Abstract
The use of protein cross-linking agents during bonding procedures has been recently proposed to improve bond durability. This study aimed to use zymography and in situ zymography techniques to evaluate the ability of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) cross-linker to inhibit matrix metalloproteinase (MMP) activity. The hypotheses tested were that: (1) bonding procedures increase dentin gelatinolytic activity and (2) EDC pre-treatment prevents this enzymatic activity. The zymographic assay was performed on protein extracts obtained from dentin powder treated with Optibond FL or Scotchbond 1XT with or without 0.3M EDC pre-treatment. For in situ zymography, adhesive/dentin interfaces were created with the same adhesives applied to acid-etched dentin slabs pre-treated or not with EDC conditioner. Zymograms revealed increased expression of dentin endogenous MMP-2 and -9 after adhesive application, while the use of EDC as a primer inactivated dentin gelatinases. Results of in situ zymograpy showed that hybrid layers of tested adhesives exhibited intense collagenolytic activity, while almost no fluorescence signal was detected when specimens were pre-treated with EDC. The correlative analysis used in this study demonstrated that EDC could contribute to inactivate endogenous dentin MMPs within the hybrid layer created by etch-and-rinse adhesives.
Keywords: human dentin, collagen cross-linker, dentin bonding agents, adhesive systems, endogenous proteinases, biochemical assays
Introduction
The durability of dentin bonding systems is affected by the degradation of the resin compounds occurring via hydrolysis of suboptimally polymerized hydrophilic resins and degradation of collagen matrices by matrix metalloproteinases (MMPs) and cysteine cathepsins (Breschi et al., 2008).
MMPs and cathepsins have been shown to be present in dentin (Mazzoni et al., 2007, 2009, 2011; Tersariol et al., 2010; Niu et al., 2011) and seem to be responsible for the slow hydrolysis of the collagen fibrils in hybrid layers that anchor resin composites to the underlying mineralized dentin (Tjäderhane et al., 2013). To prolong the durability of resin-dentin bonds, inactivation of these proteases has been recommended over the use of synthetic MMP inhibitors (Breschi et al., 2010a,b; Liu et al., 2011; Almahdy et al., 2012), quaternary ammonium methacrylates, or benzalkonium chloride (Tezvergil-Mutluay et al., 2011a,b). Moreover, other approaches have been proposed, including remineralization, ethanol wet-bonding, and the use of collagen-cross-linkers (Tay and Pashley, 2009; Bedran-Russo et al., 2010; Tjäderhane et al., 2013).
The use of collagen cross-linkers in adhesive procedures has gained increased popularity in recent years (Liu et al., 2011; Tjäderhane et al., 2013). The use of proanthrocyanidin, glutaraldehyde, genipin, riboflavin, and carbodiimide has been proposed to enhance the mechanical and structural stability of dentin collagen, leading to a stable dentin matrix network that, after resin infiltration, should provide a durable hybrid layer (Al-Ammar et al., 2009; Macedo et al., 2009; Cova et al., 2011; Mazzoni et al., 2013b). In addition, collagen cross-linkers have been reported to improve the resistance of uncross-linked or mildly cross-linked collagen matrices to degradation by bacterial collagenases (Avila and Navia, 2010; Ma et al., 2010), potentially contributing to the stabilization of the resin-dentin interface over time.
In addition to the proven efficacy of collagen cross-linkers in chemical or physical modification of the dentin collagen substrate, the clinical applicability of these solutions is desirable. Accordingly, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) has been recently proposed as an effective collagen-cross-linker in the preservation of bond strength over time and in the inhibition of matrix-bound MMPs (Bedran-Russo et al., 2010; Tezvergil-Mutluay et al., 2012; Mazzoni et al., 2013b).
The aim of the study was to evaluate the ability of an EDC-cross-linker-containing primer to inhibit dentin endogenous MMP activity by means of a zymographic assay (to determine the types and nature of proteinases) and an in situ zymography technique to determine the three-dimensional localization of MMP activity within the hybrid layer (HL). The tested hypotheses were that: (1) HLs created with etch-and-rinse adhesives are affected by gelatinolytic activity; and (2) 0.3M EDC pre-treatment inactivates this endogenous enzymatic activity, regardless of the adhesives tested.
Materials & Methods
Reagents were purchased from Sigma Chemical (St. Louis, MO, USA) unless otherwise specified and were used as received.
Zymographic Analysis
Zymographic analysis was performed in accordance with procedures described by Mazzoni et al. (2013a). Mineralized dentin powder was obtained from 14 human third molars, after approval by the Ethical Committee of the College of Dental Medicine, Georgia Regents University (Augusta, GA, USA). Powder was obtained by freezing the dentin in liquid nitrogen and triturating it by means of a Retsch mill (Model MM400, Retsch GmbH, Haan, Germany). Aliquots of mineralized dentin powder were divided into 7 groups: G1, left mineralized (control); G2, demineralized with 10 wt% phosphoric acid for 10 min; G3, demineralized as for G2 and treated with 0.3M EDC for 30 min; G4, demineralized as for G2 and treated with Optibond FL (OFL; Kerr, Orange, CA, USA) for 30 min; G5, demineralized as for G2 and treated with 0.3M EDC followed by OFL application; G6, demineralized as for G2 and treated with Scotchbond 1XT (3M ESPE, St. Paul, MN, USA); and G7, demineralized as for G2 and treated with 0.3M EDC followed by SB1XT application. The adhesive was extracted from each dentin-treated powder with 1 mL of acetone and centrifuged (20,800 g for 20 min), after which dentin powder was re-suspended in acetone and re-centrifuged 2 more times for removal of additional unpolymerized comonomers (Mazzoni et al., 2012).
For protein extraction, dentin powder aliquots were re-suspended in extraction buffer (50 mM Tris-HCl pH 6, containing 5 mM CaCl2, 100 mM NaCl, 0.1% Triton X-100, 0.1% non-ionic detergent P-40, 0.1 mM ZnCl2, 0.02% NaN3) for 24 hrs at 4°C and sonicated for 10 min (at ≈ 30 pulses), centrifuged for 20 min at 4°C (20,800 g), after which the supernatant was removed and re-centrifuged. The protein content was further concentrated in a Vivaspin centrifugal concentrator (10,000 KDa cut-off; Vivaspin Sartorius Stedim Biotech, Goettingen, Germany) for 30 min at 4°C (15,000 g, 3 times). Total protein concentration of dentin extracts was determined by Bradford assay (Bio-Rad, Hercules, CA, USA). Dentin protein aliquots (60 µg) were diluted in Laemmli sample buffer at a 4:1 ratio and subjected to electrophoresis under non-reducing conditions in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) containing 1 mg/mL fluorescently labeled gelatin. Pre-stained low-molecular-weight SDS-PAGE standards (Bio-Rad) were used as molecular-weight markers. After electrophoresis, the gels were washed for 1 hr in 2% Triton X-100, and then were incubated in zymography activation buffer (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, pH 7.4) for 48 hrs. Proteolytic activity was evaluated and registered under long-wave UV light scanner (ChemiDoc Universal Hood, Bio-Rad). Gelatinase activity in the samples was analyzed in duplicate by gelatin zymography.
Zymographic bands were identified and quantified with Bio-Rad Quantity One Software (Bio-Rad).
In situ Zymography of the Hybrid Layer
Twenty freshly extracted non-carious human third molars were selected for in situ zymography. Enamel and cementum were removed, and 1-mm-thick disks of middle/deep coronal dentin were obtained from each tooth by means of a slow-speed saw (Micromet, Remet, Casalecchio di Reno, Italy). A standardized smear layer was created with 600-grit wet silicon-carbide paper, and dentin was etched for 15 sec with 35% phosphoric-acid gel (3M ESPE, St. Paul, MN, USA) and rinsed with continuous water irrigation for 30 sec. Etched dentin specimens were then equally divided into 4 groups and treated as follows. Group 1 dentin was pre-treated with 0.3M EDC water solution for 1 min, and the excess was gently blown off with air, then bonded with OFL; in Group 2, OFL was applied to untreated etched dentin as per the manufacturer’s instructions; Group 3 dentin was pre-treated with 0.3M EDC as described in Group 1, then bonded with SB1XT; and in Group 4, SB1XT was applied to untreated etched dentin as per the manufacturer’s instructions. A 1-mm-thick flowable composite (Filtek Flow; 3M ESPE) was applied to the resin-bonded disks and light-cured for 20 sec with a quartz-tungsten-halogen light-curing unit (Curing Light 2500, 3M ESPE). Bonded specimens were then cut vertically into 1-mm-thick slabs to expose the adhesive/dentin interfaces by means of a slow-speed saw (Micromet); slabs were glued to glass slides and ground down to obtain specimens ca. 50 µm thick.
In situ zymography was performed with self-quenched fluorescein-conjugated gelatin as the MMP substrate (E-12055, Molecular Probes, Eugene, OR, USA) in accordance with Mazzoni et al. (2012). In brief, the fluorescent gelatin mixture was placed on top of each slab and covered with a coverslip, and the slides were light-protected and incubated in humidified chambers at 37°C for 24 hrs. The hydrolysis of quenched fluorescein-conjugated gelatin substrate, indicative of endogenous gelatinolytic enzyme activity, was assessed by examination with a confocal laser scanning microscope [excitation (ex), 488 nm; and emission (em), lp530 nm; Nikon A1-R, Tokyo, Japan]. The 2-D images obtained were then combined to create 3D-like structural images to provide additional information regarding the depth of gelatinolytic activity.
Negative control sections were incubated as described above, except that: (1) 250 mM ethylenediaminetetraacetic acid (EDTA) was dissolved in the mixture of quenched fluorescein-conjugated gelatin or (2) 2 mM 1,10-phenanthroline or (3) standard non-fluorescent instead of fluorescent-conjugated gelatin was used. EDTA and 1,10-phenanthroline were used as negative controls because they are well-known MMP inhibitors.
Results
Zymographic Analysis
Results of both zymographic analysis and the densitometric evaluation of bands (expressed as percentage increase/decrease of MMP activity among the different treatment groups compared with mineralized dentin, considered as baseline) are shown in Fig. 1. Mineralized dentin showed the presence of MMP-2 pro- and active-form (72- and 66-kDa, respectively) and pro-MMP-9 (95 kDa) and of an additional band around 50 kDa (Fig. 1, lane 1). Proteins extracted from dentin powder demineralized with 10% phosphoric acid showed similar presence of MMP-2 pro- and active-form, expression of MMP-9 and of the additional band at 50 kDa (Fig. 1, lane 2). The incubation of demineralized dentin with 0.3M EDC resulted in complete inactivation of dentinal gelatinases (Fig. 1, lane 3). Demineralized dentin powder treated with OFL resulted in enzymatic activation (Fig. 1, lane 4), whereas the pre-treatment with EDC followed by the application of OFL resulted in complete inactivation of dentinal gelatinases (Fig. 1, lane 5). Treatment of demineralized dentin powder with SB1XT resulted in MMP-2 and -9 activation (Fig. 1, lane 6), while pre-treatment with 0.3M EDC followed by SB1XT resulted in almost complete inhibition of dentinal gelatinases, although a band with molecular weight slightly higher than that of other bands, indicated as activated MMP-2, was present (68 kDa; Fig. 1, lane 7). This band could represent the intermediate form of MMP-2 activation, and respective (even if obviously less clear) bands can also be seen in lanes 4 and 6.
Figure 1.
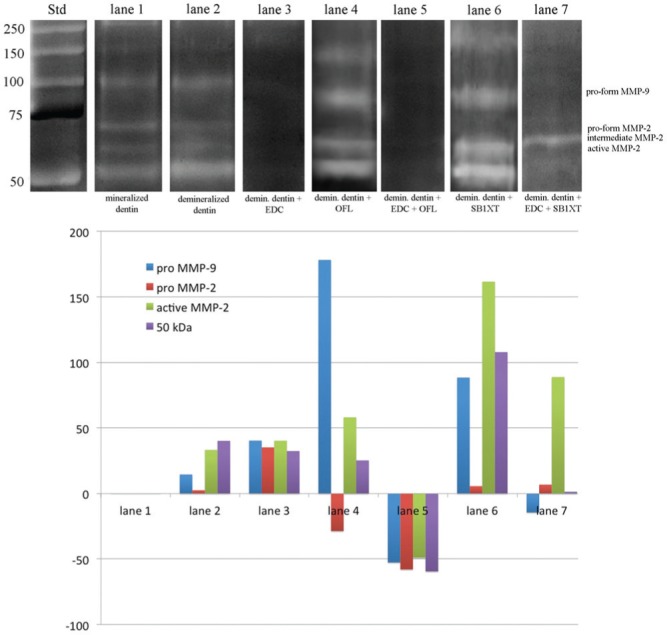
Zymographic analysis of proteins extracted from dentin powder and densitometric evaluation of bands expressed as percentage increase/decrease of MMPs activity among the different treatment groups compared with mineralized dentin (considered as baseline). Std: Standards (Std) are reported in lane Std. Lane 1: Mineralized dentin showing the presence of MMP-2 pro- and active-form (72- and 66-kDa, respectively) and pro-MMP-9 (95 kDa) and an additional band around 50 kDa. Lane 2: Proteins extracted from dentin powder demineralized with 10% phosphoric acid, showing similar presence of MMP-2 pro-form and an increase in the expression of pro-MMP-9, MMP-2 active-form, and of the additional band at 50 kDa. Lane 3: Demineralized dentin powder after incubation with 0.3M EDC showing complete inactivation of dentinal MMPs. Lane 4: Demineralized dentin powder treated with Optibond FL (OFL) showing enzymatic activation of both MMP-2 and -9 and of the additional band at approx. 50 kDa. Lane 5: Proteins extracted from demineralized dentin powder pre-treated with 0.3M EDC followed by OFL application showing complete inactivation of dentinal gelatinases. Lane 6: Demineralized dentin powder treated with Adper Scotchbond 1 XT (SB1XT) showing enzymatic activation of both MMP-2 and -9 and of the additional band at approx. 50 kDa. Lane 7: Proteins extracted from demineralized dentin powder pre-treated with 0.3M EDC followed by SB1XT application showing reduced activation of MMP-9, MMP-2, and of the 50-kDa band, and presence of the MMP-2 intermediate form compared with lane 6.
Control zymograms incubated with 5 mM EDTA or 2 mM 1,10-phenanthroline showed no enzymatic activity (data not shown).
In situ Zymography of the Hybrid Layer
For control specimens bonded with OFL and SB1XT, the in situ zymography revealed an intense green fluorescence within the HL after incubation, indicating that the fluorescein-conjugated gelatin was strongly hydrolyzed at these sites (Figs. 2a, 2b, 3a, 3b, respectively). The 3D in situ zymography obtained from multiple images stacked together showed very intense activity within the dentinal tubules and at the bottom of the HL (Figs. 4a-4c, respectively), representing the partially demineralized, poorly resin-infiltrated collagen matrix.
Figure 2.
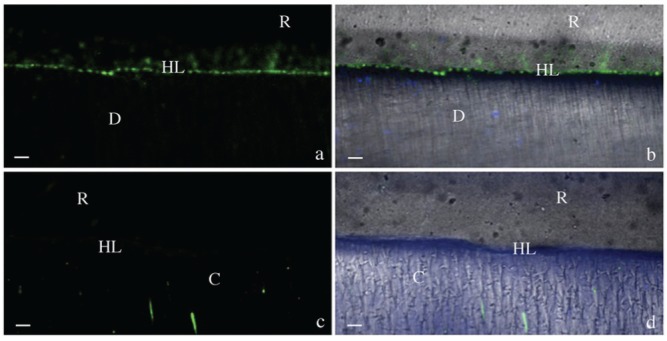
Resin-bonded dentin interfaces prepared with Optibond FL (OFL) with or without EDC pre-treatment, incubated with quenched fluorescein-labeled gelatin. D = Dentin; HL = Hybrid Layer; R = Resin Composite; bar = 5 µm. (a) Acquired image in green channel, showing fluorescence within the HL created with OFL. (b) Image of OFL without EDC pre-treatment, obtained by merging differential interference contrast image (showing the optical density of the resin-dentin interface) and image acquired in green channel (showing enzymatic activity). (c) Image acquired in green channel of hybrid layer created with OFL applied to acid-etched dentin pre-treated with EDC showing absence of fluorescence. (d) Image of HL created with OFL after EDC pre-treatment obtained by merging differential interference contrast image and image acquired in green channel.
Figure 3.
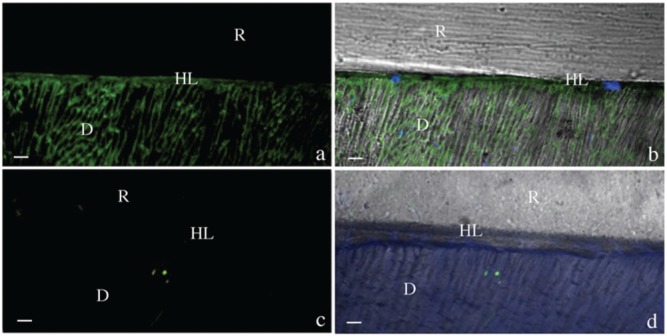
Resin-bonded dentin interfaces prepared with Adper Scotchbond 1 XT (SB1XT) with or without EDC pre-treatment, incubated with quenched fluorescein-labeled gelatin. D = Dentin; HL = Hybrid Layer; R = Resin Composite; bar = 5 µm. (a) Image acquired in green channel, showing fluorescence (identifying intense endogenous enzymatic activity) in dentinal tubules and within the hybrid layer (HL) created with SB1XT without EDC pre-treatment. (b) Image of SB1XT without EDC pre-treatment, obtained by merging differential interference contrast image (showing the optical density of the resin-dentin interface) and image acquired in green channel (showing enzymatic activity). (c) Image acquired in green channel of hybrid layer created with SB1XT applied to acid-etched dentin pre-treated with EDC showing absence of fluorescence. (d) Image of HL created with SB1XT after EDC pre-treatment obtained by merging differential interference contrast image and image acquired in green channel.
Figure 4.
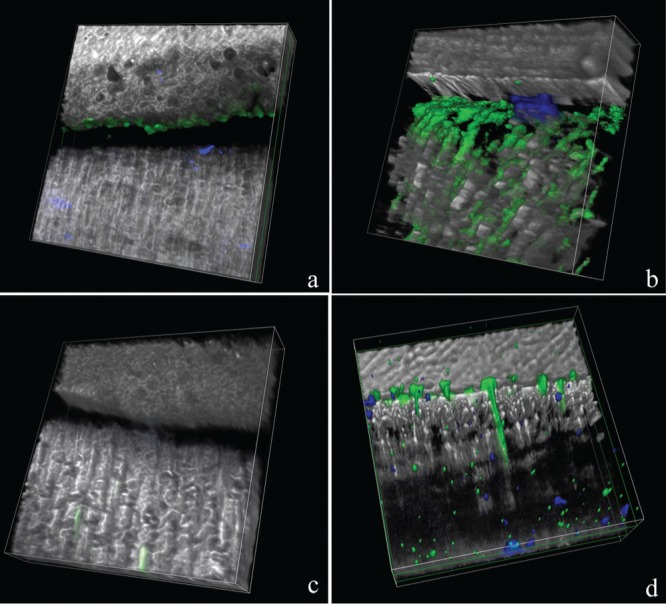
Three-dimensional surface-shaded reconstruction of the acquired images. Optibond FL (OFL) and Adper Scotchbond 1 XT (SB1XT) 3D reconstructions showing intense fluorescence (evidence of gelatin hydrolysis due to endogenous proteases), throughout the hybrid layer (a and b, respectively), while reduced fluorescence was recorded when OFL and SB1XT were applied to 0.3M EDC pre-treated dentin (c and d, respectively).
Hybrid layers created by either OFL or SB1XT, which were pre-treated with 0.3M EDC-containing primer, exhibited almost no fluorescence signal at the adhesive-dentin interface (Figs. 2c, 2d, 3c, 3d, 4b-4d, respectively).
No fluorescence was detected in negative controls, i.e., (1) EDTA-treated, (2) 2 mM 1,10-phenanthroline-treated, or (3) specimens incubated with standard non-fluorescent gelatin (data not shown).
Discussion
The results of this study showed that the application of adhesives to acid-etched dentin resulted in activation of MMP-2 and -9, while the use of a 0.3M EDC pre-treatment before the bonding procedure produced nearly complete inactivation of dentinal gelatinases for both adhesives. These results require acceptance of both null hypotheses.
Dentin collagen is strengthened by native inter- and intramolecular cross-links, which increase its resistance to thermal denaturing and enzymatic degradation (Liu et al., 2011). The use of collagen cross-linkers increases the longevity of resin-dentin bonds (Bedran-Russo et al., 2010; Cova et al., 2011; Mazzoni et al., 2013b). However, since dentin collagen is already highly cross-linked, doubts were raised regarding the ability of cross-linking agents to increase the durability of resin-dentin bonds in clinically relevant treatments (Liu et al., 2011). Cross-linkers, in fact, have also been reported to possess the ability to inactivate MMPs and reduce their collagenolytic activity (Calero et al., 2002; Matchett et al., 2005; Cova et al., 2011; Tezvergil-Mutluay et al., 2012; Mazzoni et al., 2013b). The results of the present study confirmed these previous findings, since gelatin zymography of proteins extracted from dentin powder revealed the presence of MMP-2 and -9, while treatment with 0.3M EDC resulted in complete inactivation of dentinal gelatinases (Fig. 1).
EDC contains a functional group with the formula RN=C=NR. The carbodiimide reacts with ionized carboxyl groups in proteins to form an O-acylisourea intermediate. This intermediate reacts with a non-proteinated amino group and an adjacent protein chain to form a stable covalent amide bond between the 2 proteins, with the only product being urea (Tezvergil-Mutluay et al., 2012). It is considered one of the least cytotoxic cross-linkers, and these cross-links are very stable (Tjäderhane et al., 2013). The proposed mechanism to explain MMP inactivation by cross-linkers is based on conformational changes in the enzyme 3D structure that may be achieved via irreversible changes induced within the catalytic domain or allosteric inhibition of other modular domains that co-participate in collagen degradation (Sela-Passwell et al., 2010; Liu et al., 2011).
Evidence of collagenolytic and gelatinolytic activities in dentin treated with both etch-and-rinse and self-etch denting bonding agents was previously published, confirming the involvement of these endoproteases in the disruption of collagen fibrils within hybrid layers being responsible for the poor durability of resin-dentin bond durability over time (Mazzoni et al., 2006; Nishitani et al., 2006; Breschi et al., 2008). Interestingly, with gelatin zymography used in the present study, the assessment of effectiveness of EDC as an inactivator of MMPs was seen when it was used as a primer before the application of OFL and SB1XT. Treatment of demineralized dentin powder with the above-mentioned etch-and-rinse adhesives resulted in increased MMP-2 and -9 activity (Fig. 1), confirming previous findings on the ability of dentin-bonding agents to increase dentinal protease activity (Mazzoni et al., 2006, 2011, 2012, 2013a; Nishitani et al., 2006). Conversely, when demineralized dentin powder was pre-treated with 0.3M EDC before the OFL application was performed, complete inactivation of dentinal gelatinases was obtained. Similar inhibitory findings were recorded for SB1XT after pre-treatment with the EDC-containing primer, although an intermediate form of MMP-2 activation could be seen (Fig. 1, lane 7). This intermediate form (68-kDa) can be considered as a transient phase (Atkinson et al., 1995) infrequently seen in zymography (because it is rapidly changed into active form with slightly lower molecular weight), and its activation from intermediate to fully active MMP-2 may be due to autolytic activity by other MMPs, or may be controlled by tissue inhibitors of metalloproteinases (TIMPs). This may indicate the cross-linking between MMP and TIMP in dentin powder, rendering the enzyme inactive (Liu et al., 2011).
Because homogenization of tissues for gelatin zymography is mandatory, localization of enzyme activity by this technique was precluded. For this reason, we additionally performed an in situ zymography technique to obtain precise localization of the MMP activity within the HL created by the tested etch-and-rinse adhesives. In situ zymography is, in fact, an adaptation of substrate zymography that does not require previous extraction of enzymes from the tissue; hence the analysis is made in situ (Mazzoni et al., 2012). Gelatinolytic activity was clearly detectable within the HLs and along the tubular wall dentin extending from the dentinal tubules. Furthermore, the location of the activity well correlates with the demineralized uninfiltrated collagen layer simplified etch-and- rinse adhesives at the bottom of the HL, an area also known for nanoleakage expression and the presence of naked collagen fibrils (Breschi et al., 2008), as well as with the effectiveness of a 0.3M EDC primer applied before bonding to inactivate protease activity within the HL.
Based on the results of the present study, the effectiveness of EDC in inactivating dentin MMPs has been demonstrated. In fact, although the effects of cross-linking agents on stabilizing dentin matrix degradation have been attributed to their capacity to increase the stiffness of dentin collagen, the results of the present study support the hypotheses that EDC can also inactivate dentinal MMP activity through direct cross-linking of MMPs. Thus, we believe that the use of EDC for 1 min could contribute to the stabilization of the hybrid layers over time, due to inactivation of endogenous proteases. Future studies are also needed to validate its effectiveness in association with self-etch adhesives as well to support the use of EDC in vivo.
Acknowledgments
The authors thank Mr. Aurelio Valmori for photographic assistance.
Footnotes
The study was funded by grants from MIUR (Italy): FIRB RBAP1095CR to L. Breschi (P.I.), PRIN 2009SAN9K5 to L. Breschi (P.I.), and PRIN 2009FXT3WL to R. Di Lenarda (P.I.), R21DE091213 (P.I. FRT), and R01DE015306 from the NIH/NIDCR (P.I. DHP).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Al-Ammar A, Drummond JL, Bedran-Russo AK. (2009). The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res B Appl Biomater 91:419-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almahdy A, Koller G, Sauro S, Bartsch JW, Sherriff M, Watson TF, et al. (2012). Effects of MMP inhibitors incorporated within dental adhesives. J Dent Res 91:605-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson SJ, Crabbe T, Cowell S, Ward RV, Butler MJ, Sato H, et al. (1995). Intermolecular autolytic cleavage can contribute to the activation of progelatinase A by cell membranes. J Biol Chem 270:30479-30485. [DOI] [PubMed] [Google Scholar]
- Avila MY, Navia JL. (2010). Effect of genipin collagen crosslinking on porcine corneas. J Cataract Refract Surg 36:659-664. [DOI] [PubMed] [Google Scholar]
- Bedran-Russo AK, Vidal CM, Dos Santos PH, Castellan CS. (2010). Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. J Biomed Mater Res B Appl Biomater 94:250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. (2008). Dental adhesion review: aging and stability of the bonded interface. Dent Mater 24:90-101. [DOI] [PubMed] [Google Scholar]
- Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, et al. (2010a). Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater 26:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. (2010b). Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater 26:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero P, Jorge-Herrero E, Turnay J, Olmo N, López de Silanes I, Lizarbe MA, et al. (2002). Gelatinases in soft tissue biomaterials. Analysis of different crosslinking agents. Biomaterials 23:3473-3478. [DOI] [PubMed] [Google Scholar]
- Cova A, Breschi L, Nato F, Ruggeri A, Carrilho M, Tjäderhane L, et al. (2011). Effect of UVA-activated riboflavin on dentin bonding. J Dent Res 90:1439-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. (2011). Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res 90:953-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DH, Lai JY, Cheng HY, Tsai CC, Yeh LK. (2010). Carbodiimide cross-linked amniotic membranes for cultivation of limbal epithelial cells. Biomaterials 31:6647-6658. [DOI] [PubMed] [Google Scholar]
- Macedo GV, Yamauchi M, Bedran-Russo AK. (2009). Effects of chemical cross-linkers on caries-affected dentin bonding. J Dent Res 88:1096-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchett MD, MacKinnon SL, Sweeney MI, Gottschall-Pass KT, Hurta RA. (2005). Blueberry flavonoids inhibit matrix metalloproteinase activity in DU145 human prostate cancer cells. Biochem Cell Biol 83:637-643. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, et al. (2006). Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials 27:4470-4476. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. (2007). Zymographic analysis and characterization of MMP-2 and -9 isoforms in human sound dentin. J Dent Res 86:436-440. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Tay FR, Gobbi P, Orsini G, Ruggeri A, et al. (2009). Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: correlative FEI-SEM/TEM analysis. J Biomed Mater Res A 88:697-703. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Papa V, Nato F, Carrilho M, Tjäderhane L, Ruggeri A, et al. (2011). Immunohistochemical and biochemical assay of MMP-3 in human dentine. J Dent 39:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Nascimento FD, Carrilho M, Tersariol I, Papa V, Tjäderhane L, et al. (2012). MMP activity in the hybrid layer detected with in situ zymography. J Dent Res 91:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Scaffa P, Carrilho M, Tjäderhane L, Di Lenarda R, Polimeni A, et al. (2013a). Effects of etch-and-rinse and self-etch adhesives on dentin MMP-2 and MMP-9. J Dent Res 92:82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Angeloni V, Apolonio FM, Scotti N, Tjäderhane L, Tezvergil-Mutluay A, et al. (2013b). Effect of carbodiimide (EDC) on the bond stability of etch-and-rinse adhesive systems. Dent Mater 29:1040-1047. [DOI] [PubMed] [Google Scholar]
- Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, et al. (2006). Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci 114:160-166. [DOI] [PubMed] [Google Scholar]
- Niu LN, Zhang L, Jiao K, Li F, Ding YX, Wang DY, et al. (2011). Localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in human coronal dentine. J Dent 39:536-542. [DOI] [PubMed] [Google Scholar]
- Sela-Passwell N, Rosenblum G, Shoham T, Sagi I. (2010). Structural and functional bases for allosteric control of MMP activities: Can it pave the path for selective inhibition? Biochim Biophys Acta 1803:29-38. [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2009). Biomimetic remineralization of resin-bonded acid-etched dentin. J Dent Res 88:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, et al. (2010). Cysteine cathepsins in human dentin-pulp complex. J Endod 36:475-481. [DOI] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M. (2011a). The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res 90:535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Mutluay MM, Gu L-S, Zhang K, Agee KA, Carvalho RM, et al. (2011b). The anti-MMP activity of benzalkonium chloride. J Dent 39:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogullari-Dirihan R, Hoshika T, Cadenaro M, et al. (2012). Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs, in vitro. J Dent Res 91:192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. (2013). Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater 29:116-135. [DOI] [PMC free article] [PubMed] [Google Scholar]


