Abstract
BACKGROUND
Heart failure is the leading cause for hospital readmission, the reduction of which is a priority under the Affordable Care Act. Digoxin reduces 30-day all-cause hospital admission in chronic systolic heart failure. Whether digoxin is effective in reducing readmission after hospitalization for acute decompensation remains unknown.
METHODS
Of the 5153 Medicare beneficiaries hospitalized for acute heart failure and not receiving digoxin, 1054 (20%) received new discharge prescriptions for digoxin. Propensity scores for digoxin use, estimated for each of the 5153 patients, were used to assemble a matched cohort of 1842 (921 pairs) patients (mean age, 76 years; 56% women; 25% African American) receiving and not receiving digoxin, who were balanced on 55 baseline characteristics.
RESULTS
30-day all-cause readmission occurred in 17% and 22% of matched patients receiving and not receiving digoxin, respectively (hazard ratio {HR} for digoxin, 0.77; 95% confidence interval {CI}, 0.63–0.95). This beneficial association was observed only in those with ejection fraction <45% (HR, 0.63; 95% CI, 0.47–0.83), but not in those with ejection fraction ≥45% (HR, 0.91; 95% CI, 0.60–1.37; p for interaction, 0.145), a difference that persisted throughout first 12-month post-discharge (p for interaction, 0.019). HRs (95% CIs) for 12-month heart failure readmission and all-cause mortality were 0.72 (0.61–0.86) and 0.83 (0.70–0.98), respectively.
CONCLUSIONS
In Medicare beneficiaries with systolic heart failure, a discharge prescription of digoxin was associated with lower 30-day all-cause hospital readmission, which was maintained at 12 months, and was not at the expense of higher mortality. Future randomized controlled trials are needed to confirm these findings.
Keywords: Digoxin, heart failure, hospital readmission
Heart failure is the leading cause of hospital admission and readmission for Medicare beneficiaries in the United States.1 Under the 2010 Patient Protection and Affordable Care Act, hospitals are collectively facing billions of dollars in penalties for excessive 30-day all-cause readmissions.2 Since October 1, 2012, heart failure is one of the three conditions along with acute myocardial infarction and pneumonia for which the law is currently being enforced.2–4 Despite limitations of the cost-driven metric of 30-day all-cause hospital readmission,5,6 the fact remains that over a quarter of heart failure patients are readmitted within 30 days of hospital discharge,1 and that there is a need for interventions to improve this outcome. Studies of transition of care strategies in heart failure are based on single center reports, post hoc analyses, and observational studies, and have shown variable and inconsistent associations with 30-day all-cause hospital readmission.7
Heart failure is a clinical syndrome characterized by fluid retention and shortness of breath, exacerbation of which often precede hospitalization.8,9 Digoxin has favorable hemodynamic and neuroendocrine effects in patients with heart failure.10–12 Findings from the Randomized Assessment of Digoxin on Inhibitors of Angiotensin-Converting Enzyme (RADIANCE) trial and the Prospective Randomized Study of Ventricular Failure and the Efficacy of Digoxin (PROVED) trial, the two major randomized controlled trials of digoxin withdrawal in heart failure conducted in the early 1990s demonstrated the beneficial effect of digoxin in reducing heart failure symptoms.13,14 These findings were subsequently confirmed in the randomized controlled Digitalis Investigation Group (DIG) trial that demonstrated that digoxin reduced the risk of hospitalization due to worsening heart failure in ambulatory patients with systolic heart failure during 37 months of average follow-up and in diastolic heart failure during the first 2 years of follow-up.15,16
Findings from post hoc analyses of the main DIG trial demonstrated that digoxin reduced 30-day all-cause hospital admission among ambulatory older patients with systolic heart failure,17 and that the beneficial effect of digoxin on hospital admission in heart failure may be more pronounced in high-risk subsets of patients.18 Based on these observations and that most evidence-based heart failure therapies that reduce hospital admission also reduce readmission,19,20 we hypothesized that discharge prescription of digoxin will be associated with lower 30-day all-cause readmission in older heart failure patients hospitalized for acute decompensation. Therefore, the objective of the current study was to test the hypothesis that digoxin use is associated with lower 30-day all-cause hospital readmission.
MATERIALS AND METHODS
Data Source and Study Patients
The current study is based on the Alabama Heart Failure Project, the details of which have been described previously.21,22 Briefly, 9649 medical records of 8555 unique fee-for-service Medicare beneficiaries discharged with a primary discharge diagnosis of heart failure from 106 Alabama hospitals between 1998 and 2001 were abstracted by trained technicians at the Clinical Data Abstraction Center. For patients with multiple hospitalizations, charts from the first hospitalization were used.23 A diagnosis of heart failure was based on the International Classification of Diseases, 9th Revision, Clinical Modification codes for heart failure.23 Of the 8555 patients, 8049 were discharged alive.
New Use of Digoxin: Assembly of an Inception Cohort
Data on admission and discharge digoxin use were collected by chart abstraction. Because prevalent drug use may cause bias through effects on baseline characteristics and by left censoring,24,25 we excluded 2896 patients who were receiving digoxin at the time of hospital admission. Of the remaining 5153 patients without prior digoxin use, 1054 (20%) received a new discharge prescription for digoxin. Extensive data on other baseline characteristics including demographics, medical history, use of medications, hospital course, and discharge disposition were also collected by chart abstraction.23
Propensity Matching: Assembly of a Balanced Cohort
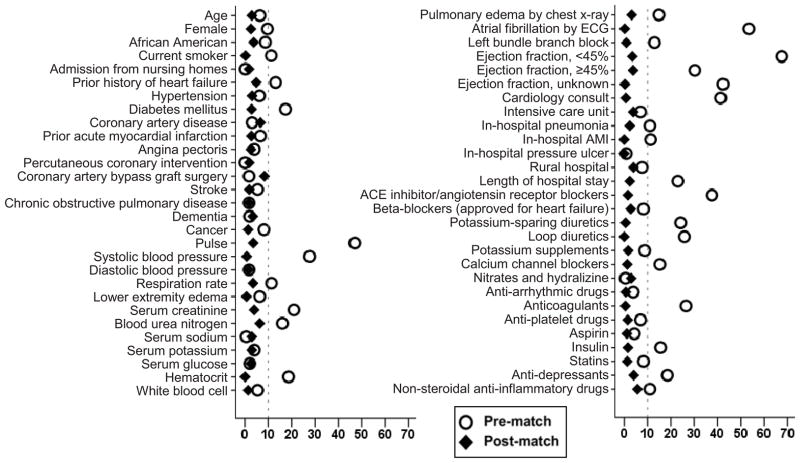
We used propensity score for the receipt of a discharge digoxin prescription to assemble a balanced matched cohort of patients receiving and not receiving digoxin.26,27 Propensity scores for digoxin use were estimated for each of the 5153 patients using a non-parsimonious multivariable logistic regression model in which the digoxin use was the dependent variable and 55 baseline characteristics were used as covariates.28–30 Using a greedy matching protocol described elsewhere,31 we matched 921 (87% of the 1054) patients receiving digoxin with 921 patients not receiving digoxin with similar propensity scores. Post-match balance in baseline characteristics was assessed by estimating absolute standardized differences, the results of which were presented as a Love plot.32 An absolute standardized difference of 0% indicates no residual bias, and differences <10% are considered inconsequential.
Hospitalization and Mortality Data
The primary outcome of the current analysis was hospital readmissions due to all-causes during 30 days after discharge from the index hospitalization. Secondary outcomes included hospital readmissions due to heart failure, all-cause mortality, and composite end point of all-cause mortality or all-cause readmissions during the 30 days post-discharge. We also examined the association of digoxin use with these outcomes at 3, 6 and 12 months after index hospital discharge. Data on all outcomes and time to first occurrence of each outcome were obtained from the Medicare Provider Analysis and Review (MedPAR) File and the Inpatient Standard Analytical File.21–23
Statistical Analysis
Baseline characteristics were compared using Pearson’s Chi-square and Wilcoxon rank-sum tests for pre-match, and McNemar’s test and paired sample t-test for post-match comparisons, as appropriate.21,22 The association of digoxin use with 30-day all-cause readmission was examined using Kaplan-Meier and Cox regression analyses, censoring all patients without an event at 30 days. Similar Cox models were used for secondary outcomes. The proportional hazards assumptions were checked using log-minus-log survival plots and the model assessment method proposed by Lin et al.33 A formal sensitivity analysis was conducted to quantify the degree of a hidden bias that would be required to explain away a significant association of digoxin use with the main outcome among matched patients.34 For 3-, 6-and 12-month outcomes, we used a single Cox model per outcome in which those without the event of interest during the first 12 months were censored. Because the proportional hazards assumption held for these models, the constant hazard ratio (HR) over the entire 12 month time frame is a viable assumption. Thus, we did not fit separate Cox models for events at 3 and 6 months post-discharge. We also examined the association of digoxin with 30-day outcomes in the pre-match cohort using three different approaches: (1) unadjusted; (2) multivariable-adjusted, using all 55 baseline characteristics; and (3) propensity score-adjusted. Finally, the association of digoxin use with 30-day all-cause readmission was examined in various clinically significant subgroups. Considering the concerns regarding the role of digoxin in women,35–37 and in those with preserved ejection fraction we also examined the association of digoxin use with 12-month all-cause readmission by sex and ejection fraction. All statistical tests were two-tailed with a p-value <0.05 considered significant. SPSS for Windows version 21 (IBM Corp., Armonk, NY) and SAS for Window version 9.2 (Cary, NC) were used for data analyses.
RESULTS
Baseline Characteristics
Matched patients had a mean age (±SD) of 76 (±11) years, 56% were women, and 25% were African American. Those receiving digoxin were more likely to be white men, have low ejection fraction and pulmonary edema, and receive diuretics and angiotensin-converting enzyme inhibitors on discharge (Table 1 and Figure 1). These and other imbalances in baseline characteristics were attenuated to inconsequential levels after matching.
Table 1.
Baseline characteristics of Medicare beneficiaries hospitalized for heart failure and not receiving prior digoxin therapy, by the receipt of a new discharge prescription for digoxin, before and after propensity score matching
| n (%) or mean (±SD) | Pre-match (N=5153)
|
P value | Post-match (N=1842)
|
P value | ||
|---|---|---|---|---|---|---|
| Use of digoxin
|
Use of digoxin
|
|||||
| No (n=4099) | Yes (n=1054) | No (n=921) | Yes (n=921) | |||
| Age (years) | 75 (±11) | 76 (±11) | 0.06 | 75 (±11) | 76 (±11) | 0.53 |
| Female | 2444 (60) | 578 (55) | 0.005 | 505 (55) | 517 (56) | 0.61 |
| African American | 1117 (27) | 247 (23) | 0.012 | 234 (25) | 219 (24) | 0.44 |
| Current smoker | 465 (11) | 160 (15) | 0.001 | 134 (15) | 135 (15) | 1.00 |
| Nursing home residents | 282 (7) | 72 (7) | 0.96 | 58 (6) | 62 (7) | 0.77 |
| Left ventricular ejection fraction (%) | ||||||
| <45% | 1167 (29) | 635 (60) | 533 (58) | 517 (56) | ||
| ≥45% | 1530 (37) | 248 (24) | <0.001 | 221 (24) | 236 (26) | 0.73 |
| Unknown | 1402 (34) | 171 (16) | 167 (18) | 168 (18) | ||
| Past medical history | ||||||
| History of heart failure | 2708 (66) | 629 (60) | <0.001 | 582 (63) | 560 (61) | 0.31 |
| Hypertension | 2956 (72) | 730 (69) | 0.07 | 631 (69) | 644 (70) | 0.53 |
| Diabetes mellitus | 1838 (45) | 383 (36) | <0.001 | 352 (38) | 339 (37) | 0.57 |
| Coronary artery disease | 2113 (52) | 527 (50) | 0.37 | 495 (54) | 464 (50) | 0.16 |
| Myocardial infarction | 880 (22) | 256 (24) | 0.049 | 231 (25) | 220 (24) | 0.59 |
| Percutaneous coronary intervention | 577 (14) | 148 (14) | 0.98 | 136 (15) | 130 (14) | 0.74 |
| Coronary artery bypass graft | 881 (22) | 219 (21) | 0.61 | 221 (24) | 189 (21) | 0.09 |
| Left bundle branch block | 437 (11) | 158 (15) | <0.001 | 136 (15) | 139 (15) | 0.90 |
| Atrial fibrillation | 649 (16) | 409 (39) | <0.001 | 306 (33) | 307 (33) | 1.00 |
| Stroke | 864 (21) | 199 (19) | 0.12 | 179 (19) | 172 (19) | 0.73 |
| Chronic obstructive pulmonary disease | 1393 (34) | 367 (35) | 0.61 | 307 (33) | 315 (34) | 0.73 |
| Dementia | 365 (9) | 100 (10) | 0.56 | 78 (9) | 87 (9) | 0.53 |
| Cancer | 73 (2) | 32 (3) | 0.010 | 25 (3) | 23 (3) | 0.88 |
| Clinical findings | ||||||
| Pulse (beats per minute) | 88 (±21) | 99 (±25) | <0.001 | 96 (±23) | 97 (±24) | 0.41 |
| Systolic blood pressure (mmHg) | 154 (±34) | 145 (±29) | <0.001 | 146 (±31) | 146 (±30) | 0.87 |
| Diastolic blood pressure (mmHg) | 81 (±20) | 81 (±19) | 0.61 | 81 (±19) | 81 (±19) | 0.74 |
| Respiration, breaths per minute | 24 (±6) | 24 (±7) | 0.001 | 25 (±7) | 24 (±7) | 0.47 |
| Lower extremity edema | 2910 (71) | 717 (68) | 0.06 | 624 (68) | 627 (68) | 0.92 |
| Pulmonary edema by chest x-ray | 2719 (66) | 771 (73) | <0.001 | 683 (74) | 670 (73) | 0.52 |
| Laboratory values | ||||||
| Serum sodium (mEq/L) | 139 (±5) | 139 (±5) | 0.87 | 138 (±5) | 139 (±5) | 0.53 |
| Serum potassium (mEq/L) | 4.2 (±0.7) | 4.2 (±0.6) | 0.24 | 4.2 (±0.6) | 4.2 (±0.6) | 0.53 |
| Serum creatinine (mEq/L) | 1.7 (±1.6) | 1.4 (±1.1) | <0.001 | 1.5 (±1.0) | 1.5 (±1.1) | 0.40 |
| Hematocrit (%) | 36 (±6) | 37 (±6) | <0.001 | 37 (±6) | 37 (±6) | 0.99 |
| In-hospital events | ||||||
| Pneumonia | 1027 (25) | 316 (30) | 0.001 | 285 (31) | 275 (30) | 0.64 |
| Acute myocardial infarction | 156 (4) | 66 (6) | <0.001 | 56 (6) | 56 (6) | 1.00 |
| Pressure ulcer | 344 (8) | 91 (9) | 0.80 | 75 (8) | 75 (8) | 1.00 |
| Hospital and care characteristics | ||||||
| Rural hospital | 1295 (32) | 296 (28) | 0.028 | 283 (31) | 266 (29) | 0.42 |
| Cardiology consult | 1953 (48) | 713 (68) | <0.001 | 609 (66) | 606 (66) | 0.92 |
| Intensive care unit | 151 (4) | 54 (5) | 0.033 | 51 (6) | 43 (5) | 0.46 |
| Length of stay (days) | 6 (±5) | 7 (±5) | <0.001 | 7 (±6) | 7 (±5) | 0.61 |
| Discharge medications | ||||||
| ACE inhibitors or ARBs | 2176 (53) | 748 (71) | <0.001 | 635 (69) | 628 (68) | 0.75 |
| Beta-blockers | 1284 (31) | 326 (31) | 0.81 | 292 (32) | 276 (30) | 0.46 |
| Loop diuretics | 3135 (77) | 911 (86) | <0.001 | 785 (85) | 785 (85) | 1.00 |
| Potassium-sparing diuretics | 451 (11) | 207 (20) | <0.001 | 164 (18) | 166 (18) | 0.95 |
| Calcium channel blockers | 1148 (28) | 226 (21) | <0.001 | 195 (21) | 200 (22) | 0.81 |
| Potassium supplements | 1749 (43) | 496 (47) | 0.010 | 438 (48) | 430 (47) | 0.74 |
| Nitrates and hydralazine | 128 (3) | 32 (3) | 0.89 | 33 (4) | 28 (3) | 0.60 |
| Anti-arrhythmic drugs | 477 (12) | 136 (13) | 0.26 | 112 (12) | 114 (12) | 0.95 |
| Anti-coagulants | 701 (17) | 296 (28) | <0.001 | 232 (25) | 230 (25) | 0.96 |
| Aspirin | 1530 (37) | 416 (40) | 0.20 | 371 (40) | 366 (40) | 0.85 |
Figure 1.
Love plot displaying absolute standardized differences for 55 baseline characteristics between heart failure patients receiving and not receiving a new discharge prescription for digoxin, before and after propensity score matching
Digoxin and 30-Day All-Cause Hospital Readmission
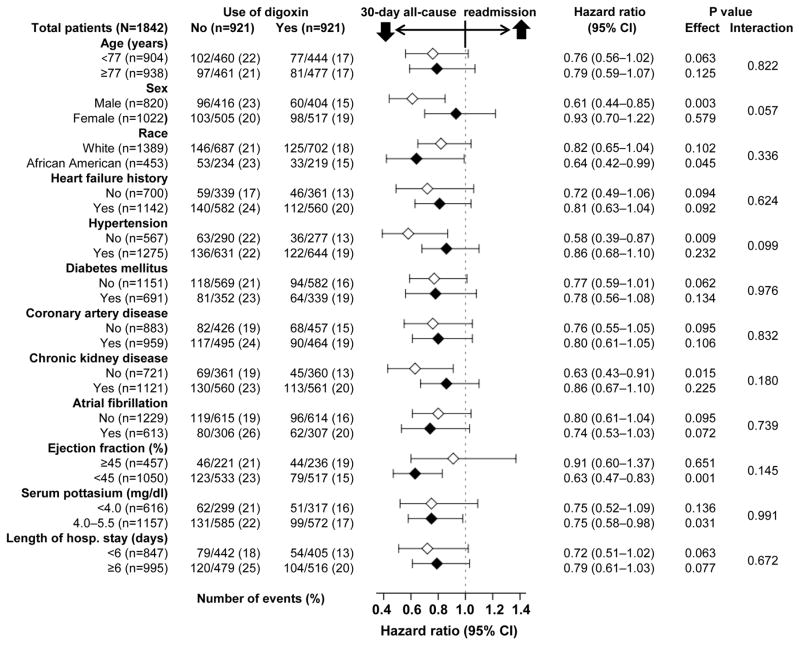
During 30-day after index hospitalization, all-cause hospital readmission occurred in 17% (158/921) and 22% (199/921) of matched patients receiving and not receiving a discharge prescription for digoxin, respectively (HR associated with digoxin prescription, 0.77; 95% confidence interval {CI}, 0.63–0.95; Table 2 and Figure 2). Although this association was generally homogeneous across various subgroups, it appeared to be significant only among men and in those with reduced ejection fraction (Figure 3). Among the 5153 pre-match patients, unadjusted, multivariable-adjusted and propensity score adjusted HRs (95% CIs) for 30-day all-cause readmission associated with digoxin prescription were 0.79 (0.67–0.92), 0.79 (0.66–0.95) and 0.80 (0.67–0.96), respectively.
Table 2.
Association between a new discharge prescription for digoxin and 30-day post-discharge outcomes in a propensity-matched cohort of Medicare beneficiaries hospitalized for heart failure
| Outcomes | % (events)
|
Absolute risk diff.* | Hazard ratio† (95% confidence interval) | |
|---|---|---|---|---|
| New discharge prescription for digoxin
| ||||
| No (n=921) | Yes (n=921) | |||
| All-cause hospital readmission | 22% (199) | 17% (158) | −5% | 0.77 (0.63–0.95) |
| Hospital readmission due to heart failure | 7% (67) | 6% (57) | −1% | 0.85 (0.59–1.20) |
| All-cause mortality | 6% (55) | 6% (59) | 0% | 1.07 (0.74–1.55) |
| All-cause mortality or all-cause rehospitalization | 26% (235) | 22% (200) | −4% | 0.83 (0.69–1.00) |
Absolute risk differences were calculated by subtracting percent events in patients receiving no digoxin from those receiving those drugs
The hazard ratios compared patients receiving digoxin versus those not receiving digoxin. These hazard ratios were calculated by treating patients without events during the first 30 days as censored
Figure 2.
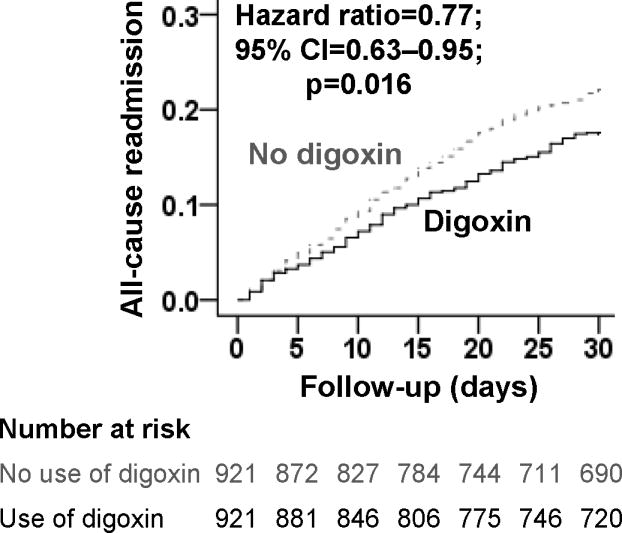
Kaplan-Meier plots for 30-day all-cause hospital readmission in a propensity-matched cohort of older heart failure patients receiving and not receiving a new discharge prescription for digoxin (CI=confidence interval)
Figure 3.
Association of new discharge prescriptions for digoxin with 30-day all-cause hospital readmission in subgroups of propensity-matched older heart failure patients
During 30-day post-discharge, 114 (6% of 1842) matched patients died; among the 1728 survivors, 30-day all-cause hospitalization occurred in 16% (141/862) and 21% (180/866) of patients receiving and not receiving digoxin, respectively (HR, 0.77; 95% CI, 0.61–0.95) suggesting that the lower 30-day readmission in the digoxin group was not at the expense higher mortality. Digoxin had no association with 30-day mortality; consequently, its association with the combined end point of 30-day all-cause mortality or all-cause readmission was attenuated (Table 2).
Digoxin use had no association with 30-day heart failure hospitalization (Table 2). However, among the 1050 matched patients with ejection fraction <45%, heart failure readmission occurred in 5.6% (29/517) and 8.6% (46/533) of patients receiving and not receiving digoxin, respectively (HR, 0.63; 95% CI, 0.40–1.01). In contrast, among the 457 matched patients with ejection fraction ≥45%, heart failure readmission occurred in 5.5% (13/236) and 5.0% (11/221) of patients receiving and not receiving digoxin, respectively (HR, 1.14; 95% CI, 0.51–2.54).
Digoxin and 12-Month Outcomes
During one-year post-discharge, digoxin use was associated with a significant 21% lower risk of all-cause readmission (HR, 0.79; 95% CI, 0.70–0.89; Table 3). Similar associations were observed at 3 and 6 months post-discharge. The association with 12-month all-cause readmission was similar for both sexes (p for interaction, 0.506) – with HRs of 0.75 (95% CI, 0.63–0.90) and 0.82 (95% CI, 0.70–0.96) among men and women, respectively. However, it was significantly different between those with ejection fraction <45% and ≥45% (p for interaction, 0.019) with HRs of 0.71 (95% CI, 0.61–0.83) and 1.00 (95% CI, 0.79–1.27) for patients with ejection fraction <45% and ≥45%, respectively.
Table 3.
Association between a new discharge prescription for digoxin and one-year post-discharge outcomes in a propensity-matched cohort of Medicare beneficiaries hospitalized for heart failure
| Outcomes | % (events)
|
Absolute risk diff.* | Hazard ratio† (95% confidence interval) | |
|---|---|---|---|---|
| New discharge prescription for digoxin
| ||||
| No (n=921) | Yes (n=921) | |||
| All-cause hospital readmission | 64% (591) | 57% (525) | −7% | 0.79 (0.70–0.89) |
| Hospital readmission due to heart failure | 31% (282) | 24% (219) | −7% | 0.72 (0.61–0.86) |
| All-cause mortality | 33% (301) | 28% (259) | −5% | 0.83 (0.70–0.98) |
| All-cause mortality or all-cause rehospitalization | 75% (690) | 66% (611) | −7% | 0.79 (0.71–0.88) |
Absolute risk differences were calculated by subtracting percent events in patients receiving no digoxin from those receiving those drugs
The hazard ratios compared patients receiving digoxin versus those not receiving digoxin. These hazard ratios were calculated by treating patients without events during the first 12 months as censored. Because the proportional hazards assumption held for the entire 12 months duration, we assumed that the hazard rate remained constant over the entire 12 month time frame and as such separate hazard ratios for 3 and 6 months were not calculated
A discharge prescription of digoxin was associated with a significant 28% lower risk of heart failure readmission (HR, 0.72; 95% CI, 0.61–0.86; Table 3). This association was similar for both men and women (p for interaction, 0.454), but not between those with reduced (HR, 0.63; 95% CI, 0.50–0.79) and preserved (HR, 1.11; 95% CI, 0.76–1.63) ejection fraction (p for interaction, 0.012). A discharge prescription of digoxin was associated with a significant 17% lower risk of all-cause mortality (HR, 0.83; 95% CI, 0.70–0.98; Table 3). This association was similar between the sexes (p for interaction, 0.587) and those with ejection fraction <45% versus ≥ 45% (p for interaction, 0.228).
DISCUSSION
Findings from the current study demonstrate that among a well-balanced cohort of Medicare beneficiaries hospitalized for acute decompensation of heart failure, a new discharge prescription for digoxin was associated with a significant lower risk of 30-day all-cause hospital readmission. Digoxin use was also associated with a lower risk for all-cause readmission, heart failure readmission, all-cause mortality and the combined end points at all times throughout the 12 months post-discharge, suggesting that the early benefit of digoxin was not at the cost of higher mortality or subsequent higher readmissions. To the best of our knowledge, this is the first report of a significant association of digoxin use with lower 30-day all-cause readmission in Medicare beneficiaries hospitalized for acute heart failure. These findings, based on a rigorously conducted propensity-matched inception-cohort study, taken together with those from the main DIG trial,17 suggest that digoxin may potentially serve as an inexpensive tool for the reduction of 30-day all-cause hospital readmission for heart failure patients, a vexing problem for the nation’s health care system.
The effect of digoxin on reduction of heart failure symptoms and hospital admission in patients with heart failure and reduced ejection fraction is well known.13–15 It is now also known that the effect of digoxin is early and broad so that in the DIG trial it also significantly reduced the risk of 30-day all-cause admission by 34%.17 This was likely mostly mediated by the reduction in 30-day heart failure hospitalization, which digoxin significantly reduced by 60%.17 Although the rate of 30-day all-cause admission in the DIG trial was low (8% in the placebo group), over half of these hospitalizations were due to worsening heart failure. Nearly 50% of Medicare beneficiaries with heart failure have ≥5 chronic comorbidities.38 It is possible that an improved hemodynamics in the digoxin group may also have helped those patients better cope their other comorbidities, thus reducing the risk of non-heart failure-related admissions. However, among real world hospitalized heart failure patients in the current study, we found no evidence of clinical effectiveness of digoxin in lowering 30-day heart failure readmission. Reasons for early readmission after a heart failure hospitalization are often complex in real world older patients and unlike in randomized trials, their documentation may be influenced by coding and billing practices. Only about a third of the readmissions in our study were due to heart failure. However, low event rates are unlikely to explain this lack of association as the overall rate of 30-day all-cause readmission was high (22% in the non-digoxin group). Older heart failure patients are known to restrict their mobility and activities to avoid symptoms.39 If patients not receiving digoxin were more symptomatic but avoided symptoms by limiting activities that may potentially explain the attenuated between-group differences in heart failure symptoms and readmissions.
The Role of Digoxin in Heart Failure and Preserved Ejection Fraction
Another potential explanation for the lack of a significant association with 30-day heart failure readmission in the overall sample is the inclusion of heart failure patients with preserved ejection fraction, as in our study, the use of digoxin was associated with a 37% lower risk of 30-day heart failure readmission among those with reduced ejection fraction. To further understand the differential effect of digoxin in heart failure patients with preserved versus reduced ejection fraction, we have recently analyzed data from older patients with heart failure and preserved ejection fraction in the ancillary DIG trial. Findings from that analysis suggest that digoxin increased the risk of all-cause admission at 30 days but not at 3 and 12 months after randomization.40 This is in sharp contrast to the findings from the main DIG trial in which digoxin reduced both short-and long-term risks of all-cause admission in older patients with heart failure and reduced ejection fraction.17 However, this is consistent with findings form the ancillary DIG trial that suggested lack of association with overall hospital admission.16 In the ancillary DIG trial, although there was a trend toward reduced risk of heart failure hospitalization in the digoxin group, this was negated by a trend toward increased risk of hospitalization due to unstable angina.16 Taken together with the findings from DIG trial,17,40 current data suggest that digoxin should not be prescribed for the purpose of reducing 30-day all-cause readmission in hospitalized older patients with heart failure with preserved ejection fraction. Future prospective studies need to clarify the role of digoxin on early readmission in patients with heart failure and preserved ejection fraction.
The Role of Digoxin in Women
Although digoxin use was associated with lower risk of all-cause hospital readmission among women throughout the first 12 months of post-discharge follow-up that included the first month and there was no significant sex interaction, the association was more modest in women than in men. However, the association of digoxin with 30-day all-cause readmission appeared different between the sexes, with benefit in men but not women (p for interaction, 0.057; Figure 3). The effect of digoxin on 30-day hospital admission in the main DIG trial was also modest among women, though there was no significant digoxin-sex interaction.17 These findings highlight the importance of using digoxin in low doses for women. The use of digoxin in low doses is likely to result in low serum digoxin concentrations.41 Despite early reports of higher mortality among women receiving digoxin in the DIG trial,35 it is now known that as in men,42 digoxin at serum digoxin concentrations between 0.5 to 0.9 ng/ml also reduced heart failure hospitalization in women (HR, 0.70; 95% CI, 0.53–0.94) without increasing mortality (HR, 0.84; 95% CI, 0.62–1.13).36
Association with Mortality
The association of digoxin use with lower 12-month all-cause mortality in our cohort of real-world older heart failure patients is rather intriguing. One potential explanation is that older adults are more likely to have more advanced heart failure and thus more likely to die from pump failure than sudden death.43 In the DIG trial, although digoxin had no effect on all-cause mortality, it reduced the risk of death due to pump failure by a near-significant 12% (HR, 0.88; 95% CI, 0.77–1.01) during the average follow-up of 37 months.15 In addition, digoxin reduced the risk of heart failure death by a significant 20% (HR, 0.80; 95% CI, 0.66–0.97) in the high-risk subset during the first 2 years of follow-up,18 and a significant 34% (HR, 0.66; 95% CI, 0.52–0.85) in all patients during the first year after randomization.44 However, given the overall lack of effect on mortality in the DIG trial, this observational association needs to be interpreted with caution as bias due to unmeasured confounders is possible.
Clinical and Public Health Implications
Digoxin is an inexpensive and relatively safe drug at low doses, which is approved by the United States Food and Drug Administration and recommended by major national chronic heart failure guidelines.45,46 Yet there has been a major recent decline in the use of digoxin in heart failure,47,48 and only about 20% of patients in our study not receiving prior digoxin therapy were given discharge prescriptions for digoxin. Although relative risk reductions from therapeutic interventions are often modest in older adults, absolute risk reductions are more substantial due to high event rates, an important consideration given the high readmission rates for heart failure.1 Findings from the current study suggest that digoxin may play an important role in improving clinical outcomes and help hospitals achieve readmission goals for heart failure. However, these findings need to be replicated in prospective studies in contemporary heart failure patients before they are broadly adopted into clinical practice.
Limitations
Several potential limitations of are study merit discussion. Patients in our study were restricted to fee-for-service Medicare beneficiaries from a single state during 1999–2001 with only 30% receiving beta-blockers, very few receiving aldosterone antagonists, and presumptively none receiving cardiac resynchronization therapy, which may limit generalizability to current practice. We had no data on dose, serum concentration of digoxin or incidence of digoxin toxicity. Although we had no post-discharge adherence data, prospective data from other heart failure studies suggest high rates of post-discharge prescription filling.49,50 Further, regression dilution from crossover of treatment during follow-up is likely to underestimate true associations.51
Findings from our sensitivity analysis suggest that the association of digoxin with our main outcome of 30-day all-cause readmissions was sensitive to potential confounding by an unmeasured covariate.34 A binary covariate that is a near-perfect predictor of our main outcome could potentially explain away this association if it would increase the odds of discharge prescription of digoxin by a relatively small percentage. However, sensitivity analysis cannot determine if such an unmeasured socio-demographic or clinical confounder exists. Further, to act as a confounder of our observed associations, an unmeasured covariate could not be strongly correlated with any of the 55 measured baseline covariates, which is unlikely. Loss of patients during matching process may limit generalizability. However, we were able to match nearly 90% of the patients receiving digoxin, and our propensity-matched associations were similar to risk-adjusted associations based on pre-match data.
CONCLUSIONS
Medicare beneficiaries with heart failure and reduced ejection fraction hospitalized for acute decompensation who received discharge prescriptions for digoxin had lower risk of 30-day all-cause hospital readmission. This benefit of digoxin extended throughout 12 months of follow-up and was not at the cost of higher mortality. Findings of efficacy of digoxin in reducing 30-day all-cause hospital admission in the DIG trial and clinical effectiveness to lower 30-day all-cause hospital readmission in the real world in the current study suggest that digoxin may have a role in reducing 30-day all-cause hospital readmission in hospitalized patients with heart failure, a challenging and growing public health problem and a target for reduction of Medicare cost under the new U.S. health care reform law. Future prospective randomized trials are needed to replicate these findings before they are broadly adopted into clinical practice.
Digoxin use was associated with lower risk of 30-day all-cause readmission without higher mortality in Medicare beneficiaries hospitalized for acute heart failure
This benefit of digoxin was observed throughout the first 12 months after discharge but appeared to be restricted to those with ejection fraction <45%
Acknowledgments
Funding: Dr. Ahmed was in part supported by the National Institutes of Health (NIH) through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama. Dr. Allman is supported in part by the grant number UL1 TR000165 from NIH.
Footnotes
Conflict of interest: None of the authors reported conflicts of interest related to this work.
Authors’ roles: AA conceived the study hypothesis and design in collaboration with the coauthors. AA and KP wrote the first draft. AA and KP performed statistical analyses in collaboration with IBA, TEL, and CJM. All authors interpreted the data, participated in critical revision of the paper for important intellectual content, and approved the final version of the article. IA, AA, CJM, and KP had full access to data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. The New England journal of medicine. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Rau J. The New York Times. New York: 2012. [Access date: December 2, 2012]. Hospitals Face Pressure to Avert Readmissions. http://www.nytimes.com/2012/11/27/health/hospitals-face-pressure-from-medicare-to-avert-readmissions.html?_r=0. [Google Scholar]
- 3.Stone J, Hoffman GJ. Prepared for Members and Committees of Congress, ed. , editor. Congressional Research Service Report for Congress. Washington, DC: 2010. [Access date: September 7, 2012]. Medicare Hospital Readmissions: Issues, Policy Options and PPACA. http://www.ncsl.org/documents/health/Medicare_Hospital_Readmissions_and_PPACA.pdf. [Google Scholar]
- 4.Sommers C, Cunningham PJ. Physician Visits After Hospital Discharge: Implications for Reducing Readmissions. The National Institute for Health Care Reform; Washington, DC: 2011. [Access date: May 9, 2012]. http://www.nihcr.org/Reducing_Readmissions.pdf. [Google Scholar]
- 5.Konstam MA. Heart Failure in the Lifetime of Musca Domestica (The Common Housefly) JACC: Heart Failure. 2013;1:178–180. doi: 10.1016/j.jchf.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Butler J, Kalogeropoulos A. Hospital strategies to reduce heart failure readmissions: where is the evidence? J Am Coll Cardiol. 2012;60:615–617. doi: 10.1016/j.jacc.2012.03.066. [DOI] [PubMed] [Google Scholar]
- 7.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99:549–553. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer P, Ekundayo OJ, Adamopoulos C, Mujib M, Aban I, White M, et al. A propensity-matched study of elevated jugular venous pressure and outcomes in chronic heart failure. Am J Cardiol. 2009;103:839–844. doi: 10.1016/j.amjcard.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Hall V, Lakier JB, Goldstein S. Comparative hemodynamic and neurohormonal effects of intravenous captopril and digoxin and their combinations in patients with severe heart failure. J Am Coll Cardiol. 1989;13:134–142. doi: 10.1016/0735-1097(89)90561-5. [DOI] [PubMed] [Google Scholar]
- 11.van Veldhuisen DJ, Man in‘t Veld AJ, Dunselman PH, Lok DJ, Dohmen HJ, Poortermans JC, et al. Double-blind placebo-controlled study of ibopamine and digoxin in patients with mild to moderate heart failure: results of the Dutch Ibopamine Multicenter Trial (DIMT) J Am Coll Cardiol. 1993;22:1564–1573. doi: 10.1016/0735-1097(93)90579-p. [DOI] [PubMed] [Google Scholar]
- 12.Newton GE, Tong JH, Schofield AM, Baines AD, Floras JS, Parker JD. Digoxin reduces cardiac sympathetic activity in severe congestive heart failure. J Am Coll Cardiol. 1996;28:155–161. doi: 10.1016/0735-1097(96)00120-9. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Gheorghiade M, Young JB, Costantini PJ, Adams KF, Cody RJ, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med. 1993;329:1–7. doi: 10.1056/NEJM199307013290101. [DOI] [PubMed] [Google Scholar]
- 14.Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol. 1993;22:955–962. doi: 10.1016/0735-1097(93)90403-n. [DOI] [PubMed] [Google Scholar]
- 15.The Digitalis Investigation Group Investigators. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourge RC, Fleg JL, Fonarow GC, Cleland JG, McMurray JJ, van Veldhuisen DJ, et al. Digoxin Reduces 30-day All-cause Hospital Admission in Older Patients with Chronic Systolic Heart Failure. Am J Med. 2013;126:701–708. doi: 10.1016/j.amjmed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheorghiade M, Patel K, Filippatos G, Anker SD, van Veldhuisen DJ, Cleland JG, et al. Effect of oral digoxin in high-risk heart failure patients: a pre-specified subgroup analysis of the DIG trial. Eur J Heart Fail. 2013;15:551–559. doi: 10.1093/eurjhf/hft010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess G, Preblick R, Hill J, Plauschinat CA, Yaskin J. Effects of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy after hospital discharge on subsequent rehospitalization for acute myocardial infarction and heart failure. Congest Heart Fail. 2009;15:170–175. doi: 10.1111/j.1751-7133.2009.00092.x. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. doi: 10.1016/j.jacc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed A, Fonarow GC, Zhang Y, Sanders PW, Allman RM, Arnett DK, et al. Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. 2012;125:399–410. doi: 10.1016/j.amjmed.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A, Rich MW, Zile M, Sanders PW, Patel K, Zhang Y, et al. Renin-angiotensin inhibition in diastolic heart failure and chronic kidney disease. Am J Med. 2013;126:150–161. doi: 10.1016/j.amjmed.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feller MA, Mujib M, Zhang Y, Ekundayo OJ, Aban IB, Fonarow GC, et al. Baseline characteristics, quality of care, and outcomes of younger and older Medicare beneficiaries hospitalized with heart failure: findings from the Alabama Heart Failure Project. Int J Cardiol. 2012;162:39–44. doi: 10.1016/j.ijcard.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175:250–262. doi: 10.1093/aje/kwr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel K, Fonarow GC, Kitzman DW, Aban IB, Love TE, Allman RM, et al. Angiotensin receptor blockers and outcomes in real-world older patients with heart failure and preserved ejection fraction: a propensity-matched inception cohort clinical effectiveness study. Eur J Heart Fail. 2012;14:1179–1188. doi: 10.1093/eurjhf/hfs101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 27.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 28.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. Primer on statistical interpretation or methods report card on propensity-score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:62–67. doi: 10.1161/CIRCOUTCOMES.108.790634. [DOI] [PubMed] [Google Scholar]
- 31.Mujib M, Patel K, Fonarow GC, Kitzman DW, Zhang Y, Aban IB, et al. Angiotensin-converting Enzyme Inhibitors and Outcomes in Heart Failure and Preserved Ejection Fraction. Am J Med. 2013;126:401–410. doi: 10.1016/j.amjmed.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wahle C, Adamopoulos C, Ekundayo OJ, Mujib M, Aronow WS, Ahmed A. A propensity-matched study of outcomes of chronic heart failure (HF) in younger and older adults. Arch Gerontol Geriatr. 2009;49:165–171. doi: 10.1016/j.archger.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 34.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. Vol. 1. New York: Springer-Verlag; 2002. pp. 105–170. [Google Scholar]
- 35.Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403–1411. doi: 10.1056/NEJMoa021266. [DOI] [PubMed] [Google Scholar]
- 36.Adams KF, Jr, Patterson JH, Gattis WA, O’Connor CM, Lee CR, Schwartz TA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005;46:497–504. doi: 10.1016/j.jacc.2005.02.091. [DOI] [PubMed] [Google Scholar]
- 37.Krumholz HM. DIGging In: Three Reasons Why the Recent Digoxin Study Is Not Relevant to Readmission. [Accessed July 27, 2013];CardioExchange: An NEJM Practice Community. 2013 http://www.cardioexchange.org/voices/digging-in-three-reasons-why-the-recent-digoxin-study-is-not-relevant-to-readmission/
- 38.The Centers for Medicare & Medicaid Services. Chronic Conditions among Medicare Beneficiaries, Chart Book. Baltimore, MD: 2011. [Access date: August 28, 2012]. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Downloads/2011Chartbook.pdf. [Google Scholar]
- 39.Ahmed A. Chronic heart failure in older adults. Med Clin North Am. 2011;95:439–461. ix. doi: 10.1016/j.mcna.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashim T, Elbaz S, Patel K, Morgan CJ, Fonarow GC, Fleg JL, McGwin G, Cutter GR, Allman RM, Prabhu SD, Zile MR, Bourge RC, Ahmed A. Digoxin and 30-day All-cause Hospital Admission in Older Patients with Chronic Diastolic Heart Failure. Am J Med. 2013 doi: 10.1016/j.amjmed.2013.08.006. pii: S0002-9343(13)00711-0. doi: 10.1016/j.amjmed.2013.08.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed A. Digoxin and reduction in mortality and hospitalization in geriatric heart failure: importance of low doses and low serum concentrations. J Gerontol A Biol Sci Med Sci. 2007;62:323–329. doi: 10.1093/gerona/62.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 43.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed A, Waagstein F, Pitt B, White M, Zannad F, Young JB, et al. Effectiveness of digoxin in reducing one-year mortality in chronic heart failure in the Digitalis Investigation Group trial. Am J Cardiol. 2009;103:82–87. doi: 10.1016/j.amjcard.2008.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 46.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 47.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 48.Castagno D, Petrie MC, Claggett B, McMurray J. Should we SHIFT our thinking about digoxin? Observations on ivabradine and heart rate reduction in heart failure. Eur Heart J. 2012;33:1137–1141. doi: 10.1093/eurheartj/ehs004. [DOI] [PubMed] [Google Scholar]
- 49.Butler J, Arbogast PG, Daugherty J, Jain MK, Ray WA, Griffin MR. Outpatient utilization of angiotensin-converting enzyme inhibitors among heart failure patients after hospital discharge. J Am Coll Cardiol. 2004;43:2036–2043. doi: 10.1016/j.jacc.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 50.Curtis LH, Mi X, Qualls LG, Check DK, Hammill BG, Hammill SC, et al. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165:979–986. doi: 10.1016/j.ahj.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]