitive period (PN8): (A) pups approach (Y-maze) the natural maternal odor or learned attachment odor produced by pairing a novel odor with either stroking or .5 mA shock. (B–D) The natural maternal odor, as well as the odor previously paired with either shock or stroking supported interactions with the mother. Specifically, if the natural maternal odor is removed, pups show little interactions with the mother (B), such as the age-specific behavior of probing (pushing nose into mother’s fur) and nipple attachment (C–D). However, an airstream of either maternal odor or the odor previously paired with stroke or shock (B) enhances interactions with the mother and also (C–D) reinstates nipple attachment. Unpaired and odor-only presentations do not support approach response, mother interactions, or nipple attachment, indicating the importance of learning in producing the maternal odor. *p < .05 between groups (n = 5–6 for all groups). PN, postnatal day.
Pups’ Interactions with the Mother
Removal of maternal odor from the mother’s ventrum disrupts pups’ nipple attachment (Figures 1B–D) (30,31) but is reinstated if natural or learned maternal odor is blown into the chamber [time probing F(6,41) = 1.500, p = .207; latency to attach F(6,41) = 635.7, p < .0001; time attached F(6,41) = 228.9, p < .0001]. Thus, learned attachment odors, including those associated with pain, support social behaviors that occur naturally in response to maternal odor. Control odor (unpaired, odor-only groups) did not elicit approach.
Converging Neural Circuit Activity
We used c-Fos immunohistochemistry to measure learning-associated changes in the olfactory bulb (area of plasticity during sensitive period learning) and amygdala (medial amygdala is important for adult social behavior) of rat pups that naturally learned the maternal odor and experimentally learned the attachment odors produced with odor-.5 mA shock and odor-stroke conditioning during the sensitive period.
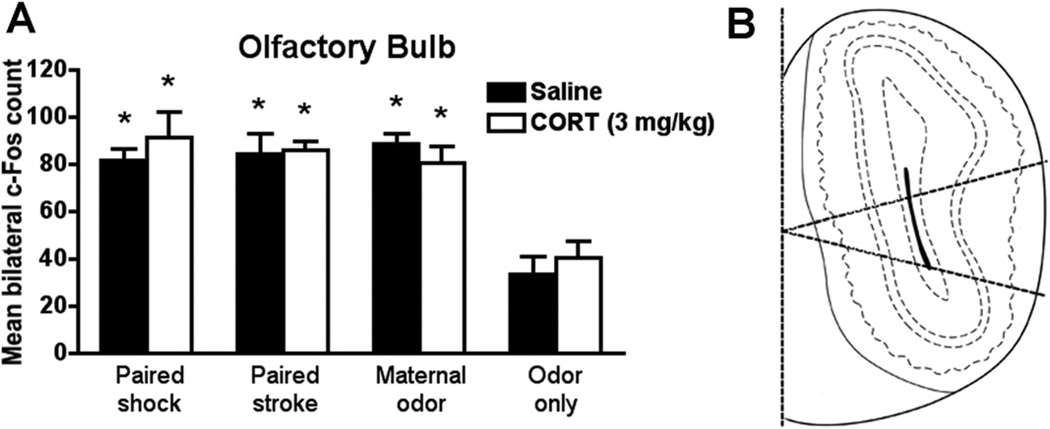
Olfactory Bulb
Our olfactory bulb neural activity results (Figure 2; saline groups) show that presentation of natural maternal odor or odors paired with either shock or stroke increased olfactory bulb neural activity.
Figure 2.
During sensitive period (PN8), (A) natural maternal odor and odors previously paired with shockor stroke increased c-Fos expression in the glomerular layer of the olfactory bulb. Systemic CORT injection (3.0 mg/kg) 30 minutes before the odor presentation did not change c-Fos expression. Bars represent the number (mean ± SEM) of c-Fos-positive cells counted bilaterally in the glomerular layer of the olfactory bulb. *p < .05 between groups (n= 5–6 for all groups). (B) Schematic representation of the division (midlateral) of the olfactory bulb analyzed. CORT, corticosterone; PN, postnatal day.
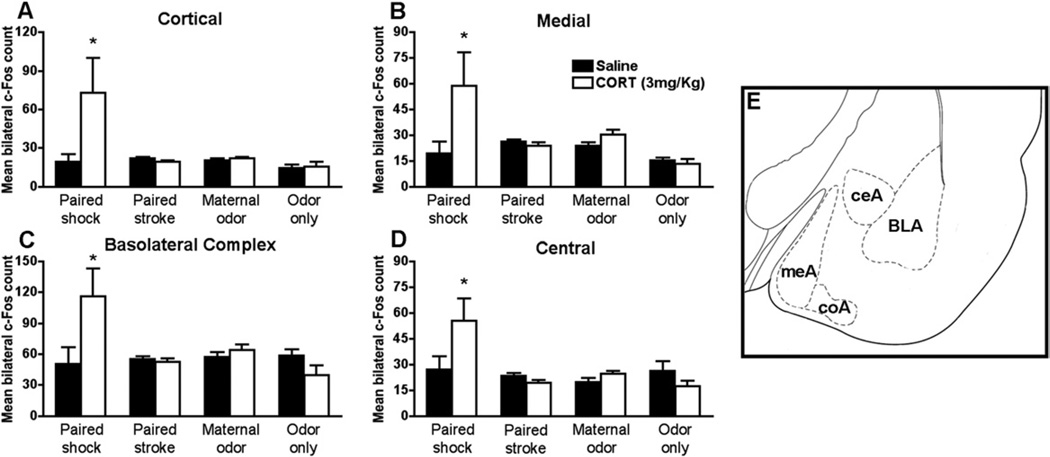
Amygdala
Our amygdala neural activity results (Figure 3; saline groups) show that presentation of maternal odor or odors paired with either shock or stroke during sensitive period do not increase neural activity in any of the nuclei analyzed.
Figure 3.
During sensitive period (PN8), systemic CORT injection (3.0 mg/kg) 30 minutes before the odor presentation increased significantly c-Fos only in the paired odor-shock group. Bars represent the number (mean ± SEM) of c-Fos-positive cells counted bilaterally in each amygdala nuclei: (A) cortical, (B) medial, (C) basolateral complex, (D) central. *p< .05 between groups (n= 4–7 for all groups). (E) Schematic representation of amygdala nuclei analyzed. BLA, basolateral complex; ceA, central; coA, cortical; CORT, corticosterone; meA, medial; PN, postnatal day.
Experiment 2
CORT-Induced Divergence in the Neurobiology of Typical-Attachment versus Abusive-Attachment Odors
Clinically, stress appears to exacerbate differences in typical and abusive attachment (25–28). Here, we questioned whether CORT could produce a divergence in typical- and abusive-attachment pups. We repeated Experiment 1 but pups’ CORT levels were increased (3.0 mg/kg, intraperitoneal) to uncover potential differences in attachment social behaviors and supporting neural circuitry.
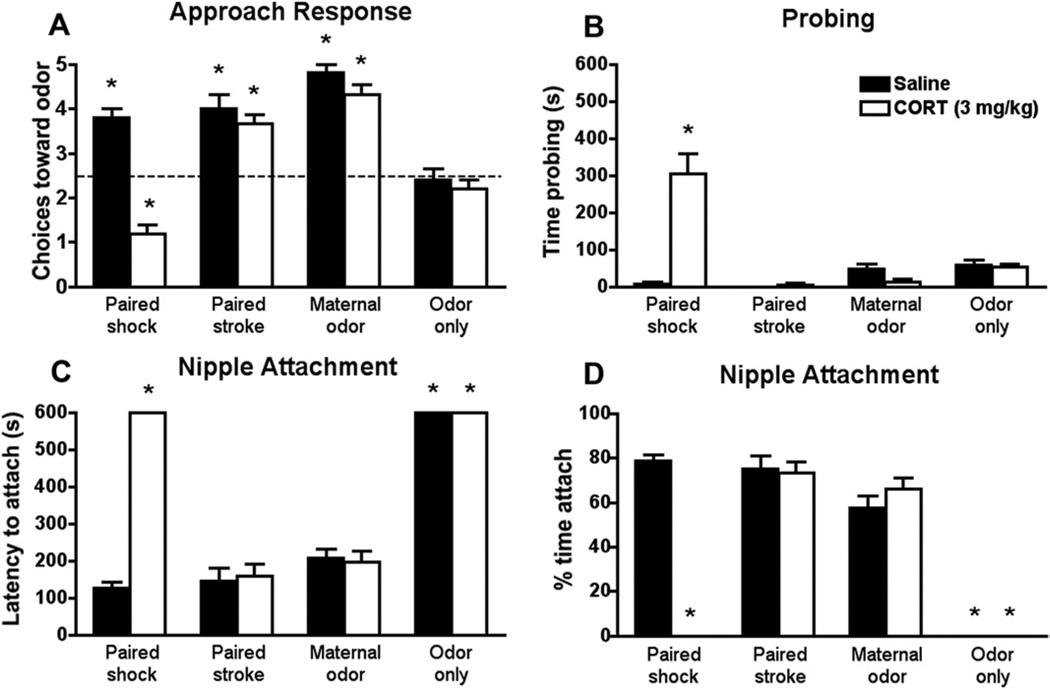
CORT Injection Produced Divergence of Social Attachment Behaviors
Our results show that a systemic CORT injection uncovers differences in the social behavior associated with typical and abusive attachment. Specifically, only paired odor-.5 mA shock pups receiving CORT injection 30 minutes before testing switched normal odor approach to avoidance [interaction between condition and drug F(3,36) = 13.027, p < .0001; Figure 4A]. These pups also showed significantly altered interactions with the mother (Figure 4B–D), spending more time nipple searching [interaction between condition and drug for probing, F(3,32) = 25.556, p < .0001] and less time nursing [interaction between condition and drug for latency to attach F(3,32) = 57.228, p < .0001; and time attached F(3,32) = 55.101, p < .0001]. Corticosterone injections did not disrupt behaviors of pups receiving natural maternal odor, paired odor-stroke, or control pups. For clarity, test performance of unpaired control pups is not presented in the graph, although these pups did not significantly differ from control pups (Y-maze: unpaired saline 2.67 ± .33 and CORT 2.33 ± .33).
Figure 4.
During the sensitive period (PN8), a systemic CORT injection (3.0 mg/kg) 30 minutes before Y-maze test (A) switches the normal odor approach (preference) to odor avoidance only in paired odor-shock pups. CORT injection also increased the time spent probing (B), increased the latency to nipple attach (C), and decreased the time spent nipple attached (D) in paired odor-shock pups. CORT injections had no effects on paired odor-stroke, maternal odor, and odor-only groups. *p < .05 between groups (n= 5 for all groups). CORT, corticosterone; PN, postnatal day.
Neural Circuitry Activity Under CORT During Testing
Olfactory Bulb
Systemic CORT injection does not change the pattern of olfactory bulb neural activity (Figure 2A). Both paired odor-shock and paired odor-stroke, as well as natural maternal odor, increases olfactory bulb neural activity after odor presentation compared with odor-only, with or without CORT injection [interaction between condition and drug treatment F(3,37) = .532, p = .663; condition F(3,37) = 22.500, p < .0001]. Olfactory bulb c-Fos of unpaired control pups is not presented in the graph, although these pups did not significantly differ from control pups (unpaired saline 26.80 ± 1.16 and CORT 23.68 ± 3.27).
Amygdala
Corticosterone injection significantly increases neural activity in the basolateral complex, medial, cortical, and central nuclei (Figure 3) only in abuse-attached pups (paired odor-shock), with no effects on typical-attachment pups (paired odor-stroke, natural maternal odor) or control pups [interaction between condition and drug treatment for cortical F(3,38) = 6.567, p < .001; medial F(3,38) = 5.493, p < .003; basolateral complex F(3,38) = 5.990, p < .002; central F(3,38) = 4.755, p < .006]. Amygdala c-Fos of unpaired control pups is not presented in the graph, although these pups did not significantly differ from control pups (cortical: unpaired saline 16.13 ± 4.30 and CORT 23.73 ± 10.72; medial: unpaired saline 16.98 ± 4.61 and CORT 24.35 ± 9.93; basolateral complex: unpaired saline 62.92 ± 7.76 and CORT 57.84 ± 19.94; central: unpaired saline 23.85 ± 4.77 and CORT 25.97 ± 9.41).
Experiment 3
Ecological Relevance of Abusive Attachment and CORT
Here, we used a more naturalistic approach to assess both the significance of CORT increase and abuse directly from the mother, rather then shock, to verify the ecological relevance of our paradigm. Specifically, pups were reared by either a stressed-induced abusive mother handling pups roughly or reared by a normal mother. This stress-mother procedure involved providing the mother with insufficient bedding for nest building from PN3 to PN8, which results in the mothers engaging in frequent and repeated nest building and pup transportation to the new nest site (Table 1; 32,33,36). The attachment social behavior tests used in experiments 1 and 2 were used also used in this experiment.
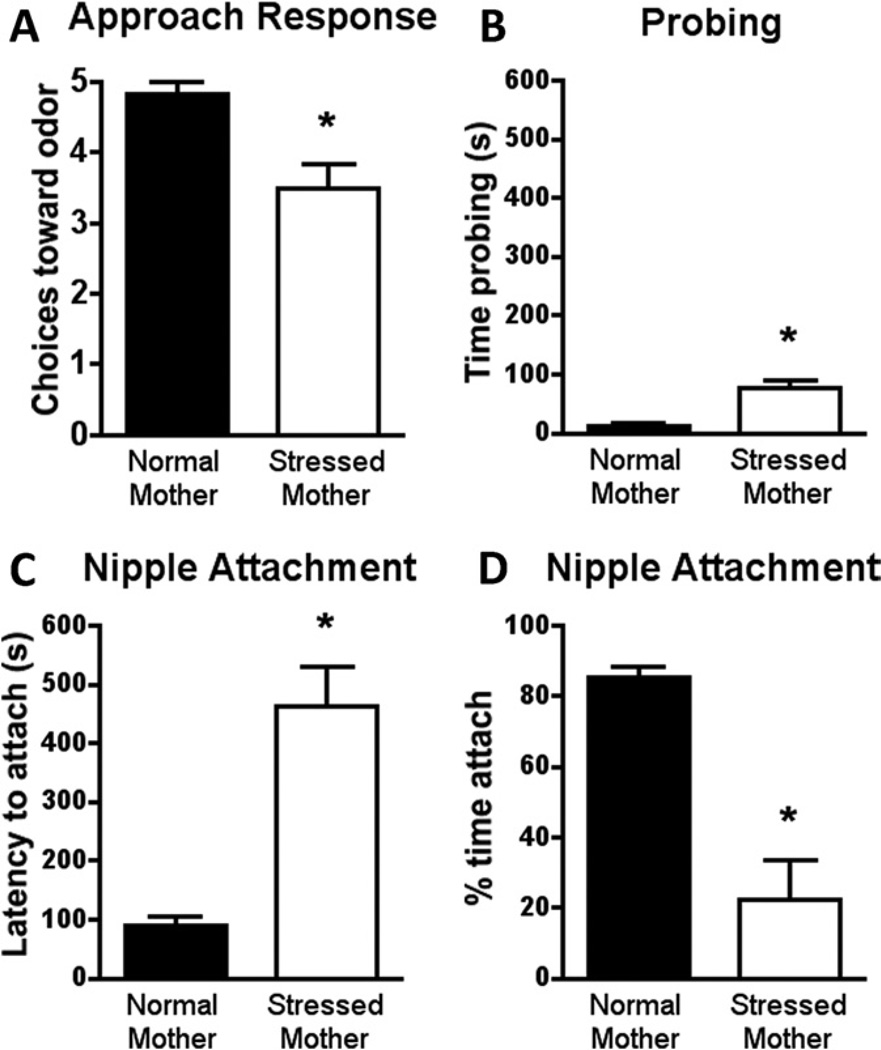
The naturalistic maternal stress procedure does not alter body weight [t(14) = .173, p = .865] but increases CORT levels [t (11) = 2.216, p < .05] through pup adrenal CORT and mother’s milk (Table 1; 32,33,36,37). Moreover, social attachment behaviors using this naturalistic stress paradigm parallel those found with CORT injection in odor-shock attachment learning. Specifically, compared with normal rearing, PN8 pups reared with the insufficient bedding exhibited reduced maternal odor preference [t(10) = 3.508, p < .006; Figure 5A], as well as significantly decreased time nursing [t(10) = 5.409, p < .0003; Figure 5D], and increased latency to nipple attach [t(10) = 5.354, p < .0003; Figure 5C] and time spent probing the nipple (t(10) = 4.954, p < .0006; Figure 5B).
Figure 5.
Stress-mother reared pups (from PN3 to PN8) present a decreased approach to maternal odor in the Y-maze test (A), increased the time probing the mother’s ventrum during nipple searching (B), increased the latency to nipple attach (C), and decreased the time nipple attached (D) in the mother-pup interaction test. *p < .05 between groups (n= 6 for all groups). PN, postnatal day.
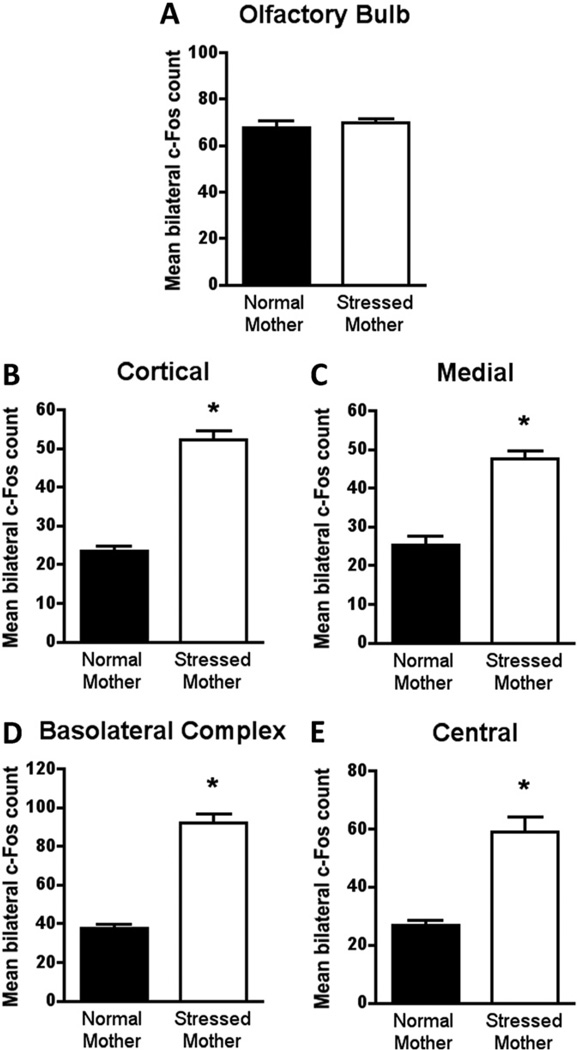
The neural activity of stress-mother reared pups mirrors abuse-attached (paired odor-shock) pups with CORT injection. Specifically, olfactory bulb neural activity (Figure 6A) of stress-mother reared and normal-mother reared pups did not differ in response to maternal odor presentation [t(8) = .505, p = .626]. However, amygdala activity did differ with stress-mother reared pups, showing a significant increase [cortical t(8) = 10.78, p < .0001; medial t(8) = 7.168, p < .0001; basolateral complex t(8) = 9.826, p < .0001; central t(8) = 5.843, p < .0004; Figure 6B–E).
Figure 6.
Stress-mother reared pups (from PN3 to PN8) do not change the expression of c-Fos in the olfactory bulb (A) after maternal odor presentation. c-Fos expression is significantly increased in amygdala nuclei analyzed in stress-mother reared pups. Bars represent the number (mean ± SEM) of c-Fos-positive cells counted bilaterally in each olfactory bulb and amygdala nuclei: (B) cortical, (C) medial, (D) basolateral complex, (E) central. *p < .05 between groups (n= 5 for all groups). PN, postnatal day.
Discussion
Our behavioral and neural results suggest that abusive and typical attachments produce similar social behaviors in infancy, including attachment odor learning producing an odor that takes on the characteristics of the natural maternal odor. However, under stress (increased CORT), differences emerge. Specifically, natural maternal odor learning and odor-stroke attachment odor learning with CORT injection continue to show normal social behavior with the mother and normal activation of the attachment circuit within the brain. However, stress-mother reared pups and odor-shock attachment odor learning with CORT injection show disrupted social behavior and recruitment of the amygdala. These results suggest that the typical and abusive attachments, while appearing to have similar supporting neural circuits, have divergent neural circuits that can be uncovered by CORT by recruitment of the amygdala. Adult animals may not need stress/CORT to uncover the effects of early-life abuse because infant paired odor-shock rats show adult deficits in the forced swim test (Y. Sevelinges, unpublished data) and decreased fear learning (38), while infant unpaired animals show increased adult anxiety-like behaviors (39).
Convergence in Social Attachment Behavior of Abusive and Typical Attachment
The present results suggest that abuse- and typical-attachment odor learning take on characteristics of the natural maternal odor and control social attachment behavior in infancy. The maternal odor controls pup behavior by enabling pups to maintain contact with the mother through the odor preference inducing proximity-seeking behavior. Removal of the maternal odor causes decreased interaction between pup and mother, greatly attenuated nipple attachment, resulting in little chance of survival (8,12,30,31,40). Normal pup social behavior and nipple attachment can be reinstated by simply infusing maternal odor into the air (presence on nipples not required), indicating that maternal odor organizes pups’ motor responses for nipple attachment, which are then guided by somatosensory stimuli from the mother’s ventrum (41).
Importantly, odor learning is critical for infant rats because the natural maternal odor is learned at birth through tactile stimulation and relearned repeatedly throughout the postnatal period (8,9). The maternal odor can also be learned through classical conditioning, as was used in the present studies. Here, the approach responses and mother-pup interactions are similarly controlled by learned attachment odors (odor paired with stroke or shock) at a level similar to the natural maternal odor. These results replicate work showing the ability of stroke to support learned odor approaches (42) and nipple attachment (9); nevertheless, these results indicate that painful stimuli such as shock also support learned odor approaches (10,11,16) and nipple attachment (present data). Our results suggest the learned attachment odors, produced with either shock or stroking, take on characteristics of natural maternal odor and control pups’ interactions with the mother.
Convergence in Neural Circuitry of Abusive and Typical Attachment
Assessment of pup neural activity found similarly increased levels of neural activity (c-Fos expression) in the olfactory bulb for all experimental groups. Specifically, the attachment odor, whether naturally learned (maternal odor) or learned with stroking or shock pairings, produced enhanced olfactory bulb response. This replicates previous data that showed learning-induced anatomical and physiological changes within the olfactory bulb (12,17,22,29,35,43).
Alternatively, amygdala neural activity results show that natural maternal odor or odors paired with either shock or stroke during the sensitive period do not increase neural activity in the amygdala, which is critical for pups’ normal interaction with the mother and odor approach learning (17–19,21,22). In fact, the inability of odor-.5 mA shock conditioning to support odor avoidance in young pups is a result of the amygdala’s failure to become incorporated into the early learning circuit and exhibit learning-induced plasticity (17,19,21). However, only a few days later, as the sensitive period terminates, the same procedure is able to induce neural activity within the amygdala that supports an odor avoidance response (17–19,21). In adult animals, the amygdala is regarded as a key brain area controlling social behavior, including emotional evaluation of the social situation (44–46). Indeed, the engagement of the amygdala in threatening social situations has been repeatedly documented in the literature (46–48). However, engagement of the amygdala in infant social behavior has been shown to disrupt nonhuman primates’ interactions with the mother (49–51).
CORT Uncovers Differences in Pup Social Attachment Behavior Based on Attachment Quality
By injecting CORT in rat pups, we were able to uncover detrimental effects of early life abuse in the expression of social attachment behavior, while typical attached pups were unaffected. Stressful situations appear to highlight differences in typical and abusive attachment in children. Clinical research has shown that life trauma/stress are associated with compromised social behavior and mental health during childhood (1,2,52–54), which is exacerbated by stress (25–28). This suggests that the context of trauma is also important, as trauma experienced during attachment can put the child at particular risk for psycho-pathologies (55).
Early life stressful experiences have powerful effects on overall brain development—effects that can last throughout the life span and influence behavior, brain function, and risk for a host of mental disorders (32,56–58). The precocious stress response resulting in increased CORT levels is significant because it indicates that early life stress is capable of prematurely terminating the stress hyporesponsive period, which is characterized by greatly attenuated hypothalamus-pituitary-adrenal axis functioning and blunted CORT release in response to stressful stimuli (i.e., shock) that would normally elicit CORT elevations in adults (59–61). The reduced stress reactivity experienced by neonates is hypothesized to protect the developing organism from the negative effects of stress hormones (62–64).
CORT Uncovers Differences in Pup Neural Circuitry Supporting Social Attachment Behavior Based on Attachment Quality
Presentation of attachment odor, learned naturally or experimentally, enhances olfactory bulb neural activity, which does not change with CORT injection. This suggests that, despite the quality of the attachment (typical or abusive), pups detect and process attachment odors even when stressed.
On the other hand, presentation of the attachment learned odor engaged the amygdala only in abuse-attached (odor-.5 mA shock) pups that received CORT. This is in sharp contrast to our amygdala results in typical-attachment pups that received CORT, where the amygdala did not appear to participate in pups’ attachment social behaviors. These results suggest that the amygdala is not associated with infant social attachment behavior but becomes engaged under stressful situations that increase CORT levels to disrupt social behavior in abusive attachment. These results parallel those seen in nonhuman primates (49–51). Moreover, effects of early life stressful experiences appear to be mediated primarily by changes occurring in brain areas such as the amygdala, hippocampus, and prefrontal cortex (38,56,57,65). However, the mechanisms by which early life experiences alter neural circuits that may mediate vulnerability to mood disorders and emotional regulation are not fully understood.
The role of CORT in disrupting social attachment behavior and its supporting neural circuitry may not be direct. While CORT has been strongly implicated in mediating the effects of early life trauma, corticotropin-releasing hormone (CRH) has also been implicated. Corticosterone increase induces an enhancement in the CRH messenger RNA expression into the amygdala (66,67). However, it has recently become abundantly clear that there are multiple pathways to pathology in early life, with CORT, CRH, serotonin, and dopamine being implicated (68–71).
Naturalistic Abusive Attachment
Rearing pups with a stress-mother increased CORT levels (present results, 32,33,36) and permitted the replication of both the disrupted behavior and amygdala activity found with CORT and odor-.5 mA shock. Specifically, stress-mother reared pups exhibited reduced maternal odor preference, as well as dysfunctional mother-pup interactions such as decreased time nursing, increased latency to nipple attach, and more time spent probing for the nipple. Our c-Fos data showed that the olfactory bulb response to attachment odors did not differ between stress-mother and normal-mother reared pups, although statistical differences were found in the amygdala. These stress-mother pups’ results mirror those found in CORT odor-shock pups, including the cortical, medial, basolateral complex, and central amygdala nuclei. These results suggest that engagement of the amygdala in infancy may disrupt interactions with the mother (49–51).
The Importance of Animal Models for Understanding Human Attachment
Bowlby (52) suggested that infants’ attachment to the caregiver is characterized by infant proximity seeking to the caregiver, despite abusive and rough treatment by an abusive caregiver. Striking similarities exist between human attachment behavior described by Bowlby (52) and other species, including nonhuman primates and rodents. This is not surprising because Bowlby’s (52) original attachment theory was strongly based on a paradigm-shifting integration of clinical observations and basic research using animal models. Indeed, tolerating caregiver abuse has widespread phylogenetic representation: in chicks tolerating shock during imprinting (72) and infant dogs tolerating pain from a handler (73,74), as well as nonhuman primates (58,75,76) and human children remaining attached to an abusive parent (77). A potential evolutionary explanation suggests selection pressures supported infants that remained attached because it increased the probability of survival. From an adaptive point of view, perhaps it is better for an altricial animal to remain attached to an abusive caregiver than receive no care.
Bowlby (52) postulated a biological attachment circuit in children, although this circuit has not been identified. However, due to the strong phylogenetic representation of attachment in humans and myriad species, animal models may be particularly useful in understanding the neurobiology of normal and abuse attachment (58,70,76–79).
Implications
In altricial animals, including humans, the infant’s primary environment and social world revolves around the caregiver. This social world can vary in quality, as determined by infant-caregiver interactions such as typical and abusive attachment, and programs infants’ emotional and cognitive development to adapt to later life environments (3–5,76). Compared with typical attachment, infants in abusive attachment have a greater probability of experiencing later life mental dysfunction and physical health problems (3–5,76). Our results suggest that abusive attachment may have unique underlying neural processes that contribute to this pathway to pathology. We suggest that our infant rat model may provide insight to unique characteristics of attachment learning in humans where attachments are formed under varied conditions of care. Knowing the neurobiological origins of psychopathologies will facilitate the development of treatments/ interventions.
Acknowledgments
This work was funded by National Institutes of Health DC009910, National Science Foundation IOB-0850527, Leon Levy Foundation, and Hope for Depression Foundation to RMS and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Brazil) to CR.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Carlson V, Cicchetti D, Barnett D, Braunwald K. Finding order in disorganization: Lessons from research on maltreated infant’s attachment to their caregivers. In: Cicchetti D, Carlson V, editors. Child Maltreatment: Theory and Research on the Causes and Consequences of Child Abuse and Neglect. New York: Cambrige University Press; 1989. pp. 494–528. [Google Scholar]
- 2.Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiatr Clin N Am. 2003;12:271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 3.Gunnar MR. Integrating neuroscience and psychological approaches in the study of early experiences. Ann N Y Acad Sci. 2003;1008:238–247. doi: 10.1196/annals.1301.024. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 5.Teicher MN, Andersen SL, Polcari A, Andersen CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: Clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 7.Leon M. Maternal pheromone. Physiol Behav. 1974;13:441–453. doi: 10.1016/0031-9384(74)90098-5. [DOI] [PubMed] [Google Scholar]
- 8.Leon M. Dietary control maternal pheromone in the lactating rat. Physiol Behav. 1975;14:311–319. doi: 10.1016/0031-9384(75)90039-6. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. J Exp Psychol Anim Behav Process. 1982;8:329–341. [PubMed] [Google Scholar]
- 10.Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Res Dev Brain Res. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- 13.Johanson IB, Teicher MH. Classical conditioning of an odor preference in 3-day-old rats. Behav Neural Biol. 1980;29:132–136. doi: 10.1016/s0163-1047(80)92596-0. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan RM, Taborsky-Barba S, Mendoza R, Itano A, Leon M, Cotman CW, et al. Olfactory classical conditioning in neonates. Pediatrics. 1991;87:511–518. [PMC free article] [PubMed] [Google Scholar]
- 15.Blozovski D, Cudennec A. Passive avoidance learning in the young rat. Dev Psychobiol. 1980;13:513–518. doi: 10.1002/dev.420130510. [DOI] [PubMed] [Google Scholar]
- 16.Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- 17.Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: Similar behaviors but divergent ages of functional amygdala emergence. Learn Mem. 2009;16:114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth T, Sullivan RM. Endogenous opioids and their role in odor preference acquisition and consolidation following odor-shock conditioning in infant rats. Dev Psychobiol. 2001;39:188–198. doi: 10.1002/dev.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth T, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Barr GA. Ontogeny of nociception and antinociception. NIDA Res Monogr. 1995;158:172–201. [PubMed] [Google Scholar]
- 24.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 25.Crittenden PM. Children’s strategies for coping with adverse home environments: An interpretation using attachment theory. Child Abuse Negl. 1992;16:329–343. doi: 10.1016/0145-2134(92)90043-q. [DOI] [PubMed] [Google Scholar]
- 26.Gunnar MR, Broderson L, Nachmias M, Buss K, Rigatuso J. Stress and attachment security. Dev Psychobiol. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald HZ, Beeghly M, Grant-Knight W, Augustyn M, Woods RW, Cabral H, et al. Longitudinal association between infant disorganized attachment and childhood posttraumatic stress symptoms. Dev Psychopathol. 2008;20:493–508. doi: 10.1017/S0954579408000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spangler G, Grossmann KE. Biobehavioral organization in securely and insecurely infants. Child Dev. 1992;64:1439–14550. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan RM, Wilson DA. The role of norepinephrine in the expression of learned olfactory neurobehavioral responses in infant rats. Psy-chobiology. 1991;19:308–312. doi: 10.3758/bf03332084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofer MA, Shair H, Singh P. Evidence that maternal ventral skin substances promote suckling in infant rats. Physiol Behav. 1976;17:131–136. doi: 10.1016/0031-9384(76)90279-1. [DOI] [PubMed] [Google Scholar]
- 31.Teicher MH, Blass EM. First suckling response of newborn albino rat: The role of olfaction and amniotic fluid. Science. 1977;198:635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- 32.Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paxinos G, Tork I, Tecott LH, Valentino KL. Atlas of the Developing Rat Brain. San Diego: Academic Press; 1991. [Google Scholar]
- 35.Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Res. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- 36.Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early life stress disrupts attachment learning: The role of amygdala corticosterone, locus coeruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci. 2009;29:15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh KY. Corticosterone concentrations in the serum and milk of lactating rats: Parallel changes after induced stress. Endocrinology. 1984;115:1364–1370. doi: 10.1210/endo-115-4-1364. [DOI] [PubMed] [Google Scholar]
- 38.Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, et al. Enduring effects of infant memories: Infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol Psychiatry. 2007;62:1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Tyler K, Moriceau S, Sullivan RM, Greenwood-Van Meerveld B. Long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs predictable shock. Neurogastroenterol Motil. 2007;19:761–768. doi: 10.1111/j.1365-2982.2007.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leon M. Neuroethology of olfactory preference development. J Neurobiol. 1992;23:1557–1573. doi: 10.1002/neu.480231012. [DOI] [PubMed] [Google Scholar]
- 41.Polan HJ, Hofer MA. Maternally directed orienting behaviors of newborn rats. Dev Psychobiol. 1999;34:269–279. doi: 10.1002/(sici)1098-2302(199905)34:2<269::aid-dev3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 42.Yuan Q, Harley CW, Darby-King A, Neve RL, McLean JH. Early odor preference learning in the rat: Bidirectional effects on cAMP response element-binding protein (CREB) and mutant CREB support a causal role for phosphorylated CREB. J Neurosci. 2003;23:4760–4765. doi: 10.1523/JNEUROSCI.23-11-04760.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson DA, Sullivan RM, Leon M. Single-unit analysis of postnatal olfactory learning: Modified olfactory bulb output response patterns to learned attractive odors. J Neurosci. 1987;7:3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baron-Cohen S. The Essential Difference: Men, Women and the Extreme Male Brain. London: Penguin; 2003. [Google Scholar]
- 45.Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 46.Amaral DG. The primate amygdala and the neurobiology of social behavior: Implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 47.Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- 48.Phelps E, LeDoux J. Contributions of the amygdala to emotions processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 49.Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: Implications from non-human primate studies. Genes Brain Behav. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 50.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behaviors following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 52.Bowlby J. Attachment. New York: Basic Books; 1965. [Google Scholar]
- 53.Helfer ME, Kempe RS, Krugman RD. The Battered Child. Chicago: University of Chicago Press; 1997. [Google Scholar]
- 54.Johnson EO, Kamilaris TC, Calogero AE, Gold PW, Chrousos GP. Effects of early parenting on growth and development in a small primate. Pediatr Res. 1996;39:999–1005. doi: 10.1203/00006450-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Briere J. The long-term clinical correlates of childhood sexual victimization. Ann N Y Acad Sci. 1988;528:327–334. doi: 10.1111/j.1749-6632.1988.tb50874.x. [DOI] [PubMed] [Google Scholar]
- 56.Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi SJ. Early life stress a risk factor for mental health: Role of neurotrophins form rodents to non-humans primates. Neurosci Biobehav Rev. 2009;33:573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 58.Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 59.Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. Blockade of alpha 2-adrenoreceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocinology. 1994;9:2549–2557. doi: 10.1210/endo.135.6.7988443. [DOI] [PubMed] [Google Scholar]
- 60.Levine S. Plasma free corticosterone response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- 61.Rosenfeld P, Shchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neurosci Biobehav Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- 62.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hypore-sponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 63.Bohn MC. Glucocorticoid-induced teratologies of the nervous system. In: Yanai J, editor. Neurobehavioral Teratology. New York: Elsevier; 1984. pp. 365–387. [Google Scholar]
- 64.Hu Z, Yuri K, Ichikawa T, Kawata M. Exposure of neonatal rats to glucocorticoids suppresses the development if choline acetyltrans-ferase-immunreactive neurons: Role of adrenal steroids in the development if forebrain cholinergic neurons. J Chem Neuroanat. 1996;10:1–10. doi: 10.1016/0891-0618(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 65.Moriceau S, Raineki C, Holman JD, Holman JG, Sullivan RM. Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Front Behav Neurosci. 2009;3:22. doi: 10.3389/neuro.08.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: An in situ hybridization analysis. Brain Res. 1998;788:305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 67.Korosi A, Baram TZ. The central corticotropin releasing factor system during development and adulthood. Eur J Pharmacol. 2008;583:204–214. doi: 10.1016/j.ejphar.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barr GA, Moriceau S, Shionoya K, Musny K, Gao P, Wang S, et al. Transitions on infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12:1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 70.Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of the stress-regulating brain regions. J Neurosci. 2006;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA. Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res. 2009;1305:47–63. doi: 10.1016/j.brainres.2009.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hess E. Ethology: An approach to the complete analysis of behavior. In: Brown R, Galanter E, Hess E, Mendler G, editors. New Directions in Psychology. New York: Holt, Rinehart and Winston; 1962. pp. 159–199. [Google Scholar]
- 73.Fisher AE. The effects of differential early treatment on the social and exploratory behavior of puppies [doctoral dissertation] University Park, PA: Pennsylvania State University; 1955. [Google Scholar]
- 74.Stanley WE. Differential human handling as reinforcing events and as treatments influencing later social behavior in Basenji puppies. Psychol Rep. 1962;10:775–788. [Google Scholar]
- 75.Harlow H, Harlow M. The affectional system. In: Schrier A, Harlow H, Stollnitz F, editors. Behavior of Non-human Primates. New York: Academic Press; 1965. [Google Scholar]
- 76.Suomi SJ. Early determinants of behavior: Evidence from primate studies. Br Med Bull. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- 77.Rajecki D, Lamb M, Obmascher P. Towards a general theory of infantile attachment: A comparative review of aspects of the social bond. Behav Brain Sci. 1978;1:417–435. [Google Scholar]
- 78.Hanson JL, Davidson RJ, Pollak SD. The correlates of early experience on structural brain development. [Accessed January 27, 2010];Abstr Soc Neurosci. 2009 Available at: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=7847ce50-22e0-4087-a349-327e89721453&cKey=eb600141-db7e-4ee7-b09f-6dc97d58e11c.
- 79.Sánchez MM, Styner M, McCornack K, Graff A, Zhang X, Maestripieri D, et al. Decreased left amygdala volume is related to early life stress in an animal model of poor maternal care. [Accessed January 27, 2010];Abstr Soc Neurosci. 2009 Available at: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=e47fd295-1d68-4615-8637-066c7f17b1dc&cKey=a8ad288c-de2d-48ea-a9da-d1446bba135b.