Abstract
Enamel formation produces the most highly mineralized tissue in the human body. The growth of enamel crystallites is assisted by enamel proteins and proteinases. As enamel formation progresses from secretory to maturation stages, the composition of the matrix with its mineral and non-mineral components dynamically changes in an inverse fashion. We hypothesized that appropriately calibrated micro-computed tomography (µCT) technology is suitable to estimate the mineral content (weight and/or density) and volume comparable in accuracy with that for directly weighed and sectioned enamel. Different sets of mouse mandibular incisors of C57BL/6 mice were used for dissections and µCT reconstructions. Calibration phantoms corresponding to the range of enamel mineral densities were used. Secretory-stage enamel contained little mineral and was consequently too poor in contrast for enamel volumes to be accurately estimated by µCT. Maturation-stage enamel, however, showed remarkable correspondence for total mineral content per volume where comparisons were possible between and among the different analytical techniques used. The main advantages of the µCT approach are that it is non-destructive, time-efficient, and can monitor changes in mineral content of the most mature enamel, which is too physically hard to dissect away from the tooth.
Keywords: enamel biomineralization/formation, mineralized tissue/development, micro-computed tomography, dental enamel, tooth, minerals, hydroxyapatite
Introduction
Among the mineralized tissues of the human body, enamel has the highest concentration of mineral. Although mature enamel consists almost solely of hydroxyapatite, it does contain minute amounts of non-mineral components in the form of residual organic material and water (enamel fluid) within residual microspaces. This composition provides the unique durability and resistance to mature enamel needed to resist occlusal forces during mastication. Both the volume and the quantity of mineral present in enamel are controlled by columnar ameloblasts of epithelial origin. The prerequisites for mineralization events are the deposition of both enamel proteins and proteinases (Caterina et al., 2002; Fukumoto et al., 2004; Hu et al., 2008; Simmer et al., 2009; Smith et al., 2011). During the secretory stage, the enamel layer gains simultaneously in volume and in mineral content through the apposition, cleavage, and removal of enamel proteins, and the elongation of crystallites (Smith et al., 1989). The volume and shape of the enamel layer are controlled by ameloblasts that outline a confined space against the mineralized dentin. The initial precipitation and later volumetric growth of hydroxyapatite crystallites during the maturation stage are monitored by ameloblasts through pathways of ion metabolism (Smith, 1998; Lacruz et al., 2013). The removal of organic material and the accumulation of mineral across the plasma membrane of ameloblasts are coupled (Smith and Nanci, 1996), and culminate in fully mature enamel (Smith et al., 1989).
Direct measurements of mineral content and enamel volume are complicated, since enamel is bonded to the dentin to form a bilayered biomineral across the crown of the tooth. Invasive and non-invasive means have been devised to determine these parameters. A validated model for forming enamel and ameloblast stages is the rodent incisor in combination with microdissection of enamel, since it displays all developmental stages as it erupts continuously (Robinson et al., 1971, 1977; Smith and Nanci, 1989). In this approach, enamel is lifted from dentin in 1-mm–wide strips from secretory through maturation stages (Smith and Nanci, 1989). Weight changes measured before and after heating to a specific temperature (ashing) provide information on the mineral and non-mineral content related to normal and pathological conditions during the course of amelogenesis (Smith et al., 2009). This technique is destructive, extremely time-consuming, and lacks reproducible inter-examiner demands on hand-skills.
Micro-computed x-ray tomography (µCT) is a non-destructive method that exploits differences in x-ray intensity before and after passing through an object (Zou et al., 2011). Differences in x-ray attenuation represent an object’s density, but thickness can affect these assessments as well (Fajardo et al., 2009). During assessment of mineral density, the attenuation differences of an object are converted to generate an image, and each pixel is assigned a grayscale value corresponding to the linear attenuation coefficient (LAC). From the LAC, the density of an object at any location can be calculated (Fajardo et al., 2002). In the past, the enamel had to be thinly sectioned for a 2-dimensional (2D) image to be obtained, but current desktop µCT systems can reconstruct intact objects in 3 dimensions with microscopic pixel sizes. The validity of density measurements relies on calibration with high-density hydroxyapatite (HA) standards (= phantoms) (Schweizer et al., 2007). It is technically challenging to fabricate phantoms of densities comparable with those of enamel because, at these high densities, the material is predominantly mineral, and specialized equipment is required to bond the crystals at high heat and/or high pressure. In the past, investigators were forced to assume that enamel mineral is equivalent to stoichiometric HA or to derive enamel density from chemical formulations (Angmar et al., 1963; Robinson et al., 1971; Elliott et al., 1998). Until recently, the ashing method, which is considered the gold standard, and µCT have not been compared for enamel. The hypothesis of this study was that the volume, mineral weight, and mineral density of forming enamel can be analyzed by calibrated polychromatic µCT technology and that results from µCT correspond well to direct measurements done by ashing of enamel strips and backscattered electron microscopy.
Materials & Methods
Protocol Approval
Protocols for the use and handling of animals were approved by the IACUC of the University of Texas Health Science Center at San Antonio and the University of Michigan. This study complied with ARRIVE guidelines for pre-clinical studies.
Animals
In total, 23 wild-type mice (C57BL/6 background) at 7 wks old were used. They were fed hard chow (7012 Teklad L-M485, Harlan Laboratories, Indianapolis, IN, USA) and maintained on a standard 12-hour light/dark cycle. Animals for µCT scanning were intracardially perfused with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) with 0.2% glutaraldehyde, pH 7.4, and the hemi-mandibles were removed and stored at 4°C in PBS containing 0.2% PFA, pH 7.4. Hemi-mandibles from different animals were used for sections (n = 3), ash weight (n = 12), and µCT (n = 8) experiments (Appendix Fig. 1).
Microdissection, Ashing, Sectioning, and Calculation of Enamel Mineral Density
Data on enamel mineral weights per mm and the ashing technique used to obtain them have been reported previously (Smith et al., 2009). To estimate enamel volumes per mm, we embedded hemi-mandibles from 3 wild-type mice in epoxy resin and cut them into eight 1-mm–long segments, starting near the apical loop. Enamel segments (ES) containing the incisor cross-sections were polished with up to 1,200-grit carbide paper and finished with a diamond suspension having a particle size of 1 µm (South Bay Technology, San Clemente, CA, USA). Images of the incisor cross-sections were obtained in backscattered mode with a Hitachi S-3000 N scanning electron microscope (15 kV, 20 Pa) (BSE). The area of the enamel was outlined (CellSens, Olympus, Center Valley, PA, USA), and the enamel volumes were calculated between 2 cross-sections in 1-mm intervals (= integrated volume). We calculated mineral density per mm by dividing ashed mineral weight by the volume estimated from the BSE measurements for the same location.
µCT Scanning, Object Reconstruction, Contouring of Enamel, and Calibration
Hemi-mandibles with attached soft tissues were scanned in 70% ethanol in a desktop SkyScan 1172 (Bruker SkyScan, Aartselaar, Belgium) system. Each specimen was positioned with the incisal edge pointing superiorly, and the tube was sealed with Parafilm (American National Can, Greenwich, CT, USA). Samples were scanned at 60 kV, 167 µA beam intensity, 5-µm image pixel size, a 0.35° rotation step, 7 frame averaging, and a 1,090-millisecond exposure time at each step. A 0.5-mm aluminum filter was used during scans (Kovács et al., 2009). A polynomial correction was also used to reduce beam-hardening effects during reconstructions (Kovács et al., 2009; Zou et al., 2011). These 2 protocols reduced the beam-hardening artifact (Appendix Figs. 2, 3). The images were reconstructed with NRecon (Bruker SkyScan, Aartselaar, Belgium) with a Feldkamp cone-beam algorithm (Feldkamp et al., 1984).
Successive 1-mm–long volumes of interest were created, starting at 75% of the distance between the opening of the apical foramen and the distal boundary of the last mandibular molar at the level of the cement-enamel junction (Fig. 1B). In each volume of interest (VOI), the enamel boundary was manually outlined, with non-enamel objects excluded. Volumes of interest correspond to ES. Enamel volumes were determined from the manually drawn volumes of interest. Intra-operator error and inter-operator differences in volumes of interest were determined for the early, mid-, and late maturation stages (Appendix Table 1). HA phantoms (0.25 and 0.75 g/cm3 (SkyScan), and 2.927 g/cm3 (Himed, Bethpage, NY, USA) were used to calibrate mineral density analyses (Appendix Table 2; Appendix Fig. 4).
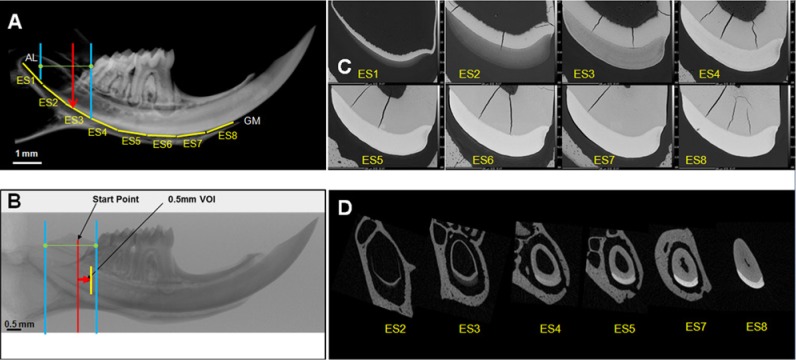
Figure 1.
Enamel of mouse mandibular incisors gains thickness and mineral density (from left to right). (A) Hemi-mandibles in seven-week-old mice are a little over 11 mm in total length. The region containing developing enamel was sawed into eight 1-mm–long cross-sectional ESs (yellow horizontal lines, ES1-8) from near the apical loop (AL) to the gingival margin (GM). The average volumes per ES were then estimated from sequential area measurements of the cross-sectioned enamel faces. (B) For µCT, a series of 0.5-mm-long enamel volumes of interest (VOI) started at 75% of the distance between the apical loop (blue line, left) and the distal aspect of the third molar crown (blue line, right). From the start point (red line), the first VOI (yellow line) was generated (red arrow). (C) Examples of eight 1-mm–long ESs imaged by BSE. (D) Examples of µCT reconstructed trans-axial images of incisor enamel.
Volumetric Percent of Mineral and Non-mineral Components
Parameters that were directly measured from mice included the (1) gross weight of enamel mineral per mm (by ashing), (2) enamel volume per mm (by BSE), and (3) mineral density and estimated enamel volume per mm (by µCT). Several derived values were also calculated, as explained in the footnotes to the Table.
Statistical Analyses
Means and standard deviations were calculated in Excel and GraphPad InStat (La Jolla, CA, USA). Unpaired t tests and analyses of variance (ANOVAs) were used to compare volume and mineral density measurements. Paired t tests were used to assess inter-operator differences. The intra-operator error was expressed by the coefficient of variation. Alpha was set to 0.05.
Results
Enamel Volume
Incisor enamel volume increased across the secretory stage (ES1-2), as seen in BSE images (Fig. 1). Sectioned and µCT-imaged enamel volumes showed overall agreement; the µCT-based enamel volumes were 4 to 12% lower than the sectioned volume estimates (Fig. 2). Volume peaked in the mid-maturation stage (ES4) for sectioned and µCT-based analyses (Table). Across the maturation stage, enamel volume shrank steadily, losing about 5% to 8% of total volume in the most mature enamel (ES8).
Figure 2.
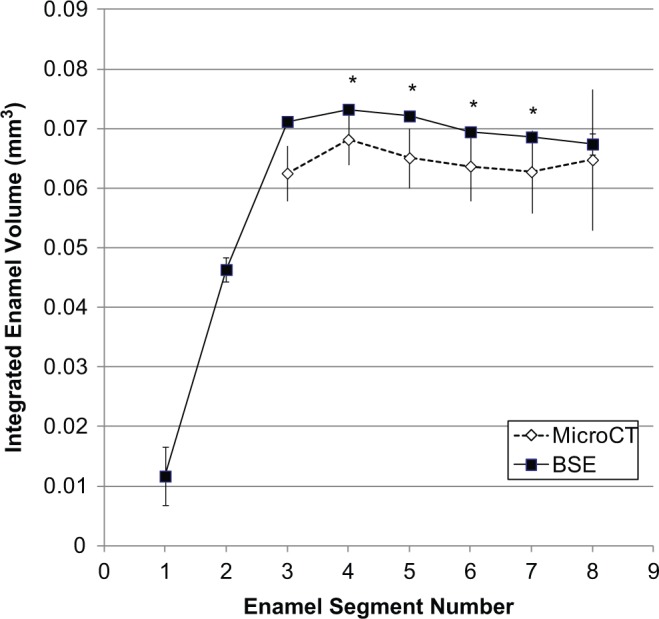
Comparison of integrated incisor enamel volume determined by BSE and µCT. BSE- and µCT-determined volumes of interest show agreement in the course of enamel formation. The earliest enamel mineral detected by µCT was at volume number 3. The volumes in ES 4-7 were statistically different. *p ≤ .05.
Table.
Enamel Volume and Mineral Content by Ashing Combined with BSE Compared with µCT Alone
| Stage | ESS | M/LS | EM | MM | MM | LM | LM | LM |
|---|---|---|---|---|---|---|---|---|
| Enamel Segment (1 mm) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| “Classic” % Mineral1 | 30 | 50 | 75 | 90 | 95 | 95 | 95 | 95 |
| BSE n = 3 | ||||||||
| Estimated Volume (mm3) | ||||||||
| Mean | 0.01165 | 0.04634 | 0.07120 | 0.07324 | 0.07211 | 0.06945 | 0.06860 | 0.06741 |
| SD | 0.00492 | 0.00204 | 0.00094 | 0.00012 | 0.00024 | 0.00027 | 0.00040 | 0.00176 |
| Expected Mineral Weight2 (µg) | 11.0 | 73.1 | 168.5 | 208.0 | 216.2 | 208.2 | 205.7 | 202.1 |
| Ashing n = 12 | ||||||||
| Measured Mineral Weight (µg) | ||||||||
| Mean | 2.27192 | 16.76667 | 42.54167 | 81.16333 | 133.54167 | CND | CND | CND |
| SD | 1.45047 | 5.82757 | 12.99094 | 23.63171 | 20.95564 | |||
| BSE-Ashing | ||||||||
| Estimated Non-mineral Components | ||||||||
| Volume3 (mm3) | 0.00924 | 0.03571 | 0.05323 | 0.04467 | 0.02757 | – | – | – |
| % Volume4 | 79.31 | 77.06 | 74.76 | 60.99 | 38.23 | – | – | – |
| % Mineral5 | 20.69 | 22.94 | 25.24 | 39.01 | 61.77 | |||
| Estimated Mineral Weight6 (µg) | 1.54 | 7.69 | 14.32 | 35.18 | 86.83 | – | – | – |
| MicroCT n = 8 | ||||||||
| Computed Volume3 (mm3) | ||||||||
| Mean | – | – | 0.06251 | 0.06817 | 0.06509 | 0.06369 | 0.06275 | 0.06477 |
| SD | 0.01068 | 0.00489 | 0.00462 | 0.00427 | 0.00496 | 0.00574 | ||
| Computed Mineral Weight (µg) | ||||||||
| Observed | – | – | 44.48067 | 85.91591 | 133.93322 | 168.64554 | 182.44505 | 192.12524 |
| SD | 11.19431 | 23.91890 | 17.26646 | 9.734022 | 12.12689 | 16.35890 | ||
| Non-mineral Components | ||||||||
| Volume (mm3) | – | – | 0.04601 | 0.04001 | 0.02477 | 0.01210 | 0.00709 | 0.00320 |
| % Volume4 | 73.60 | 58.69 | 38.05 | 19.00 | 11.30 | 4.94 | ||
| Mineral | ||||||||
| % Mineral5 | – | – | 26.40 | 41.31 | 61.95 | 81.00 | 88.70 | 95.06 |
“Classic” % mineral was derived from historical evidence. The expected mineral weight (µg) was derived from the volume and the estimated % mineral. ESS, early secretory stage; MS, mid-secretory stage; LS, late secretory stage; EM, early maturation stage; MM, mid-maturation stage; LM, late maturation stage; and CND, could not dissect, enamel too hard.
Calculations
“Classic”% mineral was derived from previously published ashing data.
Expected mineral weight (µg) was derived from the measured volumes and the estimated % mineral.
Estimated non-mineral components represents organic material and water. It reflects the ratio of observed to expected mineral weight in consideration of the average volume for an enamel slice, according to:
Percentage volume of non-minerals was calculated as:
% Mineral was estimated as:
For microCT, the measured parameters were volume and mineral density, from which expected mineral weights could be computed as:
Estimated mineral weight was derived from:
Enamel Mineral Weight, % Mineral, and % Non-mineral Components
Mineral weight measured from enamel strips ranged between 2.27 ± 1.45 µg (ES1) and 133.54 ± 20.95 µg (ES5). The enamel could not be dissected from the underlying dentin after ES5; the enamel was too hard to remove. The mineral content by volume increased from 20.69% (ES1) to 61.77% (ES5), with the greatest accumulation occurring after the secretory stage when the ameloblasts transitioned into maturation stage. The percentage of non-mineral components by volume correspondingly decreased from 79.31% in ES1 to 38.23% in ES5. µCT-determined volumes corresponded with ashing-derived volumes across the maturation stage (ES3 onward). The calculated mineral weight increased from 44.48 ± 11.19 µg (ES3) to 192.12 ± 16.35 µg in mature enamel near the gingival margin (ES8). This represented increases in the % mineral by volume from 26.40% to 95.06%.
Enamel Mineral Density
Filtration and post-processing protocols effectively reduced the influence of a beam-hardening cupping artifact (Appendix Figs. 2, 3). Enamel mineral density increased in a sigmoidal pattern from the secretory (ES1+2) (Ash/BSE) to the late-maturation stage (ES4-8) (Fig. 3). Enamel mineral density peaked at 2.97 ± 0.05 mg HA/mm3 near the gingival margin (ES8) (µCT/µCT) (Appendix Table 3). Enamel mineral densities in ES 3-5 were statistically similar between ash/BSE and µCT/µCT, but µCT estimates of enamel mineral density were slightly higher. Enamel mineral density discrepancies fell to 2%, 5%, and almost 1% for these same segments when ash/µCT and µCT/µCT enamel mineral densities were compared. This latter comparison highlights the similarity in the mineral content calculation between the ash and the µCT methods.
Figure 3.
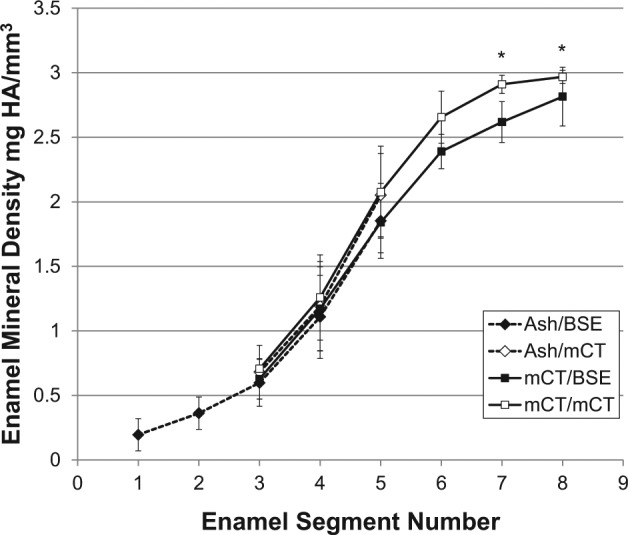
Changes in mineral density of forming enamel by ashing (n = 12) combined with BSE or µCT compared with µCT combined with BSE or µCT alone (n = 8). Mineral density was calculated from mineral weight by ashing or µCT combined with enamel volume derived by either BSE or µCT. *p ≤ .05. For numeric mineral density values, see Appendix Table 3.
Inter- and intra-operator variability was minimal (Appendix Table 3). Also, the effect of a VOI radial pixel peel to minimize partial volume boundary effects showed small but significant differences among peel settings (Appendix Fig. 5).
Discussion
Enamel microdissection was first developed to measure the bulk mineral content in mature enamel (Weidmann et al., 1967) and was later modified to sample developing enamel from rodent incisors (Robinson et al., 1977; Smith and Nanci, 1989). While this technique is the gold standard, it is inherently destructive, is time-consuming (Appendix Table 4), and requires advanced technical skills to process small and frail enamel strips. µCT mineral estimates correspond very well with the ashing technique. Comparisons of results (ash/BSE - µCT/µCT and ash/µCT - µCT/µCT) obtained by 3 different approaches (ash, BSE, µCT) support this argument (Fig. 3). Ash/BSE and µCT/µCT results are statistically similar but showed actual percentage differences of 15%, 12%, and 11% for ES 3-5. These differences in mineral density are the result of volumetric differences between BSE and µCT, since they were reduced to 2%, 5%, and approximately 1% when mineral content estimates from ashing and µCT were divided by µCT-based volumes. Moreover, this similarity suggests that the significant differences observed between µCT/BSE and µCT/µCT densities in segments 7 and 8 originate from volume measurement discrepancies rather than differences in mineral weight.
µCT-based mineral density values showed a sigmoidal pattern through incisor development. The values ranged between 0.7 mg HA/mm3 and 2.97 mg HA/mm3. This range could be measured because we used phantoms with a broad range of densities (Schweizer et al., 2007). It is important to note that the 2.97 mg/mm3 reached in the late maturation stage was lower than previously reported enamel densities based on assumptions about the similarity between enamel and hydroxyapatite (3.15 g/cm3; Angmar et al., 1963) or estimates based on the chemical composition of hydroxyapatite with bound water (2.99 g/cm3; Elliott et al., 1998). This peak value, however, is higher than reports on human (Clementino-Luedemann and Kunzelmann, 2006) and rat enamel (Wong et al., 2000), based on aluminum wire reference material. Recently, murine enamel incisor mineral density encased in a methylmethacrylate block was reported to be 2.7 g/cm3, based on calibrations with hydroxyapatite phantoms ranging from 0 to 1.2 g/cm3 (Bronckers et al., 2013). Given this context, the values reported here sit firmly within the range of reported mature enamel densities.
Enamel maturation is a slow process. To reach the final mineral content, ameloblasts devote 65% of their time to the maturation stage. The % mineral in enamel by volume has been reported to range from 86.2% (vol) (Angmar et al., 1963) to 92.9% (vol) (Elliott et al., 1998). These values are greatly influenced by the model used for enamel composition and mineral density (Angmar et al., 1963; Elliott et al., 1998). By combining volumetric measurements from ground enamel sections and calibrated µCT, we bypassed the need for modeling the enamel mineral, arriving at 95.06% mineral by volume in mature enamel.
The influence of beam-hardening on µCT-based measurements of mineral density is well-known (Burghardt et al., 2008; Nazarian et al., 2008; Fajardo et al., 2009; Kovács et al., 2009; Zou et al., 2011; Hamba et al., 2012). Filtration aims to eliminate low-energy photons emitted by the polychromatic source that contribute to the beam-hardening artifact (Zou et al., 2011). Similar to Kovács et al. (2009), a 0.5-aluminum filter was used to reduce beam-hardening effects in teeth. The aluminum filter combined with the polynomial correction reduced beam hardening in test images (Appendix Figs. 3, 4) and reduced noise (Meganck et al., 2009). Others have recommended the use of copper or aluminum-copper filters for the analysis of teeth and bones (Meganck et al., 2009; Hamba et al., 2012); however, these filters increased scan time over four-fold while providing minimal improvement over aluminum in terms of beam-hardening reduction.
Manual contouring of VOIs for µCT analysis is subject to inter- and intra-operator error. VOI precision is critical in these analyses. Values for enamel mineral density were highly repeatable within and between operators. Enamel volume measurements were more sensitive to user error. The greatest variation was found during the early maturation stage due to low quantity and low mineralization of enamel, resulting in poor boundary contrast in µCT images. These results suggest that µCT protocols for contouring tissue interfaces should be performed by a single operator. Future work may require a second set of scan parameters for better imaging of the secretory stage.
µCT offers many advantages over conventional methodologies for estimating enamel density. The time to scan and analyze one sample was approximately 4 hr, a ten-fold reduction in labor time compared with that necessary for microdissection and ashing (Appendix Table 4). µCT can perform volumetric analyses as opposed to 2D estimates of 3D volumes. It is a non-destructive modality, and the same samples can be used for multiple assays. Finally, erupted enamel is the endpoint of enamel formation and is critical to mastication. µCT is capable of analyzing this highly mineralized enamel, which is not feasible with microdissection. However, µCT is limited in its ability to analyze secretory-stage enamel due to the similarity in densities between enamel and dentin. Furthermore, the protocol described here requires that animals be euthanized, which restricts longitudinal studies.
The murine incisor offers an opportunity for the study of genetic mutations in enamel formation that are modeling human amelogenesis imperfecta (Wright et al., 2009). Manual methods such as ashing and the imaging of sectioned samples have been the gold standard for mineral analyses of this tissue. µCT offers a suitable alternative that can accurately quantify mineral content and density while providing many time, cost, and specimen advantages (potentially multiple use). It is concluded that properly calibrated µCT images are as accurate as labor-intensive methods for estimating mineral content and density.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by K08 DE022800 (YPC) and S10 RR025687-01A1 (RJF) from the National Institutes of Health, Bethesda, MD, USA. This work was also sponsored by the UTHSCSA Department of Periodontics and the UTHSCSA program for Research Core Laboratories.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Angmar B, Carlström D, Glas JE. (1963). Studies on the ultrastructure of dental enamel. IV. The mineralization of normal human enamel. J Ultrastruct Res 8:12-23. [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Gueneli N, Lüllmann-Rauch R, Schneppenheim J, Morau AP, Himmerkus N, et al. (2013). The intramembrane protease SPPLA is critical for tooth enamel formation. J Bone Miner Res 28:1622-1630. [DOI] [PubMed] [Google Scholar]
- Burghardt AJ, Kazakia GJ, Laib A, Majumdar S. (2008). Quantitative assessment of bone tissue mineralization with polychromatic micro-computed tomography. Calcif Tissue Int 83:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, et al. (2002). Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 277:49598-49604. [DOI] [PubMed] [Google Scholar]
- Clementino-Luedemann TN, Kunzelmann KH. (2006). Mineral concentration of natural human teeth by a commercial micro-CT. Dent Mater J 25:113-119. [DOI] [PubMed] [Google Scholar]
- Elliott JC, Wong FS, Anderson P, Davis GR, Dowker SE. (1998). Determination of mineral concentration in dental enamel from x-ray attenuation measurements. Connect Tissue Res 38:61-72. [DOI] [PubMed] [Google Scholar]
- Fajardo RJ, Ryan TM, Kappelman J. (2002). Assessing the accuracy of high-resolution x-ray computed tomography of primate trabecular bone by comparisons with histological sections. Am J Phys Anthropol 118:1-10. [DOI] [PubMed] [Google Scholar]
- Fajardo RJ, Cory E, Patel ND, Nazarian A, Laib A, Manoharan RK, et al. (2009). Specimen size and porosity can introduce error into microCT-based tissue mineral density measurements. Bone 44:176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkamp LA, Davis LC, Kress JW. (1984). Practical cone-beam algorithm. J Opt Soc Am A 1:612-619. [Google Scholar]
- Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, et al. (2004). Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol 167:973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamba H, Nikaido T, Sadr A, Nakashima S, Tagami J. (2012). Enamel lesion parameter correlations between polychromatic micro-CT and TMR. J Dent Res 91:586-591. [DOI] [PubMed] [Google Scholar]
- Hu JC, Hu Y, Smith CE, McKee MD, Wright JT, Yamakoshi Y, et al. (2008). Enamel defects and ameloblast-specific expression in Enam knock-out/lacz knock-in mice. J Biol Chem 283:10858-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács M, Danyi R, Erdélyi M, Fejérdy P, Dobó-Nagy C. (2009). Distortional effect of beam-hardening artefacts on microCT: a simulation study based on an in vitro caries model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:591-599. [DOI] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Kurtz I, Hubbard MJ, Paine ML. (2013). New paradigms on the transport functions of maturation-stage ameloblasts. J Dent Res 92:122-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganck JA, Kozloff KM, Thornton MM, Broski SM, Goldstein SA. (2009). Beam hardening artifacts in micro-computed tomography scanning can be reduced by x-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone 45:1104-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Snyder BD, Zurakowski D, Müller R. (2008). Quantitative micro-computed tomography: a non-invasive method to assess equivalent bone mineral density. Bone 43:302-311. [DOI] [PubMed] [Google Scholar]
- Robinson C, Weatherell JA, Hallsworth AS. (1971). Variation in composition of dental enamel within thin ground tooth sections. Caries Res 5:44-57. [DOI] [PubMed] [Google Scholar]
- Robinson C, Lowe NR, Weatherell JA. (1977). Changes in amino-acid composition of developing rat incisor enamel. Calcif Tissue Res 23:19-31. [DOI] [PubMed] [Google Scholar]
- Schweizer S, Hattendorf B, Schneider P, Aeschlimann B, Gauckler L, Müller R, et al. (2007). Preparation and characterization of calibration standards for bone density determination by micro-computed tomography. Analyst 132:1040-1045. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Hu Y, Lertlam R, Yamakoshi Y, Hu JC. (2009). Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J Biol Chem 284:19110-19121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE. (1998). Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128-161. [DOI] [PubMed] [Google Scholar]
- Smith CE, Nanci A. (1989). A method for sampling the stages of amelogenesis on mandibular rat incisors using the molars as a reference for dissection. Anat Rec 225:257-266. [DOI] [PubMed] [Google Scholar]
- Smith CE, Nanci A. (1996). Protein dynamics of amelogenesis. Anat Rec 245:186-207. [DOI] [PubMed] [Google Scholar]
- Smith CE, Pompura JR, Borenstein S, Fazel A, Nanci A. (1989). Degradation and loss of matrix proteins from developing enamel. Anat Rec 224:292-316. [DOI] [PubMed] [Google Scholar]
- Smith CE, Wazen R, Hu Y, Zalzal SF, Nanci A, Simmer JP, et al. (2009). Consequences for enamel development and mineralization resulting from loss of function of ameloblastin or enamelin. Eur J Oral Sci 117:485-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Richardson AS, Hu Y, Bartlett JD, Hu JC, Simmer JP. (2011). Effect of kallikrein 4 loss on enamel mineralization: comparison with mice lacking matrix metalloproteinase 20. J Biol Chem 286:18149-18160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann SM, Weatherell JA, Hamm SM. (1967). Variations of enamel density in sections of human teeth. Arch Oral Biol 12:85-97. [DOI] [PubMed] [Google Scholar]
- Wong FS, Elliott JC, Davis GR, Anderson P. (2000). X-ray microradiotomographic study of mineral distribution in enamel of mandibular incisors. J Anat 196(Pt 3):405-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JT, Hart TC, Hart PS, Simmons D, Suggs C, Daley B, et al. (2009). Human and mouse enamel phenotypes resulting from mutation or altered expression of AMEL, ENAM, MMP20 and KLK4. Cells Tissues Organs 189:224-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Hunter N, Swain MV. (2011). Application of polychromatic microCT for mineral density determination. J Dent Res 90:18-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.