Abstract
The prevalence of morbidly obese individuals is rising rapidly. Being overweight predisposes patients to multiple serious medical comorbidities including type two diabetes (T2DM), hypertension, dyslipidemia, and obstructive sleep apnea. Lifestyle modifications including diet and exercise produce modest weight reduction and bariatric surgery is the only evidence-based intervention with sustainable results. Biliopancreatic diversion (BPD) produces the most significant weight loss with amelioration of many obesity-related comorbidities compared to other bariatric surgeries; however perioperative morbidity and mortality associated with this surgery are not insignificant; additionally long-term complications including undesirable gastrointestinal side effects and metabolic derangements cannot be ignored. The overall quality of evidence in the literature is low with a lack of randomized control trials, a preponderance of uncontrolled series, and small sample sizes in the studies available. Additionally, when assessing remission of comorbidities, definitions are unclear and variable. In this review we explore the pros and cons of BPD, a less well known and perhaps underutilized bariatric procedure.
1. Introduction
Global obesity, defined as a body mass index (BMI) greater than 30 kg/m2, is on the rise. Over the last thirty years mean BMI in individuals aging 20 years or older has increased at an escalating rate of 0.4 kg/m2 per decade [1]. This disease is a complex multisystem condition, associated with increased comorbidities including type two diabetes (T2DM), dyslipidemia, hypertension, obstructive sleep apnea, heart disease, stroke, asthma, bone and joint problems, cancer, and depression [2]. Cumulatively this has negative implications on health and longevity with the potential to reverse life-expectancy gains in high-income nations [3]. In fact, obesity is the fifth leading risk factor for mortality worldwide [4]. Lifestyle modifications, including diet and exercise, have been shown to be ineffective in treating obesity long term. Bariatric surgery has been shown to produce significant sustainable weight loss in obese patients. In addition, bariatric surgery has been effective not just for weight loss but also for improvement or remission of obesity-related comorbidities [5]. Of the various bariatric surgical procedure, biliopancreatic diversion (BPD) is considered the most effective in producing marked weight loss and comorbidity reduction [5]. However, concerns remain regarding the pros and cons with this surgical intervention in obese patients. This review will explore the literature supporting BPD and focus on the limitations of this technique.
2. Bariatric Surgery
The exponential rise in obesity has been matched by advances in surgical techniques of bariatric procedures [6, 7]. Traditionally, surgical procedures have been divided into restrictive, malabsorptive, or a combination of both. Restrictive procedures include vertical banded gastroplasty, laparoscopic adjustable gastric banding (LAGB), and laparoscopic sleeve gastrectomy (LSG). These techniques limit the size of the stomach thereby limiting caloric intake. Malabsorptive techniques divert biliopancreatic secretions, limiting the absorption of nutrients in the intestine. Jejunoileal bypass, popular in the 1960s and 1970s, is defined as a purely malabsorptive procedure. It has been abandoned due to significant morbidity and mortality [8, 9]. Biliopancreatic diversion, with (BPD/DS) or without (BPD) duodenal switch, and Roux-en-Y gastric bypass (RYGB) are defined as combination procedures, having both restrictive and malabsorptive features. Additionally, recent evidence suggests that gastrointestinal hormonal, inflammatory, central nervous system, and gut microbial factors contribute to overall benefits of these procedures [10–13].
3. Biliopancreatic Diversion
BPD was originally described by Scopinaro in 1979 as an alternative to jejunoileal bypass for severely obese patients [14]. The BPD procedure consists of: (1) partial distal gastrectomy in which the duodenal stump is closed (or bypass of the distal part of the stomach), (2) transection of the small bowel approximately halfway between the ligament of Treitz and the ileocecal valve, (3) Roux-en-Y gastroenterostomy from the gastric pouch to the distal bowel loop creating an alimentary limb, and (4) a biliopancreatic limb anastomosed with the alimentary limb 50 cm before the ileocecal valve forming a common channel [14]. The addition of the duodenal switch (DS), in which a vertical sleeve gastrectomy is combined with a duodenoenterostomy, was termed the “second generation BPD” [15]. DS involves preservation of the lesser curvature, antrum, pylorus, and first part of the duodenum along with lengthening of common channel lengths from 50 cm to 100 cm or more (Figure 1). These modifications were created to control for complications associated with Scopinaro's original description including marginal ulceration, vomiting, diarrhea, dumping syndrome, and micronutrient deficiencies. As such, Marceau et al. in a retrospective cohort study showed decreased necessity for revisional surgery secondary to aforementioned complications, specifically a 50% reduction in emesis and overall favourable micronutrient profiles [16]. Nonetheless, BPD/DS is one of the most complex and highest risk bariatric surgeries utilized today.
Figure 1.
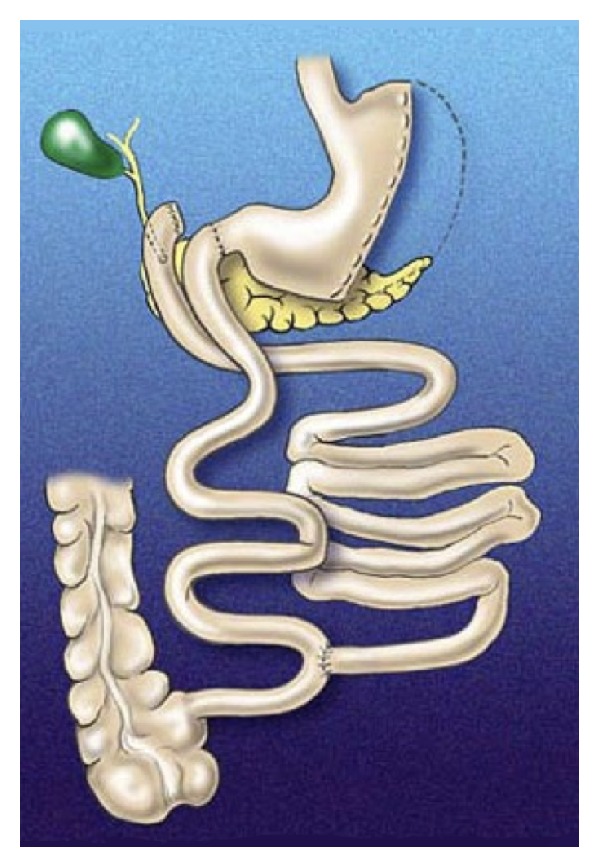
Illustration of the biliopancreatic diversion with duodenal switch procedure.
4. BPD/DS-Associated Weight Loss
BPD/DS has proven to be successful in achieving and maintaining significant weight loss in the superobese population (BMI >50 kg/m2). Buchwald's systematic review which included 48 studies (3 nonrandomized controlled trials, 6 comparative retrospective, 21 uncontrolled case series, 14 single-arm retrospective, and 3 observational studies) for a total of 1565 patients compared bariatric surgical procedures. This review suggests that BPD/DS is the most effective operation with a percentage of excess body weight loss (EBWL), defined as (preoperative BMI−current BMI)/(preoperative BMI−25) × 100, of 73% at 2 years follow-up, followed by gastric bypass (63%), gastroplasty (56%), and gastric banding (49%) [17]. Sovik et al. randomized 60 superobese patients (BMI 50–60 kg/m2) to undergo either RYGB or BPD/DS. Two years after surgery percentage of EBWL was found to be 31.2% following RYGB compared to 44.8% following BPD/DS. Additionally, health-related quality of life, as measured by the Short Form Health—36 survey, improved equally in both groups [18]. Similarly, Prachand et al. retrospectively analyzed 350 superobese patients who underwent either BPD/DS or RYGB. Preoperative BMI was significantly greater in the BPD/DS group compared to the RYGB group (58.8 kg/m2 versus 56.4 kg/m2, P = 0.0014). Percentage of EBWL was found to be significantly greater in the BPD/DS group compared to RYGB (12 months, 64.1% versus 55.9%; 18 months, 71.9% versus 62.8%; 24 months, 71.6% versus 60.1%; 36 months, 68.9% versus 54.9%) [19]. Contrarily, Deveney et al. compared weight loss after 1 and 2 years in super-obese patients who underwent RYGB or BPD/DS and reported percentage of EBWL to be similar between the 2 groups: 54% versus 53% at 1 year and 67% versus 64% at 2 years, with longer length of stay and higher rates of anastomotic leak in the BPD/DS group. It is important to note that those patients undergoing BPD/DS had higher BMI (59 kg/m2 versus 55 kg/m2, P < 0.05); therefore percentage of EBWL would be proportionally smaller when compared to smaller patients in the RYGB group. Percentage of EBWL being equal signifies greater overall weight loss in the BPD/DS group [20].
Biertho et al. in an uncontrolled series of 810 morbidly obese patients with mean initial BMI of 44.2 ± 3.6 kg/m2 showed a EBWL of 76% maintained at 8.6 years follow-up concluding that BPD/DS was appropriate for non-super-obese patients [21]. Concordantly, Anthone et al. in an uncontrolled series including 701 BPD/DS patients with preoperative BMIs ranging from 34 kg/m2 to 95 kg/m2, found a EBWL of 69% after 1 year, 73% after 3 years, and 66% after 5 or more years of follow-up [22]. Overall, several uncontrolled series investigating BPD/DS suggest similar results with EBWL ranging from 61% to 85% with moderate-term follow-up [23–29].
5. BPD/DS and Cardiometabolic Risk Factors
BPD and BPD/DS have a marked effect on obesity-related comorbidities, specifically T2DM (Table 1) [17, 21, 24, 25, 30–38]. Mingrone et al. randomized 60 morbidly obese patients with T2DM to receive medical therapy (lifestyle modifications and hypoglycemic agents) or surgical therapy (RYGB or BPD). They reported no remission of T2DM in the medical therapy group, compared to 75% in the RYGB group and 95% in the BPD group after 2 years of follow-up [30]. Supportively, both Iaconelli et al.'s uncontrolled series including 50 patients and Tsoli et al.'s nonrandomized trial including 24 patients showed resolution of T2DM in all BPD patients 12 months after surgery [31, 32]. A recent systematic review and metaanalysis confirmed that diabetes resolution was the greatest for patients undergoing BPD/DS, followed by RYGB, and least for banding procedures [17]. Bariatric surgery has now been recommended for management of T2DM for select obese patients by the International Diabetes Federation [39]. Astiarraga et al. recently assessed the effect of BPD/DS on T2DM in nonobese patients demonstrating marked amelioration (improved glycemia) of metabolic control and remission (HbA1C <6.5% and normal oral glucose tolerance test) in 1/3 of patients, suggesting a weight-independent effect of the operation, as only modest weight loss (−12 kg at 2 mo, −14 kg at 1 yr) was observed in this nonobese patient population [33].
Table 1.
Effect of biliopancreatic diversion with duodenal switch on diabetes.
| Study n (number of patients) |
DM incidence preoperatively | Resolution of DM | Measure of resolution |
|---|---|---|---|
|
Buchwald et al. [17] 31 studies of BPD n = 2502 |
30% | 95% | Clinical and laboratory manifestations of diabetes—not otherwise specified (different measures for individual studies) |
|
| |||
|
Biertho et al. [21] n = 810 |
28% | 93% | Discontinuation of diabetic treatment (oral hypoglycemic agents/insulin) |
|
| |||
|
Crea et al. [24] n = 540 |
Discontinuation of diabetic treatment (oral hypoglycemic agents/insulin) within 1 yr | ||
| A-BPD: 287 | A-35% | A-98% | |
| B-DS: 253 | B-36% | B-64% | |
|
| |||
|
Papadia et al. [25] n = 68 |
3% | 100% | Fasting glucose < 125 mg/dL |
|
| |||
|
Mingrone et al. [30] n = 60 |
Discontinuation of diabetic treatment (oral hypoglycemic agents/insulin), fasting glucose < 100 mg/dL, and HbA1C < 6.5% | ||
| A-medical: 20 | A-100% | A-0% | |
| B-RYGB: 20 | B-100% | B-75% | |
| C-BPD: 20 | C-100% | C-95% | |
|
| |||
|
Iaconelli et al. [31] n = 50 |
Not defined | ||
| A-medical: 28 | A-100% | A-45% | |
| B-BPD: 22 | B-100% | B-100% | |
|
| |||
|
Tsoli et al. [32] n = 24 |
Oral glucose tolerance test | ||
| A-BPD: 12 | A-100% | A-100% | |
| B-SG: 12 | B-100% | B-100% | |
|
| |||
|
Astiarraga et al. [33] n = 30 |
50% | 40% | HbA1C < 6.5% and normal oral glucose tolerance test |
|
| |||
|
Marceau et al. [34] n = 1423 |
28% | 92% | Discontinuation of oral hypoglycemic agents Discontinuation of insulin |
| 98% | |||
|
| |||
|
Vage et al. [35] n = 80 |
100% | 94% | Fasting glucose < 7 mmol/L and HbA1C < 6.5% |
|
| |||
|
Dorman et al. [36] n = 329 |
Self-reported | ||
| A-BPD/DS: 173 | A-36% | A-82% | |
| B-RYGB: 139 | B-31% | B-64% | |
|
| |||
|
Prachand et al. [37] n = 350 |
Discontinuation of the medications used for treatment with the absence of symptoms | ||
| A-BPD/DS: 198 | A-24% | 100% | |
| B-RYGB: 152 | B-36% | 60% | |
|
| |||
|
Pata et al. [38] n = 874 |
35% | 97% | Discontinuation of oral hypoglycemic agents Discontinuation of insulin |
| 67% | |||
DM: diabetes mellitus.
BPD: biliopancreatic diversion.
DS: duodenal switch.
RYGB: Roux-en-Y gastric bypass.
SG: sleeve gastrectomy.
Other cardiometabolic risk factors, including hypertension (Table 2) and dyslipidemia (Table 3), have also shown marked improvement following BPD/DS [21, 24, 25, 28, 29, 31, 32, 35, 36, 38, 40]. Additionally, obstructive sleep apnea was resolved in the majority of patients (Table 4) [21, 24, 29, 34, 38].
Table 2.
Effect of biliopancreatic diversion with duodenal switch on hypertension.
| Study n (number of patients) |
Hypertension incidence preoperatively | Resolution of hypertension | Measure of resolution |
|---|---|---|---|
| Biertho et al. [21] n = 810 |
37% | 60% | Discontinuation of antihypertensive medications |
|
| |||
| Crea et al. [24] n = 540 |
Not defined | ||
| A-BPD: 287 | A-55% | A-93% | |
| B-DS: 253 | B-52% | B-94% | |
|
| |||
| Papadia et al. [25] n = 68 |
49% | 82% | Discontinuation of antihypertensive medications and blood pressure < 140/85 mmHg |
|
| |||
|
Baltasar et al. [29] n = 125 |
8% | 90% | Not defined |
|
| |||
| Iaconelli et al. [31] n = 50 |
Blood pressure < 130/85 mmHg | ||
| A-medical: 28 | A-71% | A-25% | |
| B-BPD: 22 | B-64% | B-73% | |
|
| |||
| Vage et al. [35] n = 80 |
84% | 54% | Discontinuation of antihypertensive medications and blood pressure < 140/90 mmHg |
|
| |||
| Dorman et al. [36] n = 329 |
Self-reported | ||
| A-BPD/DS: 190 | A-58% | A-67% | |
| B-RYGB: 139 | B-57% | B-39% | |
|
| |||
| Prachand et al. [37] n = 350 |
Discontinuation of the medications used for treatment with the absence of symptoms | ||
| A-BPD/DS: 198 | A-67% | A-68% | |
| B-RYGB: 152 | B-37% | B-39% | |
|
| |||
| Pata et al. [38] n = 874 |
57% | 95% | Discontinuation of antihypertensive medications and blood pressure < 140/90 mmHg |
BPD: biliopancreatic diversion.
DS: duodenal switch.
RYGB: Roux-en-Y gastric bypass.
Table 3.
Effect of biliopancreatic diversion with duodenal switch on dyslipidemia.
| Study n (number of patients) |
Dislipidemia incidence preoperatively | Resolution of dyslipidemia | Measure of resolution |
|---|---|---|---|
| Crea et al. [24] | A-Hypercholesterolemia 87% | A-Hypercholesterolemia 98% | Hypercholesterolemia/hypertriglyceridemia—Laboratory values within the normal range (not defined) |
| n = 540 | A-Hypertriglyceridemia 53% | A-Hypertriglyceridemia 97% | |
| A-BPD: 287 | B-Hypercholesterolemia 85% | B-Hypercholesterolemia 99% | |
| B-DS: 253 | B-Hypertriglyceridemia 55% | B-Hypertriglyceridemia 99% | |
|
| |||
| Papadia et al. [25] n = 68 |
16% | 100% | Serum cholesterol < 200 mg/dL |
|
| |||
| Vage et al. [35] n = 80 |
48% (on treatment) | 92% | LDL Hyperlipidemia—discontinuation of hypolipidemic agents with LDL < 2.6 mmol/L |
|
| |||
| Dorman et al. [36] n = 329 |
Hyperlipidemia—not defined | ||
| A-BPD/DS: 190 | A-54% | A-81% | |
| B-RYGB 139 | B-44% | B-55% | |
|
| |||
| Prachand et al. [37] n = 350 |
Dyslipidemia—discontinuation of the medications used for treatment with the absence of symptoms | ||
| A-BPD/DS: 198 | A-31% | A-72% | |
| B-RYGB: 152 | B-36% | B-26% | |
|
| |||
| Pata et al. [38] n = 874 |
Hypercholesterolemia 87% Hypertriglyceridemia 53% |
Hypercholesterolemia 98% Hypertriglyceridemia 96% |
Laboratory values within the normal range (cholesterol 120–200 mg/dL, triglycerides < 150 mg/dL) |
BPD: biliopancreatic diversion.
DS: duodenal switch.
Medical: medical management of weight loss/comorbidities.
Tcholesterol: total cholesterol.
TG: triglycerides.
HDL: high-density lipoprotein.
LDL: low-density lipoprotein.
RYGB: Roux-en-Y gastric bypass.
Table 4.
Effect of biliopancreatic diversion with duodenal switch on obstructive sleep apnea.
| Study n (number of patients) |
Obstructive sleep apnea incidence preoperatively | Resolution of obstructive sleep apnea | Measure of resolution |
|---|---|---|---|
| Biertho et al. [21] n = 810 |
25% | 98% | Discontinuation of breathing apparatus |
|
| |||
| Crea et al. [24] n = 540 |
Discontinuation of CPAP apparatus | ||
| A-BPD: 287 | A-7% | A-100% | |
| B-DS: 253 | B-6% | B-100% | |
|
| |||
| Baltasar et al. [29] n = 125 |
6% | 100% | Not defined |
|
| |||
| Marceau et al. [34] n = 1423 |
40% | 90% | Discontinuation of CPAP apparatus |
|
| |||
| Pata et al. [38] n = 874 |
9% | 100% | Discontinuation of breathing apparatus |
BPD: biliopancreatic diversion.
DS: duodenal switch.
CPAP: continuous positive airway pressure.
6. BPD/DS Related Complications
A recent paper by Buchwald and Oien, which surveyed International Federation for the Surgery of Obesity and Metabolic Disorders member nations with an 84% response rate, revealed that the proportion of BPD/DS procedures in relation to all bariatric surgeries declined from 6.1% to 4.9% to 2.1% in 2003, 2008, and 2011, respectively. Trends vary geographically. Overall, more BPD/DS procedures were executed in 2011 compared to 2008 and 2003; however other bariatric surgeries were performed preferentially [7]. This raises questions about why the procedure with the greatest-weight loss, evidence of lasting effect, and reversal of obesity-related comorbidities is the least performed bariatric surgery worldwide. The answer is likely multifactorial and complex. Firstly, the technical complexity of this procedure is a consideration, with the operation being time consuming and requiring a skilled surgeon. A laparoscopic approach, introduced by Gagner in 1999, sought the benefits of BPD/DS weight loss and reduced morbidity associated with laparoscopic surgery [41, 42]. In some studies this has proven to be true, with lower postoperative complication rates [43, 44]; however others show no difference when compared to an open procedure [45]. Likely, learning curve and operative volume may be important considerations, with a majority of BPD/DS being performed at focused speciality centers [46, 47].
6.1. Perioperative Mortality and Morbidity
In a recent meta-analysis of 361 studies including 85 048 patients overall mortality within 30 days of bariatric surgery was found to be 0.28%. BPD/DS had the highest early mortality with a rate of 0.29% to 1.23% for open and 0.0% to 2.7% for laparoscopic procedures [44]. Postoperative mortality is most commonly associated with pulmonary embolism, respiratory failure, and anastomotic leaks. It is important to acknowledge that BPD/DS is the procedure of choice for the most extremely obese patients; therefore it can be assumed that surgical risk in this group is higher at baseline. This was demonstrated in the Longitudinal Assessment of Bariatric Surgery Consortium, a prospective multicenter observational study, which included 4776 patients analyzing 30-day outcomes after bariatric surgery. It showed that extreme values of BMI were significantly associated with increased risk of major adverse outcomes (death; venous thromboembolism; percutaneous, endoscopic, or operative reintervention; and length of stay greater than 30 days) [48].
One-year complication rates as reported in the US Bariatric Outcomes Longitudinal Database are 4.6%, 10.8%, 14.9%, and 25.7%, respectively, after LAGB, LSG, RYGB, and BPD/DS [49]. This includes minor complications such as gastrointestinal side effects including flatulence, malodorous stools, and steatorrhea. Of major complications, gastrointestinal anastomotic leak is the most common, serious, early surgical complication. Hamoui et al. reviewed 701 BPD/DS cases performed over a ten-year period and reported that 5% of patients developed complications necessitating revisional surgery. Protein malnutrition was the most common indication for reoperation. A postoperative complication rate of 15% was then seen in their revisional surgery group, with wound infections being the most common complication in this group [50]. Biertho et al. analyzed a series of 1000 BPD/DS patients, in which major complications occurred in 7% of patients. They showed no difference in complication rate when comparing laparoscopic to open BPD/DS. Rehospitalization was required in 12.7% of patients and reoperations occurred in 6% of patients [43].
In a randomized trial of 60 patients, Sovik et al. compared mean operating time, median length of stay, and complication rates between RYGB and BPD/DS. On average RYGB required 91 min compared to 206 min for BPD/DS. Median length of stay was 2 days post-RYGB and 4 days post-BPD/DS. Perioperative complication rates were comparable between groups; however this study was likely underpowered and larger studies are necessary to truly draw conclusions [51]. It can not be understated that a greater volume of less complex bariatric procedures could be performed in the time that it takes to complete this operation. With the overwhelming burden of obesity and need for bariatric surgical procedures mindful resource allocation is crucial. A staged procedure with BPD/DS following LSG for select patients may allow for better utilization of resources in those patients who would benefit most from this complex surgery.
6.2. Metabolic Related Complications
BPD/DS is the bariatric procedure associated with some of the greatest perioperative malnutrition/metabolic related complications. All patients begin supplementation postoperatively; however there is no standardized approach to replacement and sometimes deficiencies are refractory to dietary supplements. Following BPD/DS patients can consume normal nutritional meals and continue to be malnourished [52]. Iron-deficiency anemia, protein calorie malnutrition, hypocalcemia, and deficiency of fat soluble vitamins, vitamin B1, vitamin B12, and folate are common [53]. BPD/DS has proven to be more malabsorptive compared to other bariatric surgeries; thus close follow-up is essential. As an example, the regimen implemented by Marceau et al. in their uncontrolled case series with fifteen-year follow-up was as follows: iron 300 mg, calcium 500 mg, vitamin D 50 000 IU, vitamin A 20 000 IU, 1 multivitamin tablet, and yogurt probiotics. Adjustments were made as appropriate; consequently severe anemia and vitamin deficiencies were uncommon [34]. Supplementation is of paramount importance; unfortunately in this patient population compliance is lacking [54].
Aasheim et al. randomized 60 super-obese patients to receive either RYGB or BPD/DS comparing 25-hydroxyvitamin D, vitamin A, and vitamin B1 up to 1 year postoperatively. BPD/DS patients had lower mean 25-hydroxyvitamin D and vitamin A concentrations, as well as a steeper decline in vitamin B1 compared to RYGB [55]. Decreased vitamin D and calcium levels with associated secondary hyperparathyroidism have been demonstrated [56–58]. Marceau et al., after prospectively analyzing 33 patients utilizing iliac crest bone biopsy, bone mineral density, and biochemical investigations, proclaimed that despite serum abnormalities in vitamin D, calcium, and PTH overall bone mineral density and fracture risk were unchanged 10 years after BPD/DS [54]. A population-based retrospective cohort study out of the United Kingdom confirmed these results, concluding that bariatric surgery (60% gastric band, 29% RYGB, 11% other—BPD/DS was not separated) did not significantly effect fracture risk with a mean follow-up of 2.2 years. Additionally, facture risk was independent of the specific surgical technique. Longer-term studies are necessary to ensure that results are enduring [59]. Clinically there have been case reports of BPD/DS-related vitamin A deficiency and associated night-blindness [60, 61], post-BPD/DS peripheral neuropathies associated with B12 deficiencies [61], and Wernicke's encephalopathy as a result of B1 deficiencies [62, 63].
7. Conclusion
Current evidence suggests that BPD/DS produces the greatest weight loss in obese individuals with the most significant improvement in obesity-related comorbidities. However, the utilization of this bariatric surgical procedure is limited compared to other surgical options. The technical complexity of BPD/DS and lack of knowledge may only partly explain the decreased utilization by surgeons. Concern regarding severe metabolic disturbances and malnutrition may also be implicated. Currently, BPD/DS remains a reasonable surgical option for severely obese patients, especially those with a BMI > 50 kg/m2 when performed by expert surgeons in high volume speciality centers with close follow-up. Additionally, BPD/DS should be considered as a staged procedure following LSG for select patients who would benefit most from this complex surgery. There continues to be a need to educate surgeons regarding this viable bariatric surgical procedure to foster accurate knowledge regarding BPD/DS as the potentially positive metabolic effects outweigh the known risks as supported by evidence available in the body of literature.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. The Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. The Journal of the American Medical Association. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. The New England Journal of Medicine. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization; 2009. [Google Scholar]
- 5.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: systematic review and meta-analysis. The Journal of the American Medical Association. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 6.Pallati P, Buettner S, Simorov A, Meyer A, Shaligram A, Oleynikov D. Trends in adolescent bariatric surgery evaluated by UHC database collection. Surgical Endoscopy. 2012;26(11):3077–3081. doi: 10.1007/s00464-012-2318-0. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obesity Surgery. 2013;23(4):427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 8.Halverson JD, Wise L, Wazna MF, Ballinger WF. Jejunoileal bypass for morbid obesity. A critical appraisal. The American Journal of Medicine. 1978;64(3):461–475. doi: 10.1016/0002-9343(78)90233-4. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Buchwald JN. Evolution of operative procedures for the management of morbid obesity 1950–2000. Obesity Surgery. 2000;12(5):705–717. doi: 10.1381/096089202321019747. [DOI] [PubMed] [Google Scholar]
- 10.Korner J, Leibel RL. To eat or not to eat—how the gut talks to the brain. The New England Journal of Medicine. 2003;349(10):926–928. doi: 10.1056/NEJMp038114. [DOI] [PubMed] [Google Scholar]
- 11.Huda MSB, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obesity Research. 2006;7(2):163–182. doi: 10.1111/j.1467-789X.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson B, Switzer NJ, Almamar A, Shi X, Birch DW, Karmali S. The impact of laparoscopic sleeve gastrectomy on plasma ghrelin levels: a systematic review. Obesity Surgery. 2013;23(9):1476–1480. doi: 10.1007/s11695-013-0999-7. [DOI] [PubMed] [Google Scholar]
- 14.Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Biliopancreatic by-pass for obesity. II. Initial experience in man. British Journal of Surgery. 1979;66(9):618–620. doi: 10.1002/bjs.1800660906. [DOI] [PubMed] [Google Scholar]
- 15.van Hee RH. Biliopancreatic diversion in the surgical treatment of morbid obesity. World Journal of Surgery. 2004;28(5):435–444. doi: 10.1007/s00268-004-7364-x. [DOI] [PubMed] [Google Scholar]
- 16.Marceau P, Biron S, Hould F-S, et al. Duodenal switch improved standard biliopancreatic diversion: a retrospective study. Surgery for Obesity and Related Diseases. 2009;5(1):43–47. doi: 10.1016/j.soard.2008.03.244. [DOI] [PubMed] [Google Scholar]
- 17.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. The American Journal of Medicine. 2009;122(3):248–256. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 18.Sovik TT, Aasheim ET, Taha O, et al. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch. Annals of Internal Medicine. 2011;155(5):281–291. doi: 10.7326/0003-4819-155-5-201109060-00005. [DOI] [PubMed] [Google Scholar]
- 19.Prachand VN, DaVee RT, Alverdy JC. Duodenal switch provides superior weight loss in the super-obese (BMI ≥ 50 kg/m2) compared with gastric bypass. Annals of Surgery. 2006;244(4):611–619. doi: 10.1097/01.sla.0000239086.30518.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deveney CW, MacCabee D, Marlink K, Welker K, Davis J, McConnell DB. Roux-en-Y divided gastric bypass results in the same weight loss as duodenal switch for morbid obesity. The American Journal of Surgery. 2004;187(5):655–659. doi: 10.1016/j.amjsurg.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Biertho L, Biron S, Hould F-S, Lebel S, Marceau S, Marceau P. Is biliopancreatic diversion with duodenal switch indicated for patients with body mass index <50 kg/m2? Surgery for Obesity and Related Diseases. 2010;6(5):508–514. doi: 10.1016/j.soard.2010.03.285. [DOI] [PubMed] [Google Scholar]
- 22.Anthone GJ, Lord RV, DeMeester TR, Crookes PF. The duodenal switch operation for the treatment of morbid obesity. Annals of Surgery. 2003;238(4):618–627. doi: 10.1097/01.sla.0000090941.61296.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topart P, Becouarn G, Salle A. Five-year follow-up after biliopancreatic diversion with duodenal switch. Surgery for Obesity and Related Diseases. 2011;7(2):199–205. doi: 10.1016/j.soard.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Crea N, Pata G, Di Betta E, et al. Long-term results of biliopancreatic diversion with or without gastric preservation for morbid obesity. Obesity Surgery. 2011;21(2):139–145. doi: 10.1007/s11695-010-0333-6. [DOI] [PubMed] [Google Scholar]
- 25.Papadia FS, Adami GF, Marinari GM, Camerini G, Scopinaro N. Bariatric surgery in adolescents: a long-term follow-up study. Surgery for Obesity and Related Diseases. 2007;3(4):465–468. doi: 10.1016/j.soard.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Archives of Surgery. 147(9):847–854. doi: 10.1001/archsurg.2012.1654. [DOI] [PubMed] [Google Scholar]
- 27.Strain GW, Gagner M, Inabnet WB, Dakin G, Pomp A. Comparison of effects of gastric bypass and biliopancreatic diversion with duodenal switch on weight loss and body composition 1-2 years after surgery. Surgery for Obesity and Related Diseases. 2007;3(1):31–36. doi: 10.1016/j.soard.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Nelson D, Beekley A, Carter P, Kjorstad R, Sebesta J, Martin M. Early results after introduction of biliopancreatic diversion/duodenal switch at a military bariatric center. The American Journal of Surgery. 2011;201(5):678–684. doi: 10.1016/j.amjsurg.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Baltasar A, Bou R, Bengochea M, et al. Duodenal switch: an effective therapy for morbid obesity—intermediate results. Obesity Surgery. 2001;11(1):54–58. doi: 10.1381/096089201321454114. [DOI] [PubMed] [Google Scholar]
- 30.Mingrone G, Panunzi S, de Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. The New England Journal of Medicine. 2012;366(17):1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 31.Iaconelli A, Panunzi S, de Gaetano A, et al. Effects of bilio-pancreatic diversion on diabetic complications: a 10-year follow-up. Diabetes Care. 2011;34(3):561–567. doi: 10.2337/dc10-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsoli M, Chronaiou A, Kehagias I, Kalfarentzos F, Alexandrides TK. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study. Surgery for Obesity and Related Diseases. 2013;9(5):667–677. doi: 10.1016/j.soard.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Astiarraga B, Gastaldelli A, Muscelli E, et al. Biliopancreatic diversion in nonobese patientients with type 2 diabetes: impact and mechanisms. The Journal of Clinical Endocrinology & Metabolism. 2013;98(7):2765–2773. doi: 10.1210/jc.2013-1476. [DOI] [PubMed] [Google Scholar]
- 34.Marceau P, Biron S, Hould F-S, et al. Duodenal switch: long-term results. Obesity Surgery. 2007;17(11):1421–1430. doi: 10.1007/s11695-008-9435-9. [DOI] [PubMed] [Google Scholar]
- 35.Vage V, Nilsen RM, Berstad A, et al. Predictors for remission of major components of the metabolic syndrome after biliopancreatic diversion with duodenal switch (BPDDS) Obesity Surgery. 2013;23(1):80–86. doi: 10.1007/s11695-012-0775-0. [DOI] [PubMed] [Google Scholar]
- 36.Dorman RB, Rasmus NF, al-Haddad BJ, et al. Benefits and complications of the duodenal switch/biliopancreatic diversion compared to the Roux-en-Y gastric bypass. Surgery. 2012;152(4):758–765. doi: 10.1016/j.surg.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Prachand VN, Ward M, Alverdy JC. Duodenal switch provides superior resolution of metabolic comorbidities independent of weight Loss in the super-obese (BMI ≥ 50 kg/m2) compared with gastric bypass. Journal of Gastrointestinal Surgery. 2012;14(2):211–220. doi: 10.1007/s11605-009-1101-6. [DOI] [PubMed] [Google Scholar]
- 38.Pata G, Crea N, Di Betta E, Bruni O, Vassallo C, Mittempergher F. Biliopancreatic diversion with transient gastroplasty and duodenal switch: long-term results of a multicentric study. Surgery. 2013;153(3):413–422. doi: 10.1016/j.surg.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 39.Dixon JB, Zimmet P, Alberti KG, Rubino F. Bariatric surgery: an IDF statement for obese type 2 diabetes. Journal of Diabetes. 2011;55(6):367–382. doi: 10.1590/s0004-27302011000600003. [DOI] [PubMed] [Google Scholar]
- 40.Benetti A, Del Puppo M, Crosignani A, et al. Cholesterol metabolism after bariatric surgery in grade 3 obesity: differences between malbsorptive and restrictive procedures. Diabetes Care. 2013;36(6):1443–1447. doi: 10.2337/dc12-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obesity Surgery. 2000;10(6):514–523. doi: 10.1381/096089200321593715. [DOI] [PubMed] [Google Scholar]
- 42.de Csepel J, Burpee S, Jossart G, et al. Laparoscopic biliopancreatic diversion with a duodenal switch for morbid obesity: a feasibility study in pigs. Journal of Laparoendoscopic and Advanced Surgical Techniques A. 2001;11(2):79–83. doi: 10.1089/109264201750162293. [DOI] [PubMed] [Google Scholar]
- 43.Biertho L, Lebel S, Marceau S, et al. Perioperative complications in a consecutive series of 1000 duodenal switches. Surgery for Obesity and Related Diseases. 2013;9(1):63–68. doi: 10.1016/j.soard.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142(4):621–635. doi: 10.1016/j.surg.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Kim WW, Gagner M, Kini S, et al. Laparoscopic versus open biliopancreatic diversion with duodenal switch: a comparative study. Journal of Gastrointestinal Surgery. 2003;7(4):552–557. doi: 10.1016/S1091-255X(02)00149-X. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen NT, Paya M, Stevens CM, Mavandadi S, Zainabadi K, Wilson SE. The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Annals of Surgery. 2004;240(4):586–594. doi: 10.1097/01.sla.0000140752.74893.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ballantyne GH, Belsley S, Stephens D, et al. Bariatric surgery: low mortality at a high-volume center. Obesity Surgery. 2008;18(6):660–667. doi: 10.1007/s11695-007-9357-y. [DOI] [PubMed] [Google Scholar]
- 48.Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. The New England Journal of Medicine. 2009;361(5):445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demaria EJ, Winegar DA, Pate VW, Hutcher NE, Ponce J, Pories WJ. Postoperative outcomes of metabolic surgery to treat diabetes from sites participating in the ASMBS bariatric surgery center of excellence program as reported in the bariatric outcomes longitudinal database. Annals of Surgery. 2010;252(3):559–566. doi: 10.1097/SLA.0b013e3181f2aed0. [DOI] [PubMed] [Google Scholar]
- 50.Hamoui N, Chock B, Anthone GJ, Crookes PF. Revision of the duodenal switch: indications, technique, and outcomes. Journal of the American College of Surgeons. 2007;204(4):603–608. doi: 10.1016/j.jamcollsurg.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Sovik TT, Taha O, Aasheim ET, et al. Randomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesity. British Journal of Surgery. 2010;97(2):160–166. doi: 10.1002/bjs.6802. [DOI] [PubMed] [Google Scholar]
- 52.Faintuch J, Matsuda M, Cruz ME, et al. Severe protein-calorie malnutrition after bariatric procedures. Obesity Surgery. 2004;14(2):175–181. doi: 10.1381/096089204322857528. [DOI] [PubMed] [Google Scholar]
- 53.Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. ASMBS allied health nutritional guidelines for the surgical weight loss patient. Surgery for Obesity and Related Diseases. 2008;4(5, supplement):S73–S108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Marceau P, Biron S, Lebel S, et al. Does bone change after biliopancreatic diversion? Journal of Gastrointestinal Surgery. 2002;6(5):690–698. doi: 10.1016/s1091-255x(01)00086-5. [DOI] [PubMed] [Google Scholar]
- 55.Aasheim ET, Bjorkman S, Sovik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. The American Journal of Clinical Nutrition. 2009;90(1):15–22. doi: 10.3945/ajcn.2009.27583. [DOI] [PubMed] [Google Scholar]
- 56.Sinha N, Shieh A, Stein EM, et al. Increased PTH and 1.25(OH)2D levels associated with increased markers of bone turnover following bariatric surgery. Obesity. 2011;19(12):2388–2393. doi: 10.1038/oby.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. Journal of Gastrointestinal Surgery. 2004;8(1):48–55. doi: 10.1016/j.gassur.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 58.Balsa JA, Botella-Carretero JI, Peromingo R, et al. Chronic increase of bone turnover markers after biliopancreatic diversion is related to secondary hyperparathyroidism and weight loss. Relation with bone mineral density. Obesity Surgery. 2010;20(4):468–473. doi: 10.1007/s11695-009-0028-z. [DOI] [PubMed] [Google Scholar]
- 59.Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fractures after bariatric surgery in the United Kingdom: population based, retrospective cohort study. British Medical Journal. 2012;345 doi: 10.1136/bmj.e5085.e5085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aasheim ET, Sovik TT, Bakke EF. Night blindness after duodenal switch. Surgery for Obesity and Related Diseases. 2008;4(5):685–686. doi: 10.1016/j.soard.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Stroh C, Weiher C, Hohmann U, Meyer F, Lippert H, Manger T. Vitamin A deficiency (VAD) after a duodenal switch procedure: a case report. Obesity Surgery. 2010;20(3):397–400. doi: 10.1007/s11695-009-9913-8. [DOI] [PubMed] [Google Scholar]
- 62.Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Annals of Surgery. 2008;248(5):714–720. doi: 10.1097/SLA.0b013e3181884308. [DOI] [PubMed] [Google Scholar]
- 63.Primavera A, Brusa G, Novello P, et al. Wernicke-Korsakoff encephalopathy following biliopancreatic diversion. Obesity Surgery. 1993;3(2):175–177. doi: 10.1381/096089293765559548. [DOI] [PubMed] [Google Scholar]


