Abstract
Resolvins are potent anti-inflammatory mediators derived from ω-3 fatty acids. Results from our previous studies indicated that resolvin D1 (RvD1) blocks pro-inflammatory responses in salivary glands. Furthermore, RvD1 enhances salivary epithelial integrity, demonstrating its potential use for the restoration of salivary gland function in Sjögren’s syndrome (SS). We investigated whether the RvD1 biosynthetic machinery (e.g., cytosolic phospholipase A2, calcium-independent phospholipase A2, 12/15 and 5-lipoxygenase) is expressed in mouse submandibular glands (mSMG), using qPCR and Western blot analyses. Additionally, we determined the localization of RvD1 biosynthetic machinery in mSMG and human minor salivary glands (hMSG), with and without SS, using confocal microscopy. Finally, we measured RvD1 levels in cell supernatants from mSMG cell cultures and freshly isolated mSMG cells, with and without SS, using ELISA. Our results indicate that: (1) RvD1 machinery is expressed in mouse and human salivary glands; (2) polar distribution of RvD1 biosynthetic machinery is lost in hMSG with SS; (3) RvD1 levels in mSMG cell culture supernatants increased with time; and (4) RvD1 levels in mSMG cell supernatants, with and without SS, were similar. These studies demonstrate that the RvD1 biosynthesis machinery is expressed and functional in salivary glands with and without SS.
Keywords: Sjögren’s syndrome, inflammation, cell biology, epithelia, lipids, resolvins
Introduction
Sjögren’s syndrome (SS) is a chronic inflammatory autoimmune disease characterized by dry mouth and dry eyes. Although extensive investigation has been done to understand the etiopathogenesis of SS, the exact causes of or cures for the disease are still unclear (Fox and Speight, 1996; Jonsson et al., 2002; Delaleu et al., 2005). Therefore, it is necessary to explore alternative mechanisms involved in SS progression and to develop novel therapies to restore secretory function.
Previous studies demonstrated that mammalian cells convert ω-3 polyunsaturated fatty acids (PUFAs) into resolvins, which are highly potent anti-inflammatory agents that regulate inflammation (Hong et al., 2003; Serhan et al., 2008). Compounds derived from eicosapentaenoic acid (EPA) are designated as resolvins of the E series, while those formed from the precursor docosahexaenoic acid (DHA) are denoted as resolvins of the D series (Serhan et al., 2002, 2008; Hong et al., 2003). We have shown that 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid (RvD1) (Sun et al., 2007) binds to the resolvin receptor ALX/FPR2 to promote resolution of salivary gland inflammation (Odusanwo et al., 2012; Nelson et al., 2014). Specifically, RvD1 blocked pro-inflammatory cytokine-mediated inflammation and enhanced epithelial integrity (i.e., increases barrier function and cell polarity) in the rat parotid gland cell line, Par-C10 (Odusanwo et al., 2012). More recently, we showed that RvD1 elicits similar effects in primary salivary cells (Nelson et al., 2014). Given that pro-inflammatory cytokines are known to be up-regulated in SS patients (Fox et al., 1994), RvD1 has the potential to resolve inflammation associated with SS and restore secretory function.
RvD1 is produced in resolving exudates in vivo by transcellular biosynthesis involving members of phospholipase A2 (PLA2) and lipoxygenases (LOX) (Serhan et al., 2002; Ariel and Serhan, 2007). More than 30 members of the PLA2 family have been identified in humans and mice within the following groups: (1) cytosolic PLA2 (cPLA2), (2) calcium-independent PLA2 (iPLA2), and (3) secretory PLA2 (sPLA2) (Burke and Dennis, 2009). RvD1 biosynthesis involves the release of DHA from membrane phospholipids by PLA2 (Shikano et al., 1993, 1994) (see Appendix Discussion and Appendix Fig. 1). Free DHA is also transported to the site of inflammation via lipoproteins coinciding with edema generation. (Kasuga et al., 2008). Thus, there are both membrane- and exudate-dependent mechanisms to produce the substrate used for RvD1 biosynthesis (Appendix Fig. 1). DHA is oxygenated via 12/15-LOX in mice (Kelavkar et al., 2004) or 15-LOX type-1 in humans (Hong et al., 2003; Kelavkar et al., 2004). This oxygenation is followed by the release of 17S-hydroperoxy-DHA from epithelial or endothelial cells to the extracellular environment and is captured by polymorphonuclear neutrophils (Krishnamoorthy et al., 2010). Finally, 17S-hydroperoxy-DHA is converted into RvD1 by the enzymatic action of 5-LOX, epoxidation, and a final hydrolysis (Hong et al., 2003, 2005) (Appendix Fig. 1).
RvD1 biosynthesis has been previously studied in mammalian whole blood, retina, and brain (Hong et al., 2003, 2005). However, RvD1 biosynthesis has not been previously described in salivary glands. In this paper, we explore the RvD1 biosynthetic machinery including its enzymes (i.e., cPLA2, iPLA2, 12/15 LOX, 15-LOX type-1, and 5-LOX) and product (RvD1) in salivary glands under normal and inflammatory conditions. We believe that a better understanding of pro-resolution pathways in salivary glands will allow us to develop new therapies for secretory hypofunction associated with SS.
Materials & Methods
Experimental Animals
Female mice were anesthetized with 80 to 100 mg/kg ketamine + 5 mg/kg xylazine and euthanized by abdominal exsanguination. Then, mSMG were removed and processed as described in the Appendix Methods. All animal usage, anesthesia, and surgeries were conducted under the protocol and approval of the State University of New York at Buffalo Institutional Animal Care and Use Committee (IACUC).
Human Specimens
Frozen human minor salivary glands (hMSG) were obtained from the SICCA Repository, University of California, San Francisco, and processed as described in the Appendix Materials & Methods. The use of the de-identified samples was approved by the Health Sciences Institutional Review Board under the exempt criterion 45 CFR 46.101(b)(4). Histological sections (10 µm) were prepared at The University at Buffalo, Histological Services, Department of Pathology and Anatomical Sciences.
qPCR Analyses
A customized PrimePCR assay was used to detect RvD1 biosynthetic machinery gene expression as recommended by the manufacturer (Bio-Rad, Hercules, CA, USA) and detailed in the Appendix Methods.
Western Blot Analyses
Western blot analyses were performed as described previously (McCall et al., 2013) and detailed in the Appendix Methods.
Confocal Microscopy Analyses
hMSG and mSMG sections were stained as previously described (McCall et al., 2013) and detailed in the Appendix Materials & Methods.
Quantification of RvD1 Levels
An enzyme-linked immunosorbent competition-based assay (ELISA) was used to detect RvD1 levels in the cell culture supernatant as described by the manufacturer (Cayman Chemical Company, Ann Arbor, MI, USA). This assay is based on the competition between free RvD1 (contained in cell culture medium) and an RvD1 tracer linked to acetylcholinesterase.
Statistical Analyses
Data are means ± SEM of results from 3 or more determinations. Data were analyzed by unpaired two-tailed t test or one-way analysis of variance (ANOVA), followed by linear trend analysis where p < .05 represents significant differences.
Results
RvD1 Biosynthetic Machinery Expression in Mouse Submandibular Gland Cells
To investigate whether enzymes involved in RvD1 biosynthesis were expressed in C57BL/6 mSMG, we performed qPCR and Western blot analyses. As shown in Figs. 1 and 2, all the enzymes involved in RvD1 biosynthesis were expressed in mSMG at the gene and protein levels.
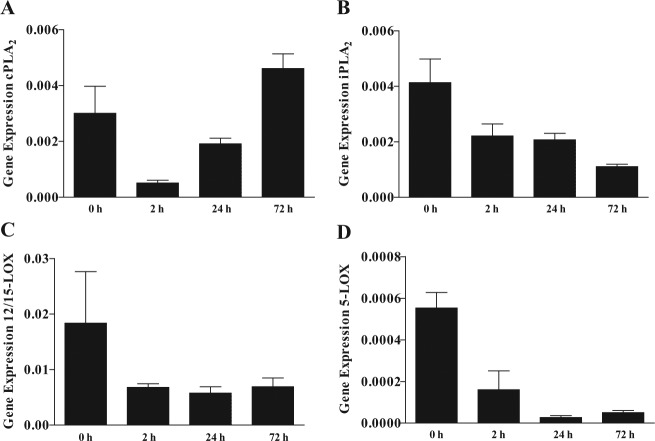
Figure 1.
Resolvin D1 (RvD1) biosynthetic machinery gene expression in mouse submandibular gland (mSMG) cells. C57BL/6 mSMG cells were cultured for 0 to 72 hr. Then, RNA was extracted as described in the Appendix Materials & Methods, and gene expression of (A) cPLA2, (B) iPLA2, (C) 12/15-LOX, and (D) 5-LOX was detected by means of a custom PrimePCR array (Bio-Rad). Data represent means ± SEM of at least 3 independent experiments, where p < .05 represents significant differences. Linear trend values are as follows: (A) R = 0.0003, R2 = 0.1672, p = .0389; (B) R = -0.0005, R2 = 0.6262, p = .0032; (C) R = -0.0018, R2 = 0.2178, p = .1349; and (D) R = <-0.0001, R2 = 0.6527, p = .0.003.
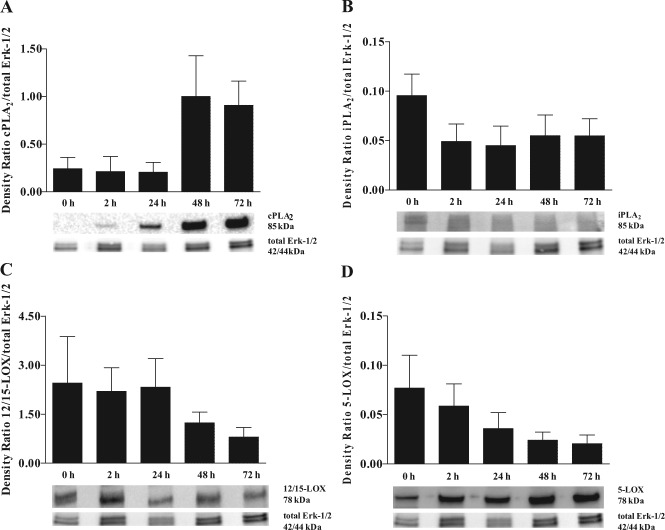
Figure 2.
Resolvin D1 (RvD1) biosynthetic machinery protein expression in mouse submandibular gland (mSMG) cells. C57BL/6 mSMG cells were subjected to lysis, and protein expression of (A) cPLA2, (B) iPLA2, (C) 12/15-LOX, (D) 5-LOX, and total Erk-1/2 was detected by Western blot analyses as described in the Appendix Materials & Methods. Data are expressed as the means ± SEM of results from 3 or more experiments, where p < .05 represents significant differences. Linear trend values are as follows: (A) R = 0.2120, R2 = 0.2104, p = .0109; (B) R = -0.0076, R2 = 0.0435, p = .2311; (C) R = -0.4263, R2 = 0.1432, p = .1282; and (D) R = -0.0147, R2= 0 .1734, p = .0297. Linear contrast enhancements of the images were made for aesthetic purposes, and scoring was performed on the original Western blot images.
To determine if time in culture affects the expression levels of enzymes involved in RvD1 biosynthesis, we used a short-term cell-culture system (Odusanwo et al., 2012). As shown in Figs. 1A and 2A, cPLA2 displayed an increasing linear trend between 0 and 72 hr in both gene (p = .0389) and protein expression (p = .0109). As shown in Figs. 1B and 2B, iPLA2 is expressed at low levels in culture, with a significant decreasing linear trend in gene expression levels with time in culture (p = .0032). However, no significant differences in iPLA2 protein expression (p = .2311) were observed between the different time points in culture.
12/15-LOX did not significantly change in gene (p = .1349) or protein expression (p = .1282) with time in culture (Figs. 1C, 2C). 5-LOX showed a significant linear decrease in gene (p = .0003) and protein expression (p = .0297) with time in culture (Figs. 1D, 2D). To determine whether the RvD1 biosynthetic machinery is altered during salivary gland inflammation (i.e., SS), we studied whole mSMG tissue from age-matched C57BL/6 and NOD/ShiLtJ strains. However, we found no significant differences in RvD1 machinery expression between these strains (Appendix Fig. 2 and Appendix Results).
RvD1 Biosynthetic Machinery Localization in Mouse and Human Salivary Glands
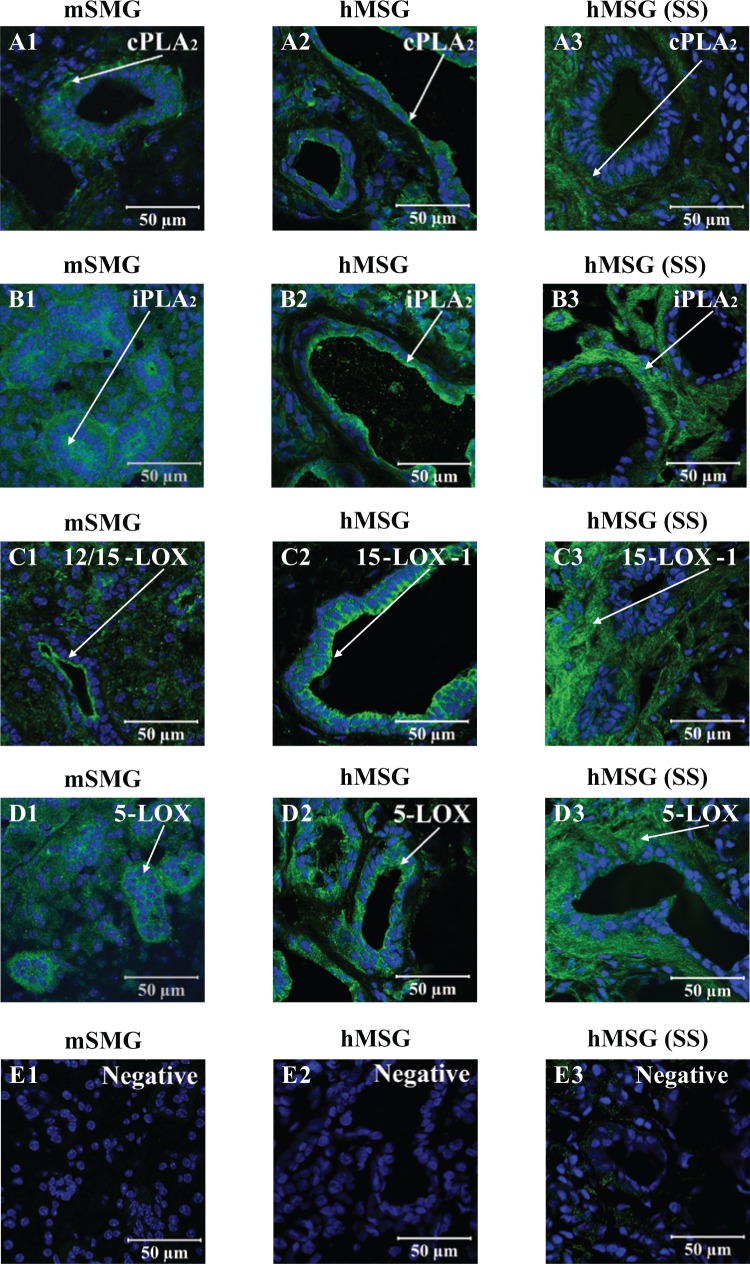
We examined the localization of cPLA2, iPLA2, 12/15-LOX (mouse tissues), 15-LOX type-1 (human tissues), and 5-LOX in mSMG from the C57BL/6 strain and hMSG from non-SS patients. As shown in Figs. 3A1 and 3A2, cPLA2 was distributed throughout the cytosol but was localized to the basolateral membrane in mSMG and to the apical membrane in hMSG. The enzyme iPLA2 showed cytosolic localization in mSMG (Fig. 3B1). In hMSG, iPLA2 showed cytosolic localization but also was strongly localized to the apical membrane (Fig. 3B2). The enzymes 12/15-LOX and 15-LOX type-1 both showed cytosolic expression and apical localization in mSMG (Fig. 3C1) and hMSG (Fig. 3C2), respectively. Finally, 5-LOX showed cytosolic localization in both mSMG (Fig. 3D1) and hMSG (Fig. 3D2). However, apical localization was observed in hMSG only (Fig. 3D2).
Figure 3.
Resolvin D1 (RvD1) biosynthetic machinery localization in salivary glands with and without Sjögren’s syndrome (SS). Frozen sections from mouse submandibular gland and human minor salivary glands with and without SS were subjected to immunofluorescent staining as described in the Appendix Materials & Methods. Sections were stained (A1, A2, and A3; green) with goat anti-rabbit anti-cPLA2, (B1, B2, and B3; green) goat anti-rabbit anti-iPLA2, (C1, C2, and C3; green) goat anti-rabbit anti-12/15-LOX, (D1; green) goat anti-sheep anti-15-LOX type-1, and (D2 and D3; green) goat anti-rabbit anti-5-LOX, followed by Hoechst or Propidium Iodide Nucleic Acid Stain (all images; blue). Images were obtained and analyzed with a Zeiss LSM 510 confocal microscope. White arrows indicate enzyme localization.
We also studied the expression of cPLA2, iPLA2, 15-LOX type-1, and 5-LOX in hMSG from SS patients. As shown in Fig. 3A3, cPLA2 displayed cytosolic localization in hMSG, which was in contrast to the apical expression observed in non-SS controls (Fig. 3A2). Similarly, iPLA2, 15-LOX type-1, and 5-LOX (Figs. 3B3, 3C3, and 3D3, respectively) were distributed through the cytosol and lacked the apicobasal polarity observed in hMSG without SS.
RvD1 Levels in Mouse Submandibular Gland Cell Supernatant
We measured RvD1 levels in C57BL/6 mSMG culture supernatants. As shown in Fig. 4A, RvD1 levels displayed a significant linear trend with time in culture (p = .0033). We also compared RvD1 levels in mSMG cell supernatants from age-matched C57BL/6 and NOD/ShiLtJ mice. As shown in Fig. 4B, the quantity of RvD1 in cell supernatants did not significantly change between C57BL/6 and NOD/ShiLtJ mice (p = .7341, Fig. 4B).
Figure 4.
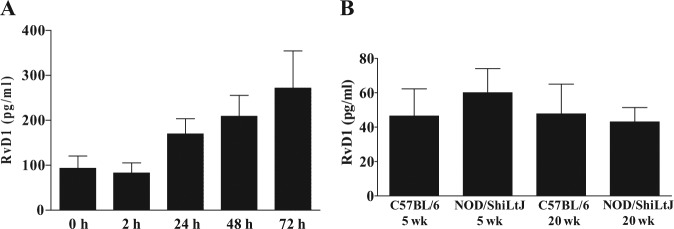
Resolvin D1 (RvD1) levels in mouse submandibular gland (mSMG) cell supernatants. RvD1 levels were detected in cell supernatants from (A) freshly isolated mSMG from C57BL/6 mice cultured for 0 to 72 hr and (B) freshly isolated mSMG from age-matched C57BL/6 and NOD/ShiLtJ mice. RvD1 levels were detected by ELISA as described in Materials & Methods. Data represent means ± SEM, where p < .05 represents significant differences. Linear trends are indicated by a red line with the following values: (A) R = 48.3500, R2 = 0.2486, p = .0033. t test analyses resulted in the following values: (B) 5 wk vs. 5 wk t(4) = 0.6442, p = .8816; 20 wk vs. 20 wk t(4) = 0.2435, p = .3787.
Discussion
Microbial challenges and/or physical injury causes up-regulation of pro-inflammatory cytokines, leading to inflammatory responses. One of these responses is the activation of PLA2 (Sun et al., 2004), which causes the release of DHA and allows for resolvin biosynthesis (Sun et al., 2007). Resolvins are agonists that promote resolution of inflammation by halting the recruitment of leukocytes (Serhan and Chiang, 2008). In SS, salivary glands fail to resolve inflammation, leading to autoimmunity and hypofunction (Voulgarelis and Tzioufas, 2010). In this study, we investigated RvD1 biosynthetic machinery in salivary glands. We observed that RvD1 biosynthetic machinery is expressed in mSMG and hMSG (Figs. 1-3). Furthermore, RvD1 biosynthesis increases with time in mSMG cell culture (Fig. 4A). These results are significant, given resolvins’ role in blocking cytokine signaling in several tissues, including corneal, renal, and salivary epithelium (Hassan and Gronert, 2009; Li et al., 2010; Odusanwo et al., 2012).
The enzymes PLA2 and LOX have been widely studied in many cell systems (Appendix Discussion). Investigating their expression patterns will allow us to understand resolvin biosynthesis in salivary glands. We used antibodies to detect resolvin biosynthetic enzymes at the group level, not at the individual subtype. This should be noted, since changes at the subtype level could have an effect that was not observed here. PLA2s are responsible for the cleavage of the acyl groups in the sn-2 position of membrane phospholipids. Specifically, cPLA2 has been shown to preferentially release arachidonic acid, and iPLA2 seems to mediate the release of DHA in rat neurons (Strokin et al., 2003). While there is evidence for PLA2 use in cleaving DHA from the cell membrane, a conclusive answer as to which group is needed for RvD1 biosynthesis is unknown. We examined 2 types of PLA2, cPLA2 and iPLA2, which are involved in the first step of lipid mediator synthesis (i.e., cleavage of DHA from cell membrane). The enzyme sPLA2 was not examined, since a broad-spectrum antibody was not commercially available at the time of this study. The enzyme cPLA2 was expressed in mSMG freshly isolated cells (Figs. 1A, 2A) and correlated with increasing levels of RvD1 with time in culture (Fig. 4A). These results suggest that cPLA2 up-regulation occurs in response to tissue damage and stress during culture. Interestingly, in whole-tissue lysates, we found that cPLA2 levels do not correlate with disease onset (i.e., whole mSMG tissue lysates 20-week NOD/ShiLtJ) (Appendix Fig. 2A). iPLA2 gene expression significantly decreased with time in culture (Fig. 1B). However, iPLA2 protein expression did not significantly change with time in culture (Fig. 2B). Similarly, in whole-tissue lysates, we found that iPLA2 levels do not correlate with disease onset (Appendix Fig. 2B). The differences observed in cPLA2 and iPLA2 expression in freshly isolated cells vs. whole-tissue lysates indicate that our in vitro model does not recapitulate the in vivo conditions.
The enzymes 12/15-LOX and 5-LOX were expressed in mSMG cells (Figs. 1, 2C, 2D), indicating that all the components necessary for RvD1 biosynthesis are present. In contrast to cPLA2, the expression of 12/15-LOX and 5-LOX decreased with time in culture and was not correlated with RvD1 levels (Fig. 4A). These results suggest cPLA2 as an important rate-limiting enzyme that catalyzes PUFAs release and initiates lipid mediator biosynthesis (Leslie, 1997). Therefore, cPLA2 activity is crucial for substrate availability while LOXs can still produce lipid mediators at low expression levels, since enzymatic activities can vary depending on different environmental factors such as substrate concentration (i.e., DHA). Further studies will be necessary to confirm this notion, since enzymatic activities were not studied here.
Localization of RvD1 machinery was determined in normal mSMG and hMSG. However, we did not observe any noteworthy differences between the 2 species, except for the localization of PLA2 isoforms (Figs. 3A2, 3B2). Regarding the RvD1 biosynthetic machinery localization, we observed a relatively strong fluorescent signal in the ducts. One possible explanation for the intense fluorescence in salivary ducts is that resolvins could be synthesized in ductal cells and directly transported into the oral cavity to promote resolution of inflammation. Further investigation detailing the presence of resolvins and lipoxins in human and mouse whole saliva needs to be conducted.
We observed a lack of polar distribution of the RvD1 biosynthetic machinery in hMSG with SS as compared with hMSG without SS (Figs. 3A3-3D3). These results are important, since the RvD1 biosynthetic enzymes are expressed in hMSG. However, the changes in protein localization in hMSG with SS may indicate that resolvins are being produced but not delivered to target cells.
We were able to detect RvD1 levels in supernatants of mSMG cell cultures (Fig. 4A), suggesting that RvD1 is biosynthesized and released by salivary cells. The present evidence supports the original biosynthetic scheme that utilizes DHA and EPA during the resolution of inflammation (Serhan et al., 2002). These studies led to the structural elucidation of RvD1 and its important role in enhancing the pro-resolution status (Sun et al., 2007). The environmental triggers for RvD1 production and release into our culture system are unknown; however, the stress of salivary cells in culture may trigger RvD1 release. It is important to note that not all RvD1-machinery protein expression correlates with RvD1 levels. This effect might be due to changes in the enzymatic activities of RvD1-related biosynthetic machinery (which were not measured in this study).
We observed no significant differences in RvD1 production (Fig. 4B) between C57BL/6 and NOD/ShiLtJ freshly isolated mSMG, indicating that the RvD1 biosynthetic machinery remains intact in SS despite the presence of chronic inflammation. These results do not explain why we still have chronic inflammation in SS. We speculate that other parts of the resolution systems are being affected, such as failure of the resolvin receptors to signal correctly. It is also possible that in glands with SS, RvD1 is produced but is released to the wrong site as the machinery localization loses its polar distribution in humans (Figs. 3A3-3D3). These results indicate that the polar distribution of RvD1 machinery might be important for the resolution of inflammation.
In summary, we were able to demonstrate for the first time that the RvD1 biosynthetic machinery is expressed and active in salivary epithelium under both normal and inflammatory conditions. These results, together with those from our previous studies [showing RvD1 potent anti-inflammatory and pro-resolving actions in salivary glands (Odusanwo et al., 2012; Nelson et al., 2014)], are highly significant in light of the current lack of effective treatment for salivary inflammation associated with SS. In addition, a timely induction of this pro-resolving environment will limit fibrosis (Fredman and Serhan, 2011), which often occurs in patients with permanent loss of salivary function (Avouac et al., 2006). Understanding resolvin biosynthesis will allow future studies to enhance RvD1 production in vitro and in vivo.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the NIH-NIDCR grants R21-DE19721-01A1, 1R01DE022971-01, and 1R01DE021697-01A (to OJB). Data and specimens used in this study are from the Sjögren’s International Collaborative Clinical Alliance (SICCA), funded under contract N01 DE-32636 by the National Institute of Dental and Craniofacial Research, with funding support from the National Eye Institute and Office for Research in Women’s Health. The new ACR criteria (Arthritis Care & Research, Vol. 64, No. 4, April 2012, pp. 475-487) were used for sample classification.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ariel A, Serhan CN. (2007). Resolvins and protectins in the termination program of acute inflammation. Trends Immunol 28:176-183. [DOI] [PubMed] [Google Scholar]
- Avouac J, Sordet C, Depinay C, Ardizonne M, Vacher-Lavenu M, Sibilia J, et al. (2006). Systemic sclerosis-associated Sjögren’s syndrome and relationship to the limited cutaneous subtype: results of a prospective study of sicca syndrome in 133 consecutive patients. Arthritis Rheum 54:2243-2249. [DOI] [PubMed] [Google Scholar]
- Burke JE, Dennis EA. (2009). Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 50(Suppl):S237-S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaleu N, Jonsson R, Koller MM. (2005). Sjögren’s syndrome. Eur J Oral Sci 113:101-113. [DOI] [PubMed] [Google Scholar]
- Fox PC, Speight PM. (1996). Current concepts of autoimmune exocrinopathy: immunologic mechanisms in the salivary pathology of Sjögren’s syndrome. Crit Rev Oral Biol Med 7:144-158. [DOI] [PubMed] [Google Scholar]
- Fox RI, Kang HI, Ando D, Abrams J, Pisa E. (1994). Cytokine mRNA expression in salivary gland biopsies of Sjögren’s syndrome. J Immunol 152:5532-5539. [PubMed] [Google Scholar]
- Fredman G, Serhan CN. (2011). Specialized pro-resolving mediators: wiring the circuitry of effector immune and tissue homeostasis. Endodontic Topics 24:39-58. [Google Scholar]
- Hassan IR, Gronert K. (2009). Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J Immunol 182:3223-3232. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. (2003). Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 278:14677-14687. [DOI] [PubMed] [Google Scholar]
- Hong S, Tjonahen E, Morgan EL, Lu Y, Serhan CN, Rowley AF. (2005). Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins—mediator lipidomic analysis. Prostaglandins Other Lipid Mediat 78:107-116. [DOI] [PubMed] [Google Scholar]
- Jonsson R, Moen K, Vestrheim D, Szodoray P. (2002). Current issues in Sjögren’s syndrome. Oral Dis 8:130-140. [DOI] [PubMed] [Google Scholar]
- Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, et al. (2008). Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol 181:8677-8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelavkar UP, Glasgow W, Olson SJ, Foster BA, Shappell SB. (2004). Overexpression of 12/15-lipoxygenase, an ortholog of human 15-lipoxygenase-1, in the prostate tumors of TRAMP mice. Neoplasia 6:821-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. (2010). Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci USA 107:1660-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie CC. (1997). Properties and regulation of cytosolic phospholipase A2. J Biol Chem 272:16709-16712. [DOI] [PubMed] [Google Scholar]
- Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. (2010). Resolvin e1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther 26:431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall AD, Nelson JW, Leigh NJ, Duffey ME, Lei P, Andreadis ST, et al. (2013). Growth factors polymerized within fibrin hydrogel promote amylase production in parotid cells. Tissue Eng Part A 19:2215-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J, Leigh N, Mellas R, McCall A, Aguirre A, Baker OJ. (2014). The ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am J Physiol Cell Physiol 306:C178-C185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. (2012). Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. Am J Physiol Cell Physiol 302:C1331-C1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. (2008). Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol 153(Suppl 1):S200-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. (2002). Resolvins – a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196:1025-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. (2008). Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikano M, Masuzawa Y, Yazawa K. (1993). Effect of docosahexaenoic acid on the generation of platelet-activating factor by eosinophilic leukemia cells, Eol-1. J Immunol 150(8 Pt 1):3525-3533. [PubMed] [Google Scholar]
- Shikano M, Masuzawa Y, Yazawa K, Takayama K, Kudo I, Inoue K. (1994). Complete discrimination of docosahexaenoate from arachidonate by 85 kDa cytosolic phospholipase A2 during the hydrolysis of diacyl- and alkenylacylglycerophosphoethanolamine. Biochim Biophys Acta 1212:211-216. [DOI] [PubMed] [Google Scholar]
- Strokin M, Sergeeva M, Reiser G. (2003). Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+. Br J Pharmacol 139:1014-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Simonyi A. (2004). Phospholipase A2 in the central nervous system implications for neurodegenerative diseases. J Lipid Res 45:205-213. [DOI] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, et al. (2007). Resolvin D1 and its aspirin-triggered 17R epimer stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem 282:9323-9334. [DOI] [PubMed] [Google Scholar]
- Voulgarelis M, Tzioufas AG. (2010). Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat Rev Rheumatol 6:529-537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.